Abstract
The kidney is arguably the most important target of microvascular damage in diabetes. At least half of all patients with type 2 diabetes mellitus and one-third of those with type 1 diabetes will develop kidney disease owing to their disease and/or other comorbidity, including hypertension, dyslipidaemia, obesity, intra-renal vascular disease, glomerular atherosclerosis, renal ischaemia or ageing-related nephron loss. The presence and severity of chronic kidney disease (CKD) identify individuals who are at increased risk for adverse health outcomes and premature mortality. Consequently, preventing and managing CKD in patients with diabetes is now a key aim of their overall management. Intensive management of patients with diabetes includes controlling blood glucose levels and blood pressure as well as blockade of the renin–angiotensin–aldosterone system; these approaches will reduce the incidence of diabetic kidney disease and slow its progression. Indeed, the major decline in the incidence of diabetic kidney disease over the past thirty years and improved patient prognosis is largely attributable to improved diabetes care. However, an unmet need remains for innovative treatment strategies for preventing, arresting, treating and reversing diabetic kidney disease. In this Primer, we summarize what is now known about the molecular pathogenesis of CKD in diabetic patients and the key pathways and targets implicated in its progression. In addition, we discuss the current evidence for the prevention and management of diabetic kidney disease as well as the many controversies. Finally, we explore the opportunities to develop new interventions through urgently needed investment in dedicated and focused research. For an illustrated summary of this Primer, visit: go.nature.com/NKHDzg
ToC
Diabetic kidney disease is caused by microvascular damage sustained as a result of diabetes. Cooper et al. discuss current knowledge on this condition, arguing that novel strategies to halt and reverse disease progression are urgently needed.
Introduction
Of the long-term complications of diabetes, chronic kidney disease (CKD) imposes the highest burden, both in terms of financial cost and the effects on daily life. The presence and severity of CKD identify individuals who are at increased risk for adverse health outcomes, including frailty, reduced quality of life, end-stage renal disease (ESRD), progressive end-organ damage at other sites and premature mortality. Indeed, excess mortality associated with type 1 diabetes and type 2 diabetes is largely confined to those with CKD1–4. Consequently, preventing and managing CKD in patients with diabetes is a key aim of their overall management.
Approximately half of all patients with type 2 diabetes and one-third with type 1 diabetes will develop CKD, which is clinically defined by the presence of impaired renal function or elevated urinary albumin excretion or both5,6 (Box 1). The percentage of these patients who can be considered to have CKD as a result of their diabetes in unclear. Invariably, other contributors to renal dysfunction are also present — including hypertension, dyslipidaemia, obesity, intra-renal vascular disease, acute kidney injury (AKI), glomerular atherosclerosis, renal ischaemia and ageing-related nephron loss. Accordingly, it is seldom possible to precisely define ‘diabetic kidney disease’ (DKD) or ‘diabetic nephropathy’ in epidemiology or clinical practice, especially in patients with type 2 diabetes. Consequently, it is more appropriate to identify diabetic patients with CKD, and to undertake strategies for holistic renoprotection in patients with diabetes.
Box 1. Clinical criteria for the diagnosis of chronic kidney disease*.
Estimated GFR of <60 ml/min/1.73 m2 or
Urinary ACR of ≥30 mg g−1 or
Urinary albumin excretion rate ≥30 mg per day
*Present for >3 months and validated by repeat testing. ACR, albumin to creatinine ratio; GFR, glomerular filtration rate.
DKD was originally staged by Mogensen7 in the 1980s as a progressive disease that began with the loss of small amounts of albumin into the urine (30-300 mg per day), known as microalbuminuria, occult or incipient nephropathy. As progressively larger amounts of albumin were lost in the urine, and albuminuria became detectable by then standard dipstick urinalysis (>300 mg per day), the terms macroalbuminuria or overt nephropathy were used. This presentation was then classically followed by a relentless decline in kidney function, renal impairment and ultimately ESRD. This paradigm has proved useful in clinical studies, especially in type 1 diabetes, for identifying cohorts who are at increased risk of adverse health outcomes. However, any boundary between stages is artificial and the relationship between urinary albumin excretion and adverse health outcomes is log-linear in clinical practice8. Moreover, many patients with type 1 diabetes, and most with type 2 diabetes, do not follow this classic course in modern clinical practice. For example, many diabetic patients with renal impairment do not manifest excessive urinary albumin loss9,10. Indeed, of the 28% of the United Kingdom Prospective Diabetes Study (UKPDS) cohort who developed an eGFR of <60 ml/min/1.73 m2, half did not have preceding albuminuria11. Even in the Diabetes Control and Complications Trial (DCCT) of the 11% of patients with type 1 diabetes who developed an eGFR of <60 ml/min/1.73 m2, 40% had never experienced overt nephropathy12. At the same time, most patients with microalbuminuria do not progressively increase their urinary albumin excretion as in the classic paradigm and treatment-induced and spontaneous ‘remission’ of albuminuria are commonly observed10,13.
Epidemiology
Although improvements in diabetes management have reduced the proportion of diabetic individuals who develop CKD over any given time period14–16, their improved prognoses17,18 combined with the rising incidence of both type 1 and type 2 diabetes19 have seen the prevalence of CKD continue to grow20. Of the approximately 400 million people with type 2 diabetes worldwide19, approximately half will have evidence of CKD21. Approximately one in five adults with type 2 diabetes will have an eGFR of <60 ml/min/1.73 m2 and between 30–50% will have elevated urinary albumin excretion. In the UKPDS, for example, after a median 15 years of follow-up study, albuminuria was observed in 52% and eGFR ≤60 ml/min/1.73m2 in 28% of participants11.
The incidence of CKD in type 1 diabetes differs from that observed in type 2 diabetes. It is estimated that approximately one-third of all people with type 1 diabetes will develop CKD over the course of their lifetime15,22–24. This difference is mostly because subjects with type 1 diabetes are generally younger, healthier at diagnosis and carry fewer co-morbid conditions than those with type 2 diabetes. Consequently, the renal presentation in type 1 diabetes potentially better reflects DKD, rather than the mixed picture of CKD in type 2 diabetes that is confounded by ‘omnipresent’ other contributors, such as ageing, vascular disease, insulin resistance and obesity.
The incidence, presentation and course of CKD in patients with diabetes varies considerably across countries and settings (Figure 1)21. For example, African American, Middle Eastern, Hispanic, Asian, and Polynesian patients with diabetes have a higher prevalence of elevated urinary albumin to creatinine ratio than European populations25. Disadvantaged and minority populations also have a high prevalence of CKD and its subsequent progression. For example, the prevalence of albuminuria is nearly twice as common in Indigenous Australians in primary care when compared with non-Indigenous patients presenting to the same practice26. The reasons for ethnic differences in CKD are complex27 and include economic, social or educational disadvantage, access to and uptake of care, lower achievement of treatment goals, lower screening rates, suboptimal early treatment of complications, diet and lifestyle factors, smoking, obesity, genetic factors and developmental programming. Another important feature is the younger age-of-onset of type 2 diabetes in these at-risk groups, which might be associated with a more-malignant course including accelerated β-cell loss in the pancreas, as well as renal and cardiovascular complications28.
Figure 1. The prevalence of CKD in different populations with type 2 diabetes.
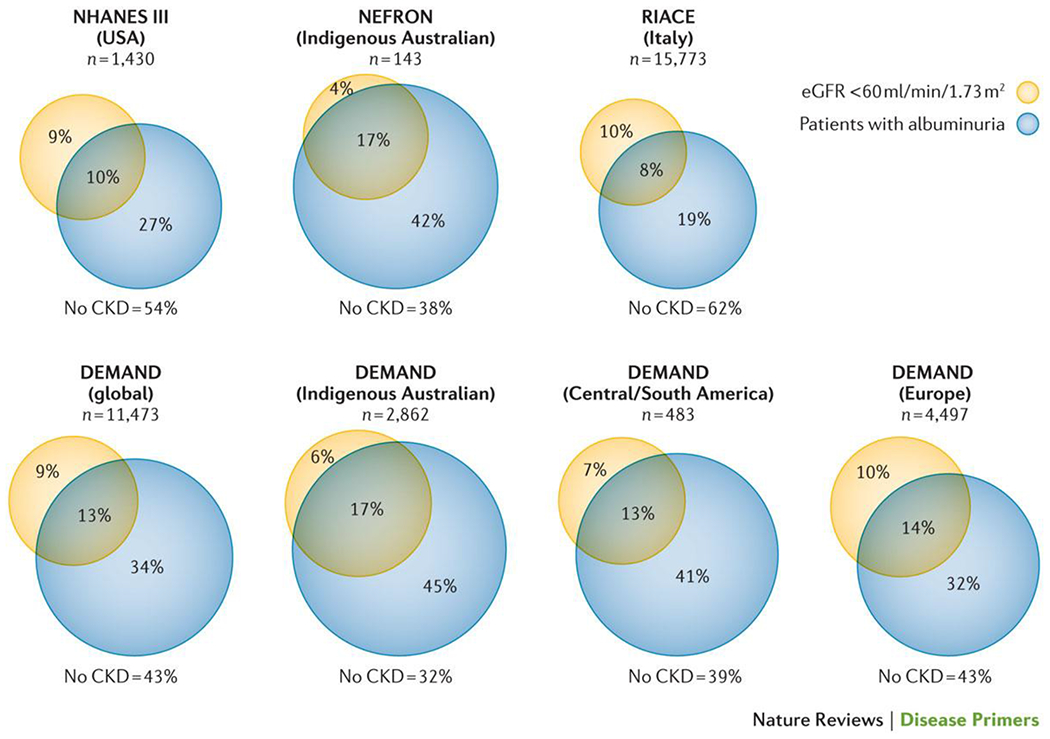
Data from patients with type 2 diabetes surveyed in the DEMAND study21, US NHANES III4, the Australian NEFRON study5 and the Italian RIACE study20. Yellow circle denotes the percentage with an eGFR of <60 ml/min/1.73 m2. Blue circle denoted patients with albuminuria. The percentage not included in either circle denotes patients without CKD.
The cumulative risk of ESRD as a result of diabetes also differs considerably between populations both between and within countries, from <1% to as high as 13%25. This variability partly relates to the competing risk of premature mortality, chiefly owing to cardiovascular disease. Many (and probably most) patients with CKD will die before they develop ESRD13,17,29. Moreover, as most patients with diabetes now reside in developing countries19, the few that reach ESRD will seldom be able to access renal replacement therapy (RRT) programmes. However, the unparalleled number of patients with diabetes makes the disease the leading single cause of ESRD. In many countries, such as the United States, diabetes is present in over half of all patients entering RRT programmes30.
Mechanisms/pathophysiology
DKD has been traditionally viewed as a microvascular disorder, clustered along with retinopathy and neuropathy, and separate from macrovascular disease that contributes to coronary heart disease (CHD), peripheral vascular disease and cerebrovascular disease. However, each disorder can be considered to be tissue-specific manifestations of the same pathogenetic process, and DKD the renal manifestation of the same glucose-driven process that occurs at susceptible sites elsewhere in the body31–34. Although all cells are chronically exposed to high plasma glucose levels in diabetic patients, only some manifest progressive dysfunction, of which the endothelial cells lining the vasculature are a prime example. Specifically, the inability of endothelial cells to down-regulate their glucose transport in response to high glucose levels35 leads to an overwhelming flux of intracellular glucose, which triggers the generation of pathogenetic mediators that contribute to the development of diabetic complications, including DKD.
Reactive oxygen species
Excessive glucose flux leads to the generation of toxic intermediates, the most important of which are thought to be reactive oxygen species (ROS). Excessive glucose flux can generate ROS in a number of different ways. Enhanced mitochondrial substrate oxidation with consequent enhanced mitochondrial membrane potential (ΔΨm) leads to the overproduction of superoxide. At the same time, increased glucose flux leads to the activation of NADPH oxidase and uncoupling of nitric oxide synthase36. ROS-mediated DNA strand breaks in the nucleus activate DNA repair mechanisms including the enzyme poly(ADP ribose)polymerase 1 (PARP-1), which inhibits the key glycolytic enzyme glyceraldehyde–3–phosphate dehydrogenase (GAPDH) by polyADP-ribosylation. Inhibition of GAPDH activity causes a ‘bottleneck’ in glycolysis, resulting in upstream accumulation of early glycolytic intermediates that are increasingly diverted into activating pathogenetic signalling pathways37,38 (Figure 2). These diversions include increased polyol pathway flux, increased hexosamine pathway activity, increased formation of the highly-reactive α-dicarbonyl methylglyoxal, increased expression of the receptor for advanced glycation end-products and its activating ligand S100A8/9, and activation of various protein kinase C (PKC) isoforms — leading to cellular dysfunction, inflammation, apoptosis, and fibrosis in cells exposed to excessive glucose flux. The central importance of ROS in initiating each of these processes is illustrated by the fact that each can be prevented when hyperglycaemia-mediated ROS generation is curtailed38.
Figure 2. The central role of ROS in diabetic complications.
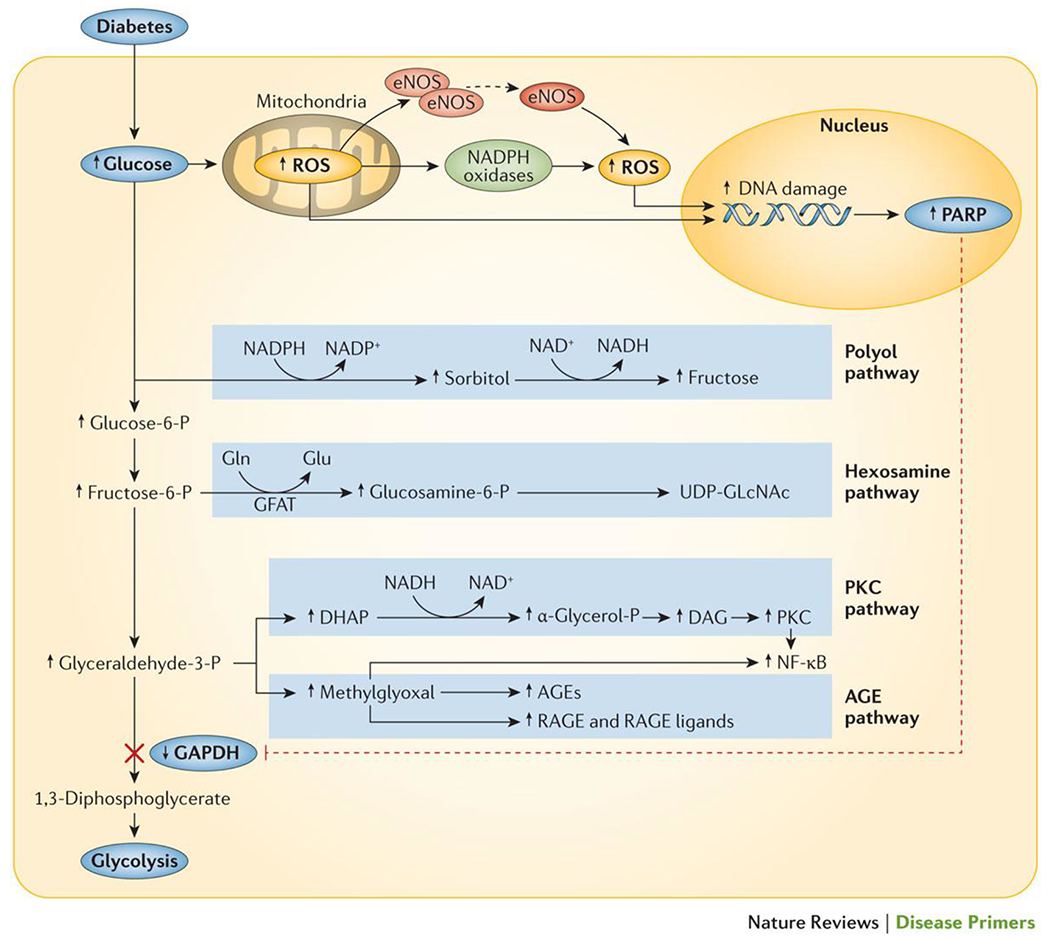
Mitochondrial production of reactive oxygen species (ROS) accelerates in response to an increase in intracellular glucose. In addition, pathogenetic ROS are also generated through the ROS-induced uncoupling of nitric oxide synthase and inactivation of NADPH oxidases. ROS go on to mediate DNA damage, which in turn activates poly(ADP ribose) polymerase (PARP). PolyADP-ribosylation of glyceraldehyde-3-dehydrogenase by PARP leads to the inhibition of this key glycolytic enzyme and a subsequent bottleneck in glycolysis. As a result, early glycolytic intermediates accumulate and are then diverted into pathogenetic signalling pathways.
Nutrient sensing pathways
Each cell has pathways that recognize and specifically respond to nutrient abundance to ensure efficient substrate use. The best known of these ‘nutrient sensors’ include the mammalian target of rapamycin (mTOR), 5’ AMP-activated protein kinase (AMPK) and the sirtuins (SIRTs). From the renal perspective, diabetes is sensed as a ‘bonanza state’ of nutrient surfeit which directly leads to changes in the expression and activity of AMPK, SIRTs and mTOR37 and downstream signalling effects on cellular homeostasis, including the down-regulation of autophagy, regeneration, mitochondrial biogenesis and other cytoprotective responses that contribute to DKD39.
In addition, podocyte-specific activation of mTOR recapitulates many features of DKD, including mesangial expansion and proteinuria40,41. These findings have led to the concept of directed interventions to simulate energy depletion (associated with increased activity of AMPK and SIRTs and reduced mTOR activity) and promote efficient cellular function. Experimental data seem to support this strategy for renoprotection39–42, and agents such as metformin, peroxisome proliferator-activated receptor (PPAR) agonists, phosphodiesterase inhibitors and resveratrol act on these pathways.
The multifactorial pathogenesis of DKD
Only a third of patients with type 1 diabetes will ever develop overt nephropathy15,22–24, whereas almost all patients with type 1 diabetes eventually develop some degree of retinopathy. This suggests that additional risk factors beyond hyperglycaemia must also be involved in DKD. Indeed, although hyperglycaemia is an essential requirement for DKD, it is seldom the only contributor. Pathogenetic pathways initiated and sustained in the kidney by elevated glucose levels can be enhanced by a number of different factors. These include a range of metabolic factors, including excess fatty acids, carbonyl and oxidative stress, as well as haemodynamic factors, including shear stress induced by transmitted systemic hypertension, impaired autoregulation, hyperperfusion and hypoperfusion and activation of the renin–angiotensin–aldosterone system (RAAS)43. On their own these factors do not cause DKD, but rather in the presence of diabetes, they feed into and enhance common pathogenetic mechanisms that include increased levels of growth factors, vasoactive hormones, cytokines and chemokines in the kidney. For example, glucose-induced endothelial dysfunction increases vascular susceptibility to shear, oxidative and other stressors. Endothelial dysfunction and subsequent microvascular rarefaction induced by hyperglycaemia also reduce blood flow while oxygen consumption is increased, leading to hypoxia. In turn, renal hypoxia induces compensatory — but ultimately maladaptive — changes in blood flow, metabolism and polar vasculosis (glomerular neoangiogenesis)44–46.
Key changes in the diabetic glomerulus
Despite the importance of the vascular endothelium in microvascular complications, many investigators view that the early changes in renal glomeruli are critical for the subsequent development of glomerulosclerosis and nephron dropout (Figure 3). Among these, the most important might be dysfunction of glomerular podocytes, highly-specialized terminally differentiated cells that cover the urinary side of the glomerular basement membrane (GBM)47. Together with glomerular endothelial cells, podocytes are responsible for the maintenance of the GBM, its charge barrier and the shape and integrity of the glomerular capillary loop, all functions that are compromised in the diabetic glomerulus. The diabetic milieu induces ‘patho-adaptive’ changes in podocytes, including cytoskeletal rearrangement, de-differentiation, apoptosis and autophagy manifested by morphological widening, retraction and flattening (known as effacement), reduced motility, increased formation of intercellular tight junctions, a decrease in slit diaphragm length, glomerular hypertrophy, detachment and dropout (Figure 3)48–50. Experimental models demonstrate that podocyte-specific injury can recapitulate a diabetes-like phenotype of glomerulosclerosis and tubulointerstitial fibrosis, even in the absence of hyperglycaemia51. Moreover, protecting podocytes from hyperglycaemia with a podocyte-specific deletion of the glucose transporter, solute carrier family 2, facilitated glucose transporter member 4 (SLC2A4; also known as GLUT4)51, or from the resulting oxidative stress52 is able to prevent diabetes-associated albuminuria without restoring normal levels of glucose. Such data place podocytes, and more particularly the dysregulation of their growth and differentiation, at the very centre of the pathogenesis of DKD. Some, but not all, studies suggest that a reduction in podocyte density may be a useful predictor for DKD and its progression53,54.
Figure 3. Glomerulopathy in diabetes.
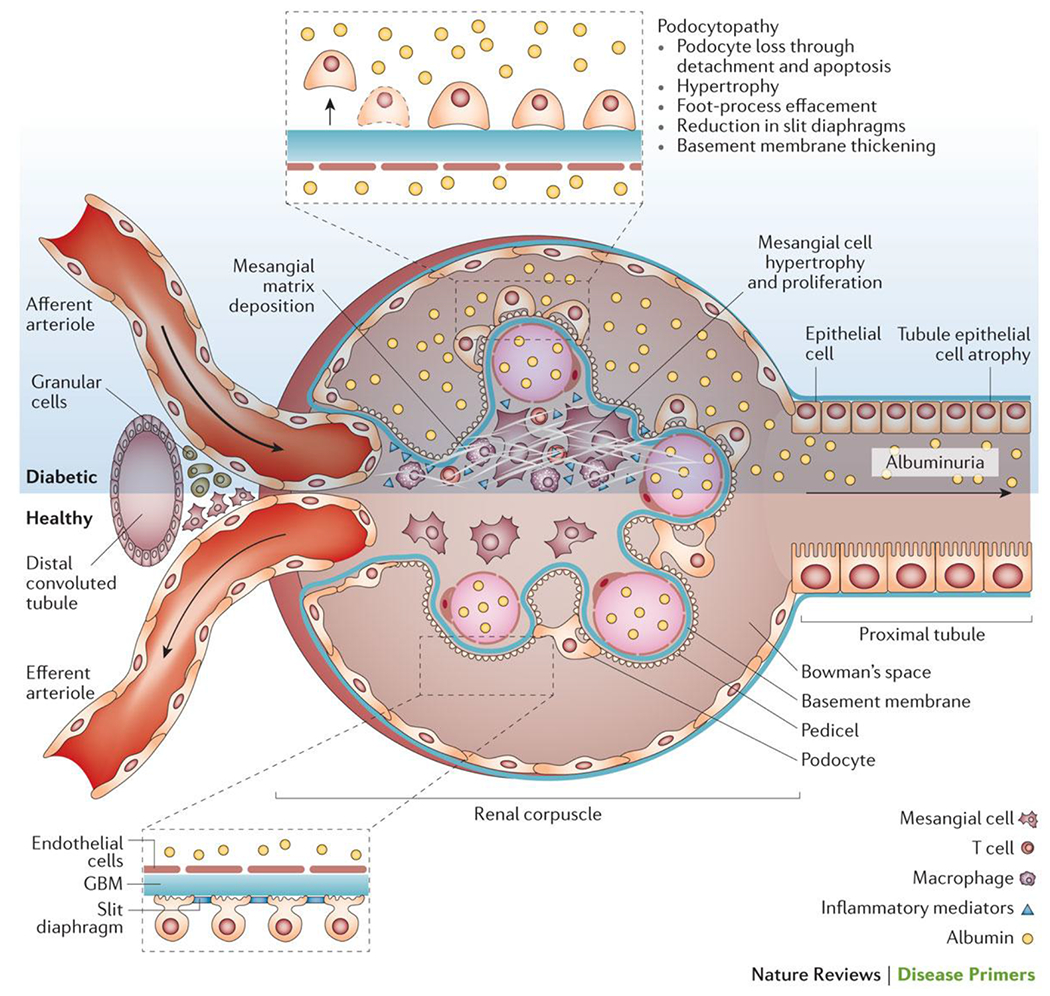
Morphological and functional alterations to renal glomeruli are one of the hallmarks of DKD.
One of the earliest and most characteristic of all glomerular changes in diabetes is homogenous thickening of the GBM53,55. Thickening of the GBM is present in almost all diabetic patients within a few years of diagnosis, although more-pronounced changes are observed in DKD56. Whether GBM thickening is a marker of podocyte or endothelial dysfunction or a mediator of progressive DKD is unclear. Certainly, changes in the composition, charge or architecture of the GBM associated with thickening could contribute to albuminuria. Stiffening of the GBM might also reduce distensibility of the pericapillary wall and compromise the subpodocyte space, facilitating glomerular injury through haemodynamic mechanisms57.
Mesangial cells are also substantially altered by diabetes, undergoing proliferation and hypertrophy while increasing their production of matrix proteins. These changes lead to the some of the unique structural features of diabetic glomerulopathy (Figure 3), including an increase in the fractional volume of the glomerulus occupied by the mesangium (mesangial expansion), focal degeneration of mesangial cells and the mesangial matrix (mesangiolysis)58 and ultimately glomerulosclerosis59. There is a strong link between mesangial matrix expansion and progression of DKD 53,55,60. However, unlike podocytes, upregulation of glucose transport into mesangial cells does not recapitulate a diabetic phenotype61, suggesting that crosstalk among podocytes, endothelial and inflammatory cells mediates mesangial matrix expansion rather than it being a direct effect of glucose exposure on mesangial cells. Although the molecular details of how diabetes alters mesangial cells are not well understood, the importance of mesangial matrix expansion for the development and progression of diabetes-associated glomerulosclerosis is clear. For example, the resulting reduction in capillary surface area as a result of the expansion of the mesangium contributes to glomerular hypertension, proteinuria and reduced glomerular filtration62.
Inflammatory cell recruitment
Diabetes is also associated with the recruitment of activated leucocytes, especially T cells and macrophages, into the glomerulus and tubulointerstitium, even in the early stages of DKD. Markers of renal and systemic inflammation correlate with albuminuria, matrix deposition and progressive decline in GFR47,63. The influx of inflammatory cells into the diabetic kidney is partly in response to tissue injury but can also act as a mediator of DKD64,65, as inflammatory cells and their products (for example, cytokines, chemokines, activated complement and ROS) transform the renal microenvironment. In experimental models inhibition of leucocyte recruitment and accumulation in the diabetic kidney protects against the development of albuminuria and progressive renal damage66,67. Indeed Rag1 knockout mice that are deficient in both T and B cells fail to develop albuminuria associated with diabetes, although renal fibrosis and hyperfiltration still occur53.
Renal tubular dysfunction and fibrogenesis
The renal tubule is also adversely affected by diabetes. Early in diabetes, the increased glucose load delivered to the proximal tubule triggers maladaptive hypertrophy and hyperplasia of the cortical tubuli68 together with up-regulation of glucose transport69, possibly to facilitate glucose reabsorption and reduce glucose wasting. However, as a consequence, sodium delivery to the macula densa is reduced and tubulo-glomerular feedback is activated —leading to increased intraglomerular pressure and hyperfiltration70–72. Chronic hyperglycaemia and other metabolic disturbances associated with diabetes also lead to progressive and cumulative atrophy of tubular epithelial cells. Up to half of the glomeruli are attached to dilated and atrophic tubules, and up to 17% of glomeruli may be ‘atubular’ in DKD73. Such tubular dysfunction results in defective uptake, transcytosis and/or lysosomal processing of filtered protein, alterations that also contribute to albuminuria74.
Tubulointerstitial fibrosis is widely considered to be the ‘final common pathway’ for loss of renal function in DKD75. Indeed, renal function and prognosis in DKD might ultimately correlate better with tubulointerstitial fibrosis than with classic and early glomerular changes75. It is generally thought that the accumulation of activated myofibroblasts is the major contributor to progressive renal scarring in diabetes. These fibrogenic cells may be derived from a number of different sources including transformation of resident fibroblasts and mesenchymal stem cells, recruitment of fibroblasts from the bone marrow, and tubuloepithelial to mesenchymal trans-differentiation (Figure 4)76.
Figure 4. Cellular contributors to myofibroblast recruitment and subsequent tubulointerstitial fibrosis in DKD.
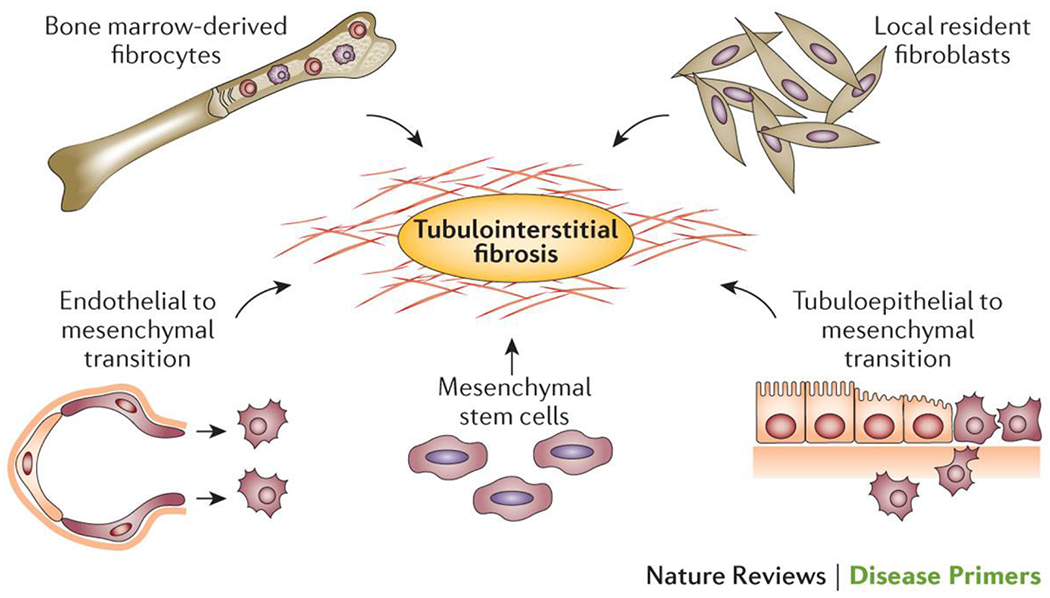
The myofibroblasts responsible for the matrix deposition that leads to tubulointerstitial fibrosis in DKD are derived from a variety of sources, with transformation of local resident fibroblasts, mesenchymal stem cells and bone marrow derived fibrocytes and the induction of endothelial to mesenchymal and tubuloepithelial to mesenchymal transitions the main contributors.239
Complex histopathology of diabetic kidney disease
The same clinical presentation of DKD can be associated with a heterogeneous range of different pathological features, including nodular or diffuse glomerulosclerosis, tubulointerstitial fibrosis, tubular atrophy and renal arteriolar hyalinosis, alone or in combination. The presence and severity of each of these features are independently associated with the risk of progressive renal disease, but not always with each other77. A histopathological staging system for glomerular lesions has been proposed (Box 2)77. However, its predictive utility remains to be established. Routine renal biopsy is not feasible or clinically appropriate outside of a research setting, and CKD remains a clinical diagnosis in most patients with diabetes.
Box 2. Proposed histological staging of diabetic glomerulopathy77.
[H]Class I: Glomerular basement membrane thickening alone
GBM >430 nm in men and >395 nm in women
[H]Class II: Mesangial expansion*
Defined by expansion present in >25% of the mesangium
[H]Class III: Nodular sclerosis
Defined by the presence of Kimmelstiel–Wilson lesions but <50% diffuse global glomerulosclerosis
[H]Class IV: Advanced diabetic glomerulosclerosis
Defined as >50% diffuse global glomerulosclerosis with or without nodules
*Previously known as diffuse diabetic glomerulosclerosis.
Diagnosis, screening and prevention
Risk factors for diabetic kidney disease
A number of different factors contribute to the development of CKD in patients with diabetes (Box 3). Some of these are potentially modifiable through optimised diabetes care, including hyperglycaemia, hypertension, weight gain and dyslipidaemia. Moreover, robust clinical data show that intensive diabetes management significantly reduces the cumulative incidence of albuminuria, renal impairment and ESRD. Indeed, the major decline in the incidence of CKD over the past 30 years is considered to be largely attributable to improved diabetes care14,15.
Box 3. Risk factors for DKD.
Non-modifiable
Advanced age
Early age at onset of diabetes28
Prolonged duration of diabetes
Genetic factors (Table 4) 113
Ethnicity
Family history of DKD, type 2 diabetes, non-diabetic CKD, hypertension or insulin resistance
Intrauterine growth retardation
Maternal gestational diabetes or developmental glucose exposure
Modifiable
Poor glycaemic control (mean, variability and maximal)
Sedentary lifestyle or low intensity of physical activity
Smoking227
Episodes of acute kidney injury230
Advanced glycation end-products231
Oral contraceptive use232
Hyperuricaemia233
Vitamin D deficiency234
CKD, chronic kidney disease; DKD, diabetic kidney disease.
Elevated blood-glucose
The most important risk factor for CKD is hyperglycaemia. Although there are some structural similarities to other renal diseases, fundamentally, the phenotype of DKD is only observed in the context of elevated glucose levels. Elliot Joslin first hypothesized a relationship between glucose and diabetic complications78. However, the defining prospective clinical study by Pirart and his Belgian colleagues unequivocally demonstrated that the degree and duration of hyperglycaemia were associated with microvascular complications, including CKD79. Subsequently, randomized controlled trials have validated this causal link in both type 180 and type 2 diabetes81,82. Nevertheless, although conventional markers of glucose levels, such as HbA1c, are associated with the incidence of microalbuminuria, it is also clear that many patients with poor glycaemic control do not develop renal complications, whereas others do despite intensive interventions and dedicated compliance (Figure 5). This discordance could partly be explained by the fact markers like HbA1c fail to capture the dynamic dysglycaemia associated with diabetes. Indeed, even in the absence of chronic hyperglycaemia, transient hyperglycaemia, transient hypoglycaemia or increased glycaemic variability around a normal mean might have long-lasting and long-term effects on the development and progression of diabetic complications, including renal disease83–87.
Figure 5. The relationship between glycaemic control and the incidence of CKD.
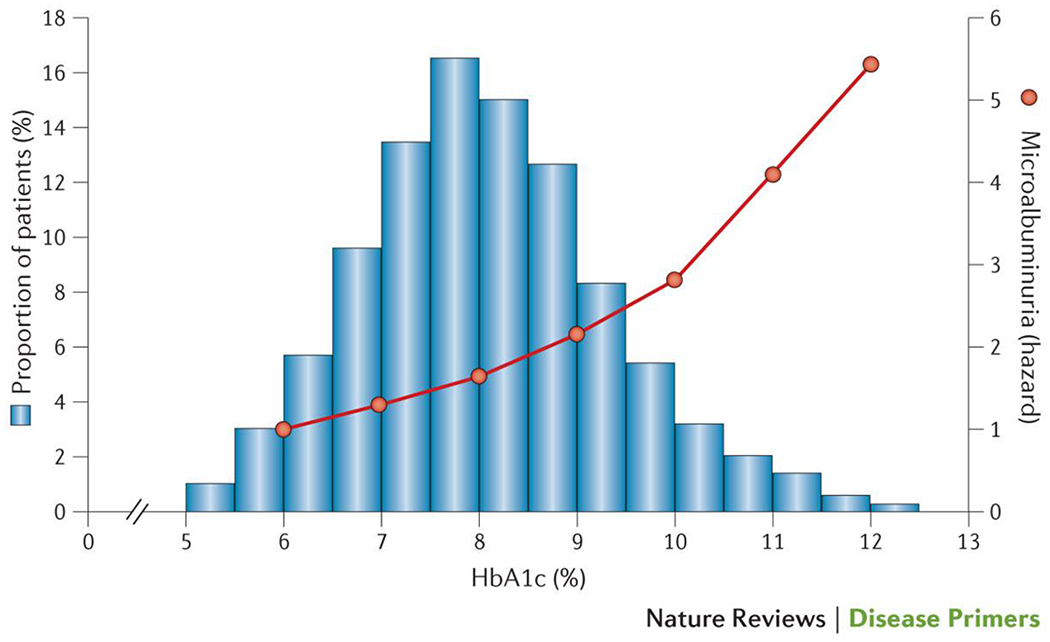
The hazard for the development of an albumin excretion rate > 30mg/day in adults from the FinnDiane study of with type 1 diabetes and no CKD (orange), and the distribution of glycaemic control (histogram) in those patients with type 1 diabetes developing microalbuminuria (bars) (P.-H.G. and M.C.T, unpublished data).
Alternatively past periods of poor glucose control, even prior to diagnosis, could also have a long-lasting legacy in the kidney, and so risk for DKD may not be represented by current or recent HbA1c levels. This phenomenon has become known as ‘metabolic memory’88,’ ‘metabolic karma’89 or the ‘legacy effect’83 and has been used to explain many clinical observations surrounding diabetes and its management, including persistent renal benefits from intensive control during the DCCT80 and UKPDS trials81,82 as well as the apparent lack of benefits in many short-term and intermediate-term trials (as patient outcomes may be significantly determined by glucose exposure prior to the commencement of the trials90). The physiological mechanism or mechanisms responsible for metabolic karma are still poorly defined, but might include epigenetic programming, remodelling and persistent post-translational modifications like advanced glycation end-products89. Further understanding of the molecular basis of a metabolic legacy in diabetes will certainly provide new targets for intervention to reduce the burden of CKD in patients with diabetes.
High blood-pressure
Elevated blood pressure is an important risk factor for the development CKD in both type 1 and type 2 diabetes91–93. In individuals with type 1 diabetes, blood pressure levels are usually normal at diagnosis, but become elevated proximate with the onset of microalbuminuria94. In type 2 diabetes, other factors contribute to the presence and severity of hypertension, which may precede CKD by many years or follow in its wake. This importance of hypertension to the pathogenesis of renal damage can be partly explained by the loss of renal autoregulation in diabetes, whereby systemic pressure is directly transmitted to vulnerable glomerular capillaries95,96. Consequently, there is no specific ‘cut-off’, above which the specific risk for CKD can be denoted or below which the therapeutic impact of blood pressure control on the development of albuminuria can be ignored in patients with diabetes.
Blood-lipid abnormalities
Dyslipidaemia is another important risk factor for the development of CKD in diabetes. In particular, elevated triglycerides, non-low-density lipoprotein cholesterol, apolipoprotein (apo) B or low high-density lipoprotein (HDL) cholesterol levels are independently associated with the development of CKD in both type 1 and type 2 diabetes97,98. However, conventional lipids and lipoprotein measurements do not fully account for the complex lipid and lipoprotein changes associated with diabetes and/or CKD. For example, HDL might not only lose its vasoprotective, antioxidant and anti-inflammatory properties in CKD, but dysfunctional HDL can be directly pathogenetic99. Detailed analysis of lipid sub-fractions have suggested that HDL3-cholesterol, sphingomyelin, apolipoprotein(a), apo-AI and apo-AII, apo-CI, and triglyceride enrichment might all be independently associated with progressive DKD100,101. Attempts have been made using lipidomics to establish a ‘lipid fingerprint’ associated with complications in diabetes102,103. However, exactly which lipids or lipoproteins are the most important in the pathogenesis of CKD in diabetes remains unclear.
Insulin resistance
Insulin resistance is also independently associated with CKD beyond its indirect links with glucose, blood pressure, weight and lipid control104–106. Insulin-sensitizing interventions (for example, thiazolidinedione therapy, exercise and weight loss) all reduce albuminuria over and above their actions on metabolic control. In podocytes, resistance to insulin signalling, arising from deletion of the insulin receptor or its downstream effectors RAC-beta serine/threonine-protein kinase (also known as protein kinaseAkt-2 ) or mTOR, leads to progressive glomerular damage similar to that observed in diabetes51. Impaired insulin sensitivity also results in altered renal cell glucose metabolism104. At the same time, increased insulin signalling as a result of compensatory hyperinsulinaemia in the setting of pathway-selective insulin resistance may also contribute to abnormal vaso-reactivity, angiogenesis, fibrogenesis and other pathways implicated in progressive renal disease107 as well as atherogenesis108.
Obesity
CKD is more prevalent and develops more rapidly in people with diabetes who are obese than their normal-weight counterparts28. This is one major reason why the cumulative incidence of CKD is greater in type 2 diabetes than type 1 diabetes109. Obesity negatively influences major risk factors associated with CKD including lipid, blood pressure and glucose control, as well as promoting insulin resistance. Obesity also has direct effects on the kidney, including changes in intraglomerular haemodynamics, increased sympathetic activity, hypertension, systemic inflammation, endothelial dysfunction, altered expression of growth factors and renal compression associated with visceral adiposity. Indeed, even in the absence of diabetes, obesity may be associated with an increased frequency and severity of albuminuria110, and obesity-related glomerulopathy has been extensively described111.
Programming for diabetic kidney disease
The majority of the variability in incident CKD remains unaccounted for by conventional risk factors. Indeed, the long-term survival of some the very first patients to be treated with insulin without the advantages or intensity of modern treatment regimens suggest that some individuals are ‘protected’. This cannot be explained as simply having the ‘right’ genes. Although an inherited predisposition for DKD is evident112 and a number of potential loci have been reproducibly associated with CKD (Table 1), most genetic variants associated with CKD lie in non-coding regions. Overall, current evidence suggests that the genetic code explains only a small amount of why some individuals develop CKD and some do not113. Furthermore, any role for of these genes, alone or in combination, in the molecular pathobiology of CKD remains to be established113.
Table 1.
Genes potentially linked to diabetic kidney disease113
| Gene | Locus | Alteration | Putative functions |
|---|---|---|---|
| Angiotensin I Converting enzyme (ACE) | rs179975 | Insertion/deletion of a 287 base pair Alu repetitive element in intron 16 of the ACE gene | Renin-angiotensin system |
| Apolipoprotein E (APOE) | rs429358 rs7412 |
T>C T>C |
Lipid metabolism, haemopoietic progenitor stem cell proliferation |
| Aldose reductase (AKR1B1) | rs759853 [AC]n microsatellite |
A>G>T Z-2 allele at an [AC]n microsatellite |
Metabolism, polyol pathway |
| 4.1 protein ezrin, radixin, moesin domain containing 3 (FRMD3) | rs10868025 rs1888747 |
G>C A>G |
Cytoskeletal integrity |
| cysteinyl-tRNA synthetase (CARS) | rs739401 rs451041 |
C>T A>G |
Protein translation |
| acetyl-CoA carboxylase beta (ACACB) | rs2268388 | C>T | Lipid metabolism |
| Myosin, heavy chain 9, non-muscle (MYH9) | rs4821480 rs2032487 rs4281481 rs3752462 |
G>T C>T T>C C>T |
Renal development |
| Sp3 transcription factor (SP3) | rs4972593 rs174162256 |
T>A G>C |
Fibrogenesis |
| AF4/FMR2 family, member 3 (AFF3) | rs7583877 | C>T | Transcriptional activation, DNA-binding/RNA-binding |
| Erb-b2 receptor tyrosine kinase 4 (ERBB4) | rs7588550 | A>G | Renal development, fibrogenesis |
| Locus between RGMA (repulsive guidance molecule family member a) and MCTP2 (multiple C2 domains, trans-membrane 2) RGMA-MCTP2 | rs12437854 | G>T | Unknown |
| Peroxisome proliferator-activated receptor gamma (PPARG) | rs1801282 | C>G | Metabolism |
Although the genetic programming for CKD remains elusive, risk can be imprinted through other means. In particular, epigenetics has emerged as an increasingly powerful paradigm to understand complex non-Mendelian diseases — including CKD. Persistent epigenetic changes can be acquired during development or in adaptations following environmental exposure (the so-called environmental footprint) including metabolic fluctuations associated with diabetes83,114–116. These epigenetic modifications — including changes in DNA methylation, histone modification and chromatin structure — store, retain and recall past experiences in a way to shape the transcription of specific genes and, therefore, cellular functions117. Technological advances now make it possible to initiate epigenome-wide association studies (EWAS)118 to identify epigenetic marks associated with disease across the whole genome118,119, with comparable resolution and throughput to genome-wide studies. For example, some studies have identified differentially methylated regions in diabetic individuals with and those without CKD120,121. Many of the genes identified were also differentially expressed, including some that had been previously linked to CKD in genome association studies. However, the broader utility of epigenetic markers to identify imprinted risk in individual patients beyond conventional risk factors remains to be established.
Some ‘risk’ programming also occurs during gestation and early development and is determined by the intrauterine environment, as well a pre-conception nutrition and health of both the mother and father122. Cells are more sensitive to this ‘epigenetic programming’ during development and differentiation, when gene regulatory regions are established. However, programming can also include constitutional or structural endowment. For example, reduction in nephron mass and filtration area associated with intrauterine growth retardation (IUGR), maternal diabetes or vitamin A deficiency can increase the risk of CKD123–126. At present, IUGR affects one-quarter of live births in developing countries, the same countries in which the risk of diabetes and CKD are also the greatest127.
Estimating the glomerular filtration rate
CKD is a clinical diagnosis made in a patient with a reduction in their eGFR to <60 ml/min/1.73 m2, a persistently elevated urinary albumin excretion or both (Box 1)5,6. The eGFR is a measure of the flux of plasma fluid filtered from the glomerular capillaries into the Bowman’s capsule per unit time (Figure 3). An eGFR <60 ml/min/1.73 m2 is used to denote a moderate to severe renal impairment, and approximates an eGFR more than two standard deviations below the mean eGFR of healthy individuals aged 20–35. The eGFR can be cheaply estimated using an appropriate mathematical formula from the serum creatinine levels, and patient age, gender, and ethnicity. This calculation is often performed automatically by clinical pathology services. The importance of this initiative is illustrated by the fact that at least half of all individuals who currently have a reported eGFR of <60 ml/min/1.73 m2 still have a serum creatinine concentration in the normal range, meaning that until recently renal impairment was frequently undetected in patients with diabetes until late in the course of their disease. However, serum creatinine is notably variable within individuals and is modified by a number of different factors (such as hydration status, physical activity and muscle mass), meaning that repeat testing is important to verify any abnormal results.
The best formula to accurately estimate GFR remains contentious128, although all widely used formulae will identify the majority of patients who have eGFR to <60 ml/min/1.73 m2. Newer methods for estimation of GFR, including cystatin C-based formulae, have some advantages129, especially in the high–normal range of GFR where serial monitoring using cystatinC can be used to accurately identify individuals with rapidly declining renal function (so called ‘progressors’) well before the eGFR declines to <60 ml/min/1.73 m2 130. There is no place for formal measurement of GFR using inulin, iothalamate or other substrates in the routine clinical assessment of renal function in patients with DKD131,132.
Estimating urinary albumin excretion
The second element to identify individuals with diabetes and CKD is to detect those with persistently elevated urinary albumin excretion (Box 1)5,6. When the kidneys are healthy, little or no intact albumin enters the urine, meaning that the presence of albumin in the urine can be used to denote abnormal kidney function. Urinary albumin excretion can also be estimated in a number of different ways. The preferred method measures the concentration of albumin in a urine sample using a sensitive assay, adjusting the result for the urinary creatinine concentration. This metric is known as the albumin to creatinine ratio (ACR) and is considered the most practical way to adjust for the void volume and urine concentration5,6. The ACR is best determined in urine collected at the first void in the morning, but can also be performed in a random manner, for example at the medical visit. Timed urine collections (for example, 4-hour, overnight or 24-hour urine collections) are also used but are time-consuming and seldom adequately performed outside of hospital settings. Spot tests of urinary albumin concentration are not recommended as the concentration of urine varies considerably from void to void. A positive urinary dipstick test or elevated urine albumin concentration is almost always associated with an abnormal ACR133. However, fewer than half of adults with both type 2 diabetes and an abnormal ACR have an elevated urinary albumin concentration or positive dipstick test133.
Owing to substantial day-to-day variability in urinary albumin excretion in any one individual (approximately 40%), any abnormal results should always be confirmed in at least one out of two additional samples collected over a 3–6-month period. If albumin excretion is within the normal range in all three initial tests, further screening is repeated on an annual basis. Any negative result, in an individual with previously negative tests, can simply be repeated annually as part of routine assessment for complications, as it is unlikely that significant CKD has been missed. However, any de novo abnormal results should be confirmed with an additional two tests during the 3–6 months5,6.
Cut-off values for defining what constitutes elevated urinary albumin excretion vary from guideline to guideline. The American Diabetes Association and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that in patients with diabetes the presence of albuminuria is defined by ACR persistently ≥30 mg g−1 in either men or women134,135. Other guidelines adjust for gender differences in urinary creatinine arising from differences in muscle mass between men and women (for example, defining albuminuria as ACR persistently >22 mg g−1 in men, and >31 mg g−1 in women), which might more accurately approximate clinical risk in patients with diabetes136. Formulae to estimate urinary albumin excretion using a single sample are also available, and as for GFR estimation, these might better adjust for demographic confounders 137.
Screening for CKD in diabetes
All patients with type 2 diabetes should have their renal function screened at least annually from diagnosis using both ACR and eGFR135, as both are independently as well as synergistically associated with mortality and progression to ESRD138. In adults with type 1 diabetes, annual screening should begin at most five years after diagnosis. More-frequent monitoring is appropriate for individuals with established renal impairment and those at increased risk for progressive kidney disease (for example, those with proteinuria of >1g/day). Critically, such screening enables the identification of susceptible individuals so that appropriate preventive actions can be taken. Indeed, identification of risk through screening must be followed by intensification of and/or changes in management, such as those detailed in the next section139.
Other biomarkers of the risk for CKD
Although screening for albuminuria and renal impairment will identify most patients who are at risk of CKD, advanced and irreparable structural damage might already be present by the time CKD is diagnosed. Indeed, an eGFR of <60 ml/min/1.73 m2 denotes a loss of renal function of >50%. At the same time, an adverse prognosis is not inevitable in patients with overt nephropathy and/or a reduced eGFR130. Developing practical ways to identify patients with good prognoses from those with poor prognoses remains important for the management of patients with diabetes and CKD, especially in the primary care setting. Some researchers have developed models incorporating additional clinical variables such as age, ethnicity and retinopathy status for risk stratification140,141, although most of the variability in these models can be predicted on the basis of eGFR and albumin excretion alone142. Nevertheless, incorporating some of these additional patient variables adds to their predictive utility. An unmet clinical need is to identify novel biomarkers that have the potential to both diagnose and risk stratify CKD in patients with diabetes earlier than current techniques. Indeed, a number of individual biomarkers have been proposed (Box 4). Other studies have attempted to more broadly identify ‘at risk’ profiles using urine proteomics143, metabolomics144, and analysis of urinary exosomes (for microRNA)145. However, none of these techniques are currently applicable to the hundreds of millions of people with diabetes worldwide.
Box 4. Potential biomarkers for progressive diabetic kidney disease235–238.
Circulating biomarkers
Soluble Tumour Necrosis Factor (TNF) α receptors,
Tumour Necrosis Factor α
Soluble Fas ligand
Fibroblast growth factor 23
Transforming growth factor β
Bone morphogenic protein 7
Inflammatory markers (e.g. C-reactive protein, fibrinogen, serum amyloid A protein, and interleukin-6, interleukin-10, intercellular adhesion molecule −1)
Uric acid
Endothelial cell-selective adhesion molecule
Urinary biomarkers
Collagen IV
Connective tissue growth factor
Angiotensin converting enzyme 2
Angiotensinogen
Filtered urinary proteins ( e.g. transferrin, ceruloplasmin)
Neutrophil gelatinase-associated lipocalin
Hepatitis A virus cellular receptor 1 (also known as kidney injury molecule-1)
Protein O-GlcNAcase
Immunoglobulin G2
Immunoglobulin A
Management
Diabetes control in patients with established CKD
Intensive management of diabetes, including concurrent control of glucose, lipids and blood pressure as well as diet and lifestyle modifications, can slow the progression of established DKD146–148,17,18. Indeed, some data suggest such approaches can even reverse early glomerulopathy. For example, pancreatic transplantation, which restores normal glucose levels in patients with type 1 diabetes, is able to ameliorate the renal histological changes associated with diabetes149. However, it takes at least ten years to observe any regression149 and metabolic control with standard therapy can seldom achieve that observed following pancreatic transplantation. Even with intensive management in the robust setting of clinical trials detailed below, many diabetic patients still experience a progressive decline in renal function. This finding has led to the suggestion that, at best current therapy simply delays the inevitable. Nonetheless, in the clinical setting, any delay in CKD has potentially profound effects on patient health.
Intensive Glucose Control.
Whether preventing hyperglycaemia is enough on its own to treat progressive CKD once it is established is uncertain. Significant reductions in albuminuria and its progression are certainly observed following intensification of glucose control using standard therapies in both type 1 and type 2 diabetes150,151. However, within the limited confines of clinical trials, no significant effect has been observed on other renal outcomes, including doubling of the serum creatinine level, ESRD or death from renal disease150. Nonetheless, 6.5 years of intensive diabetes therapy in the DCCT study was ultimately associated with a 50% reduction in the risk of renal impairment in its follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study and a modestly lower rate of decline in renal function152. Moreover, this effect seemed to be entirely attributable to improved glucose control152. In addition, over the course of the EDIC study, renal replacement therapy (RRT; haemodialysis, peritoneal dialysis or renal transplantation) was needed in only eight participants in the intensive-therapy group, whereas 16 patients in the conventional-therapy group required renal replacement therapy. Furthermore, the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study of 11,140 patients with type 2 diabetes also reported fewer patients required RRT following intensification of glucose control153 compared with a control arm. Moreover, as with EDIC, a recent 5-year follow-up of the ADVANCE study confirmed this renal benefit154. However, total ESRD events (RRT plus deaths from renal disease; Figure 6) and doubling in serum creatinine were not significantly changed. Whether intensive glycaemic control has any influence on cardiovascular or mortality outcomes when initiated late, that is, after patients have established DKD or cardiovascular disease also remains controversial90.
Figure 6. The incidence of ESRD in patients with type 2 diabetes from the ADVANCE-ON trial.
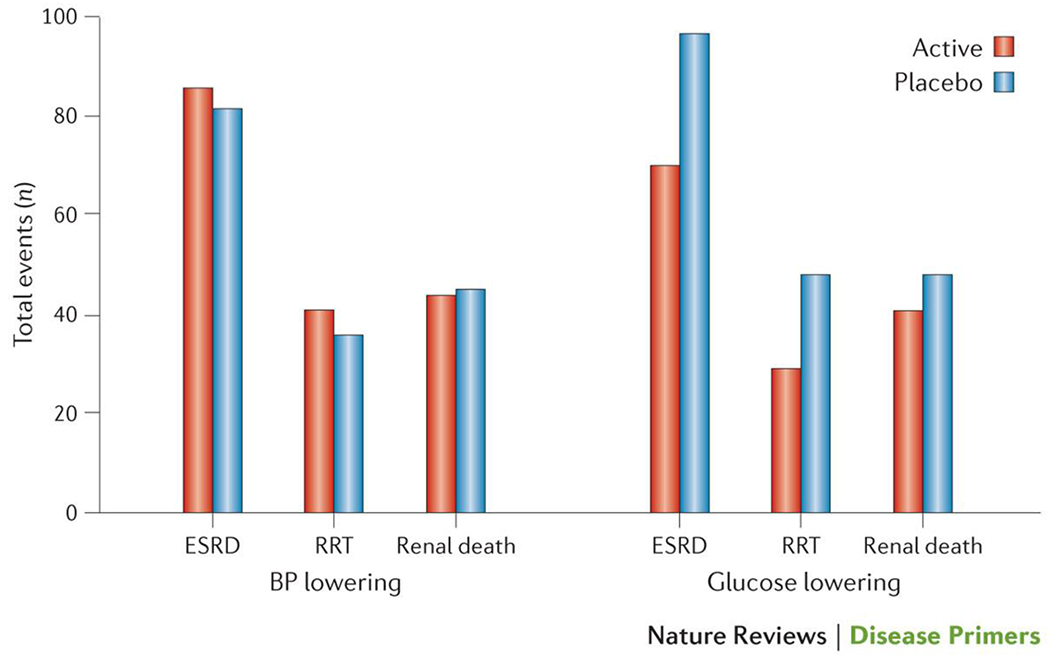
Incidence of end-stage renal disease (ESRD) stratified according to intervention arm, where patients were subjected to either blood pressure (BP) lowering or glucose lowering treatments. Data is presented for sites (n=144) that were able to follow the majority (≥85%) of patients surviving to participate in post-trial follow-up outlining the number of patients who progressed to ESRD and, of these, the number who were receiving renal replacement therapy (RRT) and the number who had died as a result of kidney disease (renal death). Neither treatment significantly reduced the incidence of ESRD154..
At the same time, intensification of glucose control in diabetic patients with CKD can be problematic as the multiple agents and high doses often required exposes patients to an increased risk of adverse drug reactions. Each class of glucose-lowering agent has some limitations (Box 5). In particular, the risk of severe hypoglycaemia is independently associated with a reduced eGFR and elevated urinary albumin excretion155. The increased risk of hypoglycaemia in patients with CKD can be determined by a number of different factors that include prescribing practices in this setting, altered insulin and drug pharmacology (including drug and metabolite accumulation, inadequate compensatory gluconeogenesis in CKD and flattening of the relationship between mean glucose control and HbA1c). Thus, careful individualized targeting, prescribing, patient education, planning and vigilance for hypoglycaemia are all important components in the management of CKD. Where possible, glucose-lowering agents not associated with hypoglycaemia are preferred, especially those not limited by renal impairment or associated co-morbid conditions such as heart failure. In some patients with CKD, less-intensive glycaemic control might be appropriate. Indeed, there may be a U-shape relationship between HbA1c and adverse outcomes including hospitalization and mortality in diabetic patients with eGFR of <60 ml/min/1.73 m2 156.
Box 5. Key limitations of glucose-lowering strategies in patients with DKD.
Metformin
Dose modification required at reduced eGFR, discontinuation at low eGFR,
increased gastrointestinal side effects, hyperlactaemia
Sulphonylureas
Increased hypoglycaemia, accumulation of parent or active metabolites (with glyburide, glimepiride), require discontinuation at low eGFR (all)
Thiazolidinediones
Fluid retention, increased risk of congestive heart failure.
Dipeptidyl peptidase 4 inhibitors
Dose modification (except linagliptin)
Glucagon-like peptide 1 agonists
Discontinuation at low eGFR (exenatide), increased gastrointestinal adverse effects
Sodium glucose co-transporter 2 inhibitors
Reduced efficacy at low eGFR, hypovolaemia, interaction with loop diuretics
Insulin
Increased hypoglycaemia, prolonged insulin half-life
DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate
A number of glucose-lowering agents are purported to have pleiotropic renoprotective actions in diabetic patients with CKD beyond glucose lowering157; these drugs include metformin, DPP-4 inhibitors158, GLP-1 analogues159, thiazolidinediones160, and sodium/glucose cotransporter 2 (SGLT2) inhibitors161. These putative renal benefits are suggested from studies in which these agents reduced or prevented albuminuria in experimental models or in which renal benefits (such as reduced albuminuria) were observed in patients with DKD. Although plausible mechanisms can explain why such agents are renoprotective, these actions remain to be established by comprehensive clinical trials with a renal focus, though some are currently in progress162,163.
Blood–pressure control
Lowering blood pressure is widely regarded the most efficacious treatment for CKD in diabetes, with many clinical trials demonstrating significant reductions in the risk of progression and increased rate of regression of albuminuria following interventions to lower systolic blood pressure164. For example, in the UKPDS trial, a reduction in systolic blood pressure from 154 mmHg to 144 mmHg was associated with a 30% reduction in microalbuminuria165. This benefit seems to occur regardless of whether patients had an elevated blood pressure to begin with166, and no evidence of a threshold for loss of efficacy or J-curve167 has been noted; the risk for albuminuria continues to decrease as the achieved blood pressure falls. Although this relationship might not be the same for mortality in patients with diabetes168, such data provide a renoprotective rationale for aggressively treating all diabetic patients with CKD with antihypertensive agents, regardless of blood pressure.
Although a target blood pressure of <130/80 mmHg has been previously recommended for diabetic patients with CKD, when achievable and tolerated, data from the ADVANCE and Action to Control Cardiovascular Risk in Diabetes (ACCORD) trials showed that intensification of blood pressure control failed to consistently improve ESRD, cardiovascular disease or other hard outcomes with the exception of stroke164. Overall, treatment of hypertension in patients with CKD at best only modestly reduces the risk of ESRD169, but exposes patients to increased drug costs, orthostatic symptomatology and potentially hypoperfusion in the setting impaired autoregulation. Indeed, an increased risk of declining renal function and incident acute kidney injury has also been reported in some studies, which may itself contribute to a progressive decline in renal function in diabetes170. Moreover, the cost and challenges of achieving this level of blood pressure control in many patients has contributed to the 2014 Joint National Council (JNC) 8 guidelines recommending a relaxed unified target of <140/90 mmHg171. However, stroke risk is also greatest in patients with CKD and the most appropriate blood pressure target continues to be the subject of avid debate.
Although large observational studies suggest that the risk of albuminuria can be reduced by blood pressure reduction, regardless of modality172, the renoprotective efficacy of blockade of the RAAS using angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) seems to be greater than that achieved by other agents with a similar degree of blood pressure reduction166,173. For example, in the Irbesartan in Diabetic Nephropathy Trial (IDNT), fewer patients receiving irbesartan (an ARB) required RRT compared with those receiving amlodipine (a calcium channel blocker)174. Yet despite these data, RAAS blockade (any and/or in adequate doses) in diabetic patients with CKD continues to be underused in routine clinical care175. No differences in the clinical efficacy of ACE inhibitors versus ARBs with respect to reduction in blood pressure are evident, although tolerability and compliance might be greater with ARBs. The combination of ACE inhibitors and ARBs is not recommended in DKD, partly because of the increased risk of acute-on-chronic renal impairment and hyperkalaemia176. The addition of the direct renin inhibitor aliskiren to conventional RAAS blockade in diabetic patients was also associated with adverse outcomes and had no effect on ESRD, although albuminuria was modestly reduced along with blood pressure levels177. Mineralocorticoid receptor antagonists (MRAs) also significantly reduce albuminuria when added to conventional RAAS blockade178, but are limited by anti-androgenic adverse effects and hyperkalaemia, especially in patients with renal impairment. Newer MRAs that reduce these adverse effects are being actively explored for the management of DKD179
Ultimately, any decision as to which blood-pressure lowering agent is best is largely academic. Even in a trial setting, most patients require three to four different antihypertensive agents to achieve acceptable blood pressure targets180. Establishing the best combination is perhaps a more appropriate clinical question. For example, in the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial, better renal outcomes (with respect to doubling of serum creatinine, starting dialysis or death) were observed in patients receiving benazepril (an ACE inhibitor) plus amlodipine (a calcium channel blocker) group than in those receiving benazepril and hydrochlorothiazide (a diuretic), despite equivalent blood pressure control181.
Blood lipid lowering
Lipid-lowering treatment is widely recommended in all patients with CKD182 to reduce the risk of cardiovascular disease and associated mortality182. Whether lipid lowering also protects the kidneys remains controversial. No clear renoprotective effect of statins in patients with diabetes is evident183,184 and some potential risks have been recently identified185. By contrast, fibrate drugs reduce albuminuria186; whether this effect is mediated by lipid lowering, pleiotropic effects mediated by activation of PPARα or trans-repression of other targets is unclear187–189. Fenofibrate is also associated with a rapid increase in serum creatinine (~10-15%) leading to a fall in eGFR, although the true GFR might be unaffected190. Nonetheless, an agent that increases serum creatinine makes its use in patients with established renal impairment challenging.
Diet and lifestyle interventions
Intensive diet and lifestyle interventions are frequently recommended to diabetic patients with CKD, including weight loss, increased physical activity, smoking cessation, Mediterranean diet and sodium restriction. Limited research supports their ability to reduce risk factors for progressive renal disease and albuminuria191–194. Indeed, the LOOK-AHEAD study reported a significant reduction in incident albuminuria following a multifactorial diet and lifestyle intervention195. However, the ability to truly modify renal progression or co-morbid vascular outcomes remains controversial and the restrictions imposed by adherence might be associated with a reduced quality of life in precisely those patients who have the shortest life expectancy. Moreover, the beneficial impacts of multi-factorial lifestyle intervention on hospitalizations and cost in the LOOK-AHEAD study were not evident among individuals with a history of cardiovascular disease (which is often the norm in DKD). Certainly, significant weight loss is associated with reduced incidence of progressive CKD in diabetes, and regression of albuminuria has been observed following bariatric surgery 195,196. Avoiding high levels of protein intake, (i.e. less than 1.3 g of protein per kilogram body weight per day or more than 20% of food energy from protein), is also appropriate for individuals with CKD197. However, formal protein restriction (<0.8 g/kgBW/day) is not generally recommended as it is difficult to apply and enjoy and might be associated with clinically important risks, including malnutrition and bone remodelling198. Some studies have suggested that dietary intake of omega-3 polyunsaturated fatty acids199 or omega-3 supplementation200 might also have beneficial effects on albuminuria in CKD.
Managing co-morbidity in patients with CKD
Diabetic patients with CKD experience an increased risk and severity of other diabetic complications, including retinopathy, neuropathy, gastroparesis, sexual dysfunction, cognitive decline, sleep and mood disorders, heart failure, atrial fibrillation, cardiovascular and foot disease. The presence of CKD in a patient with diabetes can be considered a risk marker for each of these conditions33 but it is also often an aggravating factor. The more severe the renal impairment, or the greater the albuminuria, the greater this risk of cardiovascular as well as other complications. For example, myocardial infarction and stroke are approximately twice as common in those with diabetes and CKD than in those with diabetes but without renal disease201,202, and patients with ESRD carry a cardiovascular risk that is at least ten times greater again.
Such is the complexity of the management in CKD, it is common for other diabetic complications (for example, eye or foot disease) to go undiagnosed or to be relatively neglected, even though the risk of non-renal calamity can be very high. The presence of CKD in diabetes necessitates intensive prevention, monitoring and screening and early aggressive treatment of co-morbid disease. Indeed, aggressive multifactorial intervention specifically in patients with CKD has sustained beneficial effects with respect to their other vascular complications and reduces their mortality203. Moreover, the application of such treatments and improved control of risk factors has largely been responsible for the halving of age-standardized mortality in patients with CKD over the past 20 years17. As cardiovascular and cerebrovascular diseases are the major preventable causes of death in diabetic patients with CKD, particular emphasis should be placed on reducing cardiovascular risk, including lipid lowering, treatment of hypertension, smoking cessation and lifestyle modification. Indeed, the absolute benefit from aggressive lipid lowering seems to be greatest in patients with CKD204,205. Low-dose aspirin can also be appropriate for primary prevention of cardiovascular disease in patients with CKD, as most have a 10-year risk of cardiovascular events of more than ten percent206. However, paradoxically harmful effects from anti-platelet therapy have also been reported for aspirin207 and clopidrogel in patients with CKD208. High risk patients with CKD can also be considered appropriate for screening for asymptomatic heart disease because early management can improve outcomes, although many of these patients are already maximally medically treated and the utility of cardiac screening beyond risk stratification remains unclear.
At the same time, the multi-factorial interventions needed for the management of CKD in diabetes and its associated burden of co-morbid disease frequently exposes patients to iatrogenic complications. In particular, adverse drug reactions are more commonly observed in patients with CKD — reflecting the pill burden, altered pharmacokinetics, interactions with abnormal physiology and other medications, as well as frequently inadequate dose-adjustments in this setting. Appropriate targeting, cautious prescribing, judicious dosing and close monitoring are necessary for all therapies in patients with CKD, especially when multiple practitioners are involved and renal disease is not the primary focus. Given the sheer complexity of multi-factorial management in patients with CKD, optimal care is best delivered by comprehensive multidisciplinary teams focused on individual patient needs. Such coordinated care is often limited and challenging to implement in routine clinical practice, although if only one subset of patients with diabetes could be targeted for such an intensive approach, it should be those with CKD .
Managing advanced CKD
Advanced-stage CKD is also associated with a range of complications that require specific additional management including anaemia, fluid retention, itch, electrolyte disturbances, calciphylaxis, and bone demineralization. In each case, these complications are more common have greater severity and are less well tolerated in patients with diabetes than in non-diabetic patients with a similar degree of renal impairment209. Patients with CKD are also more vulnerable to episodes of acute kidney injury (AKI), including contrast nephropathy, renal ischaemia, hypovolaemia, sepsis, surgery and NSAID-induced AKI, all of which can be avoided by vigilance, education, close follow-up monitoring and assiduous early management, including stopping RAAS blockade, diuretic use and metformin treatment when appropriate. Ultimately, progressive renal decline requires timely referral to specialist services and, when appropriate, advance care planning for some form of RRT or conservative care, before their renal impairment becomes symptomatic210,211. The optimal timing for any RRT should be determined by individual circumstances, but generally dialysis should be considered when there are signs or symptoms of uremia, inability to control hydration status or blood pressure or a progressive deterioration in nutritional status; usually noted at when eGFR falls to between 6–9 ml/min/1.73m2. Earlier asymptomatic initiation of dialysis specifically because of diabetes is not warranted, unless uremic symptoms are difficult to detect and/or close supervision is not feasible.
Quality of life
The presence and severity of CKD in any individual with diabetes is also strongly associated with their health-related quality of life (HRQOL)212,213. This is partly mediated by the presence and severity of co-morbid disease (Figure 7) and associated risk factors in these patients. At the same time, CKD can also affect on HRQOL through the burden of multi-factorial interventions necessitated by the increased risk for or presence of co-morbid disease, which often leads to a costly time-consuming round of clinical appointments with multiple practitioners across different specialities, contributing to patient confusion, poly-pharmacy and an increased risk of iatrogenic complications155. Clinically relevant improvements in HRQOL in diabetic patients with CKD can be obtained from structured management programmes that incorporate different specialties. Specific education and support programmes targeting ‘at risk’ patients with CKD can also vastly improve diabetes care; such care can be individualized or community-based care214. Formalized education of primary care physicians and other healthcare providers, as well as systematic management and decision-support programmes, can also improve outcomes for their patients, including HRQOL215–217.
Figure 7. The strong association between chronic kidney disease (CKD) and increased incidence and prevalence of other diabetic complications.
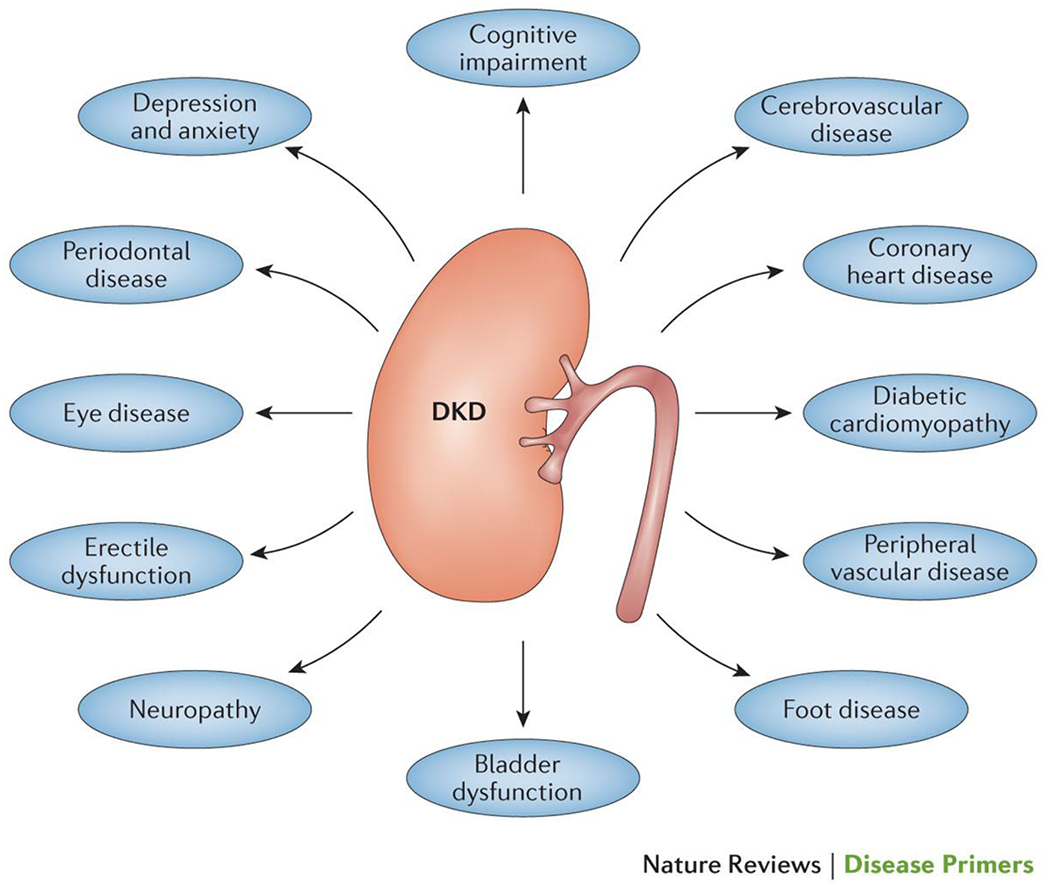
The increased risk of diabetic complications for patients with CKD means that the management of CKD is never only focused on the kidney, but must also involve the pro-active prevention, early detection and effective treatment of all diabetic complications.
Beyond its association with co-morbid disease, CKD can also directly affect HRQOL indices in individuals with diabetes through its negative effects on physical performance, fatigability, appetite, nutrition, immune function, bone mineralization, cognitive function, pruritus and fluid retention. Some of these complications are mediated by the retention of so-called ‘uraemic toxins’, which are highest when HRQOL is at its lowest. Renal anaemia might also play an important part in some patients. By the time the eGFR declines to <60 ml/min/1.73 m2 up to one in three individuals with diabetes will have anaemia218. Palliative correction of anaemia using erythropoietin receptor agonists can improve performance and quality of life, but not without considerable cost, in terms of financial cost of the medications themselves, potential for adverse effects219 and the systematic management and follow-up programme they require. Abnormal calcium phosphate homeostasis is also common in patients with CKD, as well as those with reduced HRQOL, but no clear evidence has shown that vitamin D, phosphate binders or calcimimetics improve HRQOL220. Limitations and restriction of certain foods and fluid in patients with advanced-stage CKD also places an additional burden.
However, by far the most important consideration for HRQOL in advanced CKD lies around the initiation of RRT, its appropriateness, its timing, modality and setting. It is beyond the scope of this Primer to discuss the enormous challenges of RRT in patients with diabetes. Importantly, even the finest RRT will at best achieve much less than a naturally functioning kidney, reinforcing the primacy of renoprotection in the management of diabetes. In addition, RRT will not be appropriate for some patients with diabetes and CKD, because of comorbidity, frailty, symptomatology and the anticipated excessive burden of therapy.
Outlook
The health implications of the diabetes epidemic are of unparalleled proportions, both in terms of morbidity and mortality as well as the vast health resources that they currently demand and will need in the future. The majority of these resources will be directed towards prevention and management of diabetic complications, including CKD. A portent of the coming storm can be demonstrated by the Pima Indian population, among whom an ‘outbreak’ of type 2 diabetes began in the late 1950s. Inevitably, in the 1970s, an epidemic of DKD followed that has continued into this century, with a steadily increasing burden of ESRD221. Without effective prevention and treatment, the current global epidemic of diabetes combined with improved survival from heart disease may well lead to a similar crisis of CKD, with overwhelming requirements for RRT and health care systems, particularly in developing counties that carry the greatest burden of type 2 diabetes19. Even in the past 10 years, the number of people with diabetes in RRT programmes has more than doubled30.
Intensive management of diabetes, including control of glucose and blood pressure and blockade of the RAAS, will reduce the incidence of CKD and slow its progression. Indeed, a decline in the incidence of CKD over the past thirty years14,15 and recent levelling off in the number of patients with diabetes who develop ESRD is considered to be attributable to improved diabetes care222,223. The prognosis of patients with DKD has also dramatically improved17,18. However, there remain deficiencies in implementation that need to be bridged through pragmatic guidelines and clinical pathways, phase IV studies/audit, providing adequate resources, appropriate targeting of education and support.
An unmet need also remains for innovative treatment strategies for preventing, arresting, treating, and reversing CKD in diabetes. Despite initially positive findings, clinical trials of new agents have frustratingly failed to live up to their promise224. Even the failure of early RAAS blockade to reduce the development of CKD225,226 in diabetic patients has undermined what was widely viewed to be the best means for renoprotection. However, each failure has led to evolution of our understanding of CKD, and led to newer agents, strategies and designs of clinical trials. A number of novel therapies are currently in development (Box 6)224. However, at present, even when used in optimal combination with standard medical care, renal complications seem to be only modestly reduced at best, and treatment often comes at the considerable expense of additional pill burden, cost and exposure to off-target effects. Given the primacy of CKD in clinical outcomes for those with diabetes, and the current absence of specific treatment, increased investment in CKD research is urgently required.
Box 6. Strategies currently in clinical development for DKD.
Endothelin-1 receptor antagonism
Novel mineralocorticoid receptor blockade
Pirfenidone (and other novel antifibrotics)
C-C chemokine receptor type 2 antagonists
Macrophage migration inhibitory factor antagonists
Membrane primary amine oxidase (also known as vascular adhesion protein-1 and semicarbazide sensitive amine oxidase) inhibitors
Vitamin D analogues
Xanthine dehydrogenase/oxidase inhibitors
Phosphodiesterase inhibitors
Urotensin-2 antagonists
NADPH oxidase inhibitors
factor erythroid 2-related factor 2 agonists
Advanced glycation end-products scavengers
Peroxisome proliferator activator receptor agonists
MicroRNA therapeutics
DKD, diabetic kidney disease.
To provide evidence of efficacy in CKD it is necessary to target robust clinical end points. Yet because the progression of renal disease is usually a slow process, over many years or even decades, clinical trials with hard end points such as ESRD are impractical. Although surrogate end points such as change in albuminuria and/or change in eGFR are useful indicators, individually or in combination they might not reflect the true renoprotective potential of interventions. Robust surrogate end points for progressive renal injury in diabetes are urgently needed to facilitate the testing of different strategies. Given the heterogeneous nature of CKD, a systematic panel of biomarkers and molecular phenotypes rather than a one-size-fits-all surrogate approach will likely be essential for the development of new treatments. Improving the design of clinical trials will also be important including larger risk-enriched cohorts, early selection of responders and early exclusion of those intolerant to therapy, and appropriate surrogate and end point definition.
Acknowledgments
Competing interests
M.C.T. has received honoraria for educational meetings conducted on behalf of Abbvie, Boehringer-Ingelheim, Eli-Lilly, Merck Sharpe and Dohme, Servier, Novartis, Takeda, Abbot, Allergan and AstraZeneca.
M.B. declares no competing interests.
K. Susztak has received research support from Boehringer Ingelheim and Biogen Idec for projects not related to this publication, and is on the advisory board of Abbvie. She has received research support from the NIH, JDRF and ADA.
K. Sharma has received research support from AbbVie, Boehringer Ingelheim and Stealth Peptides, Inc. for projects not related to this publication, and is on the scientific advisory board of Merck and Astellas. He is founder of Clinical Metabolomics, Inc. He has received research support from the NIH, JDRF and ADA.
K.J-D. has received research grants from Genkyotex and Boehringer Ingelheim.
S.Z. has served on the advisory board for Amgen Australia Pty Ltd, AstraZeneca Pty Ltd/Bristol-Myers Squibb Australia Pty Ltd, Merck Sharp & Dohme (Australia) Pty Ltd,
Novartis Pharmaceuticals Australia, Sanofi, and Takeda Pharmaceuticals Australia Pty Ltd. S.Z. has received consultancy fees and honoraria from AstraZeneca Pty Ltd/Bristol-Myers Squibb Australia Pty Ltd, Janssen-Cilag Pty Ltd, Merck Sharp & Dohme (Australia) Pty Ltd, and Servier Laboratories (Aust) Pty Ltd. She has received grants from NH&MRC and Heart Foundation of Australia, and undertaken institutional contract work for Bristol-Myers Squibb Australia Pty Ltd and the Commonwealth Department of Health.
P.R. has received consultancy and/or speaking fees (to his institution) from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi Aventis, Astellas, Abbvie and MSD. He has received research grants from Abbvie, Novo Nordisk and Astra Zeneca. P.R. has shares in Novo Nordisk.
P.-H.G. has received lecture honoraria from Boehringer- Ingelheim, AstraZeneca, Genzyme, Novartis, Novo Nordisk, MSD, Eli Lilly and Medscape.
M.E.C. has received honoraria and consulting fees from Abbvie, Bayer, Boehringer-Ingelheim, Eli-Lilly, Merck, Servier, Takeda, Novo-Nordisk and AstraZeneca as well as research grants from Novo-Nordisk and Abbvie.
Footnotes
For Office Use Only
Subject Categories
Health sciences / Diseases / Kidney diseases / Glomerular diseases / Diabetic nephropathy
Health sciences / Nephrology / Kidney diseases / Chronic kidney disease
Health sciences / Endocrinology / Endocrine system and metabolic diseases / Diabetes / Diabetes complications
Biological sciences / Physiology / Circulation / Hypertension
Caption for PrimeView Thumbnail
PRIMEVIEW: For an illustrated summery of this Primer, visit: go.nature.com/NKHDzg
Suggested File name for PrimeView PDF
DKD-2015.pdf
References
- 1.Groop PH, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58, 1651–1658 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orchard TJ, Secrest AM, Miller RG & Costacou T In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53, 2312–2319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 50, 941–948 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Afkarian M, et al. Kidney disease and increased mortality risk in type 2 diabetes. Journal of the American Society of Nephrology : JASN 24, 302–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME & Mathew TH The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). The Medical journal of Australia 185, 140–144 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Dwyer JP, et al. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal medicine 2, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogensen CE, Christensen CK & Vittinghus E The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32 Suppl 2, 64–78 (1983). [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Parving HH & Henning RH Microalbuminuria as an early marker for cardiovascular disease. Journal of the American Society of Nephrology : JASN 17, 2100–2105 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Thomas MC, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes care 32, 1497–1502 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, et al. Regression of microalbuminuria in type 1 diabetes. The New England journal of medicine 348, 2285–2293 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55, 1832–1839 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Molitch ME, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes care 33, 1536–1543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler AI, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney international 63, 225–232 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE & Ludvigsson J Declining incidence of nephropathy in insulin-dependent diabetes mellitus. The New England journal of medicine 330, 15–18 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Hovind P, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes care 26, 1258–1264 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Gregg EW, et al. Changes in diabetes-related complications in the United States, 1990-2010. The New England journal of medicine 370, 1514–1523 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Andresdottir G, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes care 37, 1660–1667 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Andresdottir G, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney international 87, 417–426 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Federation., I.D. IDF Diabetes Atlas, (2013). [Google Scholar]
- 20.de Boer IH, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA : the journal of the American Medical Association 305, 2532–2539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parving HH, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney international 69, 2057–2063 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Pambianco G, et al. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 55, 1463–1469 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Krolewski AS, Warram JH, Christlieb AR, Busick EJ & Kahn CR The changing natural history of nephropathy in type I diabetes. Am J Med 78, 785–794 (1985). [DOI] [PubMed] [Google Scholar]
- 24.Rossing P, Rossing K, Jacobsen P & Parving HH Unchanged incidence of diabetic nephropathy in IDDM patients. Diabetes 44, 739–743 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Sheen YJ & Sheu WH Risks of rapid decline renal function in patients with type 2 diabetes. World journal of diabetes 5, 835–846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas M, Weekes AJ & Thomas MC The management of diabetes in indigenous Australians from primary care. BMC public health 7, 303 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW & Bootsma AH Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes care 28, 2280–2288 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Group TS, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine 366, 2247–2256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsblom C, et al. Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. Journal of the American Society of Nephrology : JASN 22, 537–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Lundbaek K Diabetic angiopathy: a specific vascular disease. Lancet 266, 377–379 (1954). [DOI] [PubMed] [Google Scholar]
- 32.Root HF, Pote WH Jr. & Frehner H Triopathy of diabetes; sequence of neuropathy, retinopathy, and nephropathy in one hundred fifty-five patients. A.M.A. archives of internal medicine 94, 931–941 (1954). [DOI] [PubMed] [Google Scholar]
- 33.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T & Kofoed-Enevoldsen A Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32, 219–226 (1989). [DOI] [PubMed] [Google Scholar]
- 34.Stehouwer CD Endothelial dysfunction in diabetic nephropathy: state of the art and potential significance for non-diabetic renal disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 19, 778–781 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Kaiser N, et al. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42, 80–89 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Inoguchi T, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49, 1939–1945 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Schaffer SW, Jong CJ & Mozaffari M Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascular pharmacology 57, 139–149 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Dugan LL, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. The Journal of clinical investigation 123, 4888–4899 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoki K, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. The Journal of clinical investigation 121, 2181–2196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godel M, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. The Journal of clinical investigation 121, 2197–2209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tikoo K, Tripathi DN, Kabra DG, Sharma V & Gaikwad AB Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett 581, 1071–1078 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Cooper ME Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 352, 213–219 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Blantz RC Phenotypic characteristics of diabetic kidney involvement. Kidney international 86, 7–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takiyama Y & Haneda M Hypoxia in diabetic kidneys. BioMed research international 2014, 837421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advani A & Gilbert RE The endothelium in diabetic nephropathy. Seminars in nephrology 32, 199–207 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Reidy K, Kang HM, Hostetter T & Susztak K Molecular mechanisms of diabetic kidney disease. The Journal of clinical investigation 124, 2333–2340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartleben B, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. The Journal of clinical investigation 120, 1084–1096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman-Edelstein M, et al. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes 60, 1779–1788 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato H, et al. Wnt/{beta}-Catenin Pathway in Podocytes Integrates Cell Adhesion, Differentiation, and Survival. The Journal of biological chemistry 286, 26003–26015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coward R & Fornoni A Insulin signaling: implications for podocyte biology in diabetic kidney disease. Current opinion in nephrology and hypertension 24, 104–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Susztak K, Raff AC, Schiffer M & Bottinger EP Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006). [PubMed] [Google Scholar]
- 53.Harindhanavudhi T, Parks A, Mauer M & Caramori ML Podocyte Structural Parameters Do Not Predict Progression to Diabetic Nephropathy in Normoalbuminuric Type 1 Diabetic Patients. American journal of nephrology 41, 277–283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer TW, Bennett PH & Nelson RG Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42, 1341–1344 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Ponchiardi C, Mauer M & Najafian B Temporal profile of diabetic nephropathy pathologic changes. Current diabetes reports 13, 592–599 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Dalla Vestra M, Saller A, Bortoloso E, Mauer M & Fioretto P Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes & metabolism 26 Suppl 4, 8–14 (2000). [PubMed] [Google Scholar]
- 57.Lewko B & Stepinski J Hyperglycemia and mechanical stress: targeting the renal podocyte. Journal of cellular physiology 221, 288–295 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Wada T, et al. Nodular lesions and mesangiolysis in diabetic nephropathy. Clinical and experimental nephrology 17, 3–9 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Qian Y, Feldman E, Pennathur S, Kretzler M & Brosius FC 3rd. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57, 1439–1445 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauer SM, et al. Structural-functional relationships in diabetic nephropathy. The Journal of clinical investigation 74, 1143–1155 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weigert C, et al. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes 52, 527–535 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Lemley KV, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney international 58, 1228–1237 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC & Tesch GH Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney international 65, 116–128 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Chow FY, et al. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney international 69, 73–80 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Lim AK & Tesch GH Inflammation in diabetic nephropathy. Mediators of inflammation 2012, 146154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC & Tesch GH Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. Journal of the American Society of Nephrology : JASN 16, 1711–1722 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Kanamori H, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochemical and biophysical research communications 360, 772–777 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Osterby R & Gundersen HJ Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 11, 225–229 (1975). [DOI] [PubMed] [Google Scholar]
- 69.Rahmoune H, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54, 3427–3434 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Vallon V, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. American journal of physiology. Renal physiology 304, F156–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomson SC, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. American journal of physiology. Regulatory, integrative and comparative physiology 302, R75–83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vallon V & Thomson SC Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annual review of physiology 74, 351–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Najafian B, Kim Y, Crosson JT & Mauer M Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. Journal of the American Society of Nephrology : JASN 14, 908–917 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Russo LM, et al. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. Journal of the American Society of Nephrology : JASN 20, 489–494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bohle A, et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathology, research and practice 186, 135–144 (1990). [DOI] [PubMed] [Google Scholar]
- 76.Wang B, et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59, 1794–1802 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tervaert TW, et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology : JASN 21, 556–563 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Joslin EP Arteriosclerosis and diabetes. . Ann Clin Med 1061 (1927). [Google Scholar]
- 79.Pirart J Glycaemic control and development of diabetic nephropathy. Acta endocrinologica. Supplementum 242, 41–42 (1981). [PubMed] [Google Scholar]
- 80.de Boer IH & Group DER Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes care 37, 24–30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chalmers J & Cooper ME UKPDS and the legacy effect. The New England journal of medicine 359, 1618–1620 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Patel A, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine 358, 2560–2572 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Cooper ME & El-Osta A Epigenetics: mechanisms and implications for diabetic complications. Circulation research 107, 1403–1413 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Kowluru RA Mitochondria damage in the pathogenesis of diabetic retinopathy and in the metabolic memory associated with its continued progression. Current medicinal chemistry 20, 3226–3233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pop-Busui R, et al. DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Current diabetes reports 10, 276–282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Penno G, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes care 36, 2301–2310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waden J, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 58, 2649–2655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roy S, Sala R, Cagliero E & Lorenzi M Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proceedings of the National Academy of Sciences of the United States of America 87, 404–408 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watson AM, et al. Quinapril treatment abolishes diabetes-associated atherosclerosis in RAGE/apolipoprotein E double knockout mice. Atherosclerosis 235, 444–448 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Bianchi C & Del Prato S Metabolic memory and individual treatment aims in type 2 diabetes--outcome-lessons learned from large clinical trials. The review of diabetic studies : RDS 8, 432–440 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Derby L, Warram JH, Laffel LM & Krolewski AS Elevated blood pressure predicts the development of persistent proteinuria in the presence of poor glycemic control, in patients with type I diabetes. Diabete Metab 15, 320–326 (1989). [PubMed] [Google Scholar]
- 92.Schmitz A, Vaeth M & Mogensen CE Systolic blood pressure relates to the rate of progression of albuminuria in NIDDM. Diabetologia 37, 1251–1258 (1994). [DOI] [PubMed] [Google Scholar]
- 93.Tanaka Y, et al. Role of glycemic control and blood pressure in the development and progression of nephropathy in elderly Japanese NIDDM patients. Diabetes care 21, 116–120 (1998). [DOI] [PubMed] [Google Scholar]
- 94.Ayodele OE, Alebiosu CO & Salako BL Diabetic nephropathy--a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc 96, 1445–1454 (2004). [PMC free article] [PubMed] [Google Scholar]
- 95.Parving HH, et al. Impaired autoregulation of glomerular filtration rate in type 1 (insulin-dependent) diabetic patients with nephropathy. Diabetologia 27, 547–552 (1984). [DOI] [PubMed] [Google Scholar]
- 96.Christensen PK, Hansen HP & Parving HH Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney international 52, 1369–1374 (1997). [DOI] [PubMed] [Google Scholar]
- 97.Tolonen N, et al. Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia 52, 2522–2530 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Thomas MC, et al. Serum lipids and the progression of nephropathy in type 1 diabetes. Diabetes care 29, 317–322 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Moore KJ & Fisher EA Dysfunctional HDL takes its toll in chronic kidney disease. Immunity 38, 628–630 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Jenkins AJ, et al. Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney international 64, 817–828 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Makinen VP, et al. Triglyceride-cholesterol imbalance across lipoprotein subclasses predicts diabetic kidney disease and mortality in type 1 diabetes: the FinnDiane Study. Journal of internal medicine 273, 383–395 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Reis A, et al. Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. Journal of lipid research (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meikle PJ, Wong G, Barlow CK & Kingwell BA Lipidomics: potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacology & therapeutics 143, 12–23 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Parvanova AI, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 55, 1456–1462 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Thorn LM, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes care 28, 2019–2024 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Groop L, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 36, 642–647 (1993). [DOI] [PubMed] [Google Scholar]
- 107.Groop PH, Forsblom C & Thomas MC Mechanisms of disease: Pathway-selective insulin resistance and microvascular complications of diabetes. Nature clinical practice. Endocrinology & metabolism 1, 100–110 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Brown MS & Goldstein JL Selective versus total insulin resistance: a pathogenic paradox. Cell metabolism 7, 95–96 (2008). [DOI] [PubMed] [Google Scholar]
- 109.Yokoyama H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney international 58, 302–311 (2000). [DOI] [PubMed] [Google Scholar]
- 110.Sharma K The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney international 76, 145–148 (2009). [DOI] [PubMed] [Google Scholar]
- 111.Tsuboi N, Utsunomiya Y & Hosoya T Obesity-related glomerulopathy and the nephron complement. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 28 Suppl 4, iv108–113 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes 46, 1829–1839 (1997). [PubMed] [Google Scholar]
- 113.Thomas MC, Groop PH & Tryggvason K Towards understanding the inherited susceptibility for nephropathy in diabetes. Current opinion in nephrology and hypertension 21, 195–202 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Okabe J, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circulation research 110, 1067–1076 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Brasacchio D, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58, 1229–1236 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. The Journal of experimental medicine 205, 2409–2417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bird A Perceptions of epigenetics. Nature 447, 396–398 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Rakyan VK, Down TA, Balding DJ & Beck S Epigenome-wide association studies for common human diseases. Nature reviews. Genetics 12, 529–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guerrero-Bosagna C & Skinner MK Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol Cell Endocrinol (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ko YA, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome biology 14, R108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miao F, et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes 63, 1748–1762 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perera F & Herbstman J Prenatal environmental exposures, epigenetics, and disease. Reproductive toxicology 31, 363–373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nelson RG, Morgenstern H & Bennett PH Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 47, 1489–1493 (1998). [DOI] [PubMed] [Google Scholar]
- 124.Lemley KV A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney international. Supplement, S38–42 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Nelson RG, Morgenstern H & Bennett PH Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. American journal of epidemiology 148, 650–656 (1998). [DOI] [PubMed] [Google Scholar]
- 126.Singh GR & Hoy WE Kidney volume, blood pressure, and albuminuria: findings in an Australian aboriginal community. American journal of kidney diseases : the official journal of the National Kidney Foundation 43, 254–259 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Weil EJ, Curtis JM, Hanson RL, Knowler WC & Nelson RG The impact of disadvantage on the development and progression of diabetic kidney disease. Clinical nephrology 74 Suppl 1, S32–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rognant N, Lemoine S, Laville M, Hadj-Aissa A & Dubourg L Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes care 34, 1320–1322 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shlipak MG, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine 369, 932–943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krolewski AS, Gohda T & Niewczas MA Progressive renal decline as the major feature of diabetic nephropathy in type 1 diabetes. Clinical and experimental nephrology 18, 571–583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsu CY & Bansal N Measured GFR as “gold standard”--all that glitters is not gold? Clinical journal of the American Society of Nephrology : CJASN 6, 1813–1814 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Nori US, Pesavento TE & Hebert LA Measured GFR has limited clinical utility. American journal of kidney diseases : the official journal of the National Kidney Foundation 57, 180; discussion 180-181 (2011). [DOI] [PubMed] [Google Scholar]
- 133.Thomas MC The assessment and management of albuminuria in primary care. Diabetes research and clinical practice 80, 83–88 (2008). [DOI] [PubMed] [Google Scholar]
- 134.American Diabetes, A. 9. Microvascular Complications and Foot Care. Diabetes care 38, S58–S66 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Kdoqi. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation 49, S12–154 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Lambers Heerspink HJ, et al. Comparison of different measures of urinary protein excretion for prediction of renal events. Journal of the American Society of Nephrology : JASN 21, 1355–1360 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fotheringham J, Campbell MJ, Fogarty DG, El Nahas M & Ellam T Estimated albumin excretion rate versus urine albumin-creatinine ratio for the estimation of measured albumin excretion rate: derivation and validation of an estimated albumin excretion rate equation. American journal of kidney diseases : the official journal of the National Kidney Foundation 63, 405–414 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Amin AP, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the Kidney Early Evaluation Program (KEEP). American journal of kidney diseases : the official journal of the National Kidney Foundation 61, S12–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomas MC, Viberti G & Groop PH Screening for chronic kidney disease in patients with diabetes: are we missing the point? Nature clinical practice. Nephrology 4, 2–3 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Elley CR, et al. Derivation and Validation of a Renal Risk Score for People With Type 2 Diabetes. Diabetes care (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jardine MJ, et al. Prediction of kidney-related outcomes in patients with type 2 diabetes. American journal of kidney diseases : the official journal of the National Kidney Foundation 60, 770–778 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Thomas MC & Groop PH Diabetes: Assessing renal risk in patients with type 2 diabetes. Nature reviews. Nephrology 9, 559–560 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Roscioni SS, et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 56, 259–267 (2013). [DOI] [PubMed] [Google Scholar]
- 144.Sharma K, et al. Metabolomics Reveals Signature of Mitochondrial Dysfunction in Diabetic Kidney Disease. Journal of the American Society of Nephrology : JASN (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McClelland A, Hagiwara S & Kantharidis P Where are we in diabetic nephropathy: microRNAs and biomarkers? Current opinion in nephrology and hypertension 23, 80–86 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Gæde P, Vedel P, Parving H-H & Pedersen O Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353, 617–622 (1999). [DOI] [PubMed] [Google Scholar]
- 147.Gaede P, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England journal of medicine 348, 383–393 (2003). [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 73, 163–171 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Fioretto P, Steffes MW, Sutherland DER, Goetz FC & Mauer M Reversal of lesions of diabetic nephropathy after pancreas transplantation. The New England journal of medicine 339, 69–75 (1998). [DOI] [PubMed] [Google Scholar]
- 150.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM & Parikh CR Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Archives of internal medicine 172, 761–769 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fullerton B, et al. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. The Cochrane database of systematic reviews 2, CD009122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Group DER, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. The New England journal of medicine 365, 2366–2376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Group TAC, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine 358, 2560–2572 (2008). [DOI] [PubMed] [Google Scholar]
- 154.Zoungas S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia (2014). [DOI] [PubMed] [Google Scholar]
- 155.Zoungas S, et al. Severe hypoglycemia and risks of vascular events and death. The New England journal of medicine 363, 1410–1418 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Shurraw S, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Archives of internal medicine 171, 1920–1927 (2011). [DOI] [PubMed] [Google Scholar]
- 157.Schernthaner G, Mogensen CE & Schernthaner GH The effects of GLP-1 analogues, DPP-4 inhibitors and SGLT2 inhibitors on the renal system. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease 11, 306–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Groop PH, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes care 36, 3460–3468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tanaka T, Higashijima Y, Wada T & Nangaku M The potential for renoprotection with incretin-based drugs. Kidney international 86, 701–711 (2014). [DOI] [PubMed] [Google Scholar]
- 160.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN & Lasaridis AN Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation 55, 835–847 (2010). [DOI] [PubMed] [Google Scholar]
- 161.Kohan DE, Fioretto P, Tang W & List JF Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney international 85, 962–971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.[online], U.N.L.o.M.C.g NCT01792518. [Google Scholar]
- 163.[online], U.N.L.o.M.C.g NCT02065791. [Google Scholar]
- 164.Emdin CA, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association 313, 603–615 (2015). [DOI] [PubMed] [Google Scholar]
- 165.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj 317, 703–713 (1998). [PMC free article] [PubMed] [Google Scholar]
- 166.Strippoli GF, Craig M, Deeks JJ, Schena FP & Craig JC Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. Bmj 329, 828 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Galan BE, et al. Lowering blood pressure reduces renal events in type 2 diabetes. Journal of the American Society of Nephrology : JASN 20, 883–892 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsika EP, Poulimenos LE, Boudoulas KD & Manolis AJ The J-curve in arterial hypertension: fact or fallacy? Cardiology 129, 126–135 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS medicine 9, e1001293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Van Buren PN & Toto RD The pathogenesis and management of hypertension in diabetic kidney disease. The Medical clinics of North America 97, 31–51 (2013). [DOI] [PubMed] [Google Scholar]
- 171.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA : the journal of the American Medical Association 311, 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Casas JP, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 366, 2026–2033 (2005). [DOI] [PubMed] [Google Scholar]
- 173.Lewis EJ, Hunsicker LG, Bain RP & Rohde RD The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. The New England journal of medicine 329, 1456–1462 (1993). [DOI] [PubMed] [Google Scholar]
- 174.Atkins RC, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. American journal of kidney diseases : the official journal of the National Kidney Foundation 45, 281–287 (2005). [DOI] [PubMed] [Google Scholar]
- 175.System USRD USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases, , Bethesda, MD, 2013). [Google Scholar]
- 176.Tobe SW, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 123, 1098–1107 (2011). [DOI] [PubMed] [Google Scholar]
- 177.Parving HH, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. The New England journal of medicine 367, 2204–2213 (2012). [DOI] [PubMed] [Google Scholar]
- 178.Bomback AS, Kshirsagar AV, Amamoo MA & Klemmer PJ Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. American journal of kidney diseases : the official journal of the National Kidney Foundation 51, 199–211 (2008). [DOI] [PubMed] [Google Scholar]
- 179.Ruilope LM, et al. Rationale, Design, and Baseline Characteristics of ARTS-DN: A Randomized Study to Assess the Safety and Efficacy of Finerenone in Patients with Type 2 Diabetes Mellitus and a Clinical Diagnosis of Diabetic Nephropathy. American journal of nephrology 40, 572–581 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Bakris GL A practical approach to achieving recommended blood pressure goals in diabetic patients. Archives of internal medicine 161, 2661–2667 (2001). [DOI] [PubMed] [Google Scholar]
- 181.Bakris GL, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet 375, 1173–1181 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Association AD Standards of medical care in diabetes - 2014. Diabetes care 37, S14–S80 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Griffin SJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 378, 156–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Haynes R, et al. Effects of lowering LDL cholesterol on progression of kidney disease. Journal of the American Society of Nephrology : JASN 25, 1825–1833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zeeuw P.D.d., et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. The Lancet Diabetes & Endocrinology (2015). [DOI] [PubMed] [Google Scholar]
- 186.Jun M, et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. Journal of the American College of Cardiology 60, 2061–2071 (2012). [DOI] [PubMed] [Google Scholar]
- 187.Park CW, et al. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney international 69, 1511–1517 (2006). [DOI] [PubMed] [Google Scholar]
- 188.Ren S, et al. PPARalpha activation upregulates nephrin expression in human embryonic kidney epithelial cells and podocytes by a dual mechanism. Biochemical and biophysical research communications 338, 1818–1824 (2005). [DOI] [PubMed] [Google Scholar]
- 189.Zhao X & Li LY PPAR-alpha agonist fenofibrate induces renal CYP enzymes and reduces blood pressure and glomerular hypertrophy in Zucker diabetic fatty rats. American journal of nephrology 28, 598–606 (2008). [DOI] [PubMed] [Google Scholar]
- 190.Ansquer JC, et al. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. American journal of kidney diseases : the official journal of the National Kidney Foundation 51, 904–913 (2008). [DOI] [PubMed] [Google Scholar]
- 191.Hansen HP, Tauber-Lassen E, Jensen BR & Parving HH Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney international 62, 220–228 (2002). [DOI] [PubMed] [Google Scholar]
- 192.Chadban S, et al. The CARI guidelines. Prevention and management of chronic kidney disease in type 2 diabetes. Nephrology 15 Suppl 1, S162–194 (2010). [DOI] [PubMed] [Google Scholar]
- 193.Bello AK, et al. Impact of weight change on albuminuria in the general population. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 22, 1619–1627 (2007). [DOI] [PubMed] [Google Scholar]
- 194.Van Huffel L, et al. Dietary restriction and exercise for diabetic patients with chronic kidney disease: a systematic review. PloS one 9, e113667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Look ARG Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. The lancet. Diabetes & endocrinology 2, 801–809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carlsson LM, et al. The incidence of albuminuria after bariatric surgery and usual care in swedish obese subjects (SOS): a prospective controlled intervention trial. International journal of obesity 39, 169–175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Evert AB, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes care 37 Suppl 1, S120–143 (2014). [DOI] [PubMed] [Google Scholar]
- 198.Stevens PE, Levin A & Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine 158, 825–830 (2013). [DOI] [PubMed] [Google Scholar]
- 199.Lee CC, Sharp SJ, Wexler DJ & Adler AI Dietary intake of eicosapentaenoic and docosahexaenoic acid and diabetic nephropathy: cohort analysis of the diabetes control and complications trial. Diabetes care 33, 1454–1456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miller ER 3rd, et al. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on biomarkers of kidney injury in adults with diabetes: results of the GO-FISH trial. Diabetes care 36, 1462–1469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.de Ferranti SD, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130, 1110–1130 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Newman DJ, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health technology assessment 9, iii–vi, xiii–163 (2005). [DOI] [PubMed] [Google Scholar]
- 203.Gaede P Intensive glucose control and cardiovascular disease in type 2 diabetes--should we change the recommended target for glycated hemoglobin? Commentary to ACCORD and ADVANCE trials. Polskie Archiwum Medycyny Wewnetrznej 118, 619–621 (2008). [PubMed] [Google Scholar]
- 204.Shepherd J, et al. Intensive lipid lowering with atorvastatin in patients with coronary artery disease, diabetes, and chronic kidney disease. Mayo Clinic proceedings 83, 870–879 (2008). [PubMed] [Google Scholar]
- 205.Palmer SC, et al. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Annals of internal medicine 157, 263–275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pignone M, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation 121, 2694–2701 (2010). [DOI] [PubMed] [Google Scholar]
- 207.Kim AJ, et al. Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PloS one 9, e104179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dasgupta A, et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). The American journal of cardiology 103, 1359–1363 (2009). [DOI] [PubMed] [Google Scholar]
- 209.Johnson RJ, Freehally J & Floege J Comprehensive Clinical Nephrology, (Elservier Health Sciences, 2014). [Google Scholar]
- 210.Excellence, N.I.f.H.a.C. Management of chronic kidney disease. , (NICE, London, 2008). [Google Scholar]
- 211.KDIGO. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, (2012). [Google Scholar]
- 212.Dukes JL, Seelam S, Lentine KL, Schnitzler MA & Neri L Health-related quality of life in kidney transplant patients with diabetes. Clinical transplantation 27, E554–562 (2013). [DOI] [PubMed] [Google Scholar]
- 213.Campbell KH, et al. Association between estimated GFR, health-related quality of life, and depression among older adults with diabetes: the Diabetes and Aging Study. American journal of kidney diseases : the official journal of the National Kidney Foundation 62, 541–548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hotu C, et al. A community-based model of care improves blood pressure control and delays progression of proteinuria, left ventricular hypertrophy and diastolic dysfunction in Maori and Pacific patients with type 2 diabetes and chronic kidney disease: a randomized controlled trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 25, 3260–3266 (2010). [DOI] [PubMed] [Google Scholar]
- 215.Rayner HC, et al. Does community-wide chronic kidney disease management improve patient outcomes? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 29, 644–649 (2014). [DOI] [PubMed] [Google Scholar]
- 216.Thomas B Improving blood pressure control among adults with CKD and diabetes: provider-focused quality improvement using electronic health records. Advances in chronic kidney disease 18, 406–411 (2011). [DOI] [PubMed] [Google Scholar]
- 217.Cortes-Sanabria L, et al. Improving care of patients with diabetes and CKD: a pilot study for a cluster-randomized trial. American journal of kidney diseases : the official journal of the National Kidney Foundation 51, 777–788 (2008). [DOI] [PubMed] [Google Scholar]
- 218.Thomas MC, MacIsaac RJ, Tsalamandris C, Power D & Jerums G Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes care 26, 1164–1169 (2003). [DOI] [PubMed] [Google Scholar]
- 219.Parfrey PS Critical appraisal of randomized controlled trials of anemia correction in patients with renal failure. Current opinion in nephrology and hypertension 20, 177–181 (2011). [DOI] [PubMed] [Google Scholar]
- 220.Block GA, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. American journal of kidney diseases : the official journal of the National Kidney Foundation 62, 457–473 (2013). [DOI] [PubMed] [Google Scholar]
- 221.Nelson RG, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. The New England journal of medicine 335, 1636–1642 (1996). [DOI] [PubMed] [Google Scholar]
- 222.Burrows NR, Li Y & Geiss LS Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes care 33, 73–77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zoccali C, Kramer A & Jager K The databases: renal replacement therapy since 1989--the European Renal Association and European Dialysis and Transplant Association (ERA-EDTA). Clinical journal of the American Society of Nephrology : CJASN 4 Suppl 1, S18–22 (2009). [DOI] [PubMed] [Google Scholar]
- 224.Thomas MC Emerging drugs for managing kidney disease in patients with diabetes. Expert opinion on emerging drugs 18, 55–70 (2013). [DOI] [PubMed] [Google Scholar]
- 225.Mauer M, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. The New England journal of medicine 361, 40–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bilous R, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Annals of internal medicine 151, 11–20, W13–14 (2009). [DOI] [PubMed] [Google Scholar]
- 227.Chakkarwar VA Smoking in diabetic nephropathy: sparks in the fuel tank? World journal of diabetes 3, 186–195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hoy WE, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney international 54, 1296–1304 (1998). [DOI] [PubMed] [Google Scholar]
- 229.Nymark M, et al. Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes care 32, 1689–1693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Thakar CV, Christianson A, Himmelfarb J & Leonard AC Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clinical journal of the American Society of Nephrology : CJASN 6, 2567–2572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Forbes JM & Cooper ME Mechanisms of diabetic complications. Physiological reviews 93, 137–188 (2013). [DOI] [PubMed] [Google Scholar]
- 232.Ahmed SB, et al. Oral contraceptives, angiotensin-dependent renal vasoconstriction, and risk of diabetic nephropathy. Diabetes care 28, 1988–1994 (2005). [DOI] [PubMed] [Google Scholar]
- 233.Hovind P, Rossing P, Johnson RJ & Parving HH Serum uric acid as a new player in the development of diabetic nephropathy. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation 21, 124–127 (2011). [DOI] [PubMed] [Google Scholar]
- 234.Zoppini G, et al. Lower levels of 25-hydroxyvitamin D3 are associated with a higher prevalence of microvascular complications in patients with type 2 diabetes. BMJ open diabetes research & care 3, e000058 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Forsblom C, et al. Added value of soluble tumor necrosis factor-alpha receptor 1 as a biomarker of ESRD risk in patients with type 1 diabetes. Diabetes care 37, 2334–2342 (2014). [DOI] [PubMed] [Google Scholar]
- 236.Jha JC, Jandeleit-Dahm KA & Cooper ME New insights into the use of biomarkers of diabetic nephropathy. Advances in chronic kidney disease 21, 318–326 (2014). [DOI] [PubMed] [Google Scholar]
- 237.Fufaa GD, et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia 58, 188–198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wong MG, et al. Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney international 83, 278–284 (2013). [DOI] [PubMed] [Google Scholar]
- 239.LeBleu VS, et al. Origin and function of myofibroblasts in kidney fibrosis. Nature medicine 19, 1047–1053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


