Abstract
Background
Biologically naïve patients with inflammatory bowel disease treated with vedolizumab (VDZ) are largely underrepresented in real-world cohorts. A multi-centre, observational cohort study was performed on the effectiveness and safety of VDZ in biologically naïve subjects with Crohn’s disease (CD) and ulcerative colitis (UC).
Methods
Data of consecutive biologically naïve patients with CD and UC treated with VDZ from July 2016 to December 2019 were extracted from the cohort of the Sicilian Network for Inflammatory Bowel Disease.
Results
A total of 172 consecutive patients (CD: N = 88; UC: N = 84; median age 66.0 years) were included, with a median follow-up of 58.8 weeks. After 14 weeks, a clinical response was reported in 68.2% of patients with CD and 67.9% of patients with UC treated with VDZ, including 45.5% patients in the CD group and 46.4% patients in the UC group who achieved steroid-free remission. After 52 weeks, a clinical response was reported in 77.4% of CD and in 73.8% of UC patients treated with VDZ, including 59.7% patients in the CD group and 60.7% patients in the UC group who achieved steroid-free remission.
Conclusions
This study demonstrates the effectiveness and safety of VDZ as a first-line biological, particularly among elderly patients.
Keywords: Vedolizumab, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, IBD
Introduction
Available since mid-2016, vedolizumab (VDZ) is a humanised monoclonal antibody that targets the α4β7 integrin, typically expressed by gut-homing lymphocytes. This biological agent is approved for the treatment of patients with moderately to severely active Crohn’s disease (CD) or ulcerative colitis (UC) who had an inadequate response with or lost response to either conventional therapy or anti-tumour necrosis factor (TNF) drugs, or who were intolerant to or not suitable for these because of co-morbidities.1 The efficacy of VDZ in CD and UC has been demonstrated against placebo in three pivotal Phase III randomised controlled trials – GEMINI 1 (UC),2 GEMINI 2 (CD)3 and GEMINI 3 (CD)4 – while a favourable safety profile has been also reported.5 In the GEMINI 1 study, VDZ had a higher rate of response in UC patients who were naïve to anti-TNFs in comparison with anti-TNFs experienced at both week 6 and week 52,6 while a post-hoc analysis from both GEMINI 2 and GEMINI 3 revealed that the rate of remission in CD was numerically higher in patients naïve to anti-TNFs compared to those with experience of anti-TNFs.7 As a consequence, data from randomised controlled trials showed higher efficacy of VDZ among patients naive to anti-TNFs compared to those with experience of anti-TNFs.
Over the last four years, several real-world experiences on VDZ in inflammatory bowel disease (IBD) were published.8–27 Overall, all of these studies confirmed the efficacy and safety demonstrated by VDZ in clinical trials, even if the majority of the patients enrolled had at least one previous failure to anti-TNFs, while the proportion of patients who were naïve to biologicals was low. So, there is need to explore this setting further.
On these premises, web-based data from the cohort of the Sicilian Network for Inflammatory Bowel Disease (SN-IBD) were extracted to perform a multi-centre, real-world assessment of the effectiveness and safety of VDZ as induction and maintenance treatment among biologically naïve patients with CD and those with UC.
Methods
Patients
The SN-IBD is a regional group composed of all 16 centres licensed to prescribe biologicals in Sicily (Italy). Since January 2013, real-life prospective data on patients with IBD treated with biologicals at these centres have been entered into web-based software with the aim of monitoring the efficacy, safety and costs of these therapeutics. All consecutive biologically naïve adult patients treated with VDZ from July 2016 (the date on which the drug became available for clinical practice in Sicily) to December 2019 were extracted from the SN-IBD cohort for the purposes of this study. All biologically naive IBD patients with active disease who received at least one infusion of VDZ and with at least 14 weeks of follow-up were included. We excluded patients who were previously treated with other biologicals, as well as those with less than 14 weeks of follow-up. VDZ was used according to the recommended dosage and modalities. A fourth 10-week induction dose in patients with CD was administered in case of insufficient response to the first three doses, based on the physician’s judgement. We also included patients who had undergone treatment optimisation by reducing the administration interval to four weeks.
Data collection and measures of outcome
Study outcomes were evaluated at 14 and 52 weeks of follow-up. At each time point, the evaluation included participants who had completed follow-up, including those who had stopped treatment. The primary outcome was the clinical response, defined as a reduction of ≥3 in the Harvey–Bradshaw Index for CD and a partial Mayo Score of ≥2 for UC compared to baseline, with a concomitant reduction of ≥50% of steroid dosage among those receiving steroids at baseline. Secondary outcomes included: (a) steroid-free clinical remission – Harvey–Bradshaw Index <5 for CD and a partial Mayo Score <2 for UC without steroid use; (b) C-reactive protein (CRP) normalisation at weeks 14 and 52 in patients with a CRP above the upper limit of normal at baseline; (c) rate of secondary loss of response; (d) mucosal healing – Simple Endoscopic Score for CD (SES-CD) ≤2 for CD and endoscopic Mayo Score ≤1 for UC; (e) endoscopic response – reduction of SES-CD ≥50% compared to baseline without the achievement of mucosal healing for CD, and reduction of endoscopic Mayo Score from 3 to 2 in UC; and (f) treatment persistence at the end of follow-up.
The following data were collected for each patient at baseline: age, sex, type of disease (CD or UC), smoking habit, disease duration, CRP values (normal values: <5 mg/L), presence of extra-intestinal manifestations, presence of steroid dependency or steroid resistance and concomitant therapy with systemic steroids or immunosuppressants at baseline. For patients with CD, the following data were also collected: disease localisation and behaviour according to the Montreal classification, previous resections and disease activity evaluated clinically with the Harvey–Bradshaw Index and endoscopically with the SES-CD. For patients with UC, we also collected disease extent according to the Montreal classification and disease activity evaluated clinically with the partial Mayo Score and endoscopically with the endoscopic Mayo Score. Steroid dependency was defined as the inability to stop steroids within three months of starting therapy without experiencing a clinical relapse or relapse within three months after steroid weaning, while steroid refractoriness was defined as active disease in spite of an adequate dose and duration of prednisone therapy (prednisone, 0.75–1 mg/kg/day orally for at least two weeks; methylprednisolone, 1 mg/kg/day intravenously for one week).28
Statistics
Continuous variables are reported as medians with interquartile ranges (IQR), and categorical variables as frequency and percentage. The Mann–Whitney U-test and chi-square test (or Fisher’s exact test, where needed) were used for comparison of continuous and categorical variables, respectively. Multiple logistic regression analyses were performed to identify independent predictors of clinical response at week 14 and week 52 among patients with CD and UC. As candidate factors, we selected all the characteristics at baseline. Variable selection was performed using a stepwise backward elimination approach, based on the Akaike information criterion. Furthermore, to investigate the effect of the variables on treatment persistence, a survival analysis using a multiple Cox proportional hazard model was performed. Both logistic and Cox PH model were fitted using Firth’s bias reduction method29 to solve the problem of separation of data that can be caused by occurrence of small sample size and/or unbalanced or highly predictive risk factors.30 All statistical analyses were performed using R v3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). A p-value of ≤0.05 was considered statistically significant.
Results
Patients
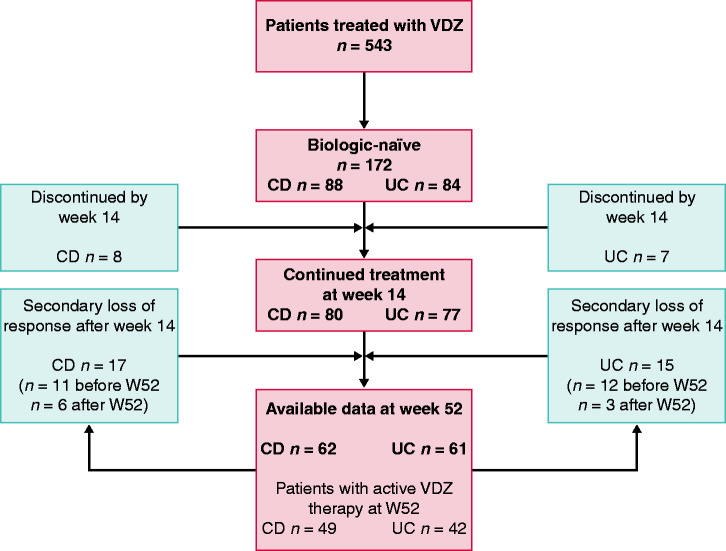
Out of 543 total patients treated with VDZ, 172 (31.7%) consecutive biologically naïve patients (CD: N = 88; UC: N = 84) were included, with a median follow-up of 58.8 weeks (IQR 30.7–101.5 weeks). A total of 123 patients (CD: n = 62; UC: n = 61) had at least 52 weeks of follow-up. Table 1 shows the baseline characteristics of patients stratified according to type of disease (CD vs. UC). The only significant difference between the two groups was in the proportion of current/former/never smokers. Of note, the median age of the patients was high (66.0 years; IQR 55.7–72.0 years), while disease activity was mainly mild at baseline, even if more than half of patients (52.9%) were taking steroids at baseline and/or were steroid dependent (65.1%) or steroid resistant (4.6%), and 52.5% had CRP levels above the upper limit of normal (normal values: <5.0 mg/L). National guidance in Italy favours TNF antagonists as first-line biologicals in IBD. The rationale for selecting VDZ within our biologically naïve cohort was as follows: advanced age in 122 (70.9%) patients, history of malignancy or a premalignant condition in 23 (13.4%) patients, previous serious infection with azathioprine or methotrexate in 11 (6.4%) patients, latent tuberculosis in eight (4.6%) patients, congestive heart failure in six (3.5%) patients and demyelinative disease in two (1.2%) patients. The study flow is depicted in Figure 1.
Table 1.
Baseline characteristics of patients, stratified according to type of disease (Crohn’s disease vs. ulcerative colitis).
| Variable | CD (N = 88) | UC (N = 84) | p-Value |
|---|---|---|---|
| Male sex, n (%) | 55 (62.5%) | 55 (65.5%) | 0.805 |
| Age (years), median (IQR) | 65.0 (52.8–71.0) | 66.9 (57.8–71.5) | 0.101 |
| Smokers, n (%) | |||
| Never | 59 (67.0%) | 51 (60.7%) | <0.001 |
| Current | 19 (21.6%) | 5 (6.0%) | |
| Former | 10 (11.4%) | 28 (33.3%) | |
| Duration of disease (years), median (IQR) | 7.00 (3.00–19.0) | 10.0 (4.0–18.5) | 0.372 |
| C-reactive protein higher than normal values, n (%) | 44 (54.3%) | 41 (50.6%) | 0.753 |
| Extra-intestinal manifestations, n (%) | 21 (23.9%) | 12 (14.3%) | 0.161 |
| Steroid dependent, n (%) | 52 (59.1%) | 60 (71.4%) | 0.124 |
| Steroid refractory, n (%) | 2 (2.3%) | 6 (7.1%) | 0.161 |
| Systemic steroids at baseline, n (%) | 43 (48.9%) | 48 (57.1%) | 0.350 |
| Concurrent therapy with immunosuppressant, n (%) | |||
| None | 83 (94.3%) | 80 (95.2%) | 1.000 |
| Azathioprine | 2 (2.3%) | 2 (2.4%) | |
| Methotrexate | 3 (3.4%) | 2 (2.4%) |
CD: Crohn’s disease; UC: ulcerative colitis; IQR: interquartile range.
Statistically significant values are shown in bold.
Figure 1.
Study flow chart.
Effectiveness at induction
CD
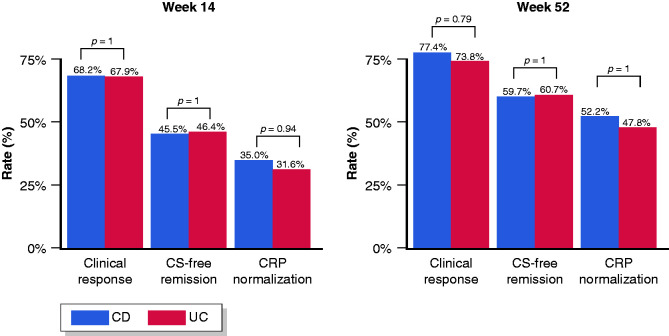
After 14 weeks, a clinical response was reported in 68.2% of patients with CD, including 45.5% patients who achieved steroid-free remission. Furthermore, CRP normalisation at week 14 was obtained in 35.0% of CD patients with elevated CRP at baseline (Figure 2). An additional week 10 infusion was administered in 38/88 (43.2%) patients with CD, while 8/88 (9.1%) patients discontinued treatment by week 14 (two due to adverse events, and six due to inefficacy). Median CRP values were not significantly different between baseline (5.4; IQR 1.8–9.6) and week 14 (5.0; IQR 2.5–8.8).
Figure 2.
Clinical effectiveness of vedolizumab among biologically naïve patients with Crohn’s disease and ulcerative colitis at 14 and 52 weeks.
UC
After 14 weeks, a clinical response was reported in 67.9% of patients with UC treated with VDZ, including 46.4% patients who achieved steroid-free remission. Furthermore, CRP normalisation at week 14 was obtained in 31.6% of UC patients with elevated CRP at baseline (Figure 2). Out of 84 patients, seven (8.3%) discontinued the treatment by week 14 (two due to adverse events, and five due to inefficacy). Median CRP values were not significantly different between baseline (5.6; IQR 2.0–10.1) and week 14 (4.9; IQR 2.5–8.8).
Effectiveness at maintenance
CD
After 52 weeks, a clinical response was reported in 77.4% of CD patients treated with VDZ, including 59.7% patients who achieved steroid-free remission. In addition, CRP normalisation at week 52 was obtained in 52.2% of CD patients with elevated CRP at baseline (Figure 2). Median CRP values were significantly reduced from baseline (5.4; IQR 1.8–9.6) to week 52 (2.7; IQR 1.2–6.5).
UC
After 52 weeks, a clinical response was reported in 73.8% of UC patients, including 60.7% patients who achieved steroid-free remission. Furthermore, CRP normalisation at week 52 was obtained in 47.8% of UC patients with elevated CRP at baseline (Figure 2). Median CRP values were significantly reduced from baseline (5.6; IQR 2.0–10.1) to week 52 (2.8; IQR 1.4–6.9).
Overall, 41/157 (26.1%) patients who started a maintenance treatment with VDZ underwent treatment optimisation, without significant differences between CD and UC (CD: 22/79 (27.8%) vs. UC: 19/78 (24.4%); p = 0.79). At the end of follow-up, loss of response following successful induction was reported in 32/157 (20.4%) patients (CD: n = 17; UC: n = 15), while 47/172 (27.3%) patients in the entire cohort discontinued treatment (CD: 28.4% vs. UC 26.2%; p = 0.88), 37 (21.5%) due to inefficacy and 10 (5.8%) due to adverse events, while four CD patients and two UC patients underwent surgery (CD: 4.5% vs. UC 2.4%; p = 0.68). Cox survival analysis showed no significant difference in the probability of treatment discontinuation between CD and UC patients (log-rank p = 0.73; Figure 3).
Figure 3.
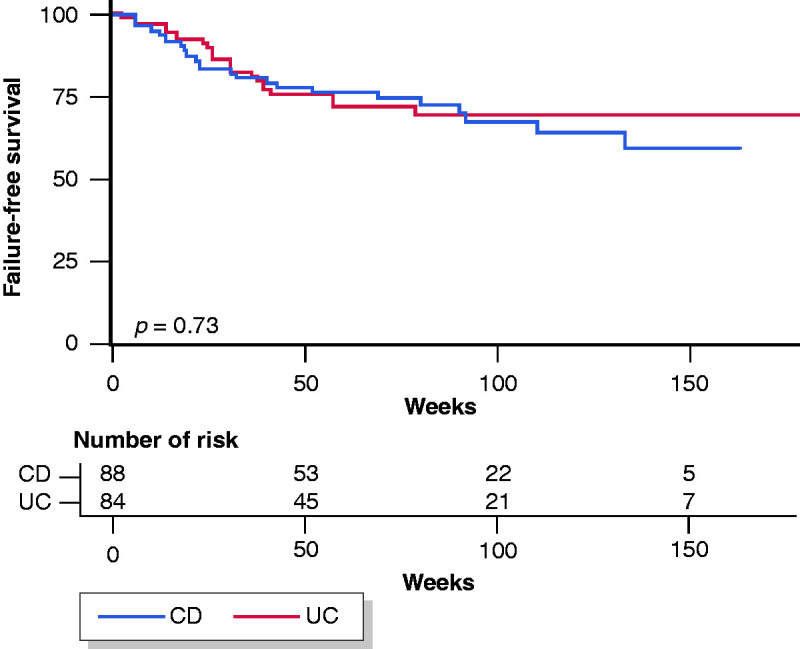
Treatment discontinuation among biologically naïve patients with Crohn’s disease and ulcerative colitis.
At the end of follow-up, 31/88 (35.2%) CD patients and 42/84 (50.0%) UC patients underwent colonoscopy (42.4% of the entire cohort) after a median of 60.1 weeks (IQR 52.0–81.6 weeks) from the beginning of VDZ treatment. Mucosal healing rates were not statistically significant between CD and UC patients (CD: 41.9% vs. UC: 57.1%; p = 0.29). An endoscopic response (a reduction of SES-CD of ≥50% compared to baseline without the achievement of mucosal healing) was reported in 10/31 (35.3%) CD patients, while a reduction of endoscopic Mayo Score from 3 to 2 was achieved by 6/42 (14.3%) UC patients.
Predictors of response at induction
CD
The presence of steroid dependence was the only factor independently associated with a lack of clinical response at week 14 by multiple logistic regression analysis (odds ratio (OR) = 0.34, 95% confidence interval (CI) 0.11–0.92, p = 0.003; Table 2).
Table 2.
Multiple logistic regression model estimates for clinical response in patients with Crohn’s disease and ulcerative colitis at week 14.
| Variable | Odds ratio | 95% CI | p-Value |
|---|---|---|---|
| Crohn’s disease | |||
| Female sex (ref: male) | 0.52 | 0.20–1.34 | 0.176 |
| Age (one-year increment) | 0.98 | 0.95–1.02 | 0.445 |
| Steroid dependency | 0.34 | 0.11–0.92 | 0.033 |
| Colic localisation (ref: ileal) | 1.33 | 0.19–15.20 | 0.780 |
| Ileo-colic localisation (ref: ileal) | 1.17 | 0.42–3.37 | 0.768 |
| Upper gastrointestinal localisation (ref: ileal) | 0.25 | 0.04–1.35 | 0.108 |
| Ulcerative colitis | |||
| Female sex (ref: male) | 0.69 | 0.23–2.01 | 0.491 |
| Age (one-year increment) | 0.96 | 0.92–1.01 | 0.100 |
| Steroid refractoriness | 0.16 | 0.01–0.97 | 0.046 |
| Proctitis (ref: extensive colitis) | 8.29 | 0.76–1173 | 0.091 |
| Left-sided colitis (ref: extensive colitis) | 1.84 | 0.67–5.27 | 0.236 |
| Partial Mayo Score (one-point increase) | 0.81 | 0.62–1.02 | 0.071 |
CI: confidence interval.
UC
The presence of steroid refractoriness was the only factor independently associated with a lack of clinical response at week 14 in patients with UC (OR = 0.16, 95% CI 0.01–0.97, p = 0.046; Table 2).
Predictors of response at maintenance
CD
The presence of upper gastrointestinal localisation was the only factor independently associated with a lack of clinical response at week 52 by multiple logistic regression analysis (OR = 0.14, 95% CI 0.02–0.84, p = 0.032; Table 3), while a multiple Cox model confirmed that the upper gastrointestinal localisation was the only factor independently associated with treatment failure (hazard ratio (HR) = 3.27, 95% CI 1.06–9.13, p = 0.044; Table 4).
Table 3.
Multiple logistic regression model estimates for clinical response in patients with Crohn’s disease and ulcerative colitis at week 52.
| Variable | Odds ratio | 95% CI | p-Value |
|---|---|---|---|
| Crohn’s disease | |||
| Female sex (ref: male) | 0.35 | 0.09–1.18 | 0.091 |
| Colic localisation (ref: ileal) | 0.78 | 0.11–9.08 | 0.817 |
| Ileo-colic localisation (ref: ileal) | 1.41 | 0.34–6.76 | 0.638 |
| Upper gastrointestinal localisation (ref: ileal) | 0.14 | 0.02–0.84 | 0.032 |
| Ulcerative colitis | |||
| Steroid refractoriness | 0.19 | 0.03–1.08 | 0.061 |
| Partial Mayo Score (one-point increase) | 0.71 | 0.50–0.95 | 0.022 |
Table 4.
Multiple Cox regression model estimates for treatment failure in patients with Crohn’s disease and ulcerative colitis at the end of follow-up.
| Variable | Hazard ratio | 95% CI | p-Value |
|---|---|---|---|
| Crohn’s disease | |||
| Extra-intestinal manifestations | 2.02 | 0.87–4.45 | 0.098 |
| Colic localisation (ref: ileal) | 1.08 | 0.11–4.47 | 0.929 |
| Ileo-colic localisation (ref: ileal) | 0.78 | 0.31–1.85 | 0.569 |
| Upper gastrointestinal localisation (ref: ileal) | 3.27 | 1.06–9.13 | 0.044 |
| Ulcerative colitis | |||
| Steroid refractoriness | 3.95 | 1.01–11.52 | 0.049 |
| Partial Mayo score (1-point increase) | 1.23 | 1.01–4.02 | 0.045 |
UC
The partial Mayo Score at baseline was the only factor independently associated with a lack of clinical response at week 52 in patients with UC (OR = 0.71 per-point increase, 95% CI 0.50–0.95, p = 0.022; Table 3), while a multiple Cox model showed that the partial Mayo Score at baseline and the presence of steroid refractoriness at baseline were independently associated with treatment failure (HR = 1.23 per-point increase, 95% CI 1.01–4.02, p = 0.045; and HR = 3.95, 95% CI 1.01–11.52, p = 0.049, respectively; Table 4).
Safety
Twenty-five (14.5%) adverse events were reported (CD: n = 18, incidence rate: 15.2/100 person-years; UC: n = 7, incidence rate: 6.2/100 person-years), of which 10 caused the interruption of the treatment. The rates of adverse events were significantly higher in patients with CD compared to those with UC (incidence rate ratio for CD = 2.36, p = 0.036). In detail, the following adverse events were reported: six articular flares, four subjective symptoms (poorly defined malaise and asthenia), three pneumonia, three cutaneous herpes zoster eruptions, two urinary tract infections, two Clostridium difficile infections, one infusion reaction, one genital mycosis, one otitis media, one abdominal abscess and one colorectal cancer.
Discussion
This multi-centre, real-world study of patients with CD and UC aimed to assess the effectiveness and safety of VDZ in patients who were naïve to biologicals. In these subjects, VDZ was used due to the presence of a contraindication to anti-TNF therapy or a safety concern that led to the choice of a non-anti-TNF biological drug, in accordance with the current VDZ indications. Overall, the rates of clinical response and steroid-free remission were high for both CD and UC at 14 and 52 weeks, and were accompanied by high rates of CRP normalisation – a sign of good control of systemic inflammation. Furthermore, the achievement of post-treatment mucosal healing was reported in approximately half of the patients who had available endoscopic data (42.4%). Another relevant point emerging from our analysis lies in the tendency towards a higher efficacy, going from 14 to 52 weeks for all clinical outcomes, reaching peaks of clinical response of 77.4% for CD patients and 73.8% for UC patients at 52 weeks – data which could reflect a slow but growing response to VDZ in clinical practice. These results may be compared to those reported by the well-conducted, multi-centre, retrospective European study by Kopylov et al.,27 which also focused on the effectiveness and safety of VDZ among biologically naïve patients. Good efficacy was also emphasised in the European study, with higher rates of clinical response and corticosteroid-free remission rates after 14 weeks compared to our cohort, while rates for clinical outcomes at maintenance were similar to ours. It should be noted that the two cohorts are only partially comparable, as the median age of the patients was lower in the Kopylov study (49 years for CD and 45 years for UC compared to 65 years for CD and 66.9 years for UC in our study), and long-term follow-up data were limited to a relatively small proportion of patients in the European study.
Taken altogether, these encouraging results – evaluated inside the rapidly evolving scenario of the current treatment of IBD – should be taken into account for the development of individualised treatment approaches. In line with this, our study could lead to considering the possibility of also using VDZ as a first-line agent, particularly in elderly patients, while these results may be not applicable to young patients because of the peculiar demographics of our cohort. Indeed, patients treated with VDZ in our cohort did not show the negative outcomes exhibited by elderly biologically naïve patients treated with anti-TNFs, as emphasised by a recent SN-IBD study, where older (≥60 years) IBD patients treated for the first time with anti-TNF agents showed lower treatment persistence than younger patients did.31 Furthermore, our results are in line with a recent study by Adar et al. showing similar – and good – effectiveness of anti-TNF and VDZ therapy in elderly (≥60 years) IBD patients.32
Our study demonstrated favourable safety outcomes, with adverse events reported in 14.5% of patients compared to a rate of 30.6% that emerged from a cumulative analysis of real-world data.33 Of note, infections were reported in 3.4% of patients, while we observed a higher rate (7.0%). These results should always be interpreted keeping in mind the advanced median age of our cohort. However, a cautious approach is always needed, particularly in patients with CD, whose incidence rate of adverse events was approximately twice that of UC patients. It is difficult to discriminate the causative agent in the occurrence of adverse events – in other words, whether VDZ was directly responsible for the adverse events or alternatively whether they were due to the severity of the disease, obviously coupled with the advanced age of the patients enrolled in our study.
This study has strengths and limitations. The main strengths lie in the choice of robust end points –evaluated with clinical scores – in order to assess the effectiveness of VDZ as objectively as possible, and in the relatively long follow-up. In addition, we focused on a specific and often underrepresented fraction of patients (i.e. elderly subjects), and this represents a relevant novel aspect. The main limitation lies in the fact that endoscopic follow-up data were available only in approximately 40% of the patients, and the reported rates could be also distorted by the possibility that a proportion of patients could have interrupted the treatment early for evidence of clinical inefficacy, without the need for a post-treatment colonoscopy. However, it should be noted that the lack of systematically collected endoscopic data is almost inevitable in all real-world studies. Furthermore, faecal calprotectin data were not available. In Italy, the National Health Service does not fund this test. As such, it is not routinely performed outside private practice.
In conclusion, this large, real-world, multi-centre study demonstrates the effectiveness and safety of VDZ as a first-line biological in patients with IBD, showing high rates of clinical response and steroid-free remission at both induction and maintenance. These results should also be kept in mind when considering the possibility of using VDZ as a first-line agent, particularly in elderly subjects, and not just in cases of a previous failure of anti-TNFs.
Appendix
STROBE Statement: Checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | V (a) Indicate the study’s design with a commonly used term in the title or the abstract |
| V (b) Provide in the abstract an informative and balanced summary of what was done and what was found | ||
| Introduction | ||
| Background/rationale | 2 | V Explain the scientific background and rationale for the investigation being reported |
| Objectives | 3 | V State specific objectives, including any pre-specified hypotheses |
| Methods | ||
| Study design | 4 | V Present key elements of study design early in the paper |
| Setting | 5 | V Describe the setting, locations and relevant dates, including periods of recruitment, exposure, follow-up and data collection |
| Participants | 6 | V (a) Give the eligibility criteria and the sources and methods of selection of participants; describe methods of follow-up |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | ||
| Variables | 7 | V Clearly define all outcomes, exposures, predictors, potential confounders and effect modifiers; give diagnostic criteria, if applicable |
| Data sources/ measurement | 8* | V For each variable of interest, give sources of data and details of methods of assessment (measurement); describe comparability of assessment methods if there is more than one group |
| Bias | 9 | V Describe any efforts to address potential sources of bias |
| Study size | 10 | Explain how the study size was arrived at |
| Quantitative variables | 11 | V Explain how quantitative variables were handled in the analyses; if applicable, describe which groupings were chosen and why |
| Statistical methods | 12 | V (a) Describe all statistical methods, including those used to control for confounding |
| V (b) Describe any methods used to examine subgroups and interactions | ||
| V (c) Explain how missing data were addressed | ||
| (d) If applicable, explain how loss to follow-up was addressed | ||
| (e) Describe any sensitivity analyses | ||
| Results | ||
| Participants | 13* | V (a) Report numbers of individuals at each stage of study – e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analysed |
| (b) Give reasons for non-participation at each stage | ||
| (c) Consider use of a flow diagram | ||
| Descriptive data | 14* | V (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders |
| V (b) Indicate number of participants with missing data for each variable of interest | ||
| (c) Summarise follow-up time (e.g. average and total amount) | ||
| Outcome data | 15* | V Report numbers of outcome events or summary measures over time |
| Main results | 16 | V (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% confidence interval); make clear which confounders were adjusted for and why they were included |
| V (b) Report category boundaries when continuous variables were categorised | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | V Report other analyses done – e.g. analyses of subgroups and interactions, and sensitivity analyses |
| Discussion | ||
| Key results | 18 | V Summarise key results with reference to study objectives |
| Limitations | 19 | V Discuss limitations of the study, taking into account sources of potential bias or imprecision; discuss both direction and magnitude of any potential bias |
| Interpretation | 20 | V Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies and other relevant evidence |
| Generalisability | 21 | V Discuss the generalisability (external validity) of the study results |
| Other information | ||
| Funding | 22 | V Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F.S.M. served as an advisory board member and/or received lecture grants from AbbVie, Biogen, MSD, Pfizer and Takeda Pharmaceuticals. W.F. served as an advisory board member and/or received lecture grants from AbbVie, Biogen, MSD, Pfizer, Mundipharma, Zambon, Janssen, Sandoz and Takeda Pharmaceuticals. S.R. served as an advisory board member for AbbVie and MSD Pharmaceuticals, and received lecture grants from AbbVie, MSD and Takeda Pharmaceuticals. M.C. served as an advisory board member for AbbVie, MSD and Takeda Pharmaceuticals, and received lecture grants from AbbVie, MSD, Chiesi and Takeda Pharmaceuticals. A.O. served as an advisory board member for AbbVie, MSD, Janssen, Pfizer and Takeda Pharmaceuticals, and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer and Takeda Pharmaceuticals.
Ethics approval: The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. As this was a retrospective observational study, no ethical approval was needed in agreement with the local legislation.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Informed consent: Informed consent has been obtained from each patient at the time of inclusion in the database of the Sicilian Network For Inflammatory Bowel Diseases (SN-IBD).
ORCID iDs
Fabio Salvatore Macaluso https://orcid.org/0000-0001-5128-3846
Anna Viola https://orcid.org/0000-0002-1733-6489
References
- 1.Armuzzi A, Gionchetti P, Daperno M, et al. Expert consensus paper on the use of vedolizumab for the management of patients with moderate-to-severe inflammatory bowel disease. Dig Liver Dis 2016; 48: 360–370. [DOI] [PubMed] [Google Scholar]
- 2.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 4.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627. [DOI] [PubMed] [Google Scholar]
- 5.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017; 66: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017; 15: 229–239. [DOI] [PubMed] [Google Scholar]
- 7.Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 2017; 23: 97–106. [DOI] [PubMed] [Google Scholar]
- 8.Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease – the Israeli real world experience. Inflamm Bowel Dis 2017; 23: 404–408. [DOI] [PubMed] [Google Scholar]
- 9.Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14: 1593–1601. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment Pharmacol Ther 2016; 43: 1090–1102. [DOI] [PubMed] [Google Scholar]
- 11.Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis 2015; 21: 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivio EE, Kanuri N, Gilbertsen JJ, et al. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J Crohns Colitis 2016; 10: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY Consortium. Am J Gastroenterol 2016; 111: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 14.Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease – a prospective multicenter observational study. Aliment Pharmacol Ther 2016; 44: 1199–1212. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson C, Marsal J, Bergemalm D, et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish national quality registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol 2017; 52: 722–729. [DOI] [PubMed] [Google Scholar]
- 16.Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017; 15: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 17.Allegretti JR, Barnes EL, Stevens B, et al. Predictors of clinical response and remission at 1 year among a multicenter cohort of patients with inflammatory bowel disease treated with vedolizumab. Dig Dis Sci 2017; 62: 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macaluso FS, Orlando R, Fries W, et al. The real-world effectiveness of vedolizumab on intestinal and articular outcomes in inflammatory bowel diseases. Dig Liver Dis 2018; 50: 675–681. [DOI] [PubMed] [Google Scholar]
- 19.Reinglas J, Gonczi L, Verdon C, et al. Low rate of drug discontinuation, frequent need for dose adjustment, and no association with development of new arthralgia in patients treated with vedolizumab: results from a tertiary referral IBD center. Dig Dis Sci 2020; 65: 2046–2053. [DOI] [PubMed] [Google Scholar]
- 20.Faleck DM, Winters A, Chablaney S, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buer LCT, Moum BA, Cvancarova M, et al. Real world data on effectiveness, safety and therapeutic drug monitoring of vedolizumab in patients with inflammatory bowel disease. A single center cohort. Scand J Gastroenterol 2019; 54: 41–48. [DOI] [PubMed] [Google Scholar]
- 22.Plevris N, Chuah CS, Allen RM, et al. Real-world effectiveness and safety of vedolizumab for the treatment of inflammatory bowel disease: the Scottish vedolizumab cohort. J Crohns Colitis 2019; 13: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 23.Cummings F, Gaya DR, Levison S, et al. A retrospective observational study of early experiences of vedolizumab treatment for inflammatory bowel disease in the UK: the REVIVE study. Medicine (Baltimore) 2019; 98: e14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amiot A, Serrero M, Peyrin-Biroulet L, et al. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther 2019; 50: 40–53. [DOI] [PubMed] [Google Scholar]
- 25.Tursi A, Mocci G, Faggiani R, et al. Vedolizumab is effective and safe in real-life treatment of inflammatory bowel diseases outpatients: a multicenter, observational study in primary inflammatory bowel disease centers. Eur J Intern Med 2019; 66: 85–91. [DOI] [PubMed] [Google Scholar]
- 26.Biemans VBC, Van Der Woude CJ, Dijkstra G, et al. Vedolizumab for inflammatory bowel disease: two-year results of the Initiative on Crohn and Colitis (ICC) registry, a nationwide prospective observational cohort study: ICC registry – vedolizumab. Clin Pharmacol Ther 2020; 107: 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naïve patients with inflammatory bowel disease – a multicenter retrospective European study. Inflamm Bowel Dis 2018; 24: 2442–2451. [DOI] [PubMed] [Google Scholar]
- 28.Gionchetti P, Rizzello F, Annese V, et al. Use of corticosteroids and immunosuppressive drugs in inflammatory bowel disease: clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease. Dig Liver Dis 2017; 49: 604–617. [DOI] [PubMed] [Google Scholar]
- 29.Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27–38. [Google Scholar]
- 30.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002; 21: 2409–2419. [DOI] [PubMed] [Google Scholar]
- 31.Porcari S, Viola A, Orlando A, et al. Persistence on anti-tumour necrosis factor therapy in older patients with inflammatory bowel disease compared with younger patients: data from the Sicilian Network for Inflammatory Bowel Diseases (SN-IBD). Drugs Aging 2020; 37: 383–392. [DOI] [PubMed] [Google Scholar]
- 32.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor α antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther 2019; 49: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD – lessons from real-world experience; a systematic review and pooled analysis. J Crohns Colitis 2018; 12: 245–257. [DOI] [PubMed] [Google Scholar]