Abstract
Background and aim
The alcoholic hepatitis histologic score has been proposed as a new prognostic tool to assess the risk of death in alcoholic hepatitis. We aimed to evaluate its prognostic value in patients with severe alcoholic hepatitis.
Methods
Liver biopsies were analysed independently by two pathologists according to the alcoholic hepatitis histologic score. The Laennec staging system was also used to evaluate fibrosis.
Results
One hundred and seven patients were included, and 89% of the patients received corticosteroids. The alcoholic hepatitis histologic score was available in 105 patients. Histologic scoring showed mild, moderate and severe scores in 10, 29 and 66 patients, respectively. Laennec staging was available for 53 patients, among whom 49 had cirrhosis, including 7 with Laennec 4A, 15 with 4B and 27 with 4C. Survival rates in mild, moderate and severe alcoholic hepatitis histologic score groups were 90%, 72% and 69% at 28 days (p = 0.6), 80%, 52% and 63% at 3 months (p = 0.3), and 70%, 41% and 58% at 6 months (p = 0.3), respectively. Within the alcoholic hepatitis histologic score, fibrosis demonstrated the best interobserver reproducibility (agreement = 100%, Κ = 1.00). Compared to patients with Laennec 4B or 4C cirrhosis, survival rates for patients without cirrhosis or with Laennec 4A cirrhosis were 100% vs 83% at 28 days (p = 0.16), 91% vs 68% at 3 months (p = 0.13), and 82% vs 64% at 6 months (p = 0.2), respectively. In multivariate analysis adjusted for age and for model for end-stage liver disease score, the alcoholic hepatitis histologic score and Laennec stage were not associated with 6-month mortality.
Conclusions
The alcoholic hepatitis histologic score is not predictive of short-term survival in this cohort of patients with severe alcoholic hepatitis.
Keywords: Alcoholic hepatitis, histology, alcoholic hepatitis histologic score, Laennec staging system
Key summary
The alcoholic hepatitis histologic score (AHHS) has been proposed as a prognostic tool to assess the risk of death in alcoholic hepatitis. Validation of this score in independent cohort studies is required.
In this study, AHHS did not correlate with 28-day, 3-month and 6-month survival.
The Laennec staging system did not correlate with 6-month survival although there was a trend toward increased survival among patients without cirrhosis and with Laennec 4A cirrhosis compared to those with Laennec 4B and 4C disease.
Further studies should evaluate whether the prognostic value of histologic parameters differs according to the severity of the underlying alcoholic liver disease.
Introduction
Alcoholic hepatitis (AH) is a clinical syndrome corresponding to clinical, biological and histological criteria. It is characterised by recent onset of jaundice, with or without other signs of liver decompensation (i.e. ascites and/or encephalopathy), in patients with chronic alcohol abuse.1 Histologically, the presence of steatosis, hepatocyte ballooning and an inflammatory infiltrate with polymorphonuclear neutrophils are the criteria required for the diagnosis of AH.1 Severe AH is defined by a modified Maddrey’s discriminant function (mDF) of 32 or higher2 and is the form of alcoholic liver disease that carries the poorest prognosis. Mortality rates as high as 50% have been reported at 3 months without treatment.3,4 As an accurate prediction of the risk of death is required for patient care, several scores have been developed to predict outcomes. In patients treated with corticosteroids, the Lille score or the combination of the Lille and the model for end-stage liver disease (MELD) scores are widely used for helping physicians to decide whether or not corticosteroids should be maintained after 1 week of treatment according to the probability of improvement in survival if corticosteroids are continued.5,6 However, the incidence of infections is increased during corticosteroid treatment, even if administered for only a short period of time, with a negative impact on patient prognosis.7 Thus, alternative predictive tools that are available at baseline are warranted.
Although histologic confirmation of AH is not mandatory in cases with typical presentation,8 the European Association for the Study of the Liver (EASL) guidelines recommend the use of histologic analysis, as exclusive reliance on clinical criteria is associated with a significant risk of misclassification.1 Recently, a new approach that focuses on histologic parameters has been proposed for assessing outcomes in AH patients. The alcoholic hepatitis histologic score (AHHS) is a combination of four histologic features (the presence of bridging fibrosis or cirrhosis, hepatocellular or canalicular/ductular bilirubinostasis, severe polymorphonuclear infiltration and megamitochondria) that allows patient classification into three groups with different risks of death within 90 days (3%, 19% and 51% in patients with favorable, intermediate and poor scores, respectively).9 In addition to this histologic score, developed for patients with AH, the Laennec staging system has shown predictive value for patients with cirrhosis.10,11 However, this score has never been evaluated in the setting of AH.
In the present study, we aimed to assess the prognostic value of the AHHS and of the Laennec staging system for predicting survival at 28 days, 3 months and 6 months in two cohorts of consecutive patients with AH seen in two tertiary centres.
Materials and methods
Patients
The study population was comprised of two cohorts. First, all patients consecutively diagnosed with histologic AH at the Lausanne University Hospital, Lausanne, Switzerland between 2010–2017 were identified using a local prospective database of AH patients and by reviewing the pathology reports from liver biopsies. To increase sample size, another prospective cohort of patients diagnosed with AH at CUB Hôpital Erasme, Brussels, Belgium was also included. These patients were included in the derivation cohort of a study aimed to establish a prognostic score combining pre-treatment clinical variables and a molecular signature in patients with AH.12
Transjugular biopsy specimens of the liver were obtained from all patients (before corticosteroid therapy in those who were treated) and archived as formalin-fixed, paraffin-embedded (FFPE) tissue blocks. To be included in the present study, patients had histologically confirmed AH and an mDF ≥32.2 Histologic criteria included the presence of steatosis, ballooned hepatocytes and neutrophilic infiltration.1 Patients with viral or autoimmune hepatitis, haemochromatosis or co-infection with hepatitis A, B, C or E virus were excluded. Patients were treated according to current guidelines. Corticosteroids were given orally (40 mg prednisone for a maximum of 28 days) in the absence of contraindication. Treatment was discontinued after 7 days in cases of non-response according to the Lille model.6
Written informed consent was obtained from all individuals. The Ethics Committee of the Lausanne University Hospital, Vaud canton, and the Ethics Committee at CUB Hôpital Erasme approved the study.
Data collection
At inclusion, data collected included demographic data (gender, age, height, weight), clinical data (alcohol consumption and tobacco use, presence of ascites and encephalopathy), past medical history (presence of oesophageal varices, past alcohol use, presence of diabetes) and biological data (bilirubin, albumin, creatinine levels, prothrombin time or international normalised ratio (INR), platelet count).
During follow-up, patients were followed as outpatients every 6 months, or more frequently if required. Data collected included clinical data (current alcohol use) and data related to the development of complications of cirrhosis, or to the occurrence of liver transplantation or death and causes of death.
Histopathological evaluation
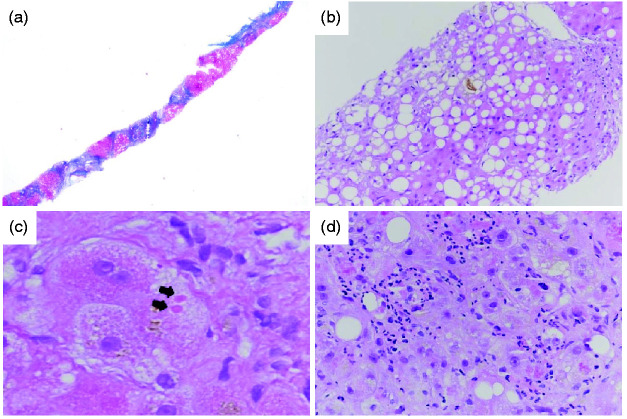
Liver biopsies were fixed in formalin and embedded in paraffin. Then, 3 μm slides were stained with haematoxylin and eosin (H&E) and Masson’s trichrome (Lausanne) or Sirius red (Brussels). Histological parameters were independently analysed by two liver pathologists. Discrepancies were resolved by co-analysis at a multi-head microscope. All patients were classified according to the AHHS score. The presence of bridging fibrosis or cirrhosis, type of bilirubinostasis, presence of severe polymorphonuclear infiltration and presence of megamitochondria (defined as small round eosinophilic structures in the cytoplasm of hepatocytes) were assessed to classify patients into mild (0–3 points), moderate (4–5 points) or severe (6–9 points) AHHS, as described by Altamirano et al.9 (Figure 1). Patients with cirrhosis were also classified according to the Laennec staging system.10 This classification is based on the thickness of the fibrous septa and the size of the nodules: A, mostly thin septa and large nodules; B, at least two broad septa and <50% of minute nodules; C, at least one very broad septum and >50% of minute nodules.
Figure 1.
Histological parameters of the alcoholic hepatitis histologic score (AHHS) and the Laennec staging system.
(a) Cirrhosis (Laennec 4C), Masson’s trichrome, original magnification X25. (b) Hepatocellular and canalicular bilirubinostasis, haematoxylin and eosin, original magnification X100. (c) Megamitochondria (arrows), haematoxylin and eosin, original magnification X400. (d) Severe polymorphonuclear infiltration, haematoxylin and eosin, original magnification X200.
Endpoints
The primary endpoint was overall survival at 6 months. Secondary endpoints were survival at 28 days and at 3 months.
Statistical analysis
Inter-group differences for continuous and categorical variables were tested using the Wilcoxon rank-sum test and Fisher’s exact test, respectively. Follow-up time was defined as the period from the first day of initiation of therapy or the day of liver biopsy to 6 months after inclusion. Data for patients who had not died, including those lost to follow-up, were censored at the date of the last follow-up visit. Patients who underwent liver transplantation were censored at the time of transplant. Histopathological interobserver agreement was determined using the kappa (K) coefficient. Area under receiver operating characteristic (AUROC) curve analyses were used to compare the prognostic value of the AHHS to the other prognostic scores. Univariable and multivariable Cox regression proportional hazard models were used to identify factors associated with death. Hazard ratios (HRs) are reported with their 95% confidence intervals (CIs). To avoid bias related to the effect of collinearity, when Child-Pugh or MELD scores were included in multivariable analysis, their constituent variables were not considered. A two-tailed p < 0.05 was considered statistically significant. All data analyses were performed using NCSS 2016 software (NCSS, Kaysville, Utah, USA).
Results
Patients
Sixty-five patients were identified at the Lausanne University Hospital. Ten patients were excluded for the following reasons: four had mDF <32, one had a missing mDF, four had detectable hepatitis C virus RNA, and one had acute hepatitis A virus infection. Thus, 55 consecutive patients were included. Fifty-two patients were included at CUB Hôpital Erasme. All patients had mDF ≥32, none had hepatitis virus infection. Thus, the whole study population was made up of 107 patients. All patients were chronic alcohol consumers (>60 g/day for males and >40 g/day for females), had recent (<90 days) onset of jaundice (bilirubin level >50 µmol/l), and less than 60 days of abstinence before the onset of jaundice.
Demographics, clinical, and biological data for included patients are summarised in Table 1. Results are provided for patients included at Lausanne, for those included at Brussels, and for the whole study population. Overall, the median age was 54 years (95% CI: 52–56) and 62% of patients were male. The mean mDF at the time of liver biopsy was 62 (95% CI: 56–67). Histologic scoring according to the AHHS was available for 53 patients included at Lausanne and for all patients included at Brussels (Table 2). The median AHHS was 7 (95% CI: 6–7). Ten patients (9%) had AHHS mild disease, 29 patients (28%) had a moderate AHHS, and 66 patients (63%) had a severe AHHS. Histologic scoring according to the Laennec staging system was available for 53 patients included in Lausanne. For patients included in Brussels, classification according to the Laennec scoring system was not feasible as it required new cutting of liver biopsies to perform Masson’s trichrome staining which was, unfortunately, not possible due to insufficient remaining histological material. Among patients included at Lausanne, 49 patients (92%) had cirrhosis. Seven patients (14%) had Laennec 4A disease, 15 patients (31%) had Laennec 4B disease and 27 patients (55%) had Laennec 4C cirrhosis.
Table 1.
Characteristics of patients at inclusion.
| Characteristics | Cohort 1 (n = 55) | Cohort 2 (n = 52) | Overall (n = 107) | p Value |
|---|---|---|---|---|
| Age (years)a | 54 (50–56) | 54 (50–58) | 54 (52–56) | 0.8 |
| Male sex (n, %) | 35 (64%) | 31 (60%) | 66 (62%) | 0.7 |
| Aspartate aminotransferase (UI/l)a | 125 (106–147) | NA | NA | – |
| Alanine aminotransferase (UI/l)a | 42 (38–49) | NA | NA | – |
| Serum bilirubin (µmol/l)a | 221 (148–273) | 248 (157–342) | 238 (188–276) | 0.2 |
| Serum albumin (g/l)a | 27 (26–28) | 27 (25–28) | 27 (26–28) | 0.5 |
| Serum creatinine (µmol/l)a | 72 (63–81) | 71 (62–80) | 71 (63–78) | 0.9 |
| INRa | 1.7 (1.5–1.8) | 1.8 (1.7–2.0) | 1.7 (1.7–1.8) | 0.003 |
| MELD scorea | 22 (20–23) | 24 (21–26) | 23 (21–24) | 0.01 |
| Child-Pugh scorea | 10 (10–11) | 11 (10–12) | 10 (10–11) | 0.005 |
| mDFa | 66 (59–73) | 56 (48–64) | 62 (56–67) | 0.2 |
| ABIC scorea | 8.1 (7.5–8.7) | 8.9 (7.9–9.2) | 8.4 (8.1–8.8) | 0.03 |
| Corticosteroid therapy (n, %) | 43 (78%) | 52 (100%) | 95 (89%) | <0.001 |
| Lille scorea | 0.32 (0.18–0.42)b | 0.32 (0.17–0.49) | 0.32 (0.21–0.43) | 0.9 |
| Responders according to the Lille score (n, %) | 28 (68%)b | 22 (58%) | 58 (62%) | 0.4 |
ABIC: age, bilirubin, INR, creatinine; AHHS: alcoholic hepatitis histologic score; CI: confidence interval; INR: international normalised ratio; mDF: modified Maddrey discriminant function; MELD: model for end-stage liver disease; NA: not available.
aData are expressed as median (95% CI); bavailable in 41 patients.
Table 2.
Histologic parameters of the study population.
| Cohort 1 (n = 53) | Cohort 2 (n = 52) | Whole cohort (n = 105) | p Value | |
|---|---|---|---|---|
| AHHS | ||||
| Stage of fibrosis | ||||
| No fibrosis, portal fibrosis, extensive fibrosis (n, %) | 3 (6%) | 4 (8%) | 7 (7%) | 0.7 |
| Bridging fibrosis or cirrhosis (n, %) | 50 (94%) | 48 (92%) | 98 (93%) | |
| Bilirubinostasis | ||||
| No or hepatocellular only (n, %) | 26 (49%) | 31 (60%) | 57 (54%) | 0.5 |
| Canalicular or ductular (n, %) | 11 (21%) | 9 (17%) | 20 (19%) | |
| Canalicular or ductular plus hepatocellular (n, %) | 16 (30%) | 12 (23%) | 58 (27%) | |
| Polymorphonuclear infiltration | ||||
| No or mild (n, %) | 38 (72%) | 29 (56%) | 67 (64%) | 0.11 |
| Severe (n, %) | 15 (28%) | 23 (44%) | 38 (36%) | |
| Megamitochondria | ||||
| No megamitochondria (n, %) | 43 (81%) | 34 (65%) | 77 (73%) | 0.08 |
| Megamitochondria (n, %) | 10 (19%) | 18 (35%) | 28 (27%) | |
| AHHS categories | ||||
| Mild (0–3) (n, %) | 3 (6%) | 7 (13%) | 10 (9%) | 0.06 |
| Moderate (4–5) (n, %) | 11 (21%) | 18 (35%) | 29 (28%) | |
| Severe (6–9) (n, %) | 39 (74%) | 27 (52%) | 66 (63%) | |
| Laennec staging system | ||||
| 4A (n, %) | 7 (14%) | NA | NA | – |
| 4B (n, %) | 15 (31%) | NA | NA | |
| 4C (n, %) | 27 (55%) | NA | NA | |
AHHS: alcoholic hepatitis histologic score; NA: not available.
Overall, 61 patients (57%) died. Among patients at Lausanne who died (n = 24) the cause of death was liver failure in 21 patients (88%), hepatocellular carcinoma in one patient (4%), and was unknown for two patients (8%). Two patients (4%) were lost to follow-up at 6 months. One patient (2%) underwent liver transplantation 3 years after inclusion. Among patients included at Brussels, all deaths (n = 37) were liver-related. One patient (2%) was lost to follow-up at 6 months. Seven patients (13%) underwent liver transplantation, five patients (10%) within the first 6 months for non-response to corticosteroids, one patient (2%) at 7 months and one patient (2%) at 3 years after inclusion. In the whole study population, the 28-day, 3-month and 6-month survival rates were 78% (95% CI: 71–86), 62% (95% CI: 53–72), and 56% (95% CI: 46–65), respectively.
Ninety-five (89%) patients were treated with corticosteroids. Of these, 58 (62%) responded to therapy according to the Lille score, categorised according to the 0.45 cut-off. Patients who did not receive corticosteroids either had spontaneous improvement of liver function tests or had infection that contraindicated corticosteroid therapy. In patients who received corticosteroids, survival rates at 6 months were better in those who were responders according to the Lille score than in those who were not responders (66% (95% CI: 53–78) vs 34% (95% CI: 19–50), p < 0.001). Alcohol intake during follow-up was available for 51 patients included at Lausanne. Among these patients, 31 (61%) returned to alcohol consumption. Abstainers had better survival rates at 6 months than consumers (90% (95% CI: 77–100) vs 55% (95% CI: 37–72), p = 0.01).
Prognostic value of the AHHS and the Laennec staging system
Survival rates in the mild, moderate and severe AHHS groups were 90% (95% CI: 71–100), 72% (95% CI: 56–89) and 69% (95% CI: 79–89) at 28 days (p = 0.6), 80% (95% CI: 55–100), 52% (95% CI: 34–70) and 63% (95% CI: 51–75) at 3 months (p = 0.3), and 70% (95% CI: 42–98), 41% (95% CI: 23–59) and 58% (95% CI: 46–70) at 6 months (p = 0.3), respectively (Figure 2). Using other cut-offs for dichotomization of the AHHS did not provide better results for predicting survival (data not shown). Among the components of the AHHS, severe polymorphonuclear infiltration was the only variable predicting 6-month survival (41% vs 63% for patients with and without severe polymorphonuclear infiltration, p = 0.01), while other parameters were not associated with survival.
Figure 2.
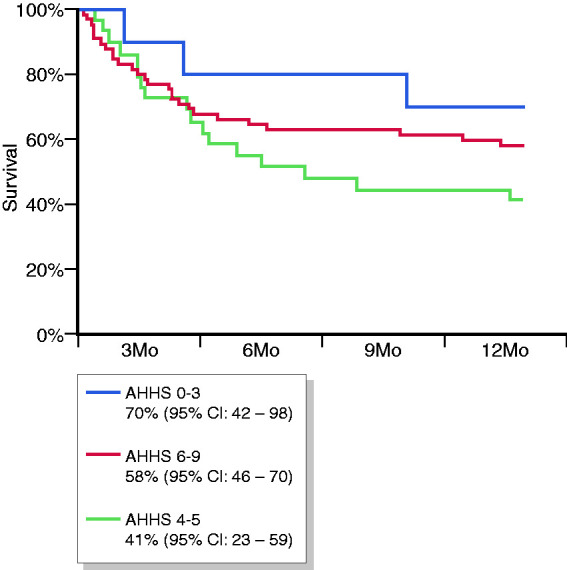
Survival curves according to the alcoholic hepatitis histologic score (AHHS). CI: confidence interval; Mo: months.
The AHHS did not predict response to corticosteroids defined with the Lille score using a 0.45 cut-off. Response rates were 50%, 73% and 58% in the mild, moderate and severe AHHS groups, respectively (p = 0.3).
The AUROC curve of AHHS for 6-month survival was 0.46 (95% CI: 0.34–0.56), lower than that of the Child-Pugh score (0.71), the MELD score (0.75) and the age, bilirubin, INR, creatinine score (ABIC) score (0.73) (p value for all comparisons ≤0.001), and similar to that of the mDF (0.50) (p = 0.24). In patients treated with corticosteroids the AUROC curve of AHHS for 6-month survival was lower than that of the Lille score (0.69) (p = 0.001).
Interobserver agreement was assessed in patients included at Lausanne. It was the best for fibrosis (agreement = 100%, Κ = 1.00). Other K values were 84% (K = 0.74) for bilirubinostasis, 96% (K = 0.9) for polymorphonuclear infiltration and 84% (K = 0.46) for megamitochondria. Overall AHHS had a 76% agreement rate with a K coefficient of 0.67.
With regard to the Laennec staging system, we considered patients without cirrhosis and with Laennec 4A cirrhosis together to avoid comparison of too many groups in the study population. Compared to patients with Laennec 4B or 4C cirrhosis, survival rates of patients without cirrhosis or with Laennec 4A cirrhosis were 100% vs 83% (95% CI: 72–95) at 28 days (p = 0.16), 91% (95% CI: 74–100) vs 68% (95% CI: 54–83) at 3 months (p = 0.13) and 82% (95% CI: 59–100) vs 64% (95% CI: 49–78) at 6 months (p = 0.2), respectively (Figure 3).
Figure 3.
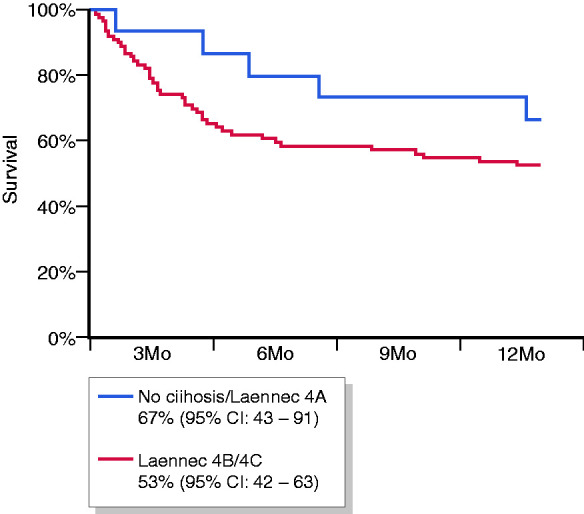
Survival curves according to the Laennec staging system. CI: confidence interval; Mo: months.
At univariable analysis, the AHHS and the Laennec staging system were not associated with the risk of death at 6 months (Table 3). In multivariate analysis adjusted for age and MELD score, the AHHS was not associated with 6-month mortality (HR: 0.94, 95% CI: 0.80–1.10, p = 0.4) (Table 3, all patients, multivariable model 1). When considering the Laennec system instead of AHHS, the presence of Laennec 4B or 4C cirrhosis was not associated with increased mortality at 6 months (HR: 4.31, 95% CI: 0.56–32.97, p = 0.16) (Table 3, all patients, multivariable model 2). In multivariate analysis including only patients treated with corticosteroids, the AHHS and the presence of Laennec 4B to 4C cirrhosis were not associated with 6-month mortality (Table 3, patients treated with corticosteroids, multivariable models 1 and 2).
Table 3.
Risk factors for death at 6 months.
| All patients |
Patients treated with corticosteroids |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable model 1a |
Multivariable model 2b |
Multivariable model 1a |
Multivariable model 2b |
|||||||
| Baseline characteristics |
Comparison group | Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) |
p-Value | Hazard ratio (95% CI) |
p-Value | Hazard ratio (95% CI) |
p-Value | Hazard ratio (95% CI) |
p-Value |
| Age | 1-Year increase | 1.04 (1.01–1.07) | 0.02 | 1.03 (0.99–1.06) | 0.13 | 1.03 (0.98–1.09) | 0.3 | 1.02 (0.98–1.05) | 0.3 | 1.02 (0.96–1.09) | 0.5 |
| Gender | Male vs female | 2.11 (0.92–4.83) | 0.06 | ||||||||
| INR | 1-Point increase | Not calculable | |||||||||
| Bilirubin | 1 µmol/l increase | 1.00 (1.00–1.00) | 0.001 | ||||||||
| Creatinine | 1 mg/dl increase | 1.00 (1.00–1.00) | <0.001 | ||||||||
| Albumin | 1 g/dl increase | 1.03 (0.95–1.12) | 0.4 | ||||||||
| mDF | 1-Point increase | 1.12 (1.06–1.19) | <0.001 | ||||||||
| Child-Pugh score | 1-Point increase | 1.49 (1.22–1.83) | <0.001 | ||||||||
| MELD score | 1-point increase | 1.19 (1.13–1.26) | <0.001 | 1.12 (1.07–1.16) | <0.001 | 1.06 (0.98–1.14) | 0.13 | 1.17 (1.10–1.23) | <0.001 | 1.07 (0.93–1.23) | 0.4 |
| ABIC score | 1-Point increase | 1.62 (1.02–2.58) | 0.04 | ||||||||
| Lille score | 1-Point increase | 11.31 (4.29–29.82) | <0.001 | 5.16 (1.70–15.69) | 0.004 | 17.30 (1.47–203.15) | 0.02 | ||||
| Fibrosis | Yes vs no | 1.01 (0.31–3.26) | 1.0 | ||||||||
| Neutrophils | Yes vs no | 0.48 (0.27–0.86) | 0.01 | ||||||||
| Bilirubinostasis | 1-Point increase | 1.30 (0.93–1.80) | 0.13 | ||||||||
| Megamitochondria | Yes vs no | 0.98 (0.51–1.86) | 0.9 | ||||||||
| AHHS | 1-Point increase | 0.97 (0.82–1.14) | 0.7 | 0.94 (0.80–1.10) | 0.4 | 1.00 (0.84–1.20) | 1.0 | ||||
| Laennec 4B/4C | Yes vs no | 1.69 (0.67–4.29) | 0.3 | 4.31 (0.56–32.97) | 0.16 | 7.47 (0.83–67.27) | 0.07 | ||||
ABIC: age, bilirubin, INR, creatinine; AHHS: alcoholic hepatitis histologic score; CI: confidence interval; INR: international normalised ratio; mDF: modified Maddrey discriminant function; MELD: model for end-stage liver disease.
aAnalysis performed on the whole study population; banalysis performed on cohort 1.
Discussion
In patients with AH, the most commonly used prognostic scores require a 1-week course of corticosteroid therapy to be calculated. Assessment of prognosis at baseline would avoid exposure to corticosteroids which increases the risk of infection, one of the most frequent causes of mortality in patients with severe AH, even if given for only a short period of time. Several attempts have been made to develop scores that provide pretreatment prognostic assessment, including recent studies focusing on genes extracted from a liver biopsy12,13 as well as older studies based on histologic findings.14–17 The last histologic approach was proposed by Altamirano et al. who developed the AHHS, a score based on four parameters, the combination of which allows classification of patients into groups with mild, intermediate or severe histologic AH.9 In the initial publication, the AHHS was strongly associated with the probability of survival at 3 months. However, validation of this score in independent cohort studies is required before it becomes widely applied. To date, two studies have aimed to validate this score.18,19 In the first study, the AHHS was associated with 90-day mortality, but this study included a very limited number of patients. In the other study, no predictive value of the AHHS was found.19 Another study that focused on the interobserver variability in scoring the four histologic parameters, observed significant interobserver variability among pathologists, limiting the usefulness of the score in clinical practice.20 Hence, the present study was needed to provide additional data on the utility of this score for patient prognostication.
The main finding of this study is that the AHHS was not correlated with survival at 28 days, 3 months or 6 months. The main explanation for this unexpected finding is likely related to the fact that only a limited number of patients belonged to the low-risk group according to the AHHS. This finding was previously observed in a population of patients with severe AH in which cirrhosis is a common finding.21 Indeed, the presence of cirrhosis, or even of extensive fibrosis, immediately adds three points to the AHHS classification and any additional points will move a patient into the intermediate severity group. Thus, the population of patients we studied may have had a more severe underlying liver disease than the population in the Altamirano et al. study, a point further supported by the fact that all patients had an mDF≥32 and by the observation of higher MELD and Lille scores in our study population. Similarly, fewer patients received corticosteroids in the Altamirano et al. study compared to our study (45% vs 89%, respectively). Finally, death rates at 3 months were lower in the Altamirano et al. study than in ours (29% vs 38%, respectively). Altogether, these findings indicate that the AHHS may be more suitable for prognostic assessment in patients with less severe AH than in a population such as the one included in our study. However, this hypothesis should be assessed in studies specifically designed to investigate this point. Another point is related to the fact that, currently, we lack factors able to select which patients should undergo treatment. Although our study was not designed to answer this question, it did not suggest a role for histologic factors in selecting which patients should be treated for AH. This point deserves additional attention in future prospective studies.
The second important finding of the present study is the absence of discriminative prognostic information between intermediate and severe AH according to the AHHS. In addition, the interobserver reproducibility of the AHHS was not perfect, especially for the criteria related to the presence of megamitochondria, a point that was already observed in the Altamirano et al. study9 and by others.20 In contrast, the extent of fibrosis demonstrated 100% interobserver reproducibility. Of note, the Laennec staging system, which is based only on the extent of fibrosis, did not correlate with 6-month survival, although there was a trend toward increased survival among patients without cirrhosis and with Laennec 4A cirrhosis compared to those with Laennec 4B and 4C. Taken together, these findings may indicate that, if histologic factors are helpful for predicting patient outcomes, their prognostic utility would likely be driven by the extent of fibrosis, a finding already observed in AH17,22 and established for other liver diseases including non-alcoholic fatty liver disease.11,23 This observation was expected as the extent of liver fibrosis is correlated to the degree of portal hypertension and related complications.24–26
We acknowledge that this study has potential limitations including its limited sample size. However, severe AH remains a rare complication among heavy drinkers.3,27 In addition, this study included a similar number of patients to the Altamirano et al. study, when compared to either the derivation cohort (n = 96) or to the validation cohort (n = 109). Hence, an effect size similar to that observed in the Altamirano et al. study should have been detected in our study population. Another limitation was that, for patients included at CUB Hôpital Erasme, classification according to the Laennec scoring system was not possible. On the other hand, our study has several strengths including the inclusion of two cohorts of consecutive patients with severe AH in two academic centres and the independent evaluation of all biopsies by two pathologists with expertise in liver disease.
In conclusion, the AHHS is not predictive of short-term survival in this cohort of patients with severe AH. Further studies should evaluate whether the prognostic value of histologic parameters differs according to the severity of liver disease.
Acknowledgments
The authors acknowledge the contribution of Sandy Field for her assistance concerning English-language editing. The following author contributions were made: MD: acquisition of data, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. AS: acquisition of data, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. ET: acquisition of data, analysis and interpretation of data; critical revision of the manuscript for important intellectual content. AM: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. JS: acquisition of data, analysis and interpretation of data; critical revision of the manuscript for important intellectual content. CM: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. CS: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision. PD: study concept and design; statistical analysis; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision.
Footnotes
Declaration of conflicting interests: The authors declare no competing interests in the present study.
Ethics approval: The Ethical Committee for the research on human beings of the state of Vaud, Switzerland, approved the study (CER-VD, number 2017-00992) the 07.18.2017. The Ethics Committee at CUB Hôpital Erasme approved the study.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from all individuals.
ORCID iD: Margaux Dubois https://orcid.org/0000-0002-9856-8379
References
- 1.Thursz M, Gual A, Lackner C, et al. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of alcohol-related liver disease. J Hepatol 2018; 69: 154–181. [DOI] [PubMed] [Google Scholar]
- 2.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75: 193–199. [PubMed] [Google Scholar]
- 3.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360: 2758–2769. [DOI] [PubMed] [Google Scholar]
- 4.Marot A, Dubois M, Trepo E, et al. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One 2018; 13: e0190823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louvet A, Labreuche J, Artru F, et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 2015; 149: 398–406 e8; quiz e16–e17. [DOI] [PubMed] [Google Scholar]
- 6.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 7.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor. Gastroenterology 2009; 137: 541–548. [DOI] [PubMed] [Google Scholar]
- 8.Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 9.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014; 146: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SU, Oh HJ, Wanless IR, et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012; 57: 556–563. [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 12.Trepo E, Goossens N, Fujiwara N, et al. Combination of gene expression signature and model for end-stage liver disease score predicts survival of patients with severe alcoholic hepatitis. Gastroenterology 2018; 154: 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deltenre P, Trepo E, Fujiwara N, et al. Gene signature-MELD score and alcohol relapse determine long-term prognosis of patients with severe alcoholic hepatitis. Liver Int Liver Int 2020; 40(3): 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chedid A, Mendenhall CL, Tosch T, et al. Significance of megamitochondria in alcoholic liver disease. Gastroenterology 1986; 90: 1858–1864. [DOI] [PubMed] [Google Scholar]
- 15.Mathurin P, Duchatelle V, Ramond MJ, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996; 110: 1847–1853. [DOI] [PubMed] [Google Scholar]
- 16.Spahr L, Rubbia-Brandt L, Genevay M, et al. Early liver biopsy, intraparenchymal cholestasis, and prognosis in patients with alcoholic steatohepatitis. BMC Gastroenterol 2011; 11: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mookerjee RP, Lackner C, Stauber R, et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol 2011; 55: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 18.Andrade P, Silva M, Rodrigues S, et al. Alcoholic hepatitis histological score has high accuracy to predict 90-day mortality and response to steroids. Dig Liver Dis 2016; 48: 656–660. [DOI] [PubMed] [Google Scholar]
- 19.Abergel A, Teilhet C, Sevigne L, et al. Evaluation rétrospective du score AHHS (Alcoholic Hepatitis Histologic Score) en routine Clinique. JFHOD, Société nationale française de gastroentérologie, abstract p21.
- 20.Horvath B, Allende D, Xie H, et al. Interobserver variability in scoring liver biopsies with a diagnosis of alcoholic hepatitis. Alcohol Clin Exp Res 2017; 41: 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno C, Deltenre P, Senterre C, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 2016; 150: 903–910. [DOI] [PubMed] [Google Scholar]
- 22.Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol 2017; 66: 610–618. [DOI] [PubMed] [Google Scholar]
- 23.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 25.Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol 2019; 70: 294–304. [DOI] [PubMed] [Google Scholar]
- 26.Kim MY, Cho MY, Baik SK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 2011; 55: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 27.Marot A, Henrion J, Knebel JF, et al. Alcoholic liver disease confers a worse prognosis than HCV infection and non-alcoholic fatty liver disease among patients with cirrhosis: An observational study. PLoS One 2017; 12: e0186715. [DOI] [PMC free article] [PubMed] [Google Scholar]