Key Points
Question
What is the clinical efficacy of co-trimoxazole (trimethoprim-sulfamethoxazole) in idiopathic pulmonary fibrosis (IPF) in terms of time to death (all causes), lung transplant, or first nonelective hospital admission?
Findings
In this randomized clinical trial, which included 343 patients with moderate or severe IPF, the incidence of the composite outcome among those treated with oral co-trimoxazole, 960 mg twice daily, vs those treated with placebo was 0.45 vs 0.38 per person-year after a median follow-up of 1.02 years; the hazard ratio was not statistically significant.
Meaning
Co-trimoxazole compared with placebo did not improve a composite clinical outcome among patients with moderate or severe IPF.
Abstract
Importance
Idiopathic pulmonary fibrosis (IPF) has a poor prognosis and limited treatment options. Patients with IPF have altered lung microbiota, with bacterial burden within the lungs associated with mortality; previous studies have suggested benefit with co-trimoxazole (trimethoprim-sulfamethoxazole).
Objective
To determine the efficacy of co-trimoxazole in patients with moderate and severe IPF.
Design, Setting, and Participants
Double-blind, placebo-controlled, parallel randomized trial of 342 patients with IPF, breathlessness (Medical Research Council dyspnea scale score >1), and impaired lung function (forced vital capacity ≤75% predicted) conducted in 39 UK specialist interstitial lung disease centers between April 2015 (first patient visit) and April 2019 (last patient follow-up).
Interventions
Study participants were randomized to receive 960 mg of oral co-trimoxazole twice daily (n = 170) or matched placebo (n = 172) for between 12 and 42 months. All patients received 5 mg of folic acid orally once daily.
Main Outcomes and Measures
The primary outcome was time to death (all causes), lung transplant, or first nonelective hospital admission. There were 15 secondary outcomes, including the individual components of the primary end point respiratory-related events, lung function (forced vital capacity and gas transfer), and patient-reported outcomes (Medical Research Council dyspnea scale, 5-level EuroQol 5-dimension questionnaire, cough severity, Leicester Cough Questionnaire, and King’s Brief Interstitial Lung Disease questionnaire scores).
Results
Among 342 individuals who were randomized (mean age, 71.3 years; 46 [13%] women), 283 (83%) completed the trial. The median (interquartile range) duration of follow-up was 1.02 (0.35-1.73) years. Events per person-year of follow-up among participants randomized to the co-trimoxazole and placebo groups were 0.45 (84/186) and 0.38 (80/209), respectively, with a hazard ratio of 1.2 ([95% CI, 0.9-1.6]; P = .32). There were no statistically significant differences in other event outcomes, lung function, or patient-reported outcomes. Patients in the co-trimoxazole group had 696 adverse events (nausea [n = 89], diarrhea [n = 52], vomiting [n = 28], and rash [n = 31]) and patients in the placebo group had 640 adverse events (nausea [n = 67], diarrhea [n = 84], vomiting [n = 20], and rash [n = 20]).
Conclusions and Relevance
Among patients with moderate or severe IPF, treatment with oral co-trimoxazole did not reduce a composite outcome of time to death, transplant, or nonelective hospitalization compared with placebo.
Trial Registration
ISRCTN Identifier: ISRCTN17464641
This randomized clinical trial compares the effects of co-trimoxazole (trimethoprim-sulfamethoxazole [TMP-SMX]) vs placebo on time to death, lung transplant, or hospital admission in patients with moderate or severe idiopathic pulmonary fibrosis (IPF).
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease with a median survival of 5.7 years,1 increasing incidence,2 and limited treatment options. Respiratory tract infection is common in patients with IPF3 and bronchial washings contain pathogenic bacteria, as identified by quantitative culture4,5 or non–culture-dependent techniques.6 Bronchoalveolar lavage studies from 2 separate groups have shown that a high bacterial load was associated with reduced lung function and death,7,8 and a lung microbiota enriched with Streptococcus species and Staphylococcus species was associated with reduced progression-free survival9 in IPF. Moreover, innate immune responses may be abnormal in IPF, potentially increasing susceptibility to infection.10,11
Co-trimoxazole (trimethoprim-sulfamethoxazole), a broad-spectrum antibiotic, was reported to improve clinical outcomes in IPF in 2 small randomized clinical trials,12,13 and was found to be cost-effective.14 An exploratory analysis suggested an improvement in health-related quality of life and oxygen requirements and, in those adhering to the study protocol, a reduction in mortality over a 12-month period; however, evidence of a survival benefit was not conclusive.13
The aim of the current study was to determine the clinical efficacy of co-trimoxazole in patients with moderate or severe IPF (defined as forced vital capacity [FVC] ≤75% predicted) in terms of the time to death (all-cause), lung transplant, or first nonelective hospital admission. Secondary aims were to assess the effects on respiratory-related outcomes, patient-reported outcomes (in terms of health-related quality of life, cough, and breathlessness), and lung function.
Methods
This was a phase 3 double-blind, placebo-controlled, parallel, randomized, multicenter study of oral co-trimoxazole added to standard care. The protocol has been published,15 and the final protocol, amendments, and statistical analysis plan are available in Supplement 1 and Supplement 2. The study was conducted according to good clinical practice, and the study protocol received ethical approval (14/LO/1800).
Patients were treated from randomization until withdrawal, death, first nonelective admission (for any reason), lung transplant, or the end of the study follow-up, with a minimum duration of 12 months and maximum of 42 months. The study was conducted in 43 specialist interstitial lung disease centers, or in sites affiliated with them, from all regions in the UK.
All participants provided written informed consent. Participants were randomized between April 2015 and April 2018, and follow-up was completed in April 2019. In May 2016, modifications removed an exclusion of participants given diagnoses more than 2 years before randomization and increased the permitted FVC predicted value from 70% to 75% to improve recruitment. An optional bronchoscopy substudy was discontinued in June 2017.
Patients were recruited to the study if they had IPF diagnosed according to contemporaneous international guidelines16 and had a modified Medical Research Council (MRC) dyspnea scale score greater than 1. They could receive licensed medication for IPF at a stable regimen. Patients were excluded if they had FVC greater than 75% predicted, a significant coexisting respiratory or other comorbidity, or a respiratory tract infection during the preceding 4 weeks or were receiving immunosuppression.
Patients were randomized on a 1:1 basis to receive 960 mg of oral co-trimoxazole (2 480-mg tablets) or 2 matched placebo tablets twice daily. The treatment allocation was generated via a computer code, using minimization for site, current use of antifibrotic therapy, and involvement in bronchoscopy substudy, under the supervision of the study statistician. All patients received 5 mg of folic acid orally once daily to prevent impaired hematopoiesis. Treatments were given in addition to standard care as defined by National Institute for Health and Clinical Excellence guidelines (https://www.nice.org.uk/CG163).
A reduction of treatment dose to 2 tablets (ie, 960 mg of co-trimoxazole or 2 placebo tablets daily) plus 5 mg of folic acid 3 times per week was permitted if a participant developed gastrointestinal adverse effects, rash, grade 1 hyperkalemia, or any other adverse event requiring dose reduction in the view of the principal investigator.
Outcomes
The primary outcome was the time to death (all causes), lung transplant, or first nonelective hospital admission for any reason. These data were obtained at each site until the date of withdrawal or the end of the study by screening hospital records and capturing details of out-of-hospital death from primary care records, if required. The primary outcome was censored at the date of withdrawal if patients withdrew consent to be followed up. Secondary outcomes included the individual components of the primary outcome. Respiratory-related events, determined by an independent committee, were analyzed separately. Spirometry17 and gas transfer (an assessment of carbon monoxide uptake by the lung that reflects the ability of the lungs to exchange gas into the bloodstream)18 were captured at baseline and at 6 and 12 months. The MRC dyspnea scale,19 5-level Euroqol 5-dimension (EQ-5D-5L) questionnaire,20 cough severity score (visual analog scale ranging from 0 [“I have not been bothered by my cough at all”] to 100 [“My cough has been the worst it can be”]), Leicester Cough Questionnaire (LCQ),21 and King’s Brief Interstitial Lung Disease (K-BILD) questionnaire22 were given at baseline and 3 and 6 months, then every 6 months throughout the study, including a final assessment at the end of the study. Sputum was obtained, when clinically relevant, and sent for local microbiological culture and antibiotic susceptibility testing. Blood was taken for complete blood cell count, urea and electrolytes, and liver function at baseline; 6 weeks; and 3, 6, 9, and 12 months, then every 6 months for the duration of the study. Adverse events were captured at each visit and assessed for severity. Blood biomarker analysis is not reported here.
Statistical Analysis
The trial was designed to have 80% power (2-sided significance level of 5%) to show a change in hospitalization-free survival from a median value of 28.8 months in the placebo group to 51.1 months in the co-trimoxazole group (hazard ratio [HR], 0.56) over this study period assuming that 330 patients were randomized and 20% withdrew from the study. This was based on a sensitivity analysis of patients from a previous study13 with reduced lung function (FVC <70% predicted) using an intention-to-treat analysis.
The primary outcome and secondary event measurements were analyzed using a Cox proportional hazards model adjusted for licensed IPF medication use as a fixed effect and site a random effect. If the model did not converge then site was included as a strata in the analysis and a sandwich-based robust cluster variance was used. The proportionality assumption was assessed using the global test with Schoenfeld residual. This had a test statistic of 1.58 and a P value of .45, thus providing no evidence that the assumption was violated. The results are presented as the Kaplan-Meier estimate with median time to outcome. The EQ-5D-5L was converted to utilities by mapping to standard health state valuations23 and the K-BILD was calculated using the logit-scoring method.24 The MRC dyspnea score was analyzed using a Mann-Whitney test at 12 months; other questionnaires and lung function measurements were analyzed using linear mixed models to compare the mean values at 12 months between the treatment and placebo groups, adjusted for licensed IPF medication use as a fixed effect and site as a random effect. A repeated-measures model was fitted for each outcome at all time points with a fixed term for licensed IPF medication, randomization group, and time, and random effects were included for site and the person identification number. For this analysis, the significance of the between-group difference at each time point was adjusted using a Bonferroni correction.
All analyses were 2-sided at the 5% level of significance and were undertaken as prespecified, including all participants analyzed in the group to which they were randomized. Additionally, prespecified per-protocol (≥80% adherence to study medication) and modified per-protocol (those who adhered to the high-dose regimen) analyses were done.
The missing end points at 12 months were imputed using the iterative chained equations approach.25 The outcomes at 12 months and baseline were included in the equations, along with randomization group, body mass index, and sex. Because the rate of missing data was high, a total of 45 imputations were created and the results model estimates combined using Rubin equations. The analysis was conducted using Stata/MP, version 16. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. The model assumptions were assessed visually by plotting the residuals, which were all approximately normally distributed.
The adverse event analysis was based on all patients who received at least 1 dose of the study drug or placebo. Data were analyzed for event rates and percentage of patients with at least 1 adverse event and were coded according to the Medical Dictionary for Regulatory Activities. Safety blood measures were compared at 12 months.
Results
A total of 1305 participants were screened at 43 sites; 349 met the inclusion criteria and 342 were randomized from 39 sites (Figure 1). One participant randomized to the intervention (co-trimoxazole) group was randomized in error and their data were not analyzed. Fifty-eight individuals (17%) withdrew from the study during follow-up without meeting an end point: 32 (19%) from the co-trimoxazole group (21 [12%] in the first 12 months) and 26 (15%) from the placebo group (16 [9%] in the first 12 months); their data are included until the point of withdrawal. A total follow-up of 395 person-years was assessed. The mean (SD) patient age was 71.3 (7.5) years, with a mean FVC of 2.25 (0.56) L or 55.7% (9.4%) predicted. Baseline characteristics and other factors were balanced between the 2 treatment groups, aside from sex and the presence of diabetes (Table 1).
Figure 1. Flow of Participants in a Study of the Effect of Co-trimoxazole vs Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Idiopathic Pulmonary Fibrosis.
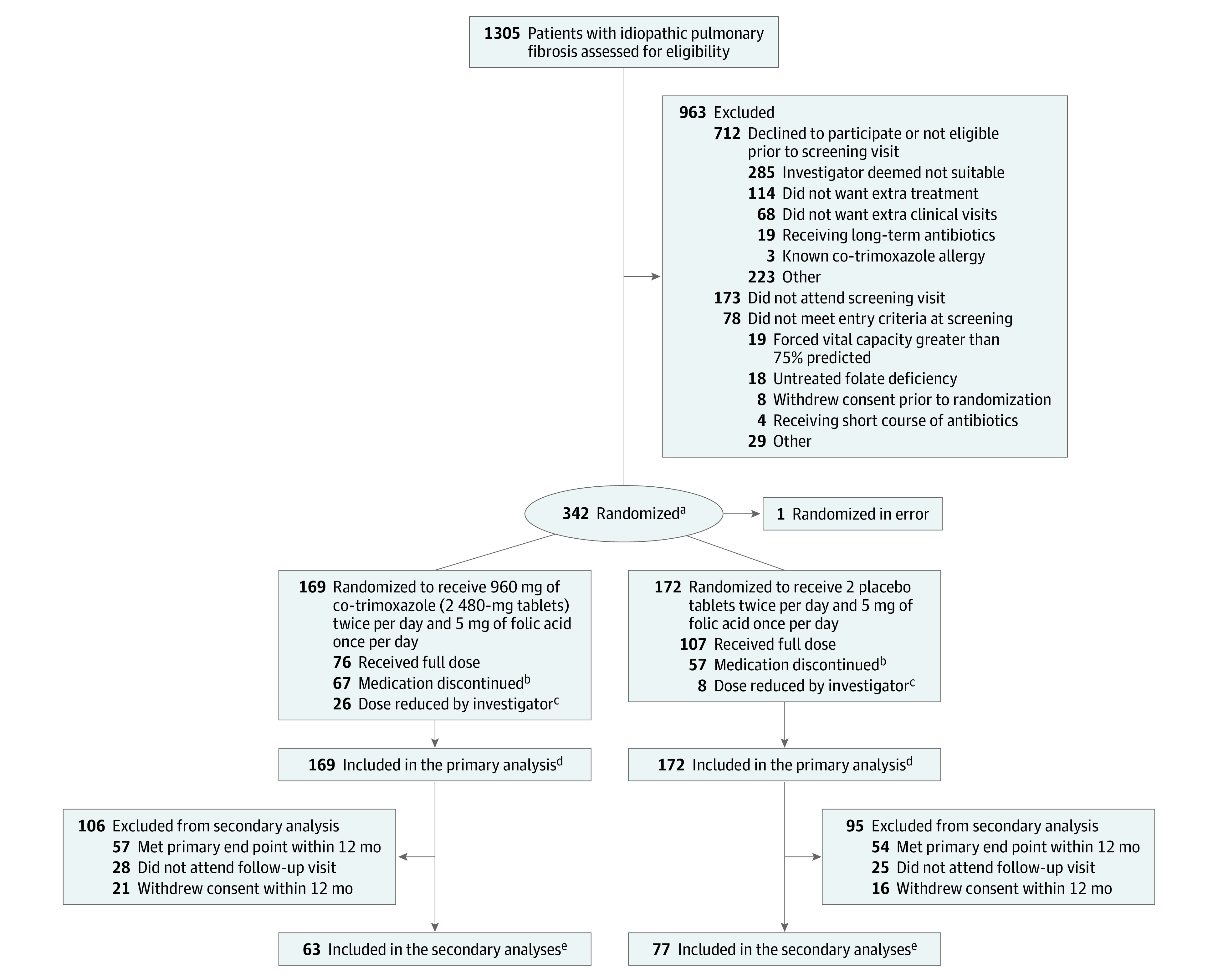
aIn a 1:1 ratio, with minimization for site and baseline fibrotic therapy.
bFive patients in the co-trimoxazole group and 8 in the control group previously had their dose reduced by the investigator.
cA reduction of the dose to 2 tablets (ie, 960 mg co-trimoxazole or 2 placebo tablets daily) plus 5 mg of folic acid 3 times weekly was permitted if a participant developed gastrointestinal adverse effects or rash, grade 1 hyperkalemia (potassium >5.0 mmol/L), or any other adverse event requiring dose reduction in the view of the principal investigator.
dA total of 32 individuals from the co-trimoxazole and 26 from the placebo group withdrew during the study, and their data are included until the point of withdrawal.
eThe secondary outcome data illustrate that of lung function.
Table 1. Baseline Characteristics of Participants in a Study of the Effect of Co-trimoxazole vs Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Idiopathic Pulmonary Fibrosis.
| Characteristic | Co-trimoxazole (n = 169a) | Placebo (n = 172a) |
|---|---|---|
| Sex, No. (%) | ||
| Men | 138 (81.7) | 157 (91.3) |
| Women | 31 (18.3) | 15 (8.7) |
| Age, mean (SD), y | 71.9 (7.8) | 70.7 (7.1) |
| Smoking status, No. (%) | ||
| Never | 59 (34.9) | 56 (32.6) |
| Ex-smoker | 109 (64.5) | 114 (66.3) |
| Current | 1 (0.6) | 2 (1.2) |
| Comorbidities, No. (%)b | ||
| Gastroesophageal reflux disease | 69 (40.8) | 62 (36.1) |
| Ischemic heart disease or angina | 38 (22.5) | 44 (25.6) |
| Diabetes | 40 (23.7) | 25 (14.5) |
| Anxiety or depression | 17 (10) | 23 (13.4) |
| Pulmonary hypertension | 13 (7.7) | 10 (5.8) |
| Osteoporosis | 11 (6.5) | 11 (6.4) |
| Chronic obstructive pulmonary disease | 6 (3.6) | 6 (3.5) |
| Bronchiectasis | 2 (1.2) | 7 (4.1) |
| Maintenance treatments, No. (%) | ||
| Proton pump inhibitor | 87 (51.5) | 78 (45.3) |
| Pirfenidone | 71 (42.0) | 66 (38.4) |
| Nintedanib | 56 (33.1) | 61 (35.5) |
| Prednisolone | 12 (7.1) | 10 (5.8) |
| N-acetylcysteine | 8 (4.7) | 7 (4.1) |
| Other antioxidant | 3 (1.8) | 5 (2.9) |
| Lung function, mean (SD)c | ||
| Absolute value | ||
| FVC, L | 2.2 (0.6) | 2.3 (0.5) |
| FEV1, L | 1.9 (0.5) | 1.9 (0.4) [n = 171] |
| FEV1/FVC ratio | 0.8 (0.1) | 0.8 (0.1) [n = 171] |
| DLCO, mmoL/min/kPa | 3.6 (1.8) [n = 123] | 3.7 (1.5) [n = 127] |
| Predicted value, % | ||
| FVC | 56.2 (8.9) | 55.2 (10.0) |
| FEV1 | 61.5 (9.3) | 60.0 (10.6) [n = 171] |
| DLCO | 43.3 (20.2) [n = 123] | 44.5 (18.0) [n = 127] |
| Outcome measures, mean (SD) | ||
| Medical Research Council dyspnea score, median (IQR)d | 3.0 (2.0-3.0) [n = 167] | 2.00 (2.00-3.00) [n = 171] |
| EQ-5D-5L utility scoree | 0.67 (0.20) [n = 168] | 0.69 (0.22) [n = 171] |
| Cough severity scoref | 39.5 (27.5) [n = 167] | 40.9 (26.6) [n = 168] |
| Leicester Cough Questionnaire scoresg | ||
| Total | 16.1 (3.6) [n = 161] | 15.8 (3.7) [n = 164] |
| Physical | 5.2 (1.1) [n = 161] | 5.1 (1.0) [n = 165] |
| Psychological | 5.4 (1.4) [n = 167] | 5.3 (1.5) [n = 166] |
| Social | 5.4 (1.4) [n = 167] | 5.4 (1.4) [n = 168] |
| King’s Brief Interstitial Lung Disease questionnaire scoresh | (n = 168) | (n = 171) |
| Total | 53.7 (9.7) | 53.6 (10.6) |
| Breathlessness | 37.7 (15.3) | 38.9 (14.3) |
| Chest | 62.9 (20.8) | 62.6 (20.7) |
| Psychological | 55.2 (14.9) | 54.9 (17.1) |
Abbreviations: DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IQR, interquartile range.
These data exclude the individual who was excluded after randomization.
Comorbidities were as detailed in the medical records.
The lung function test values were obtained at screening.
Medical Research Council dyspnea score is a 5-point scale ranging from 1 to 5 (higher values represent increasing breathlessness); a value of 3 represents walking slower than contemporaries or having to stop when walking at own pace.
The 5-level Euroqol 5-dimension questionnaire (EQ-5D-5L) utility score ranges from −0.59 to 1 (higher score indicates better health utility); a value of 0.75 indicates that the quality of life–adjusted years has been reduced by a slight amount. Utilities are calculated by mapping to standard health state valuations.
The cough severity score is a cough severity visual analog scale ranging from 0 to 100 (higher scores represent greater cough severity); a value of 40 indicates that coughing is two-fifths of the maximum perceived cough severity.
The Leicester Cough Questionnaire is a cough-related quality of life score that ranges from 3 to 21 (domain scores range from 1-7; higher values represent better quality of life); a value of 15 suggests a cough that has affected life activities a small amount of the time over the past 2 weeks. It is calculated as the sum of the individual domains.
The King’s Brief Interstitial Lung Disease questionnaire total and domain scores range from 0 to 100 (higher values indicate better health status); a value of 50 indicates that idiopathic pulmonary fibrosis has affected life activities some of the time over the past 2 weeks. It is calculated using the logit-scoring method.
The mean (SD) percentage of adherence in the co-trimoxazole group was 81.4% (22.8%), compared with 85.5% (21.7%) in the control (placebo) group. The number of participants who met the 80% treatment threshold was 120 of 167 (71.9%) in the co-trimoxazole group compared with 124 of 172 (72.1%) in the placebo group. Dose reduction occurred in 31 of 167 patients (19%) in the co-trimoxazole group and 16 of 172 (9%) in the placebo group (eTable 1 in Supplement 3).
Primary Outcome
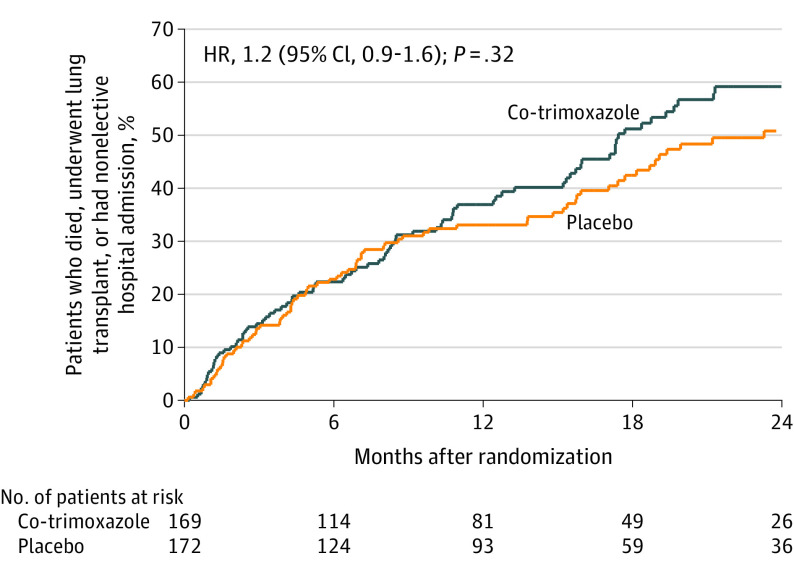
A total of 164 primary outcome events occurred (Figure 2). The incidence of events was 0.45 (84/186) per person-year in the co-trimoxazole group and 0.38 (80/209) per person-year in the placebo group (HR, 1.2 [95% CI, 0.9-1.6] for the unadjusted and adjusted analyses). The median (interquartile range) survival was 1.45 (1.28-1.78) years in the co-trimoxazole group and 1.94 (1.48-2.84) years in the placebo group. The site could not be included in this model due to model instability; a robust variance method gave an HR of 1.2 ([95% CI, 0.8-1.6]; P = .37), and an HR of 1.2 ([95% CI, 0.9-1.7]; P = .24) with stratification. There were no statistically significant differences between the groups for the per-protocol or modified per-protocol analyses for event outcomes (eTable 2 in Supplement 3).
Figure 2. Primary End Point in a Study of the Effect of Co-trimoxazole vs Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Idiopathic Pulmonary Fibrosis.
Kaplan-Meier estimates for time to event for the primary end point (death [all causes], lung transplant, or first nonelective hospital admission), analyzed according to the group to which participants were randomized. There was no significant difference between the co-trimoxazole and placebo groups. Median (interquartile range) follow-up was 12.0 (4.4-21.0) months.
Secondary Outcomes
The individual components of the primary outcome are shown in Figure 2 and eTable 2 in Supplement 3. There was no statistically significant difference in all-cause mortality (HR, 1.5 [95% CI, 0.8-2.8]; P = .17), respiratory-related death (HR, 1.4 [95% CI, 0.7-2.6]; P = .34), all-cause hospitalization (HR, 1.1 [95% CI, 0.7-1.5]; P = .75), or respiratory-related hospitalization (HR, 1.0 [95% CI, 0.7-1.6]; P = .83) between the 2 groups. There were no statistically significant differences between the 2 groups for the per-protocol or modified per-protocol analyses for event outcomes (eTable 2 in Supplement 3).
There was no statistically significant differences between the groups for the lung function or patient-reported outcomes (Table 2). In the per-protocol analysis there were no statistically significant differences between the co-trimoxazole or placebo groups for the lung function measurements or the patient-reported outcomes, other than for the chest domain of the K-BILD (adjusted and unadjusted analysis) and physiological and social domains of the LCQ (unadjusted analysis), which favored co-trimoxazole (eTable 3 in Supplement 3). The modified per-protocol analysis is shown in eTable 4 in Supplement 3.
Table 2. Secondary Outcomes at 12 Months in a Study of the Effect of Co-trimoxazole vs Placebo on Patients With Idiopathic Pulmonary Fibrosis.
| Outcome | Mean (SD)a | Adjusted for site and baseline antifibrotic therapy | Adjusted for site, baseline antifibrotic therapy, and baseline value | |||
|---|---|---|---|---|---|---|
| Co-trimoxazole | Placebo | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | |
| Lung function | ||||||
| Absolute value | ||||||
| FVC, L | 2.26 (0.53) [n = 63] | 2.23 (0.51) [n = 77] | −0.02 (−0.19 to 0.15) | .81 | −0.01 (−0.09 to 0.07) | .80 |
| FEV1, L | 1.86 (0.43) [n = 63] | 1.86 (0.42) [n = 77] | 0 (−0.14 to 0.14) | >.99 | −0.02 (−0.08 to 0.05) | .62 |
| DLCO, mmoL/min/kPa | 3.49 (1.75) [n = 50] | 3.71 (1.50) [n = 60] | 0.19 (−0.39 to 0.77 | .51 | 0.3 (−0.26 to 0.85) | .30 |
| Predicted value, % | ||||||
| FVC | 54.0 (8.9) [n = 63] | 53.6 (9.1) [n = 77] | −0.5 (−3.56 to 2.47) | .72 | −0.6 (−2.6 to 1.5) | .59 |
| FEV1 | 57.8 (9.7) [n = 63] | 58.2 (10.4) [n = 77] | 0.2 (−3.2 to 3.6 | .93 | −0.7 (−2.8 to 1.5) | .55 |
| DLCO | 40.2 (17.7) [n = 50] | 43.2 (16.3) [n = 60] | 2.5 (−3.7 to 8.7) | .43 | 3.9 (−2.4 to 10.3) | .22 |
| Medical Research Council dyspnea score, median (IQR)b | 3.0 (2.0-4.0) [n = 72] | 3.0 (2.0-4.0) [n = 86] | .94 | .29 | ||
| EQ-5D-5L utility scorec | 0.41 (0.36) [n = 103] | 0.45 (0.35) [n = 118] | 0.04 (−0.05 to 0.13) | .37 | 0.03 (−0.06 to 0.11) | .55 |
| Cough severity scored | 44.7 (27.0) [n = 72] | 49.7 (26.7) [n = 84] | 5.1 (−3.4 to 13.6) | .24 | 2.2 (−5.4 to 9.9) | .57 |
| Leicester Cough Questionnaire scorese | ||||||
| Total | 15.4 (4.09) [n = 69] | 14.6 (4.0) [n = 71] | −0.8 (−2.1 to 0.6) | .27 | −0.6 (−1.6 to 0.4) | .22 |
| Physical | 4.9 (1.2) [n = 69] | 4.7 (1.2) [n = 72] | −0.2 (−0.6 to 0.2) | .36 | −0.1 (−0.4 to 0.2) | .43 |
| Psychological | 5.2 (1.4) [n = 69] | 4.9 (1.5) [n = 75] | −0.3 (−0.8 to 0.2) | .25 | −0.3 (−0.6 to 0.1) | .17 |
| Social | 5.3 (1.5) [n = 69] | 5.0 (1.5) [n = 75] | −0.3 (−0.8 to 0.2) | .28 | −0.2 (−0.6 to 0.1) | .20 |
| King’s Brief Interstitial Lung Disease questionnaire scoresf | ||||||
| Total | 50.3 (12.3) [n = 71] | 50.7 (11.20) [n = 85] | 0.4 (−3.3 to 4.1) | .83 | 0.1 (−2.8 to 3.0) | .93 |
| Breathlessness | 34.4 (17.4) [n = 72] | 35.0 (14.55) [n = 86] | 0.9 (−4.1 to 5.9) | .73 | −0.5 (−4.4 to 3.3) | .79 |
| Chest | 59.9 (20.3) [n = 72] | 56.8 (22.82) [n = 86] | −3.4 (−10.2 to 3.4) | .33 | −2.0 (−7.8 to 3.8) | .50 |
| Psychological | 49.7 (17.9) [n = 71] | 51.9 (16.9) [n = 85] | 2.0 (−3.5 to 7.5) | .48 | 1.5 (−3.0 to 5.9) | .53 |
Abbreviations: DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IQR, interquartile range.
Data were incomplete for some patients because they did not complete the questionnaires or attend lung function assessments.
Medical Research Council dyspnea score is a 5-point scale ranging from 1 to 5 (higher values represent increasing breathlessness); a value of 3 represents walking slower than contemporaries or having to stop when walking at own pace.
eThe 5-level Euroqol 5-dimension questionnaire (EQ-5D-5L) utility score ranges from −0.59 to 1 (higher scores indicate better health utility); a value of 0.75 indicates that the quality of life–adjusted years has been reduced by a slight amount. Utilities are calculated by mapping to standard health state valuations.
The cough severity score is a cough severity visual analog scale ranging from 0 to 100 (higher score represents greater cough severity); a value of 40 indicates that coughing is two-fifths of the maximum perceived cough severity.
The Leicester Cough Questionnaire is a cough-related quality of life score that ranges from 3 to 21 (domain scores range from 1-7; higher values represent better quality of life); a value of 15 suggests a cough that has affected life activities a small amount of the time over the past 2 weeks. It is calculated as the sum of the individual domains.
The King’s Brief Interstitial Lung Disease questionnaire total and domain scores range from 0 to 100 (higher values represent better health status); a value of 50 indicates that idiopathic pulmonary fibrosis has affected life activities some of the time over the past 2 weeks. It is calculated using the logit-scoring method.
When reviewing the data at all time points, there was a statistically significant (P = .02) difference in cough severity score (15.0 [95% CI, 1.2-28.8]) at 18 months in favor of the co-trimoxazole group and, overall, the differential between the groups was statistically significant (P = .04) with a mean difference of 5.7 (95% CI, 0.1-11.2) (eTable 5 in Supplement 3). There were no statistically significant differences for the LCQ and K-BILD total or subdomain scores, MRC score, or lung function measurements between the groups, and no overall treatment effect.
Adverse Events
There were 696 adverse events (20 serious adverse events) in the co-trimoxazole group and 640 (17 serious adverse events) in the placebo group (Table 3). There were more reports of nausea in the co-trimoxazole group compared with the placebo group (89/157 vs 67/163), whereas diarrhea was reported more frequently in the placebo group (52/157 vs 84/163). There were more episodes of hyperkalemia (24/157 vs 14/163), vomiting (28/157 vs 20/163), and rash (31/157 vs 20/163) in the co-trimoxazole group than the placebo group (Table 3; eTable 6 in Supplement 3). There was no difference in the 12-month safety blood analysis between the groups (eTable 7 in Supplement 3) aside from creatinine, which was statistically significantly higher in the co-trimoxazole group.
Table 3. Adverse Events in a Study of the Effect of Co-trimoxazole vs Placebo on Death, Lung Transplant, or Hospital Admission in Patients With Idiopathic Pulmonary Fibrosis.
| Adverse eventa | No. of events | |
|---|---|---|
| Co-trimoxazole | Placebo | |
| Blood and lymphatic system disorders | 3 | 3 |
| Cardiac disorders | 6 | 4 |
| Ear and labyrinth disorders | 3 | 0 |
| Eye disorders | 5 | 6 |
| Gastrointestinal disorders | 216 | 224 |
| Nausea | 89 | 67 |
| Diarrhea | 52 | 84 |
| Vomiting | 28 | 20 |
| Constipation | 11 | 5 |
| General disorders and administration site conditions | 36 | 20 |
| Fatigue | 15 | 11 |
| Chest pain | 8 | 6 |
| Edema peripheral | 5 | 0 |
| Immune system disorders | 1 | 1 |
| Infections and infestations | 110 | 127 |
| Lower respiratory tract infection | 63 | 66 |
| Injury, poisoning, and procedural complications | 7 | 10 |
| Investigationsb | 44 | 22 |
| Weight decrease | 24 | 16 |
| Metabolism and nutrition disorders | 57 | 27 |
| Decreased appetite | 26 | 9 |
| Hyperkalemiac | 24 | 14 |
| Musculoskeletal and connective tissue disorders | 21 | 20 |
| Neoplasm/s benign, malignant, and unspecified (including cysts and polyps) | 3 | 1 |
| Nervous system disorders | 41 | 32 |
| Headache | 22 | 14 |
| Psychiatric disorders | 5 | 2 |
| Kidney and urinary disorders | 14 | 7 |
| Reproductive system and breast disorders | 0 | 2 |
| Respiratory, thoracic, and mediastinal disorders | 77 | 95 |
| Cough | 27 | 33 |
| Dyspnea | 31 | 34 |
| Skin and subcutaneous tissue disorders | 46 | 30 |
| Rash | 31 | 20 |
| Surgical and medical procedures | 1 | 2 |
| Vascular disorders | 0 | 5 |
| Total adverse events | 696 | 640 |
| ≥1 adverse event, No. (%) | 146 (86) | 142 (83) |
| ≥2 adverse events, No. (%) | 119 (70) | 121 (70) |
Adverse events were captured at each study visit and coded using Medical Dictionary for Regulatory Activities terms.
Investigations include abnormal laboratory results and weight change.
Hyperkalemia was defined as a potassium greater than 5.0 mmol/L.
Seventeen sputum samples and 1 nasal swab were obtained in total for all patient visits. Three of these grew possible relevant microbiological agents on culture: Staphylococcus aureus (n = 1), Haemophilus influenzae (n = 1), and “yeasts” (n = 1).
Discussion
In this double-blind, randomized, placebo-controlled trial, there was no significant reduction in the incidence of the composite outcome of death, lung transplant, or nonelective hospitalization with co-trimoxazole in patients with moderate or severe IPF.
In contrast to the previous smaller study that compared co-trimoxazole with placebo in patients with IPF,13 there was no reduction in mortality with co-trimoxazole. In this previous study, nearly 60% of patients were taking prednisolone (mostly at a high dose) and 30% were taking azathioprine, whereas in the current trial, those receiving immunosuppression other than low-dose corticosteroids (6% of individuals) were excluded. It is therefore plausible that, in the previous study,13 co-trimoxazole prevented infection-related adverse outcomes that were contingent on immunosuppression, which is known to result in poor outcomes in IPF.26 Furthermore, antifibrotic therapy (pirfenidone and nintedanib) was not available at the time of the previous study,13 whereas 75% of patients in the current study were receiving this treatment. Pirfenidone, for example, has been estimated to improve IPF life expectancy by 2.47 years.27 Overall, the changes between the 2 studies resulted in a doubling of the hospital-free survival (mean of 23.3 months vs 12.8 months). In addition, inclusion in the current study was restricted to people with moderate to severe IPF, as defined by FVC less than or equal to 75% predicted, whereas no such restriction pertained in the previous study. However, it is unlikely that co-trimoxazole would have been more effective in a less severe population with better lung function (FVC >75% predicted), because there was no subgroup effect of baseline disease severity in the previous study13 and neither bacterial burden28 nor response to antifibrotic therapy29,30 is known to be related to FVC in IPF.
The benefit of co-trimoxazole in terms of the cough severity score, LCQ, and the chest symptom domain of the K-BILD questionnaire (which captures chest tightness, air hunger, and wheeze) only met clinically relevant thresholds at 18 months, but support the previously identified clinical benefit of co-trimoxazole13 in respect to the “symptom” domain of the St George’s Respiratory Questionnaire31 (which captures cough, sputum, breathlessness, and wheeze). However, given the null primary outcome results and large number of secondary outcomes, the findings for cough should be considered only hypothesis-generating.
The results of this study do not disprove the hypothesis that the “lung microbiome” influences disease progression and outcomes in IPF.7,9,28 A potential antibacterial benefit of co-trimoxazole may have been lost owing to widespread bacterial resistance, despite in-therapy selection of resistance being rare for co-trimoxazole32 and acquired resistance, while not uncommon, is unlikely to have been so universal as to overwhelm a positive effect. Furthermore, the possibility of an unrecognized IPF-associated co-trimoxazole–resistant pathogen cannot be entirely dismissed, although there is no evidence to support such a hypothesis; an ongoing randomized open-label trial of co-trimoxazole or doxycycline vs standard care may provide further insight.33 However, a study of explanted lungs yielded very few bacterial 16S rRNA gene reads in the IPF interstitium compared with the airways of patients with IPF and healthy control patients. It is therefore possible that airway and lung tissue compartments are separate in IPF with different microbiota.34
This was an adequately powered, multicenter, academic clinical trial using a clinically relevant outcome with high follow-up rates and long timescales. A total of 342 individuals were recruited and evaluated from 39 geographically diverse sites of varying sizes for up to 3 years. The primary end point included unplanned hospital admission, which is financially and socially costly with a high frequency of death, and all-cause mortality, which is the most clinically meaningful primary end point.35 The study was aligned to clinical care, minimizing the research burden for patients. The event rate (164 events) was higher than anticipated (99 events), so the study likely had adequate power to detect a meaningful difference in the primary end point.
Limitations
This study has several limitations. First, there was a lack of evaluation of the lung microbiome or quantitation of the influence that co-trimoxazole had on its composition and ecology, including antimicrobial resistance. However, given the lack of efficacy, it is questionable whether any such analysis would have been clinically meaningful. Second, it is not possible to determine whether co-trimoxazole reduced infection-related events, because the numbers of respiratory tract infections were not captured; rather, “respiratory-related” events were assessed, encompassing all events related to the respiratory system. Assessing whether respiratory infection is present during acute exacerbations or other clinical settings is challenging.36 Third, a protocol exclusion was allergy to co-trimoxazole and, although few people were reported to have this allergy, this exclusion criteria and the FVC criteria may have limited the generalizability of the study. Fourth, the entry criteria were modified by removing the exclusion of participants given diagnoses more than 2 years before randomization as well as increasing the permitted FVC predicted value from 70% to 75%. Fifth, the statistical analysis plan did not include adjustment for gastroesophageal reflux disease or proton pump inhibitor usage; however, the occurrences of these were similar in both groups, and an exploratory post hoc analysis adjusting for these variables did not change the conclusion of the study.
Conclusions
Among patients with moderate or severe IPF, treatment with oral co-trimoxazole did not reduce a composite outcome of time to death, lung transplant, or nonelective hospitalization compared with placebo.
Trial protocol
Statistical analysis plan
eTable 1. Dose modifications
eTable 2. Primary outcome and secondary outcomes for individual components of primary outcome
eTable 3. Per-protocol analysis of the lung function and questionnaire outcomes at 12 months
eTable 4. Modified per-protocol analysis of the lung function and questionnaire outcomes at 12 months
eTable 5. Cough score over time by randomisation arm
eTable 6. Adverse events
eTable 7. Summary of safety blood measures at 12 months
Data sharing statement
References
- 1.Guenther A, Krauss E, Tello S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):141. doi: 10.1186/s12931-018-0845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66(6):462-467. doi: 10.1136/thx.2010.148031 [DOI] [PubMed] [Google Scholar]
- 3.Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014;108(2):376-387. doi: 10.1016/j.rmed.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009;64(8):692-697. doi: 10.1136/thx.2008.110445 [DOI] [PubMed] [Google Scholar]
- 5.Vidal S, de la Horra C, Martín J, et al. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin Microbiol Infect. 2006;12(3):231-235. doi: 10.1111/j.1469-0691.2005.01337.x [DOI] [PubMed] [Google Scholar]
- 6.Garzoni C, Brugger SD, Qi W, et al. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax. 2013;68(12):1150-1156. doi: 10.1136/thoraxjnl-2012-202917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molyneaux PL, Cox MJ, Willis-Owen SA, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(8):906-913. doi: 10.1164/rccm.201403-0541OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han MK, Zhou Y, Murray S, et al. ; COMET Investigators . Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014l;2(7):548-556. doi: 10.1016/S2213-2600(14)70069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savici D, Campbell PA, King TE Jr. Bronchoalveolar macrophages from patients with idiopathic pulmonary fibrosis are unable to kill facultative intracellular bacteria. Am Rev Respir Dis. 1989;139(1):22-27. doi: 10.1164/ajrccm/139.1.22 [DOI] [PubMed] [Google Scholar]
- 11.Samara KD, Antoniou KM, Karagiannis K, et al. Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int J Oncol. 2012;40(5):1397-1404. doi: 10.3892/ijo.2012.1374 [DOI] [PubMed] [Google Scholar]
- 12.Varney VA, Parnell HM, Salisbury DT, Ratnatheepan S, Tayar RB. A double blind randomised placebo controlled pilot study of oral co-trimoxazole in advanced fibrotic lung disease. Pulm Pharmacol Ther. 2008;21(1):178-187. doi: 10.1016/j.pupt.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 13.Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68(2):155-162. doi: 10.1136/thoraxjnl-2012-202403 [DOI] [PubMed] [Google Scholar]
- 14.Wilson EC, Shulgina L, Cahn AP, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: an economic evaluation alongside a randomised controlled trial. Pharmacoeconomics. 2014;32(1):87-99. doi: 10.1007/s40273-013-0112-z [DOI] [PubMed] [Google Scholar]
- 15.Hammond M, Clark AB, Cahn AP, et al. The efficacy and mechanism evaluation of treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole (EME-TIPAC): study protocol for a randomised controlled trial. Trials. 2018;19(1):89. doi: 10.1186/s13063-018-2453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 18.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 19.Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med. 2010;10:32. doi: 10.1186/1471-2466-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339-343. doi: 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel AS, Siegert RJ, Brignall K, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax. 2012;67(9):804-810. doi: 10.1136/thoraxjnl-2012-201581 [DOI] [PubMed] [Google Scholar]
- 23.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708-715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Sinha A, Patel AS, Siegert RJ, et al. The King’s Brief Interstitial Lung Disease (KBILD) questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res. 2019;6(1):e000363. doi: 10.1136/bmjresp-2018-000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 26.Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ; Idiopathic Pulmonary Fibrosis Clinical Research Network . Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968-1977. doi: 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher M, Nathan SD, Hill C, et al. Predicting life expectancy for pirfenidone in idiopathic pulmonary fibrosis. J Manag Care Spec Pharm. 2017;23(3-b Suppl):S17-S24. doi: 10.18553/jmcp.2017.23.3-b.s17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Invernizzi R, Barnett J, Rawal B, et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur Respir J. 2020;55(4):1901519. doi: 10.1183/13993003.01519-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan SD, Costabel U, Albera C, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44-51. doi: 10.1016/j.rmed.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 30.Noble PW, Albera C, Bradford WZ, et al. ; CAPACITY Study Group . Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760-1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 31.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 32.Lacey RW, Lord VL, Gunasekera HK, Leiberman PJ, Luxton DE. Comparison of trimethoprim alone with trimethoprim sulphamethoxazole in the treatment of respiratory and urinary infections with particular reference to selection of trimethoprim resistance. Lancet. 1980;1(8181):1270-1273. doi: 10.1016/S0140-6736(80)91732-8 [DOI] [PubMed] [Google Scholar]
- 33.Anstrom KJ, Noth I, Flaherty KR, et al. ; CleanUP-IPF Study Team . Design and rationale of a multi-center, pragmatic, open-label randomized trial of antimicrobial therapy: the study of clinical efficacy of antimicrobial therapy strategy using pragmatic design in idiopathic pulmonary fibrosis (CleanUP-IPF) clinical trial. Respir Res. 2020;21(1):68. doi: 10.1186/s12931-020-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins G. A big beautiful wall against infection. Thorax. 2018;73(5):485. doi: 10.1136/thoraxjnl-2017-211220 [DOI] [PubMed] [Google Scholar]
- 35.Wells AU, Behr J, Costabel U, Cottin V, Poletti V, Richeldi L; European IPF Consensus Group . Hot of the breath: mortality as a primary end-point in IPF treatment trials: the best is the enemy of the good. Thorax. 2012;67(11):938-940. doi: 10.1136/thoraxjnl-2012-202580 [DOI] [PubMed] [Google Scholar]
- 36.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis: an international working group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. Dose modifications
eTable 2. Primary outcome and secondary outcomes for individual components of primary outcome
eTable 3. Per-protocol analysis of the lung function and questionnaire outcomes at 12 months
eTable 4. Modified per-protocol analysis of the lung function and questionnaire outcomes at 12 months
eTable 5. Cough score over time by randomisation arm
eTable 6. Adverse events
eTable 7. Summary of safety blood measures at 12 months
Data sharing statement