This systematic review and meta-analysis investigates how deficits in social cognitive domains diverge or overlap between schizophrenia spectrum disorders and autism spectrum disorder based on the extant literature.
Key Points
Question
How do deficits in social cognition diverge or overlap between individuals with schizophrenia spectrum disorders (SSDs) and autism spectrum disorder (ASD)?
Findings
In this systematic review and meta-analysis of 36 studies directly comparing social cognitive performance in individuals with SSDs vs ASD, there were no statistically significant differences in emotion processing or theory of mind.
Meaning
Similar levels of social cognitive impairment may be present in individuals with SSDs and ASD, but cross-disorder studies probing social cognitive domains with larger samples, consistent reporting of clinical measures, and neuroimaging are needed to substantiate these findings, clarify underlying mechanisms, and parse heterogeneity.
Abstract
Importance
Schizophrenia spectrum disorders (SSDs) and autism spectrum disorder (ASD) both feature social cognitive deficits; however, these disorders historically have been examined separately using a range of tests and subdomain focus and at different time points in the life span. Moving beyond diagnostic categories and characterizing social cognitive deficits can enhance understanding of shared pathways across these disorders.
Objective
To investigate how deficits in social cognitive domains diverge or overlap between SSDs and ASD based on the extant literature.
Data Sources
Literature searches were conducted in MEDLINE, PsycInfo, Embase, and Web of Science from database inception until July 26, 2020.
Study Selection
Original research articles were selected that reported performance-based measures of social cognition in both SSDs and ASD samples. Selected articles also had to be published in English and use International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, DSM-IV, or more recent diagnostic criteria.
Data Extraction and Synthesis
This systematic review and meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses and Meta-analysis of Observational Studies in Epidemiology reporting guidelines, including data extraction and quality assessment using a modified version of the Newcastle-Ottawa Scale. Data were pooled using a random-effects model.
Main Outcomes and Measures
Effect sizes were calculated as Hedges g (SSDs vs ASD). The primary outcomes were performance on emotion processing tasks, theory of mind (ToM) tasks, and the Reading the Mind in the Eyes Test (RMET) in SSDs compared with ASD. Meta-regressions were performed for age difference, publication year, quality assessment scores, and antipsychotic medication use.
Results
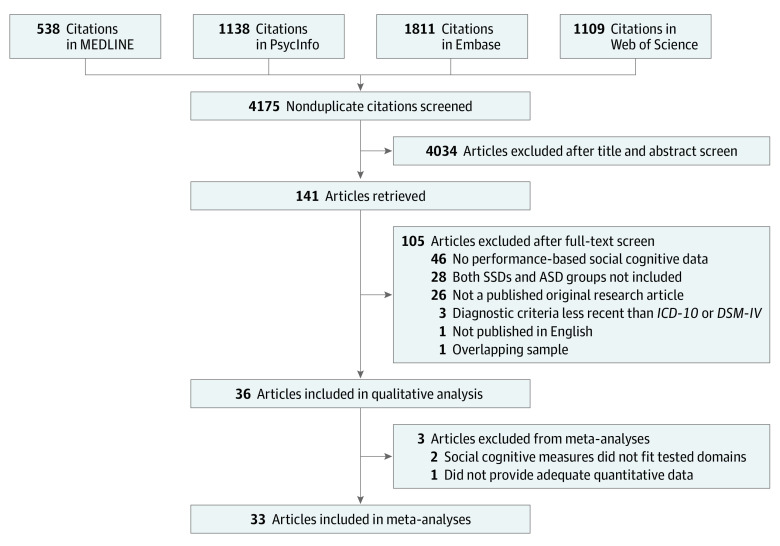
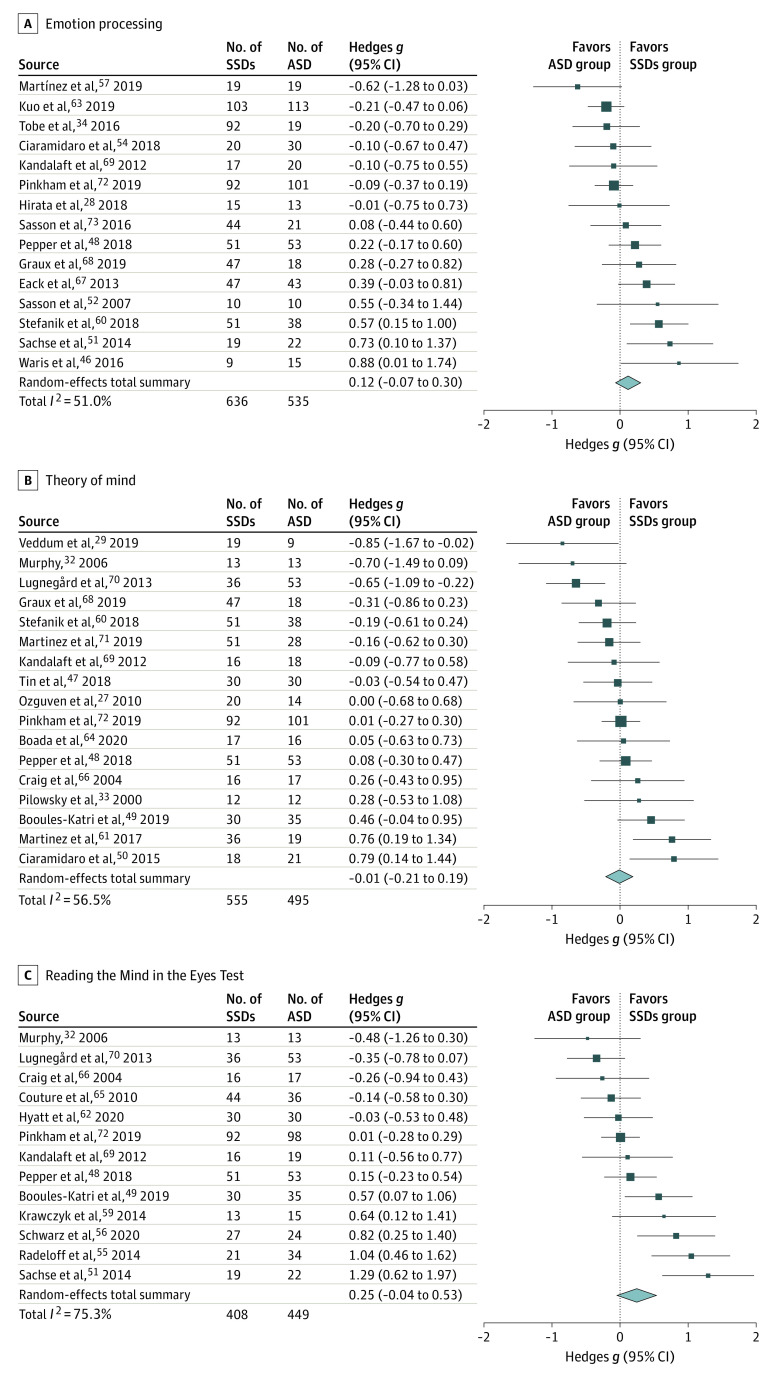
Of the 4175 screened articles, 36 studies directly comparing social cognitive performance in individuals with SSDs vs ASD were included in the qualitative analysis (n = 1212 for SSDs groups and n = 1109 for ASD groups), and 33 studies were included in the quantitative analyses (n = 1113 for SSDs groups and n = 1015 for ASD groups). Most study participants were male (number of studies [k] = 36, 72% [878 of 1212] in SSDs groups and 82% [907 of 1109] in ASD groups), and age (k = 35) was older in SSDs groups (mean [SD], 28.4 [9.5] years) than in ASD groups (mean [SD], 23.3 [7.6] years). Included studies highlighted the prevalence of small, male-predominant samples and a paucity of cross-disorder clinical measures. The meta-analyses revealed no statistically significant differences between SSDs and ASD on emotion processing measures (k = 15; g = 0.12 [95% CI, –0.07 to 0.30]; P = .21; I2 = 51.0%; 1 outlier excluded), ToM measures (k = 17; g = –0.01 [95% CI, –0.21 to 0.19]; P = .92; I2 = 56.5%; 1 outlier excluded), or the RMET (k = 13; g = 0.25 [95% CI, –0.04 to 0.53]; P = .10; I2 = 75.3%). However, SSDs vs ASD performance differences between studies were statistically significantly heterogeneous, which was only minimally explained by potential moderators.
Conclusions and Relevance
In this analysis, similar levels of social cognitive impairment were present, on average, in individuals with SSDs and ASD. Cross-disorder studies of social cognition, including larger samples, consensus batteries, and consistent reporting of measures, as well as data across multiple levels of analysis, are needed to help identify subgroups within and across disorders that may be more homogeneous in etiology and treatment response.
Introduction
Schizophrenia spectrum disorders (SSDs) and autism spectrum disorder (ASD) both feature social cognitive deficits,1,2 which contribute to disability and poor functional outcome,3,4 and have limited treatment options. Overlapping clinical symptoms in SSDs and ASD, and social impairments in particular, have long been recognized. Highly heterogeneous clinical presentation is also characteristic within and across these disorders.5,6,7 Although historically few studies have included both diagnostic groups, cross-disorder research on social cognition in people with SSDs and ASD is increasing because of a shift toward transdiagnostic research,8 use of the Research Domain Criteria framework,9 and the prioritization of improving functional outcome.10,11
Social cognition can be divided into subprocesses, including emotion processing and theory of mind (ToM [also known as mentalizing]), subserved by partially dissociable neural networks.12,13 Meta-analyses have provided evidence for impaired emotion processing and ToM in people with SSDs14,15 and those with ASD16,17 vs typically developing individuals. The onset of ASD begins within the early years of life, during the development of lower-level social cognitive processing (eg, emotion processing), whereas the onset of SSDs begins during late adolescence, when higher-level social cognitive processing (eg, ToM) is still developing.6 Despite these developmental onset differences, similar levels of ToM impairment have been shown in both clinical groups in 2 meta-analyses18,19 of studies that included people with SSDs compared with studies that included people with ASD. However, methods and sample characteristics across studies have been highly variable, making cross-disorder comparisons difficult. Investigations directly comparing social cognition in both conditions are less common, with mixed results regarding relative levels of impairment.
Only 1 meta-analysis20 has examined social cognition across studies directly comparing SSDs and ASD; it found similar ToM performance but greater emotion processing impairment in ASD vs SSDs. Since then, more than 10 studies directly comparing social cognitive performance in SSDs and ASD have been published. Including these new studies more than doubles the sample size and allows for the examination of additional moderators, such as differences in age and antipsychotic treatment, to examine their contribution to the heterogeneity of findings. Formal assessment of study quality, detection of outliers, and sensitivity analyses to evaluate robustness are also needed, which were not included in the previous meta-analysis.20
Herein, we aimed to investigate how deficits in emotion processing and ToM diverge or overlap between individuals with SSDs and ASD by conducting a comprehensive, updated systematic review and meta-analysis of studies directly comparing social cognitive performance in SSDs and ASD. Given differences in age at onset, variation in the use of antipsychotic medication, and changes in diagnostic criteria and research practices over time, we evaluated the implications of age difference, publication year, study quality assessment scores, and antipsychotic medication use as potential moderators for group differences in social cognition using meta-regressions. We hypothesized that effect sizes would be heterogeneous, with a large degree of overlap in emotion processing and ToM performance across SSDs and ASD. Based on meta-analyses to date and evidence of early-onset lower-level social cognitive challenges in ASD,17 we also hypothesized that people with ASD would be more impaired overall on emotion processing than those with SSDs but that similar levels of ToM impairment would be observed.
Methods
This systematic review and meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)21 and Meta-analysis of Observational Studies in Epidemiology (MOOSE)22 guidelines, including data extraction and quality assessment using a modified version of the Newcastle-Ottawa Scale (NOS). Data were pooled using a random-effects model.
Search Strategy
After consultation with a reference librarian, literature searches were conducted in MEDLINE, PsycInfo, Embase, and Web of Science (eMethods in the Supplement) to identify original research articles published from database inception until July 26, 2020, that reported performance-based measures of social cognition in both SSDs and ASD samples. Backward and forward citation searches were also conducted for all studies that met inclusion criteria, as well as relevant reviews and meta-analyses.5,6,18,23,24
Eligibility Criteria
Eligibility criteria for study inclusion were as follows: (1) a group with SSDs (that could include first-episode psychosis) and (2) a group with ASD according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10),25 DSM-IV,26 or DSM-51 definitions (details are provided in the eMethods in the Supplement), (3) reported data from a performance-based measure of social cognition, and (4) original research article. Additional eligibility criteria implemented during the full-text review stage were (5) articles published in English and (6) diagnoses based on ICD-10, DSM-IV, or more recent criteria. Only studies that used performance-based measures (rather than questionnaire or self-report measures) were included because of their greater validity as an index of social cognitive abilities.
References were managed using EndNote Online (Thomson Reuters). Titles and abstracts of nonduplicate publications were screened twice independently (by L.D.O., I.M.-E., and L.G.). Full texts of the potentially eligible studies were then assessed for inclusion by 2 reviewers (L.D.O. and I.M.-E.). Any uncertainties regarding eligibility (n = 135) were reconciled among the reviewers (eMethods in the Supplement).
Data Extraction
Data including social cognitive outcomes and participant demographics (eMethods in the Supplement) were extracted from included articles by 1 of 2 reviewers (L.D.O. and I.M.-E.) and subsequently cross-checked by the other. When SSDs and ASD group means and SDs were not provided, they were calculated27,28,29,30,31 or extracted from plots32,33,34 where possible (eMethods in the Supplement). Calculations and plot extractions were performed twice independently.
Quality Assessment
A modified version of the NOS35 was used to assess the quality and risk of bias of included articles (eg, group comparability) by either L.D.O. or I.M-E. (score range, 1-10 from low to high quality) (eTable 1 in the Supplement). Uncertainties were discussed between reviewers (L.D.O. and I.M-E.) until consensus was reached.
Statistical Analysis
Data were analyzed in RStudio, version 1.1.447 (R Foundation for Statistical Computing) using the metafor package.36 Effect sizes were calculated as standardized mean difference (SSDs vs ASD Hedges g).37 The primary outcomes were performance on emotion processing tasks, ToM tasks, and the RMET (Reading the Mind in the Eyes Test) in SSDs compared with ASD. Random-effects meta-analyses were conducted using inverse-variance weighting and the restricted maximum likelihood estimator.38 For each meta-analysis (emotion processing, ToM, and RMET), heterogeneity of the pooled effect sizes was assessed using the Cochran Q test. Tau2 (τ2) was calculated to estimate between-study variance. The I2 statistic was used to quantify the percentage of total variation across studies (ie, variation in effect sizes for differences between SSDs vs ASD) that was because of true between-study heterogeneity vs chance; 25%, 50%, and 75% reflect low, moderate, and high heterogeneity, respectively.39 Outliers and impactful studies were detected using studentized residuals greater than 3 (in absolute value) and leave-one-out and combinatorial meta-analyses (eMethods in the Supplement).40,41 Normality was checked for the final models using Q-Q plots. Publication bias was also assessed for each meta-analysis via visual inspection of funnel plots and Egger regression tests for funnel plot asymmetry.42 Statistical tests were performed with a significance level of .05 (2-sided).
Meta-analyses
Separate meta-analyses were conducted for emotion processing and ToM performance. A third meta-analysis was performed for the RMET43 because it was the most commonly used measure (number of studies [k] = 13) and the literature is mixed regarding whether it assesses emotion processing or ToM abilities or both.43,44,45 We defined emotion processing tasks as those assessing emotion labeling or matching (eg, emotion recognition), largely thought to involve less complex, lower-level processing, whereas ToM (or mentalizing) is thought to involve more complex, higher-level processing, including perspective taking and mental or emotional state inference.12,13,45 Social cognitive outcomes were categorized by L.D.O. and verified by coauthors (Table 1 and eMethods in the Supplement).
Table 1. Overview of 36 Studies27,28,29,30,31,32,33,34,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73 Included in the Qualitative Analysis.
| Source | No. of SSDs | No. of ASD | Male, No./total No. (%) | Age, mean (SD), y | QA scorea | Meta-analysis | Social cognitive measureb | ||
|---|---|---|---|---|---|---|---|---|---|
| SSDs | ASD | SSDs | ASD | ||||||
| Boada et al,64 2020 | 35 | 42 | 20/35 (57.1) | 37/42 (88.1) | 27.1 (5.8) | 21.4 (5.3) | 5 | ToM | Frith Happé Animations (modified Triangles Task): ToM Questions (17 SSDs and 16 ASD) |
| Bölte and Poustka,31 2003 | 21 | 35 | 15/21 (71.4) | 29/35 (82.9) | 18.1 (3.1) | 13.6 (6.3) | 7 | Emotion processing | Facial Affect Recognition Test |
| Booules-Katri et al,49 2019 | 30 | 35 | 22/30 (73.3) | 34/35 (97.1) | 28.8 (11.5) | 18.6 (6.4) | 5 | ToM | Strange Stories Test: ToM |
| RMET | RMET | ||||||||
| Ciaramidaro et al,50 2015 | 18 | 23 | 14/18 (77.8) | 21/23 (91.3) | 25.7 (4.5) | 18.7 (4.8) | 8 | ToM | Picture Sequencing Task: Communicative Intention (21 ASD) |
| Ciaramidaro et al,54 2018 | 20 | 33 | 14/20 (70.0) | 31/33 (93.9) | 24.7 (5.0) | 18.8 (5.0) | 8 | Emotion processing | Explicit Facial Affect Recognition (30 ASD) |
| Couture et al,65 2010 | 44 | 36 | 39/44 (88.6) | 29/36 (80.6) | 27.5 (6.3) | 20.9 (5.7) | 5 | RMET | RMET |
| Craig et al,66 2004 | 16 | 17 | 11/16 (68.8) | 15/17 (88.2) | 31.7 (9.9) | 24.1 (6.7) | 3 | ToM | Hinting Task |
| RMET | RMET | ||||||||
| Eack et al,67 2013 | 47 | 43 | 34/47 (72.3) | 38/43 (88.4) | 35.0 (12.5) | 24.9 (5.8) | 8 | Emotion processing | Penn Emotion Recognition Test (ER-40) |
| Graux et al,68 2019 | 47 | 18 | 34/47 (72.3) | 16/18 (88.9) | 33.9 (9.5) | 26.5 (5.8) | 1 | Emotion processing | Facial Emotion Recognition Test (TREF) |
| ToM | Movie for the Assessment of Social Cognition: ToM | ||||||||
| Hirata et al,28 2018 | 15 | 13 | 12/15 (80.0) | 12/13 (92.3) | 40.1 (15.7) | 31.7 (13.5) | 4 | Emotion processing | Emotional Face Recognition Task: Facial Emotional Matching |
| Hyatt et al,62 2020 | 30 | 30 | 19/30 (63.3) | 26/30 (86.7) | 26.0 (3.5) | 21.7 (3.4) | 8 | RMET | RMET |
| Kandalaft et al,69 2012 | 19 | 20 | 12/19 (63.2) | 16/20 (80.0) | 31.1 (5.4) | 22.9 (4.9) | 6 | Emotion processing | Ekman 60 Faces Test (Ekman 60) (17 SSDs) |
| ToM | Triangles Task: ToM Intentionality (16 SSDs and 18 ASD) | ||||||||
| RMET | RMET (16 SSDs and 19 ASD) | ||||||||
| Krawczyk et al,59 2014 | 13 | 15 | 7/13 (53.8) | 11/15 (73.3) | 30.0 (5.7) | 21.7 (4.4) | 5 | RMET | RMET |
| Kuo et al,63 2019 | 103 | 113 | 74/103 (72.0) | 92/113 (81.0) | 24.8 (5.4) | 24.8 (6.7) | 6 | Emotion processing | Mayer-Salovey-Caruso Emotional Intelligence Test: Perceiving Emotion, Faces |
| Lugnegård et al,70 2013 | 36 | 53 | 22/36 (61.1) | 26/53 (49.1) | 28.8 (4.1) | 27.3 (4.1) | 7 | ToM | Triangles Task: ToM Intentionality |
| RMET | RMET | ||||||||
| Martinez et al,61 2017 | 36 | 19 | 30/36 (83.3) | 15/19 (78.9) | 23.4 (3.5) | 22.7 (4.1) | 5 | ToM | Movie for the Assessment of Social Cognition: ToM |
| Martinez et al,71 2019 | 51 | 32 | 42/51 (82.4) | 25/32 (78.1) | 23.4 (3.6) | 22.6 (3.5) | 8 | ToM | Animated Shapes Task (modified Triangles Task): ToM Intentionality (28 ASD) |
| Martínez et al,57 2019 | 19 | 20 | 15/19 (78.9) | 16/20 (80.0) | 37.2 (10.2) | 28.9 (7.7) | 8 | Emotion processing | Penn Emotion Recognition Test (ER-40) (19 ASD) |
| Morrison et al,58 2017 | 54 | 54 | 47/54 (87.0) | 47/54 (87.0) | 28.7 (10.1) | 25.7 (7.2) | 7 | NA | Social Skills Performance Assessment |
| Murphy,32 2006 | 13 | 13 | 13/13 (100) | 13/13 (100) | 29.7 (6.2) | 35.0 (7.5) | 4 | ToM | Modified Advanced ToM Test: Second-Order |
| RMET | RMET | ||||||||
| Ozguven et al,27 2010 | 20 | 14 | 20/20 (100) | 14/14 (100) | 27.0 (4.8) | 24.4 (7.1) | 9 | ToM | Second-Order ToM Stories Task |
| Pepper et al,48 2018 | 51 | 53 | 36/51 (70.6) | 35/53 (66.0) | 21.8 (4.4) | 23.9 (7.4) | 8 | Emotion processing | Ekman 60 Faces Test (Ekman 60) |
| ToM | Picture Sequencing Task: False Belief | ||||||||
| RMET | RMET | ||||||||
| Pilowsky et al,33 2000 | 12 | 12 | 9/12 (75.0) | 11/12 (91.7) | 12.2 (1.7) | 13.0 (3.9) | 6 | ToM | False Belief Task |
| Pinkham et al,30 2008 | 24 | 12 | 24/24 (100) | 12/12 (100) | 27.2 (4.6) | 24.1 (5.7) | 6 | NA | Trustworthiness Task |
| Pinkham et al,72 2019 | 92 | 101 | 65/92 (70.7) | 90/101 (89.1) | 27.8 (7.3) | 24.2 (6.2) | 10 | Emotion processing | Penn Emotion Recognition Test (ER-40) |
| ToM | Hinting Task | ||||||||
| RMET | RMET (98 ASD) | ||||||||
| Radeloff et al,55 2014 | 21 | 34 | 16/21 (76.2) | 31/34 (91.2) | 24.7 (5.2) | 19.1 (5.1) | 9 | RMET | RMET |
| Sachse et al,51 2014 | 19 | 22 | 14/19 (73.7) | 18/22 (81.8) | 25.5 (4.9) | 20.9 (5.6) | 9 | Emotion processing | Frankfurt Test for the Recognition of Facial Affects: Face |
| RMET | RMET | ||||||||
| Sasson et al,52 2007 | 10 | 10 | 9/10 (90.0) | 10/10 (100) | 28.1 (5.07) | 23 (5.3) | 6 | Emotion processing | Modified Social Scenes Task: Face-Present |
| Sasson et al,73 2016 | 44 | 21 | 27/44 (61.4) | 18/21 (85.7) | 35.3 (10.6) | 23.4 (4.4) | 6 | Emotion processing | Emotions in Context Task: Face in Congruent Context |
| Schwarz et al,56 2020 | 27 | 25 | 18/27 (66.7) | 14/25 (56.0) | 32.4 (10.4) | 32.1 (9.6) | 9 | RMET | RMET (24 ASD) |
| Stanfield et al,53 2017 | 21 | 28 | 14/21 (66.7) | 22/28 (78.6) | NA | NA | 5 | NA | Ekman 60 Faces Test (Ekman 60; usable values not reported) |
| Stefanik et al,60 2018 | 51 | 38 | 32/51 (62.7) | 26/38 (68.4) | 26.5 (4.8) | 22.6 (4.6) | 7 | Emotion processing | TASIT-R I: Emotion Evaluation Task |
| ToM | TASIT-R III: Social Inference–Enriched | ||||||||
| Tin et al,47 2018 | 30 | 30 | 19/30 (63.3) | 23/30 (76.7) | 17.5 (1.2) | 17.0 (0.9) | 9 | ToM | Faux Pas Task: Inference of Intention |
| Tobe et al,34 2016 | 92 | 19 | 79/92 (85.9) | 17/19 (89.5) | 37.8 (10.4) | 39.4 (12.5) | 4 | Emotion processing | Penn Emotion Recognition Test (ER-40) |
| Veddum et al,29 2019 | 21 | 11 | 10/21 (47.6) | 4/11 (36.4) | 40.5 (12.4) | 25.1 (6.4) | 6 | ToM | Triangles Task: ToM Intentionality (19 SSDs and 9 ASD) |
| Waris et al,46 2016 | 10 | 15 | 2/10 (20.0) | 7/15 (46.7) | 16.2 (1.6) | 16.1 (1.6) | 6 | Emotion processing | NEPSY-II: Emotion Recognition Matching (9 SSDs) |
| ToM | NEPSY-II: ToM Verbal (9 SSDs) | ||||||||
Abbreviations: ASD, autism spectrum disorder; NA, not applicable; NEPSY, A Developmental Neuropsychological Assessment; QA, quality assessment; RMET, Reading the Mind in the Eyes Test; SSDs, schizophrenia spectrum disorders; TASIT-R, The Awareness of Social Inference Test–Revised; ToM, theory of mind.
The maximum QA score was 10 points, with lower scores reflecting greater risk of bias.
Where numbers differed by social cognitive measure, this number is indicated beside the task description.
Moderator Analyses
Meta-regressions were performed for age difference, publication year, quality assessment scores, and antipsychotic medication use. The associations of age difference (SSDs vs ASD Hedges g) and publication year were examined using multivariable meta-regressions for each meta-analysis (eMethods in the Supplement). Exploratory univariable meta-regressions were also conducted to examine the associations of quality assessment scores and medication use (SSDs vs ASD mean proportion of participants taking antipsychotic medication) for emotion processing and ToM, as well as stimulus type (verbal and visual) for ToM (emotion processing was all visual).
Sensitivity Analyses
The main meta-analyses were rerun after excluding studies that required calculations or plot extraction for the quantitative analyses27,28,29,31,32,33,34 or those with only youth participants (age range, 10-20 years)33,46,47 to ensure that these samples were not unduly altering results. Meta-analyses were also rerun after excluding articles with SSDs groups composed only of individuals with first-episode psychosis,48 because this designation does not constitute a formal SSDs diagnosis,1 and articles with SSDs groups composed only of those with schizotypal or schizoid personality disorder,49 because this classification can be considered both part of the schizophrenia spectrum and a personality disorder.
Results
Study Selection and Characteristics
Of the 4175 screened articles, the literature search (Figure 1) resulted in 36 studies directly comparing social cognitive performance in individuals with SSDs vs ASD being included in the qualitative analysis (n = 1212 for SSDs groups and n = 1109 for ASD groups) and 33 studies being included in the quantitative analyses (n = 1113 for SSDs groups and n = 1015 for ASD groups). Eleven authors were contacted for further information (eTable 2 in the Supplement). Across included studies (Table 1),27,28,29,30,31,32,33,34,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73 sample sizes of the SSDs and ASD groups tended to be small (a group with <25 participants in 22 studies; range, 10-113). Most study participants were male (k = 36, 72% [878 of 1212] in SSDs groups and 82% [907 of 1109] in ASD groups), and age (k = 35) was older in SSDs groups (mean [SD], 28.4 [9.5] years) than in ASD groups (mean [SD], 23.3 [7.6] years). IQ data reported (k = 31) were inconsistent (eg, full-scale, verbal, nonverbal, and unspecified), precluding data pooling. Antipsychotic medication use status was reported for both groups in 22 articles,27,28,30,32,34,47,48,50,51,52,53,54,55,56,57,58,60,62,67,70,72,73 10 of which reported chlorpromazine equivalents for both.28,30,50,51,52,53,54,55,56,57 All studies used ICD-10, DSM-IV, or DSM-5 criteria for SSDs and ASD diagnoses.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram Showing Study Selection.
Literature searches were conducted from database inception until July 26, 2020. ASD indicates autism spectrum disorder; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; SSDs, schizophrenia spectrum disorders.
Social Cognitive and Additional Measures
Among the studies included in the meta-analyses, 16 studies28,31,34,46,48,51,52,54,57,60,63,67,68,69,72,73 used performance-based emotion processing measures, 18 studies27,29,32,33,46,47,48,49,50,60,61,64,66,68,69,70,71,72 included ToM measures, and 13 studies32,48,49,51,55,56,59,62,65,66,69,70,72 used the RMET (Table 1). Three articles30,53,58 were excluded from the quantitative analyses (details are provided in the eMethods in the Supplement).
Reporting of measures of nonsocial cognition, clinical symptoms, everyday or adaptive functioning, and neuroimaging was uncommon and inconsistent within and between studies (eMethods in the Supplement). Nine studies27,28,29,34,46,56,59,60,74 included a measure of nonsocial cognition, but these varied widely. Clinical scores or subscores provided from each study varied; only 2 studies61,62 reported measures of schizophrenia and autistic symptoms in both groups. Measures of everyday functioning were also rare (k = 5).29,60,62,63,64 Ten articles28,30,50,53,54,55,56,57,60,62 reported neuroimaging data in conjunction with performance-based social cognition.
Quality Assessment
Five studies28,32,34,66,68 received low (<5 points), 18 studies29,30,31,33,46,49,52,53,58,59,60,61,63,64,65,69,70,73 received moderate (5-7 points), and 13 studies27,47,48,50,51,54,55,56,57,62,67,71,72 received high (8-10 points) scores on the modified NOS (Table 1). Independent validation of diagnoses was most often unreported, and additional points were lost fairly uniformly among the remaining criteria.
Quantitative Findings
Quantitative findings included emotion processing, ToM, and RMET analyses. Detailed results from the main meta-analyses and sensitivity analyses are listed in Table 2.
Table 2. Main Meta-analyses and Sensitivity Analyses.
| Variable | k | No. of SSDs | No. of ASD | Hedges g (95% CI) | P value | Q | Q P value | τ2 | I2 statistic, % | Bias coefficient | Egger P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emotion processing | |||||||||||
| Main meta-analysis | 16 | 657 | 570 | 0.21 (−0.03 to 0.44) | .08 | 48.1 | <.001 | 0.152 | 71.8 | 1.22 | .22 |
| Main meta-analysis (outlier removed) | 15 | 636 | 535 | 0.12 (−0.07 to 0.30) | .21 | 28.9 | .01 | 0.061 | 51.0 | 1.15 | .25 |
| Sensitivity analysesa | |||||||||||
| Data available | 13 | 529 | 503 | 0.15 (−0.05 to 0.36) | .14 | 27.6 | .006 | 0.071 | 56.1 | 1.30 | .19 |
| SSDs diagnosis | 14 | 585 | 482 | 0.11 (−0.09 to 0.31) | .28 | 28.3 | .008 | 0.072 | 54.2 | 1.19 | .23 |
| Adults only | 14 | 627 | 520 | 0.09 (−0.09 to 0.27) | .33 | 25.5 | .02 | 0.053 | 49.0 | 0.57 | .57 |
| Theory of mind | |||||||||||
| Main meta-analysis | 18 | 564 | 510 | −0.13 (−0.45 to 0.18) | .42 | 66.9 | <.001 | 0.360 | 83.0 | −3.18 | .002 |
| Main meta-analysis (outlier removed) | 17 | 555 | 495 | −0.01 (−0.21 to 0.19) | .92 | 35.1 | .004 | 0.091 | 56.5 | −0.08 | .94 |
| Sensitivity analysesa | |||||||||||
| Data available | 12 | 474 | 431 | 0.05 (−0.18 to 0.28) | .68 | 27.6 | .004 | 0.099 | 63.1 | 1.32 | .19 |
| SSDs diagnosis | 15 | 474 | 407 | −0.06 (−0.27 to 0.16) | .62 | 30.8 | .006 | 0.099 | 56.7 | 0.13 | .89 |
| Adults only | 15 | 513 | 453 | −0.02 (−0.25 to 0.20) | .85 | 34.6 | .002 | 0.113 | 62.4 | −0.31 | .76 |
| Reading the Mind in the Eyes Test | |||||||||||
| Main meta-analysis | 13 | 408 | 449 | 0.25 (−0.04 to 0.53) | .10 | 42.0 | <.001 | 0.200 | 75.3 | 0.85 | .39 |
| Sensitivity analysesa | |||||||||||
| Data available | 12 | 395 | 436 | 0.29 (0.00 to 0.58) | .05 | 39.3 | <.001 | 0.191 | 75.4 | 1.71 | .08 |
| SSDs diagnosis | 11 | 327 | 361 | 0.23 (−0.12 to 0.57) | .20 | 39.4 | <.001 | 0.253 | 78.1 | 0.81 | .42 |
Abbreviations: ASD, autism spectrum disorder; k, number of studies; SSDs, schizophrenia spectrum disorders.
For sensitivity analyses, “Data available” excluded studies that required calculations or plot extraction for the quantitative analyses, “SSDs diagnosis” excluded studies with SSDs groups composed entirely of individuals with schizotypal or schizoid personality disorder or only individuals with first-episode psychosis, and “Adults only” excluded studies with only youth participants (age range, 10-20 years). Heterogeneity of the pooled effect sizes are calculated using the Cochran Q test. Tau2 (τ2) provides an estimate of between-study variance, and the I2 statistic quantifies the percentage of total variation across studies because of between-study heterogeneity vs chance.
Emotion Processing
There was no statistically significant between-group difference in emotion processing, although a suggestion toward better performance in the SSDs vs ASD groups was found (k = 16; g = 0.21 [95% CI, −0.03 to 0.44]; P = .08) (eFigure 1A in the Supplement). Effect sizes were statistically significantly heterogeneous (I2 = 71.8%). One impactful outlier was detected using studentized residuals and the leave-one-out (eTable 3 in the Supplement) and combinatorial (Figure 2A) meta-analyses.31,41 After removal of 1 outlier, the between-group difference remained non–statistically significant and decreased in size (k = 15; g = 0.12 [95% CI, −0.07 to 0.30]; P = .21) (Figure 3A),28,34,46,48,51,52,54,57,60,63,67,68,69,72,73 and heterogeneity was reduced (I2 = 51.0%). Funnel plot visualization (eFigure 2A in the Supplement) and Egger regression test revealed no evidence of publication bias (Table 2).
Figure 2. Graphical Display of Study Heterogeneity (GOSH)41 Plots for Visualizing Between-Study Heterogeneity.
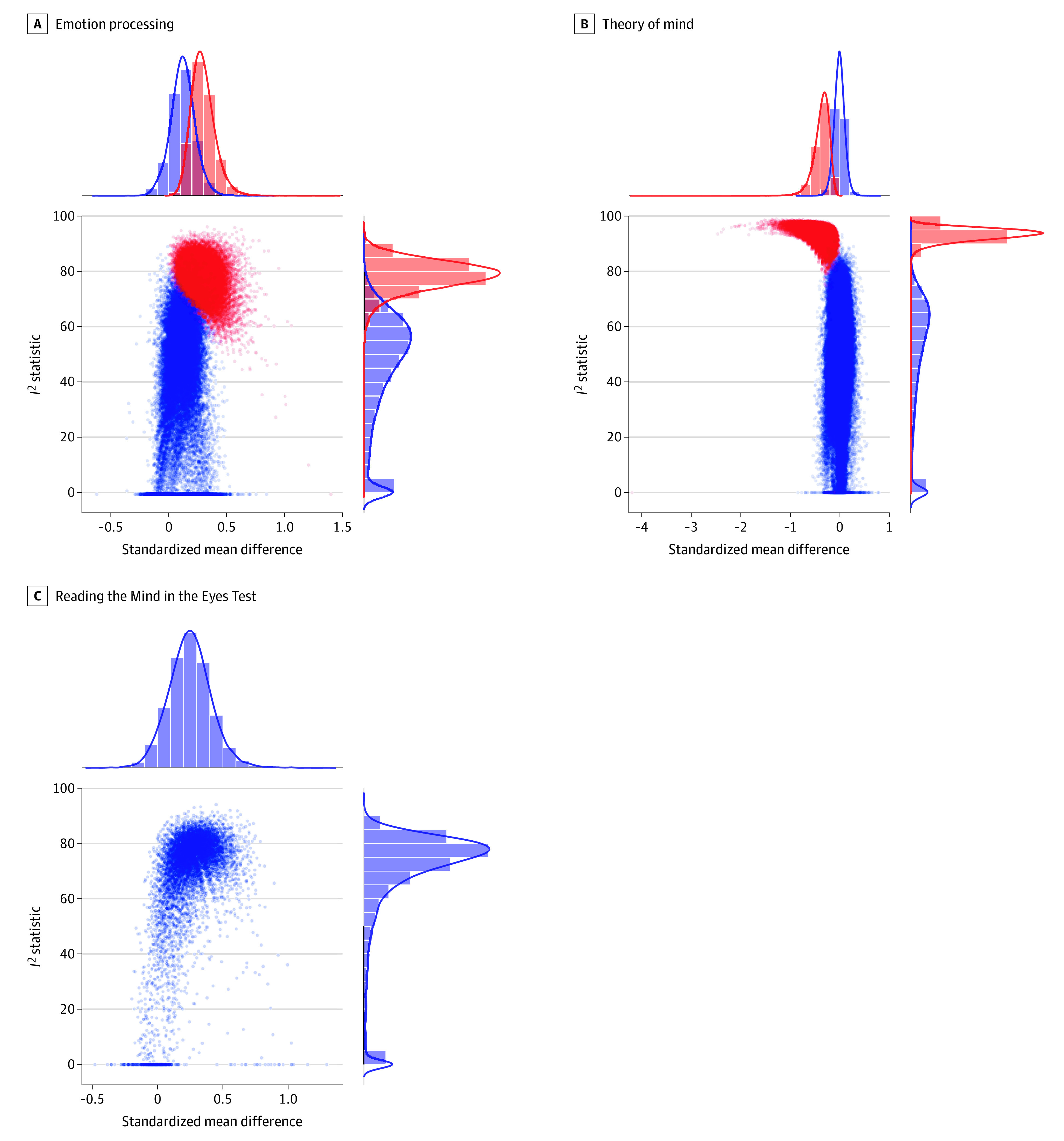
GOSH plots were generated by performing separate meta-analyses on all possible subsets of included studies (combinatorial meta-analyses) for emotion processing (A), theory of mind (B), and the Reading the Mind in the Eyes Test (C). Each plot shows overall standardized mean difference (Hedges g) and between-study heterogeneity (I2 statistic) for each of the meta-analyses performed to enable visualization of patterns between effect sizes and heterogeneity. The data points for meta-analyses that included studies detected as outliers are shown in red (with larger effect sizes and higher heterogeneity), and all others are shown in blue. Hedges g values exceeding 0 indicate that SSDs groups outperformed ASD groups. Greater I2 statistics represent higher between-study heterogeneity.
Figure 3. Forest Plots of Effect Sizes for Each Meta-analysis.
After outlier removal (where detected), forest plots for meta-analyses show standardized mean difference (Hedges g) and 95% CIs for emotion processing (A),28,34,46,48,51,52,54,57,60,63,67,68,69,72,73 theory of mind (B),28,34,46,48,51,52,54,57,60,63,67,68,69,72,73 and the Reading the Mind in the Eyes Test (C).32,48,49,51,55,56,59,62,65,66,69,70,72 Hedges g values exceeding 0 indicate that SSDs groups outperformed ASD groups. Square size is proportional to study weight in the model. The diamond signifies the overall effect size and its 95% CI.
Moderator analyses demonstrated no statistically significant associations of age difference (z = −0.27; P = .79) or publication year (z = −1.58; P = .11) with emotion processing effect sizes (k = 15; P = .26; R2 = 8.96%). Exploratory moderator analyses also revealed no statistically significant association of quality assessment scores (k = 15; z = 0.05; P = .96; R2 = 0%) or antipsychotic medication use (k = 11; z = 0.50; P = .61; R2 = 0%). The overall emotion processing effect size remained non–statistically significant in sensitivity analyses (Table 2 and eResults in the Supplement).
Theory of Mind
There was no statistically significant between-group difference in ToM performance (k = 18; g = −0.13 [95% CI, −0.45 to 0.18]; P = .42) (eFigure 1B in the Supplement). Effect sizes were highly heterogeneous (I2 = 83.0%), and 1 impactful outlier was identified (Figure 2B and eTable 4 in the Supplement).46 The between-group difference remained non–statistically significant after removal of 1 outlier (k = 17; g = −0.01 [95% CI, −0.21 to 0.19]; P = .92) (Figure 3B),28,34,46,48,51,52,54,57,60,63,67,68,69,72,73 and heterogeneity decreased but remained statistically significant (I2 = 56.5%). There was no evidence of publication bias after outlier removal based on funnel plot inspection (eFigure 2B in the Supplement) and Egger regression test (Table 2).
There were no statistically significant associations of age difference (z = 0.59; P = .55) or publication year (z = −0.16; P = .88) with ToM effect sizes (k = 17; P = .84; R2 = 0%). Exploratory meta-regressions also revealed no statistically significant association of quality assessment scores (k = 17; z = 0.22; P = .83; R2 = 0%) or stimulus type (k = 14; z = −0.54; P = .59; R2 = 0%). The between-group difference in the proportion of participants taking antipsychotic medication was statistically significantly associated with ToM effect sizes, accounting for a substantial amount of heterogeneity (k = 8; z = −1.99; P = .047; R2 = 46.40%) (eFigure 3 in the Supplement). As the proportion of individuals with SSDs vs ASD taking antipsychotic medication increased, ToM performance of the SSDs vs ASD groups worsened. The overall ToM effect size remained non–statistically significant in sensitivity analyses (Table 2 and eResults in the Supplement).
Reading the Mind in the Eyes Test
The RMET meta-analysis revealed no statistically significant difference in performance between groups, although SSDs groups performed slightly better than ASD groups (k = 13; g = 0.25 [95% CI, −0.04 to 0.53]; P = .10) (Figure 3C).32,48,49,51,55,56,59,62,65,66,69,70,72 Effect sizes were statistically significantly and highly heterogeneous (I2 = 75.3%), although no outliers were identified (Figure 2C and eTable 5 in the Supplement). Funnel plot visualization (eFigure 2C in the Supplement) and Egger regression test revealed no evidence of publication bias (Table 2).
Moderator analyses yielded no statistically significant associations of age difference (z = 0.93; P = .35) or publication year (z = 1.36; P = .17) with RMET performance (k = 13; P = .25; R2 = 3.60%). Exploratory meta-regressions also revealed no statistically significant association of quality assessment scores (k = 13; z = 1.60; P = .11; R2 = 8.55%) or antipsychotic medication use (k = 7; z = 1.11; P = .27; R2 = 0.33%). The overall RMET effect size remained non–statistically significant in sensitivity analyses (Table 2 and eResults in the Supplement).
Discussion
This systematic review and meta-analysis included studies that directly compared individuals with SSDs vs ASD on performance-based social cognitive measures to identify overlapping and divergent deficits. Findings herein suggest that similar levels of social cognitive impairment may be present in SSDs and ASD across emotion processing and ToM domains. However, heterogeneity of effect sizes was apparent and was only minimally explained by the moderators explored. Included studies highlighted the prevalence of small, male-predominant samples and a paucity of cross-disorder clinical measures.
Across the main meta-analyses comparing emotion processing, ToM, and RMET performance in individuals with SSDs vs ASD, no statistically significant group differences were identified. This finding builds on previous meta-analyses showing similar impairment levels in those with SSDs and ASD vs typically developing individuals on ToM tasks18,19 and the RMET,18 providing further evidence based on direct SSDs vs ASD comparisons. The ToM and RMET findings herein align with results from the only other meta-analysis20 to date examining direct SSDs vs ASD comparisons; the present study provides additional support for minimal differences among individuals with SSDs vs ASD and includes 9 and 5 additional studies, respectively. The previous meta-analysis20 found that participants with SSDs outperformed those with ASD on emotion processing tasks; however, heterogeneity was high, only 8 studies were included, and assessment of impactful studies was not reported. With twice the number of studies, we found no statistically significant group differences in emotion processing performance. Results herein suggest that a consistent pattern of differences in social cognitive performance between SSDs and ASD is not apparent across studies to date. However, many sample sizes were small, and the moderate to high levels of effect size heterogeneity indicate that differences in social cognitive performance between SSDs and ASD varied among studies, warranting further investigation. This heterogeneity represents variability in SSDs vs ASD performance differences rather than within-disorder performance variability.
Age difference between SSDs vs ASD and publication year (partially reflecting changes in clinical practices and diagnostic criteria over time) did not explain between-study variability observed herein in emotion processing, ToM, or RMET effect sizes. The previous meta-analysis20 found better emotion processing and RMET performance in SSDs vs ASD among studies with younger participants across groups. It may be that age difference is less relevant than whether participants are generally younger or older across diagnoses because of relative differences in neurodevelopment and pathoplastic progression (eg, earlier emerging challenges in ASD).6 The exploratory moderator analyses herein revealed that a greater proportion of people with SSDs vs ASD taking antipsychotic medication was associated with poorer ToM performance in SSDs vs ASD, accounting for a moderate amount of between-study heterogeneity. This finding may suggest that antipsychotic medication use negatively impacts ToM performance or could reflect worse performance in those receiving antipsychotic treatment potentially because of greater illness severity. Although there is some meta-analytic evidence for moderating associations of antipsychotic medication use with emotion processing performance in SSDs,15 other meta-regressions have found no association between antipsychotic medication use in SSDs and emotion processing, ToM, or RMET performance.18,75 Additional work that includes complete reporting of medication use status is needed to investigate how antipsychotic treatment and other medication use impact social cognition across SSDs and ASD.
Both SSDs and ASD are highly heterogeneous disorders, and differences in nonsocial cognition, clinical symptoms, and everyday functioning may also have contributed to between-study variability observed herein. However, reporting of such metrics was uncommon across included studies, and measures used to assess these domains were inconsistent both within and between groups. Social cognition has been consistently positively associated with nonsocial cognition and functional outcome in SSDs3 and ASD4 and inversely associated with negative symptoms in SSDs.45,76 Meta-regressions15,75 have demonstrated a negative association between emotion processing performance and negative symptoms in SSDs. Illness duration may also be an important factor. Studies that concurrently include assessments tapping these variables in both SSDs and ASD are necessary to elucidate their association and facilitate transdiagnostic comparisons.
Of the studies reviewed, those with neuroimaging alongside social cognitive measures had mixed findings and tended to include small samples and a single imaging modality. Neurobiological investigations into shared or distinct mechanisms underlying the common social cognitive deficits across SSDs and ASD are warranted. For example, hypermentalizing or hypomentalizing at a cognitive level or opposing patterns of brain activation or connectivity could both lead to impaired ToM performance,50 aligning with a diametrical hypothesis of SSDs and ASD.5 However, the degree of between-study heterogeneity may also align with evidence suggesting that subgroups with overlapping brain-behavior associations may exist transdiagnostically,60 reflecting multiple etiological pathways. Future work is needed to directly test these and alternative theories.7 Integrating data across multiple levels of analysis through approaches like network theory77 will provide additional insight into factors contributing to the emergence of psychiatric disorders, those associated with social cognition, and potential treatment targets.78
Limitations
This study has some limitations. Few of the reviewed studies focused on adolescents, limiting generalizability of these findings. This limitation is particularly important given the different developmental trajectories of SSDs and ASD and of lower-level vs higher-level social cognition.6 Future work that includes people with co-occurring diagnoses of SSDs and ASD could be informative from a transdiagnostic perspective given the high prevalence of shared traits79 and co-occurrence of these conditions.80,81 Indeed, dimensional analyses of shared symptoms are becoming more common in investigations of the overlap between SSDs and ASD.82,83,84 Many existing social cognitive tasks may also lack discriminatory power between SSDs and ASD. Finally, although most studies reviewed herein implemented at least some practices to limit bias, a standardized approach would have improved opportunities for comparisons across studies.
Conclusions
Based on meta-analyses of the extant literature, similar levels of social cognitive impairment may be present in SSDs and ASD across emotion processing and ToM. These results highlight the need for cross-disorder studies of social cognition with larger samples, including adolescents, and consistent reporting of measures that may impact outcome. Integrating data spanning multiple levels of analysis across SSDs and ASD is a critical next step to identify associations that may delineate more homogeneous subgroups with similar etiology, treatment response, and phenotypic characteristics.
eMethods. Supplemental Methods
eTable 1. Modified Newcastle-Ottawa Scale for Quality Assessment Legend
eTable 2. Additional Information Provided by Contacted Authors
eTable 3. Emotion Processing Leave-One-Out Meta-analyses
eTable 4. Theory of Mind Leave-One-Out Meta-analyses
eTable 5. Reading the Mind in the Eyes Test Leave-One-Out Meta-analyses
eFigure 1. Forest Plots of Effect Sizes for Meta-analyses Prior to Outlier Removal
eFigure 2. Funnel Plots for Each Meta-analysis
eFigure 3. Theory of Mind Meta-Regression Scatterplot With Difference in Proportion of Participants on Antipsychotics as Moderator
eResults. Supplemental Results
eReferences.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16(10):620-631. doi: 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- 3.Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212-219. doi: 10.1016/j.neubiorev.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 4.Sasson NJ, Morrison KE, Kelsven S, Pinkham AE. Social cognition as a predictor of functional and social skills in autistic adults without intellectual disability. Autism Res. 2020;13(2):259-270. doi: 10.1002/aur.2195 [DOI] [PubMed] [Google Scholar]
- 5.Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31(3):241-261. doi: 10.1017/S0140525X08004214 [DOI] [PubMed] [Google Scholar]
- 6.Sasson NJ, Pinkham AE, Carpenter KL, Belger A. The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J Neurodev Disord. 2011;3(2):87-100. doi: 10.1007/s11689-010-9068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisholm K, Lin A, Abu-Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. 2015;55:173-183. doi: 10.1016/j.neubiorev.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 8.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35. doi: 10.1002/wps.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- 10.Fleischhacker WW, Arango C, Arteel P, et al. Schizophrenia: time to commit to policy change. Schizophr Bull. 2014;40(suppl 3):S165-S194. doi: 10.1093/schbul/sbu006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren Z, Taylor JL, McPheeters ML, et al. Future research needs: interventions for adolescents and young adults with autism spectrum disorders: identification of future research needs from comparative effectiveness review No. 65. Agency for Healthcare Research and Quality; September 2012. Future Research Needs Papers No. 20. Accessed May 13, 2020. https://www.ncbi.nlm.nih.gov/books/NBK121979/ [PubMed]
- 12.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64(1):48-61. doi: 10.1016/j.biopsych.2008.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17(1):18-24. doi: 10.1177/1073858410379268 [DOI] [PubMed] [Google Scholar]
- 14.Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39(5):979-992. doi: 10.1093/schbul/sbs080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36(5):1009-1019. doi: 10.1093/schbul/sbn192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velikonja T, Fett AK, Velthorst E. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76(2):135-151. doi: 10.1001/jamapsychiatry.2018.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uljarevic M, Hamilton A. Recognition of emotions in autism: a formal meta-analysis. J Autism Dev Disord. 2013;43(7):1517-1526. doi: 10.1007/s10803-012-1695-5 [DOI] [PubMed] [Google Scholar]
- 18.Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr Bull. 2014;40(3):602-616. doi: 10.1093/schbul/sbt048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bliksted V, Ubukata S, Koelkebeck K. Discriminating autism spectrum disorders from schizophrenia by investigation of mental state attribution on an on-line mentalizing task: a review and meta-analysis. Schizophr Res. 2016;171(1-3):16-26. doi: 10.1016/j.schres.2016.01.037 [DOI] [PubMed] [Google Scholar]
- 20.Fernandes JM, Cajão R, Lopes R, Jerónimo R, Barahona-Corrêa JB. Social cognition in schizophrenia and autism spectrum disorders: a systematic review and meta-analysis of direct comparisons. Front Psychiatry. 2018;9:504. doi: 10.3389/fpsyt.2018.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 23.Cotter J, Granger K, Backx R, Hobbs M, Looi CY, Barnett JH. Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev. 2018;84:92-99. doi: 10.1016/j.neubiorev.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 24.Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6(10):e25322. doi: 10.1371/journal.pone.0025322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . International Statistical Classification of Diseases, Tenth Revision (ICD-10). World Health Organization; 1992. [Google Scholar]
- 26.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 27.Ozguven HD, Oner O, Baskak B, Oktem F, Olmez S, Munir K. Theory of mind in schizophrenia and Asperger’s syndrome: relationship with negative symptoms. Klinik Psikofarmakol Bulteni. 2010;20(1):5-13. doi: 10.1080/10177833.2010.11790628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata K, Egashira K, Harada K, et al. Differences in frontotemporal dysfunction during social and non-social cognition tasks between patients with autism spectrum disorder and schizophrenia. Sci Rep. 2018;8(1):3014. doi: 10.1038/s41598-018-21379-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veddum L, Pedersen HL, Landert AL, Bliksted V. Do patients with high-functioning autism have similar social cognitive deficits as patients with a chronic cause of schizophrenia? Nord J Psychiatry. 2019;73(1):44-50. doi: 10.1080/08039488.2018.1554697 [DOI] [PubMed] [Google Scholar]
- 30.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1-3):164-175. doi: 10.1016/j.schres.2007.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bölte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med. 2003;33(5):907-915. doi: 10.1017/S0033291703007438 [DOI] [PubMed] [Google Scholar]
- 32.Murphy D. Theory of mind in Asperger’s syndrome, schizophrenia and personality disordered forensic patients. Cogn Neuropsychiatry. 2006;11(2):99-111. doi: 10.1080/13546800444000182 [DOI] [PubMed] [Google Scholar]
- 33.Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophr Res. 2000;42(2):145-155. doi: 10.1016/S0920-9964(99)00101-2 [DOI] [PubMed] [Google Scholar]
- 34.Tobe RH, Corcoran CM, Breland M, et al. Differential profiles in auditory social cognition deficits between adults with autism and schizophrenia spectrum disorders: a preliminary analysis. J Psychiatr Res. 2016;79:21-27. doi: 10.1016/j.jpsychires.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed April 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 36.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 37.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; 1985. [Google Scholar]
- 38.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261-293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 41.Olkin I, Dahabreh IJ, Trikalinos TA. GOSH: a graphical display of study heterogeneity. Res Synth Methods. 2012;3(3):214-223. doi: 10.1002/jrsm.1053 [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241-251. doi: 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- 44.Oakley BFM, Brewer R, Bird G, Catmur C. Theory of mind is not theory of emotion: a cautionary note on the Reading the Mind in the Eyes Test. J Abnorm Psychol. 2016;125(6):818-823. doi: 10.1037/abn0000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliver LD, Haltigan JD, Gold JM, et al. Lower- and higher-level social cognitive factors across individuals with schizophrenia spectrum disorders and healthy controls: relationship with neurocognition and functional outcome. Schizophr Bull. 2019;45(3):629-638. doi: 10.1093/schbul/sby114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waris P, Tani P, Lindberg N, et al. Are there differences in neurocognition and social cognition among adolescents with schizophrenia, a pervasive developmental disorder, and both disorders? Appl Neuropsychol Child. 2016;5(4):303-310. doi: 10.1080/21622965.2015.1064001 [DOI] [PubMed] [Google Scholar]
- 47.Tin LNW, Lui SSY, Ho KKY, et al. High-functioning autism patients share similar but more severe impairments in verbal theory of mind than schizophrenia patients. Psychol Med. 2018;48(8):1264-1273. doi: 10.1017/S0033291717002690 [DOI] [PubMed] [Google Scholar]
- 48.Pepper KL, Demetriou EA, Park SH, et al. Autism, early psychosis, and social anxiety disorder: understanding the role of social cognition and its relationship to disability in young adults with disorders characterized by social impairments. Transl Psychiatry. 2018;8(1):233. doi: 10.1038/s41398-018-0282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booules-Katri TM, Pedreño C, Navarro JB, Pamias M, Obiols JE. Theory of mind (ToM) performance in high functioning autism (HFA) and schizotypal-schizoid personality disorders (SSPD) patients. J Autism Dev Disord. 2019;49(8):3376-3386. doi: 10.1007/s10803-019-04058-1 [DOI] [PubMed] [Google Scholar]
- 50.Ciaramidaro A, Bölte S, Schlitt S, et al. Schizophrenia and autism as contrasting minds: neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr Bull. 2015;41(1):171-179. doi: 10.1093/schbul/sbu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sachse M, Schlitt S, Hainz D, et al. Facial emotion recognition in paranoid schizophrenia and autism spectrum disorder. Schizophr Res. 2014;159(2-3):509-514. doi: 10.1016/j.schres.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 52.Sasson N, Tsuchiya N, Hurley R, et al. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45(11):2580-2588. doi: 10.1016/j.neuropsychologia.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanfield AC, Philip RCM, Whalley H, et al. Dissociation of brain activation in autism and schizotypal personality disorder during social judgments. Schizophr Bull. 2017;43(6):1220-1228. doi: 10.1093/schbul/sbx083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciaramidaro A, Bölte S, Schlitt S, et al. Transdiagnostic deviant facial recognition for implicit negative emotion in autism and schizophrenia. Eur Neuropsychopharmacol. 2018;28(2):264-275. doi: 10.1016/j.euroneuro.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 55.Radeloff D, Ciaramidaro A, Siniatchkin M, et al. Structural alterations of the social brain: a comparison between schizophrenia and autism. PLoS One. 2014;9(9):e106539. doi: 10.1371/journal.pone.0106539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz K, Moessnang C, Schweiger JI, et al. Transdiagnostic prediction of affective, cognitive, and social function through brain reward anticipation in schizophrenia, bipolar disorder, major depression, and autism spectrum diagnoses. Schizophr Bull. 2020;46(3):592-602. doi: 10.1093/schbul/sbz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez A, Tobe R, Dias EC, et al. Differential patterns of visual sensory alteration underlying face emotion recognition impairment and motion perception deficits in schizophrenia and autism spectrum disorder. Biol Psychiatry. 2019;86(7):557-567. doi: 10.1016/j.biopsych.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison KE, Pinkham AE, Penn DL, Kelsven S, Ludwig K, Sasson NJ. Distinct profiles of social skill in adults with autism spectrum disorder and schizophrenia. Autism Res. 2017;10(5):878-887. doi: 10.1002/aur.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krawczyk DC, Kandalaft MR, Didehbani N, et al. An investigation of reasoning by analogy in schizophrenia and autism spectrum disorder. Front Hum Neurosci. 2014;8:517. doi: 10.3389/fnhum.2014.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefanik L, Erdman L, Ameis SH, et al. Brain-behavior participant similarity networks among youth and emerging adults with schizophrenia spectrum, autism spectrum, or bipolar disorder and matched controls. Neuropsychopharmacology. 2018;43(5):1180-1188. doi: 10.1038/npp.2017.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez G, Alexandre C, Mam-Lam-Fook C, et al. Phenotypic continuum between autism and schizophrenia: evidence from the Movie for the Assessment of Social Cognition (MASC). Schizophr Res. 2017;185:161-166. doi: 10.1016/j.schres.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 62.Hyatt CJ, Calhoun VD, Pittman B, et al. Default mode network modulation by mentalizing in young adults with autism spectrum disorder or schizophrenia. Neuroimage Clin. 2020;27:102343. doi: 10.1016/j.nicl.2020.102343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo SS, Wojtalik JA, Mesholam-Gately RI, Keshavan MS, Eack SM. Establishing a standard emotion processing battery for treatment evaluation in adults with autism spectrum disorder: evidence supporting the Mayer-Salovey-Caruso Emotion Intelligence Test (MSCEIT). Psychiatry Res. 2019;278:116-124. doi: 10.1016/j.psychres.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boada L, Lahera G, Pina-Camacho L, et al. Social cognition in autism and schizophrenia spectrum disorders: the same but different? J Autism Dev Disord. 2020;50(8):3046-3059. doi: 10.1007/s10803-020-04408-4 [DOI] [PubMed] [Google Scholar]
- 65.Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. 2010;40(4):569-579. doi: 10.1017/S003329170999078X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res. 2004;69(1):29-33. doi: 10.1016/S0920-9964(03)00154-3 [DOI] [PubMed] [Google Scholar]
- 67.Eack SM, Bahorik AL, McKnight SA, et al. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr Res. 2013;148(1-3):24-28. doi: 10.1016/j.schres.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graux J, Thillay A, Morlec V, et al. A transnosographic Self-Assessment of Social Cognitive Impairments (ACSo): first data. Front Psychiatry. 2019;10:847. doi: 10.3389/fpsyt.2019.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandalaft MR, Didehbani N, Cullum CM, et al. The Wechsler ACS social perception subtest: a preliminary comparison with other measures of social cognition. J Psychoeduc Assess. 2012;30(5):455-465. doi: 10.1177/0734282912436411 [DOI] [Google Scholar]
- 70.Lugnegård T, Unenge Hallerbäck M, Hjärthag F, Gillberg C. Social cognition impairments in Asperger syndrome and schizophrenia. Schizophr Res. 2013;143(2-3):277-284. doi: 10.1016/j.schres.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 71.Martinez G, Mosconi E, Daban-Huard C, et al. “A circle and a triangle dancing together”: alteration of social cognition in schizophrenia compared to autism spectrum disorders. Schizophr Res. 2019;210:94-100. doi: 10.1016/j.schres.2019.05.043 [DOI] [PubMed] [Google Scholar]
- 72.Pinkham AE, Morrison KE, Penn DL, et al. Comprehensive comparison of social cognitive performance in autism spectrum disorder and schizophrenia. Psychol Med. 2019;1-9. doi: 10.1017/S0033291719002708 [DOI] [PubMed] [Google Scholar]
- 73.Sasson NJ, Pinkham AE, Weittenhiller LP, Faso DJ, Simpson C. Context effects on facial affect recognition in schizophrenia and autism: behavioral and eye-tracking evidence. Schizophr Bull. 2016;42(3):675-683. doi: 10.1093/schbul/sbv176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta UM, Thirthalli J, Subbakrishna DK, Gangadhar BN, Eack SM, Keshavan MS. Social and neuro-cognition as distinct cognitive factors in schizophrenia: a systematic review. Schizophr Res. 2013;148(1-3):3-11. doi: 10.1016/j.schres.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 75.Chan RC, Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178(2):381-390. doi: 10.1016/j.psychres.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 76.Pelletier-Baldelli A, Holt DJ. Are negative symptoms merely the “real world” consequences of deficits in social cognition? Schizophr Bull. 2020;46(2):236-241. doi: 10.1093/schbul/sbz095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5-13. doi: 10.1002/wps.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galderisi S, Rucci P, Mucci A, et al. ; Italian Network for Research on Psychoses . The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry. 2020;19(1):81-91. doi: 10.1002/wps.20700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou HY, Yang HX, Gong JB, et al. Revisiting the overlap between autistic and schizotypal traits in the non-clinical population using meta-analysis and network analysis. Schizophr Res. 2019;212:6-14. doi: 10.1016/j.schres.2019.07.050 [DOI] [PubMed] [Google Scholar]
- 80.Zheng Z, Zheng P, Zou X. Association between schizophrenia and autism spectrum disorder: a systematic review and meta-analysis. Autism Res. 2018;11(8):1110-1119. doi: 10.1002/aur.1977 [DOI] [PubMed] [Google Scholar]
- 81.Lai MC, Kassee C, Besney R, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):819-829. doi: 10.1016/S2215-0366(19)30289-5 [DOI] [PubMed] [Google Scholar]
- 82.Trevisan DA, Foss-Feig JH, Naples AJ, Srihari V, Anticevic A, McPartland JC. Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Front Psychiatry. 2020;11:548. doi: 10.3389/fpsyt.2020.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ziermans TB, Schirmbeck F, Oosterwijk F, Geurts HM, de Haan L; Genetic Risk and Outcome of Psychosis (GROUP) Investigators . Autistic traits in psychotic disorders: prevalence, familial risk, and impact on social functioning. Psychol Med. 2020;1-10. doi: 10.1017/S0033291720000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larson FV, Wagner AP, Chisholm K, Reniers RLEP, Wood SJ. Adding a dimension to the dichotomy: affective processes are implicated in the relationship between autistic and schizotypal traits. Front Psychiatry. 2020;11:712. doi: 10.3389/fpsyt.2020.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Modified Newcastle-Ottawa Scale for Quality Assessment Legend
eTable 2. Additional Information Provided by Contacted Authors
eTable 3. Emotion Processing Leave-One-Out Meta-analyses
eTable 4. Theory of Mind Leave-One-Out Meta-analyses
eTable 5. Reading the Mind in the Eyes Test Leave-One-Out Meta-analyses
eFigure 1. Forest Plots of Effect Sizes for Meta-analyses Prior to Outlier Removal
eFigure 2. Funnel Plots for Each Meta-analysis
eFigure 3. Theory of Mind Meta-Regression Scatterplot With Difference in Proportion of Participants on Antipsychotics as Moderator
eResults. Supplemental Results
eReferences.