Abstract
Background
There is limited evidence on the effectiveness of biological therapy in stricturing complications in patients with Crohn’s disease.
Aim
The study aims to determine the effectiveness of anti-tumor necrosis factor (TNF) agents in Crohn’s disease complicated with symptomatic strictures.
Methods
In this multicentric and retrospective study, we included adult patients with symptomatic stricturing Crohn’s disease receiving their first anti-TNF therapy, with no previous history of biological, endoscopic or surgical therapy. The effectiveness of the anti-TNF agent was defined as a composite outcome combining steroid-free drug persistence with no use of new biologics or immunomodulators, hospital admission, surgery or endoscopic therapy during follow-up.
Results
Overall, 262 patients with Crohn’s disease were included (53% male; median disease duration, 35 months, 15% active smokers), who received either infliximab (N = 141, 54%) or adalimumab (N = 121, 46%). The treatment was effective in 87% and 73% of patients after 6 and 12 months, respectively, and continued to be effective in 26% after a median follow-up of 40 months (IQR, 19–85). Nonetheless, 15% and 21% of individuals required surgery after 1 and 2 years, respectively, with an overall surgery rate of 32%. Postoperative complications were identified in 15% of patients, with surgical site infection as the most common. Starting anti-TNF therapy in the first 18 months after the diagnosis of Crohn’s disease or the identification of stricturing complications was associated with a higher effectiveness (HR 1.62, 95% CI 1.18–2.22; and HR 1.55, 95% CI 1.1–2.23; respectively). Younger age, lower albumin levels, strictures located in the descending colon, concomitant aminosalicylates use or presence of lymphadenopathy were associated with lower effectiveness.
Conclusions
Anti-TNF agents are effective in approximately a quarter of patients with Crohn’s disease and symptomatic intestinal strictures, and 68% of patients are free of surgery after a median of 40 months of follow-up. Early treatment and some potential predictors of response were associated with treatment success in this setting.
Keywords: Anti-TNF, biologic drug, Crohn’s disease, stricture, surgery
Introduction
Crohn’s disease (CD) is a chronic and disabling inflammatory disorder of the gastrointestinal tract.1 It may affect any segment of the gastrointestinal tract but most often involves the terminal ileum. The characteristic transmural inflammatory process leads to progressive structural damage of the bowel wall; as a result, certain complications like strictures and penetrating disease often arise in the long term. Stenosing lesions are a consequence of an exaggerated accumulation of collagen-rich extracellular matrix and expansion of mesenchymal cells, accompanied by a thickening of the muscularis mucosa and the muscularis propia.2–4
In a recent European inception cohort study, 21% of patients with CD already had strictures at diagnosis.5 Moreover, 10% of patients with initial non-stricturing non-penetrating disease (B1 according to the Montreal classification) developed stenosing lesions during the first 5 years.5 Previous studies have estimated that the overall lifetime risk of strictures in CD patients may be as high as 50%.4 The relevance of these findings is that they imply a high risk of requiring surgery, as these patients usually show a poor response to medical therapy.4,6
The currently approved medical treatments for CD are mainly focused on controlling the transmural inflammatory process. Although this is probably the main driver of fibrosis in the gastrointestinal tract, the efficacy of currently available drugs in fibrosis-predominant lesions is very limited.7 Hence, in this clinical situation, surgery and endoscopic balloon dilation remain the mainstay treatments, among all the possible treatment options.4,8 Anti-tumor necrosis factor (TNF) agents have shown a clinical benefit in patients with stricturing complications.9–11 A recent prospective and observational trial, the CREOLE study, evaluated the efficacy of adalimumab in 97 patients with small bowel strictures.9 After 24 weeks of follow-up, 64% of them achieved the primary endpoint of the study, and 53% were free of surgery in the long term. Nonetheless, given the high heterogeneity in the design of the remaining studies, characteristics of lesions and patients analysed, it is not possible to establish generalised recommendations about the use of biologics in these cases.12 On the other hand, our primary goal in the treatment of CD should be to halt the development of long-term bowel damage and thereby reduce the risk of intestinal surgery and hospitalisation.13,14 In this context, the aim of our study was to determine the effectiveness of first-line anti-TNF therapy in CD complicated by symptomatic intestinal strictures in a real-world setting.
Methods
All members of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) were invited to participate in the study. The study protocol followed the current evaluation process for research projects promoted by this group. All clinical data were extracted retrospectively from the medical records of each participating hospital. We included adult patients with an established diagnosis of CD, according to the European Crohn’s and Colitis Organisation (ECCO) criteria,15 with luminal or obstructive symptoms secondary to small or large bowel stenosis and who received their first anti-TNF agent for this condition. Strictures were defined as a localised and constant luminal narrowing with bowel wall thickening and pre-stricture dilatation evidenced by radiological and/or endoscopic examinations.16 We excluded patients with previous exposure to biologics or a history of bowel surgery or endoscopic therapy for these lesions. Patients receiving immunomodulators before starting anti-TNF therapy or a surgical procedure that was not related to stricturing complications (e.g. perianal) were eligible to be included in the study.
The main outcome of the study was the effectiveness of the anti-TNF agent, defined as a composite outcome combining the steroid-free persistence of the TNF antagonist, with no need for new immunomodulators and/or biologics, and no hospital admission, surgery or endoscopic therapy during follow-up. Those events not associated with a loss of response to the anti-TNF therapy (i.e. pregnancy or intolerance) were not considered as treatment failures. As secondary outcomes, we investigated the effectiveness according to the type of anti-TNF (infliximab and adalimumab), stricture location and evaluated possible clinical, analytical and radiological predictors of response to anti-TNF therapy at baseline or during follow-up.
The Spanish Agency of Medicines and Medical Devices (AEMPS) and the ethics committee of the Basque Country approved the study protocol (EPA2015025, 16/05/2018) and it was conducted according to the 1975 Declaration of Helsinki and the Good Clinical Practice guidelines. All patients signed the informed consent before their inclusion in the study.
Data were gathered on the following clinical characteristics: sex, age at diagnosis of CD, smoking history, age at diagnosis of stricturing behaviour, disease extension according to the Montreal classification,17 history of immunomodulator use, steroid use and response, surgical procedures, presence of perianal disease and its classification,18 extraintestinal manifestations and Harvey–Bradshaw index according to Harvey–Bradshaw index. We also recorded baseline laboratory test results for C-reactive protein, haemoglobin, albumin, total leucocytes and platelets in the 3 months before starting anti-TNF therapy.
We compiled information from the radiological examinations (computed tomography (CT), magnetic resonance enterography (MRE) and abdominal ultrasound) performed at the diagnosis of the stricture and also in the previous 3 months before starting the anti-TNF agent (baseline), when available. Radiological examinations during the follow-up were assessed based on the same variables. All of these examinations were reviewed by radiologists at each centre, when necessary. Bowel ultrasound was performed only in sites with high experience in the performance of this technique. The characteristics that were analysed included the type of examination, number of strictures, their location (duodenum, jejunum, proximal ileum, terminal ileum, cecum-ascending colon, transverse colon, descending colon and recto-sigmoid), bowel wall thickening, and bowel wall contrast enhancement, as well as whether there were ulcers at the stenosis, pre-stenotic dilatation, lymphadenopathies or penetrating disease (fistulae or abscess) complicating the stricture. Bowel wall thickening was defined as >4 mm thickness with luminal distension in the maximally thickened area, in an appropriately distended lumen. Pre-stenotic dilatation was defined as a luminal diameter greater than 30 mm.16 Bowel wall enhancement was classified by its pattern: mucosal (superficial layer enhancing), layered (both mucosa-submucosa and serosa enhancing, with a central band of relatively reduced enhancement) or homogeneous (all bowel wall enhancing equally).19 If data were available from an endoscopic examination at the time of diagnosis, we noted the presence of strictures and if it was possible to pass through the narrowed area and the observation of ulcers at this area.
Study data were collected and managed using the Research Electronic Data Capture (REDCap) electronic data capture tools hosted by the Spanish Gastroenterology Association (AEG).20 REDCap is a secure, web-based application designed to support data capture for research studies, providing (a) an intuitive interface for validated data entry; (b) audit trails for tracking data manipulation and export procedures; (c) automated export procedures for seamless data downloads to common statistical packages; and (d) procedures for importing data from external sources.
Statistical analysis
Patient sociodemographic characteristics, their baseline radiological features and the main outcomes during the follow-up were described, stratified by type of anti-TNF therapy. Associations between the clinical variables and anti-TNF therapy were assessed with the non-parametric Wilcoxon test (for continuous variables) and the chi-square test (or the Fisher’s exact test if required). To explore changes in the radiological findings over the subsequent examinations, generalised linear mixed models were built. For this, radiological outcomes were used as dependent variables and follow-up time as an independent variable. Finally, a multivariable Cox regression was performed to identify potential predictors which could be associated with the overall effectiveness. For this, the time to each of the events was taken as a dependent variable and variables with a p-value less than or equal to 0.20 in the univariate model as independent exposure variables. The final multivariable model was determined using a backward procedure. All effects were deemed statistically significant at p < 0.05.
Results
A total of 262 patients were treated with anti-TNF therapy between October 2001 and July 2018 at 32 hospitals in Spain and were included in the analysis. The main characteristics of these patients are summarised in Table 1. We had information available from radiological examinations at baseline in 184 patients (70%), mainly from MRE procedures (60% of them). At baseline, 22 patients (8%) had information available only from endoscopic examinations. The radiological characteristics of the strictures at baseline are summarised in Table 2. Notably, most strictures were located in the terminal ileum (N = 158, 86%) and the median number of strictures per patient was 1 (interquartile range [IQR], 1–2). Further, an endoscopic examination was performed at baseline in 101 patients (39%), with evidence of stenosis in 76% of cases (77 patients). Ulcers were present in 99% (76 cases) of these strictures and it was not possible to pass through the stenosis in 25% of these patients.
Table 1.
Patient characteristics.
| All N = 262 |
Infliximab N = 141 |
Adalimumab N = 121 |
p-value | ||
|---|---|---|---|---|---|
| Sex, male | |||||
| Mean (SD) | 139 (53) | 72 (51) | 67 (55) | 0.48 | |
| Age at diagnosis, years | |||||
| Median (IQR) | 33 (25–46) | 33 (25–47) | 33 (23–46) | 0.92 | |
| Disease duration (from diagnosis to anti-TNF), months | |||||
| Median (IQR) | 35 (7–103) | 28 (7–102) | 43 (8–105) | 0.38 | |
| Smoking habits, N (%) | 0.82 | ||||
| Never | 62 (24) | 32 (23) | 30 (25) | ||
| Former | 29 (11) | 17 (12) | 12 (10) | ||
| Active | 39 (15) | 21 (15) | 18 (15) | ||
| Time between stricture diagnosis and starting anti-TNF, months | |||||
| Median (IQR) | 5 (1–28) | 5 (1–29) | 4 (1–24) | 0.56 | |
| Disease extension,a N (%) | |||||
| Ileum (L1) | 164 (63) | 94 (67) | 70 (58) | 0.09 | |
| Colon (L2) | 9 (3) | 2 (1) | 7 (6) | ||
| Ileocolonic (L3) | 89 (34) | 45 (32) | 44 (36) | ||
| Upper GI tract (L4) | 34 (13) | 19 (13) | 15 (12) | ||
| Fistulising disease (B3) | 36 (14) | 23 (16) | 13 (11) | 0.13 | |
| Perianal disease | 35 (13) | 19 (13) | 16 (13) | 0.55 | |
| Extraintestinal manifestations | 49 (19) | 23 (16) | 26 (21) | 0.56 | |
| Number of strictures per patient | |||||
| Median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.43 | |
| Range | 1–9 | 1–8 | 1–9 | ||
| Previous treatment, N (%) | |||||
| Thiopurines | 190 (77) | 103 (73) | 87 (72) | 0.89 | |
| Steroids | 150 (61) | 86 (61) | 64 (53) | 0.21 | |
| Mesalamine | 64 (26) | 41 (29) | 23 (19) | 0.06 | |
| Methotrexate | 25 (10) | 10 (7) | 15 (12) | 0.2 | |
| Previous response to steroids, N (%) | 0.44 | ||||
| Steroid-refractory | 22 (8) | 14 (10) | 8 (7) | ||
| Steroid-dependence | 68 (26) | 40 (29) | 28 (23) | ||
| Previous surgery,b N (%) | 2 (1) | 2 (1) | 0 (0) | 0.5 | |
| Baseline laboratory parameters | |||||
| Median (IQR) | |||||
| C-reactive protein, mg/l | 5.3 (1.0–15) | 5.3 (1.0–11.9) | 3.9 (0.7–10.7) | 0.26 | |
| Haemoglobin, g/dl | 13 (12–14) | 12.7 (11.7–13.8) | 13.3 (12.3–14.2) | 0.024 | |
| Albumin, g/dl | 3.9 (3.6–4.2) | 3.9 (3.6–4.2) | 4.0 (3.7–4.3) | 0.13 | |
| Leucocytes, mm3 | 7,900 (5,900–9,800) | 7,500 (5,800–9,400) | 8,100 (6,000–9,900) | 0.37 | |
| Platelets, mm3 | 303,000 (249,000–376,000) | 322,000 (249,000–387,000) | 288,000 (245,000–350,000) | 0.017 | |
| Concomitant therapy, N (%) | |||||
| Thiopurines | 165 (63) | 98 (69) | 67 (55) | 0.003 | |
| Steroids | 70 (27) | 39 (28) | 31 (26) | 0.39 | |
| Mesalamine | 23 (9) | 16 (11) | 7 (6) | 0.27 | |
| Methotrexate | 14 (5) | 8 (6) | 6 (5) | 0.14 | |
aAccording to the Montreal classification.
bBoth patients with previous small bowel resection not related to the current stricture.
GI: gastrointestinal; IQR: interquartile range; SD: standard deviation; TNF: tumour necrosis factor.
Table 2.
Radiological characteristics of the strictures at baseline.
| All N = 184 |
Infliximab N = 101 |
Adalimumab N = 83 |
p-value | |
|---|---|---|---|---|
| Length of small bowel strictures | ||||
| Length, cm | 11 (5–18) | 11 (5–18) | 11 (5–17) | 0.9 |
| Length of colonic strictures | ||||
| Length, cm | 3 (2–5) | 5 (2–6) | 2 (1–4) | 0.31 |
| Duodenum, Number of patients (%) | ||||
| Length, cm | 2 | 0 | 2 | 0.21 |
| Median (IQR) | 3 (N/A)a | – | 3 (N/A)a | N/A |
| Jejunum, N (%) | ||||
| Length, cm | 6 | 4 | 2 | 0.69 |
| Median (IQR) | 10.5 (9–12) | 10.5 (9–12) | – | N/A |
| Proximal ileum, N (%) | ||||
| Length, cm | 26 | 15 | 11 | 0.84 |
| Median (IQR) | 6 (4–15) | 7 (5–15) | 5 (3–10) | 0.15 |
| Terminal ileum, N (%) | ||||
| Length, cm | 158 | 86 | 72 | 0.90 |
| Median (IQR) | 12 (7–20) | 10 (5–19) | 10 (4–17) | 0.92 |
| Ascending colon, N (%) | ||||
| Length, cm | 6 | 3 | 3 | 0.85 |
| Median (IQR) | 4 (2–4) | 4 (1–5) | 3 (2–4) | 0.83 |
| Transverse colon, N (%) | ||||
| Length, cm | 5 | 2 | 3 | 0.66 |
| Median (IQR) | 3 (2–5) | 4 (2–5) | 3 (1–5) | 0.7 |
| Descending colon, N (%) | ||||
| Length, cm | 4 | 1 | 3 | 0.34 |
| Median (IQR) | 2 (2–4) | 2 (2–2) | 3 (2–4) | 1.0 |
| Recto-sigmoid, N (%) | ||||
| Length, cm | 3 | 2 | 1 | 0.65 |
| Median (IQR) | 2 (1–5) | 4 (2–5) | 1 (1–1) | 0.54 |
| Number of stenosis per patient | ||||
| Median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.59 |
| Bowel wall thickening, mm | ||||
| Median (IQR) | 8 (6–9) | 8 (7–9) | 8 (6–9) | 0.82 |
| Pre-stenotic dilatation, N (%) | 101 (55) | 58 (58) | 43 (52) | 0.58 |
| Pre-stenotic dilatation, mm | ||||
| Median (IQR) | 34 (20–45) | 36 (20–45) | 30 (20–44) | 0.63 |
| Contrast enhancement,b N (%) | 0.29 | |||
| Layered | 67 (37) | 39 (39) | 28 (34) | |
| Mucosal | 48 (26) | 26 (26) | 22 (27) | |
| Homogeneous | 17 (9) | 6 (6) | 11 (13) | |
| Normal | 6 (3) | 5 (5) | 1 (1) | |
| Not available | 45 (25) | 24 (24) | 21 (25) | |
| Ulcers, N (%) | 46 (25) | 25 (25) | 21 (26) | 0.87 |
| Internal fistula, N (%) | 23 (13) | 14 (14) | 9 (11) | 0.57 |
| Lymphadenopathy, N (%) | 65 (36) | 40 (40) | 25 (30) | 0.33 |
IQR: Interquartile range.
aData available only from one patient.
bDefined as mucosal (superficial layer enhancing), layered (both mucosa-submucosa and serosa enhancing, with central band of relatively reduced enhancement) or homogeneous (all bowel wall enhancing equally).
Anti-TNF therapy
Overall, 141 patients (54%) received infliximab (74% originator and 26% biosimilar) and 121 (46%) adalimumab. Notably, as combination therapy with infliximab, a higher proportion of patients received thiopurines than adalimumab (69% versus 55%, p = 0.003).
Effectiveness of biological therapy
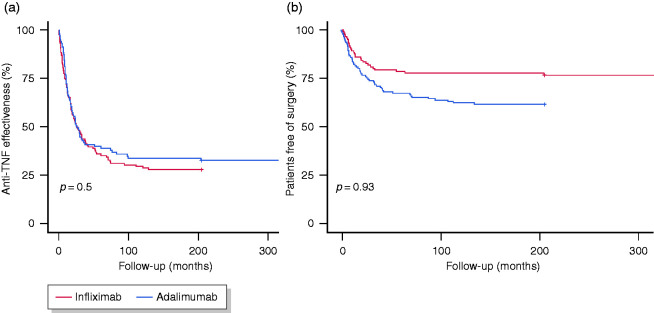
The main outcomes during follow-up are presented in Table 3 and Figure 1. After a median follow-up of 40 months (IQR, 19–85), the anti-TNF therapy was effective in 68 patients (26%) (Figure 2(a)). The median time until loss of effectiveness was 15 months (IQR, 7–30) and the treatment had been effective in 87% and 73% of patients at 6 and 12 months, respectively. During follow-up, 5% of patients showed a complete resolution of the strictures (among those with radiological data). The initiation of anti-TNF therapy in the first 18 months before the diagnosis of CD or the diagnosis of stricturing complications was associated with a higher effectiveness (hazard ratio (HR) 1.62, 95% confidence interval (CI) 1.18–2.22, p = 0.003; and HR 1.55, 95% CI 1.1–2.23, p = 0.015; respectively). We found no differences in effectiveness by stricture location (25% for the small bowel versus 27% for the colon; log-rank, p = 0.70) or type of anti-TNF (26% for both infliximab and adalimumab; log-rank, p = 0.93).
Table 3.
Main outcomes.
| All N = 262 |
Infliximab N = 141 |
Adalimumab N = 121 |
p-value | |
|---|---|---|---|---|
| Steroid-free, N (%) | 59 (84a) | 30 (77a) | 29 (94a) | 0.096 |
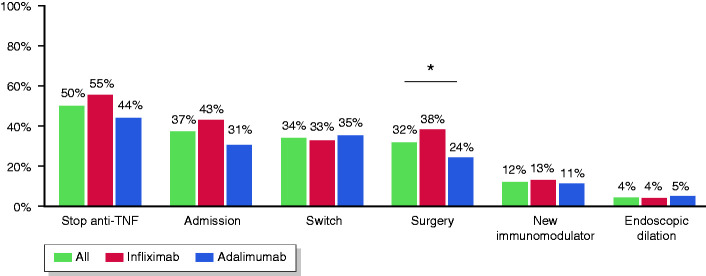
| Stop anti-TNF treatment, N (%) | 131 (50) | 78 (55) | 53 (44) | 0.08 |
| Admission, N (%) | 98 (37) | 60 (43) | 38 (31) | 0.07 |
| Surgery, N (%) | 83 (32) | 54 (38) | 29 (24) | 0.02 |
| Switch biologic, N (%) | 88 (34) | 46 (33) | 42 (35) | 0.79 |
| Adalimumab | 33 (38) | 32 (23) | 1 (1) | |
| Infliximab | 30 (34) | 3 (2) | 27 (22) | |
| Ustekinumab | 21 (24) | 8 (6) | 13 (11) | |
| Vedolizumab | 4 (5) | 3 (2) | 1 (1) | |
| New immunosuppressive therapy, N (%) | 31 (12) | 18 (13) | 13 (11) | 0.70 |
| Thiopurines | 15 (50) | 8 (6) | 7 (6) | |
| Methotrexate | 13 (43) | 9 (6) | 4 (3) | |
| Calcineurin inhibitor | 2 (7) | 0 (0) | 2 (2) | |
| Endoscopic balloon dilation, N (%) | 11 (4) | 5 (4) | 6 (5) | 0.76 |
aPercentage out of those receiving concomitant steroids at baseline.
Figure 1.
Main outcomes in patients receiving anti-TNF therapy, including anti-TNF drug survival, hospital admission, switch, surgery, use of new immunomodulators or endoscopic therapy.
Figure 2.
Kaplan–Meier estimates of the effectiveness of anti-TNF therapy (a) and surgery rates (b) during follow-up.
The anti-TNF agent was discontinued in 131 patients (50%), but after excluding cases not related to a loss of response, 108 patients (41%) were found to have stopped the treatment for lack of effectiveness after a median of 16 months (IQR, 7–33). Withdrawal rates with infliximab originator were significantly higher than with its biosimilar or adalimumab (53%, 24% and 36%, respectively; p = 0.0034). Approximately a third (34%) of patients required a switch to a new biologic, usually to a second anti-TNF (72%), while 24% started ustekinumab and 5% vedolizumab. Seventeen patients (27%) were receiving steroids at baseline and most of them were able to withdraw them completely (59, 84% among those at baseline), with no differences between infliximab and adalimumab-treated patients (77% versus 94%, respectively; p = 0.096).
Surgery rates
During anti-TNF therapy, 83 patients (32%) required surgery (Figure 2(b)). The most common indication for surgery was the persistence of symptomatic disease (66/83, 80%), followed by penetrating disease (16/83, 19%). The percentage of patients requiring surgery was 15%, 21% and 25% after 1, 2 and 3 years, respectively. No patient with colonic involvement required surgery. The rate of surgery was significantly higher in patients receiving infliximab compared with adalimumab (38% versus 24%, p = 0.02), and the difference remained significant in the multivariable analysis (OR 1.78, 95% CI 1.025–3.09). Nonetheless, in the survival analysis, the two agents were associated with similar rates of surgery (log-rank, p = 0.93).
Regarding the surgical procedure, intestinal resection was the most common (83%), while strictureplasty was performed in a small percentage of cases (4%). Most patients underwent elective surgery (80%) and usually without requiring an ostomy (89%), with no significant difference between the rates of abdominal and laparoscopic approach (49% versus 51%, respectively). Postoperative complications were recorded in 15% of patients, with wound infection being the most common diagnosis (42%). The median time between administration of the last dose of anti-TNF and the intervention was 43 days (IQR, 23–75), and this did not differ significantly between patients who did and did not develop complications (p = 0.92).
Safety
In total, 16 patients (6%) stopped the anti-TNF due to an adverse event. One male patient with ileocolonic CD was diagnosed with small bowel adenocarcinoma after 31 months of weekly adalimumab treatment in combination with thiopurines. There were no signs suggestive of malignancy in his preoperative radiological work-up. One patient was diagnosed with cholangiocarcinoma after 15 months on adalimumab and another developed recurrent renal cancer after 18 months on adalimumab.
Radiological examinations during follow-up
The findings in subsequent radiological examinations are summarised in the supplementary material.
Predictive factors of response
The results of the survival analysis are summarised in Table 4. In this analysis, we found that younger age, lower albumin levels at baseline, strictures being located in the descending colon, concomitant use of mesalamine and the presence of lymphadenopathy at the stricture were associated with lower effectiveness.
Table 4.
Predictors of non-response to anti-TNF agents in stricturing Crohn’s disease.
| Hazard ratio (95% confidence interval) | p-value | |
|---|---|---|
| Age at diagnosis | 0.99 (0.98–0.99) | 0.01 |
| Location at the descending colon | 6.57 (1.39–33.33) | 0.02 |
| Albumin at baseline | 0.48 (0.29–0.66) | 0.004 |
| Concomitant use of mesalamine | 2.77 (1.17–6.57) | 0.02 |
| Lymphadenopathy | 2.06 (1.31–3.22) | 0.002 |
Adjusted for sex and type of anti-TNF agent.
C-index = 0.67 (s.e.: 0.03).
Discussion
In our cohort, we observed that anti-TNF therapy was effective in approximately a quarter of patients with CD complicated by symptomatic intestinal strictures. Importantly, a high proportion of patients are able to avoid surgery with this therapy in the long term. Of note, we also found that starting anti-TNF therapy early after the diagnosis of CD or stricturing complications was associated with greater effectiveness. Our results are relevant in clinical practice, as patients with early disease but established bowel damage are at a higher risk of requiring intestinal surgery and hospitalisation.13 Translating our data into practice, TNF antagonists can improve the outcomes of patients with symptomatic stricturing lesions, but timely intervention appears as an important factor also when complicated disease is already present, with comparable data when it has been assessed by the Lémman index.21
CD is a chronic and relapsing disorder of the gastrointestinal tract with a progressive and disabling course.1 The great majority of patients have inflammatory lesions at the time of diagnosis, but up to a third already have some stricturing at this point.5 Further, the percentage of patients with fibrotic lesions may increase by 10% by 5 years after diagnosis.5 Strictures show a predominance of collagen band deposition and thickening of the muscularis mucosa and muscularis propria,4 but they often coexist with a variable degree of inflammation.22 Therefore, we can expect some benefit in established stricturing lesions from currently available drugs targeting any of the multiple immune mediators involved in the inflammatory process.19 But, even more importantly, observational studies have suggested that the risk of progression from inflammatory to stenosing disease may be reduced by the timely use of immunomodulators or anti-TNF agents during the early stages of the disease.6,23,24 Nevertheless, no anti-fibrotic drugs are currently approved in established bowel stenosis in patients with CD.16
Initial experience with anti-TNF agents indicated a possible deleterious effect of these drugs with a possible increment in the rate of surgery, suggesting a worsening of the fibrotic component in these lesions.25–27 Further experience with these drugs, however, has shown that they are safe and effective in this context.10,11,28 Nonetheless, there is only one prospective, observational cohort study evaluating the efficacy of anti-TNF agents in CD complicated by symptomatic intestinal strictures (CREOLE).9 In this study, all patients received open-label adalimumab, and 64% of patients reached the primary endpoint after 24 weeks of therapy, defined as the continuation of adalimumab treatment, without the use of steroids, surgery, endoscopic balloon dilation or adverse events. In the long term, after a median follow-up of 4 years, the drug continued to be effective in 29% of the initial cohort. However, important differences between the CREOLE study and our cohort should be considered when comparing the main outcomes. While the French study included patients with a long-standing disease and long-segment stenosis, with previous intestinal surgery (44%) and prior infliximab exposure in some cases (7%), our clinical scenario might be different. Our patients had shorter disease duration (median 8.8 years [3.4–14.9] versus 2.9 years [0.6–8.6], respectively) and were receiving their first-line biologic therapy with no previous surgical treatment for this condition. This allowed us to evaluate the long-term outcomes of TNF antagonists as a primary treatment early in the course of established bowel damage. Recent observational studies have demonstrated similar results in clinical practice.10,11 Although previous studies evaluating the efficacy of this treatment have produced mixed results, we note that there are consistent data supporting the observation that around one in four patients with stricturing CD treated with anti-TNF therapy shows a clinical response in the long term.12
The results from our cohort demonstrate that, after 1 year, most patients benefit from steroid-free maintenance anti-TNF therapy with no hospital admissions, new immunomodulators or biologics, surgery or endoscopic therapy. Nevertheless, there is an evident progressive loss of response to this therapy, as it continued to be effective in only 26% of patients after a follow-up of approximately 3 years. Our findings show a similar proportion of patients responding to this therapy as that previously reported by the CREOLE trial and some observational studies.9–11 On the other hand, patients with stricturing complications have a high risk of requiring surgery, and we observed that 68% of patients receiving anti-TNF therapy remained free from this outcome. Previous observations from two European cohorts have shown that the rate of surgery with this therapy may be up to 31–39% in the long term, in patients with long-segment stenosis and mainly located in the terminal ileum as in our cohort.10,11 Our results also suggest that adalimumab may show an additional numerical benefit over infliximab in terms of drug survival and surgery rates, similar to the findings of Allocca et al.,10 but neither of the studies found significant differences in the risk of surgery in the survival analysis.
We were able to identify that younger age, lower albumin levels at baseline, strictures being located in the descending colon, concomitant use of mesalamine and ulcers or lymphadenopathy at the stricture were associated with non-response to anti-TNF therapy. Some of these factors may be associated with more aggressive disease (e.g. younger age) or a pronounced inflammatory process underlying the stricturing lesions (e.g. lower albumin levels, ulcers and lymphadenopathy). Notably, we observed that the early introduction of TNF antagonists after the diagnosis of stricturing complications is associated with better outcomes in the following 12 months. Other radiologic predictors of anti-TNF treatment failure identified in the CREOLE trial and observational studies were ileocolonic location,10 small bowel stricture larger than 12 cm,9 increased stricture wall thickness,9 stricture diameter,9 pre-stenotic dilatation,9,10 marked enhancement on delayed phase9 and abdominal fistulae.9,10 These prognostic markers may be useful but further research is required to improve the decision-making process in these patients with established bowel damage and a high risk of disabling disease.
Our study has some limitations that should be addressed. The retrospective collection of data may have limited the quality of the information, thus hindering the assessment of the main outcomes. On the other hand, we chose robust clinical endpoints (anti-TNF persistence with no steroid use, use of new immunomodulators or biologics, endoscopic therapy and surgery) that were used as objective outcomes of the effectiveness of this therapy. Another limitation is that the radiological follow-up examinations were not pre-established, and hence, the time interval and the indication may have differed between patients. A strength of the study is that our results come from a relatively large cohort recruited from a considerable number of hospitals, and this may have reduced the risk of bias. Furthermore, the definition of the main outcome is comparable to the CREOLE trial, allowing possible comparisons between both cohorts.
In conclusion, this large cohort of patients shows that a high proportion of patients with CD with symptomatic intestinal strictures benefit from anti-TNF therapy and the response may be improved by early introduction of this treatment. Approximately two-thirds of these patients are able to avoid intestinal surgery in the long term. Although currently available biologics may be effective in this context, there is still an unmet need for anti-fibrotic drugs that are able to slow or even reverse the progression of stricturing CD.
Author contributions: IR-L and JPG: conceived the study and its design, analysed and interpreted the data and drafted the manuscript. All authors: compiled the clinical information. UA: performed the statistical analysis. JPG: revised the manuscript for important intellectual content. All authors have significantly contributed and accepted the final version of the manuscript.
Ethics approval: The Spanish Agency of Medicines and Medical Devices (AEMPS) and the ethics committee of the Basque Country approved the study protocol (EPA2015025, 16/05/2018) and it was conducted according to the 1975 Declaration of Helsinki and the Good Clinical Practice guidelines.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: IR-L is supported by a research grant from Biocruces Bizkaia Health Research Institute [Grant No INT-BC-2018-007].
Informed consent: All patients signed the informed consent before their inclusion in the study.
Supplementary material: Supplementary material of Radiological follow-up examinations is available online.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IR-L has received financial support for travelling and educational activities from or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Ferring, Dr. Falk Pharma and Otsuka Pharmaceutical.
AP-G: has received financial support for travelling and educational activities from Rovi.
RF-I has served as a speaker for or has received research funding from Takeda, MSD, Abbvie, Janssen, Palex, Shire Pharmaceuticals, Tillotts Pharma and Casen Recordati.
MJC has received education funding from Pfizer, Janssen, MSD, Takeda, Ferring and Abbvie.
MC has served as a speaker or has received research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, and Tillotts Pharma.
JPG has served as a speaker, consultant or advisory board member for or has received research funding from MSD, Abbvie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Sandoz, Celgene, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma.
The remaining authors have no conflicts of interest related to this manuscript.
ORCID iDs
Iago Rodríguez-Lago https://orcid.org/0000-0003-1133-4578
María José García https://orcid.org/0000-0002-6517-7005
Maite Arroyo https://orcid.org/0000-0001-8089-4398
Pedro Delgado-Guillena https://orcid.org/0000-0002-5798-5491
Víctor Morales-Alvarado https://orcid.org/0000-0003-4379-2821
Javier P. Gisbert https://orcid.org/0000-0003-2090-3445
References
- 1.Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet 2016; 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 2012; 18: 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med 2013; 5: 167sr161. [DOI] [PubMed] [Google Scholar]
- 4.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017; 152: 340–350 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: An Epi-IBD study. Gut 2019; 68(3): 423–433. [DOI] [PubMed] [Google Scholar]
- 6.Magro F, Rodrigues-Pinto E, Coelho R, et al. Is it possible to change phenotype progression in Crohn's disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol 2014; 109: 1026–1036. [DOI] [PubMed] [Google Scholar]
- 7.Bharadwaj S, Fleshner P, Shen B. Therapeutic armamentarium for stricturing Crohn's disease: Medical versus endoscopic versus surgical approaches. Inflamm Bowel Dis 2015; 21: 2194–2213. [DOI] [PubMed] [Google Scholar]
- 8.Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn's disease. Inflamm Bowel Dis 2017; 23: 133–142. [DOI] [PubMed] [Google Scholar]
- 9.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allocca M, Bonifacio C, Fiorino G, et al. Efficacy of tumour necrosis factor antagonists in stricturing Crohn's disease: A tertiary center real-life experience. Dig Liver Dis 2017; 49: 872–877. [DOI] [PubMed] [Google Scholar]
- 11.Campos C, Perrey A, Lambert C, et al. Medical therapies for stricturing Crohn's disease: Efficacy and cross-sectional imaging predictors of therapeutic failure. Dig Dis Sci 2017; 62: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-lago I, Gisbert JP. The role of immunomodulators and biologics in the medical management of stricturing Crohn's disease. J Crohns Colitis 2020; 14(4): 557–566. [DOI] [PubMed] [Google Scholar]
- 13.Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn's disease and its impact on disease outcome. J Crohns Colitis 2017; 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 14.Fiorino G, Bonifacio C, Allocca M, et al. Impact of therapies on bowel damage in Crohn's disease. United European Gastroenterol J 2020; 8(4): 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomollon F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 16.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther 2018; 48: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn's disease. Gastroenterology 2003; 125: 1508–1530. [DOI] [PubMed] [Google Scholar]
- 19.Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–440. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauriot Dit Prevost C, Azahaf M, Nachury M, et al. Bowel damage and disability in Crohn's disease: A prospective study in a tertiary referral centre of the Lemann Index and Inflammatory Bowel Disease Disability Index. Aliment Pharmacol Ther 2020; 51: 889–898. [DOI] [PubMed] [Google Scholar]
- 22.Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 23.Safroneeva E, Vavricka SR, Fournier N, et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn's disease. Aliment Pharmacol Ther 2015; 42: 977–989. [DOI] [PubMed] [Google Scholar]
- 24.Frei R, Fournier N, Zeitz J, et al. Early initiation of Anti-TNF is associated with favourable long-term outcome in Crohn's disease: 10-year-follow-up data from the Swiss IBD Cohort Study. J Crohns Colitis 2019; 13: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 25.Louis E, Boverie J, Dewit O, et al. Treatment of small bowel subocclusive Crohn's disease with infliximab: An open pilot study. Acta gastro-enterologica Belgica 2007; 70: 15–19. [PubMed] [Google Scholar]
- 26.Toy LS, Scherl EJ, Kornbluth A. Complete bowel obstruction following initial response to infliximab therapy for Crohn’s disease: A series of a newly described complication. Gastroenterology 2000; 118: A569. [Google Scholar]
- 27.Vasilopoulos S, Kugathasan S, Saeian K. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am J Gastroenterol 2000; 95: 2503. [Google Scholar]
- 28.Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn's disease. Am J Gastroenterol 2006; 101: 10s30–1038. [DOI] [PubMed] [Google Scholar]