Abstract
Common genetic polymorphisms associated with severity of COVID-19 illness can be utilized for discovering molecular pathways and cell types driving disease pathogenesis. Here, we assessed the effects of 679 COVID-19-risk variants on gene expression in a wide-range of immune cell types. Severe COVID-19-risk variants were significantly associated with the expression of 11 protein-coding genes, and overlapped with either target gene promoter or cis-regulatory regions that interact with target promoters in the cell types where their effects are most prominent. For example, we identified that the association between variants in the 3p21.31 risk locus and the expression of CCR2 in classical monocytes is likely mediated through an active cis-regulatory region that interacted with CCR2 promoter specifically in monocytes. The expression of several other genes showed prominent genotype-dependent effects in non-classical monocytes, NK cells, B cells, or specific T cell subtypes, highlighting the potential of COVID-19 genetic risk variants to impact the function of diverse immune cell types and influence severe disease manifestations.
Keywords: COVID-19, DICE, GWAS, CCR2, IL-10, Immune cells
MAIN
The clinical presentation of SARS-CoV-2 infection in humans can range from severe respiratory failure to disease that is very mild or without symptoms1. Although hyperactivation of various cellular components of the immune system have been observed in patients with severe COVID-19 illness2,3, the host genetic factors that determine susceptibility to severe COVID-19 illness are not well understood. Genome-wide association studies (GWAS) addressing this question have identified a number of genetic variants associated with COVID-19 susceptibility and severity4–7. However, their target genes and the immune cell types where their effects are most prominent are not known. The DICE database of immune cell gene expression, epigenomics and expression quantitative trait loci (eQTLs) (http://dice-database.org) was established to precisely answer these questions as well as to help narrow down functional variants in dense haploblocks linked to disease susceptibility8,9. Here, we utilize the DICE database and 3D cis-interactome maps to provide a list of target genes and cell types most affected by genetic variants linked to severity of COVID-19 illness.
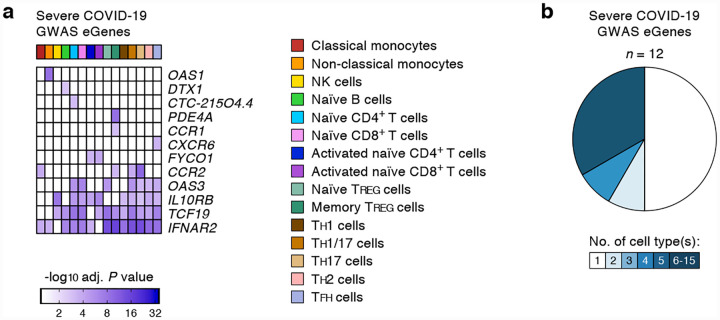
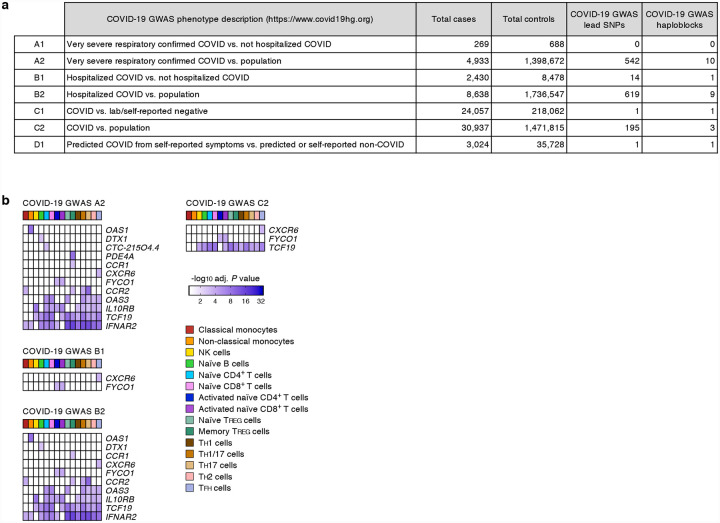
We systematically assessed the effects of 679 COVID-19-risk variants (defined by the COVID-19 Host Genetics Initiative; release 4 from 20 October 20204; GWAS association P value < 5×10−8) on gene expression in 13 different immune cell types and 2 activation conditions (Supplementary Tables 1 and 2). The expression of 11 protein-coding genes and 1 non-coding RNA (referred here as eGenes) was associated with the genetic variants linked to severe COVID-19 illness requiring hospitalization (Fig. 1a and Extended data Fig. 1). Notably, the majority of the eGenes associated with severe COVID-19 illness showed prominent effects in specific immune cell types (Fig. 1b). Applying a more liberal GWAS association P value threshold of 1×10−5, we identified 41 additional eGenes that were associated with genetic variants non-significantly linked to severe COVID-19 illness (Supplementary Table 3). Some of these variants are likely to reach statistical significance (GWAS association P value < 5×10−8) as more donors with severe COVID-19 illness are included in the subsequent analysis phases.
Figure 1. COVID-19-risk variants with eQTL activity in human immune cell types.
(a) Genes and cell types influenced by GWAS SNPs linked to severe COVID-19 illness requiring hospitalization. For each cell type (columns), the adj. association P value for the peak GWAS cis-eQTL associated with the indicated eGenes (rows) is shown. (b) Fractions of GWAS eGenes linked to severe COVID-19 illness identified in varying numbers of cell types.
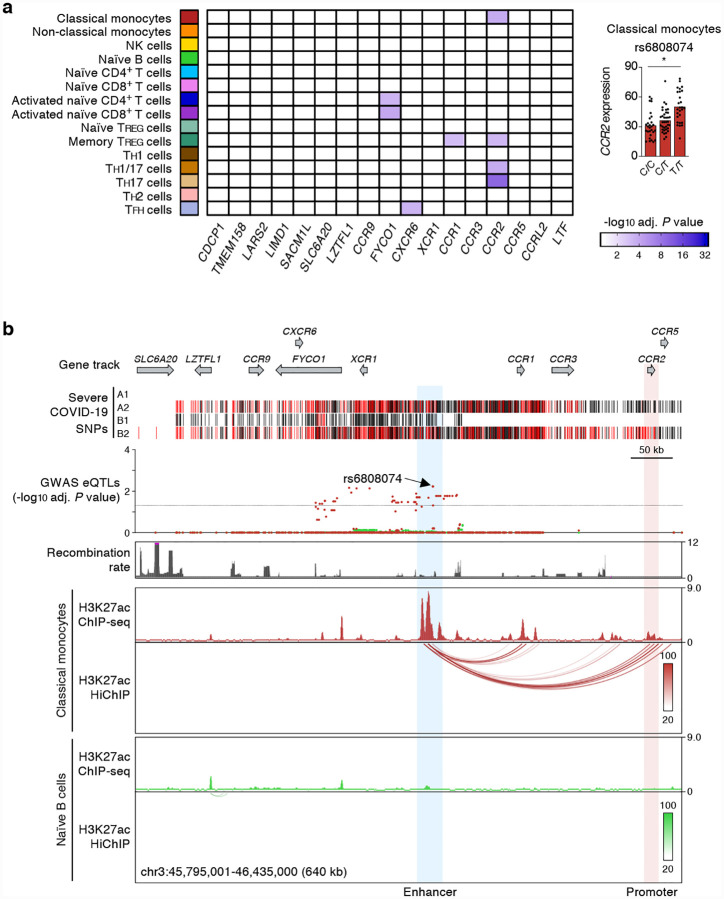
Genetic variants in the 3p21.31 locus have been linked to severity of COVID-19 illness by multiple GWAS studies4–7. These severe COVID-19-risk variants are inherited as a dense >300 kb haploblock that was shown to have entered the human population >50,000 years ago from Neanderthals10. Populations with higher frequency of this Neanderthal-origin COVID-19-risk haplotype have higher risk of severe COVID-19 illness10. The severe COVID-19-risk variants in the 3p21.31 locus contains 17 known protein-coding genes (Fig. 2a), including SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, XCR1, CCR1, CCR3, CCR2 and CCR5. Among these genes CCR2 (encoding for C-C type chemokine receptor, also known as monocyte chemoattractant protein 1 receptor) expression showed the strongest association with 3p21.31 severe COVID-19-risk variants identified by GWAS studies4 (Fig. 2a). Importantly, the risk variants were associated with expression of CCR2 in certain CD4+ memory T cell subsets (TH17, TH1/17) and classical monocytes (Fig. 1a, 2a and Supplementary Tables 1 and 2). Although the CCR2 promoter did not directly overlap the risk variants, we found that the peak eQTL (rs6808074), located >200kb upstream, directly overlapped an intergenic cis-regulatory region that specifically interacted (H3K27ac HiChIP) with CCR2 promoter in classical monocytes (Fig. 2b). These findings suggested that the severe COVID-19-risk variant (rs6808074) likely perturbs the function of a distal enhancer that is important for regulating CCR2 expression in monocytes. Thus, genetic evidence points to an important role of CCR2 pathway in the pathogenesis of COVID-19. Patients with severe COVID-19 illness were shown to have increased CCR2 expression in circulating monocytes as well as very high levels of CCR2 ligand (CCL2) in bronchoalveolar lavage fluid11, supporting the hypothesis that excessive recruitment of CCR2-expressing monocytes may drive pathogenic lung inflammation in COVID-19.
Figure 2. Promoter interacting distal cis-eQTLs regulate CCR2 promoter activity specifically in classical monocytes.
(a) Genes and cell types most susceptible to the effects of severe COVID-19-risk variants (all with GWAS association P value < 5×10−8) in the 3p21.31 locus. The adj. association P value for the peak GWAS cis-eQTL associated with the indicated eGenes in each cell type and activation condition is shown (left). Right, mean expression levels (TPM) of CCR2 gene in classical monocytes (* adj. association P value: 5.94×10−3), from subjects (n=91) categorized based on the genotype at the indicated GWAS cis-eQTL (each symbol represents an individual subject; adj. association P value calculated by Benjamini-Hochberg method). (b) WashU Epigenome browser tracks for the 3p21.31 locus, severe COVID-19-risk associated GWAS variants (based on GWAS study, see Extended Data Figure 1a; red color bars are lead GWAS SNPs, black color bars are SNPs in linkage disequilibrium), adj. association P value for GWAS cis-eQTLs associated with expression of CCR2 expression in classical monocytes (dark red) and naïve B cells (green), recombination rate tracks27,28, H3K27ac ChIP-seq tracks, and H3K27ac HiChIP interactions in classical monocytes and naïve B cells.
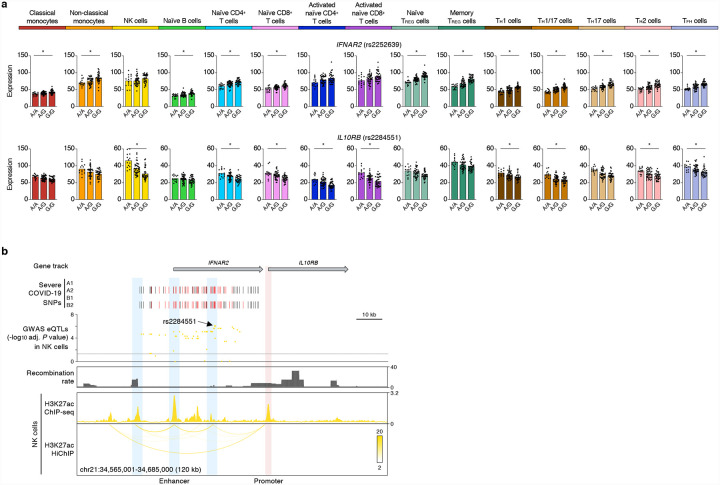
Defects in the type 1 interferon pathway have been reported in patients with severe COVID-19 illness12–15. We found many severe COVID-19-risk variants in chromosome 21, overlapping the IFNAR2 gene that encodes for interferon receptor 2, were associated with the expression of IFNAR2 in multiple immune cell types (Fig. 3a). H3K27ac HiChIP-based chromatin interaction maps in this locus showed that the severe COVID-19-risk variants overlapping the IFNAR2 gene promoter and an intronic enhancer interacted with the promoter of a neighboring gene, IL10RB and also influenced its expression levels (Fig. 3b). The effects of these risk variants were most prominent in NK cells (rs2284551, adj. association P value = 8.99×10−7) (Fig. 3a). IL10RB, encodes for IL-10 receptor beta, and given the immunomodulatory role of IL-1016, it is likely that the lower expression on the IL10RB in NK cells may perturb their responsiveness to IL-10. Thus, our findings point to a potentially important role for IL-10 signaling and NK cells in influencing susceptibility to severe COVID-19 illness.
Figure 3. COVID-19-risk variants show cell-type-restriction of their effects on gene expression.
(a) Mean expression levels (TPM) of selected severe COVID-19-risk associated GWAS eGenes (all with GWAS association P value < 5×10−8) in the indicated cell types from subjects (n=91) categorized based on the genotype at the indicated peak GWAS cis-eQTL; each symbol represents an individual subject, * adj. association P value < 0.05. (b) WashU Epigenome browser tracks for the IFNAR2 and IL10RB loci, severe COVID-19-risk associated GWAS variants (based on GWAS study, see Extended Data Figure 1a; red color bars are lead GWAS SNPs, black color bars are SNPs in linkage disequilibrium), adj. association P value for GWAS cis-eQTLs associated with expression of IL10RB in NK cells, recombination rate tracks27,28, H3K27ac ChIP-seq tracks, and H3K27ac HiChIP interactions in NK cells.
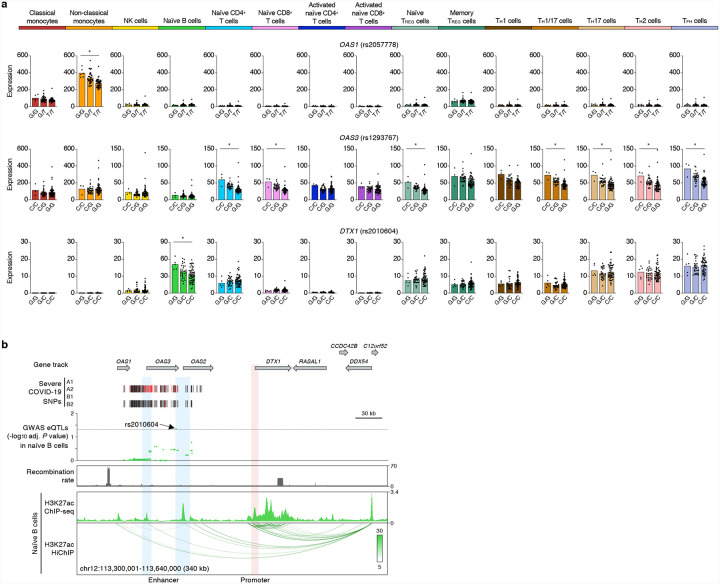
The expression of two interferon-inducible genes (OAS1 and OAS3) was also influenced by severe COVID-19-risk variants in chromosome 12. OAS1 and OAS3, encode for oligoadenylate synthase family of proteins that degrades viral RNA and activate antiviral responses17. OAS1 showed a peak COVID-19-risk eQTL (rs2057778, adj. association P value = 1.77×10−7) specifically in patrolling non-classical monocytes, whereas OAS3 showed prominent eQTLs in T cells (Fig. 4a), further highlighting cell-type-restricted effects of severe COVID-19-risk variants. Interestingly, we found that a severe COVID-19-risk variant (rs2010604, adj. association P value = 4.50×10−2) in the OAS1/OAS3 locus also influenced the expression of a neighboring gene (DTX1) specifically in naïve B cells (Fig. 4a). Active chromatin interaction maps in naïve B cells showed that a cis-regulatory region near the eQTL (rs2010604) interacts with the promoter of DTX1, located >80kb away, and likely modulates its transcriptional activity (Fig. 4b). This notion is supported by recent reports showing that promoters can interact with neighboring gene promoters and regulate their expression9,18. DTX1, encodes for a ubiquitin ligase Deltex1 that regulates NOTCH activity in B cells19. Deltex1 has also been shown to promote anergy, a functionally hypo-responsive state, in T cells20; if Deltex1 has a similar functions in B cells, then genetic modulation of DTX1 levels may have a profound impact on the function of B cells in COVID-19 illness.
Figure 4. Target genes of severe COVID-19-risk variants in chromosome 12.
(a) Mean expression levels (TPM) of selected severe COVID-19-risk associated GWAS eGenes (all with GWAS association P value < 5×10−8) in the indicated cell types from subjects (n=91) categorized based on the genotype at the indicated peak GWAS cis-eQTL; each symbol represents an individual subject, * adj. association P value < 0.05. (b) WashU Epigenome browser tracks for the extended DTX1 locus, severe COVID-19-risk associated GWAS variants (based on GWAS study, see Extended Data Figure 1a; red color bars are lead GWAS SNPs, black color bars are SNPs in linkage disequilibrium), adj. association P value for GWAS cis-eQTLs associated with expression of DTX1 in naïve B cells, recombination rate tracks27,28, H3K27ac ChIP-seq tracks, and H3K27ac HiChIP interactions in naïve B cells.
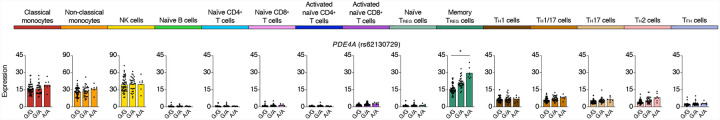
Several COVID-19 risk variants located in the promoter region of TCF19 were associated with its expression in multiple lymphocyte subsets but not in classical or non-classical monocytes (Fig. 1a). TCF19 encodes for a transcription factor TCF19 that has been shown to regulate activation of T cells21 and also involved in cell proliferation22,23. A noteworthy example of a highly cell-specific severe COVID-19-risk eGene in regulatory T cells (TREG) was PDE4A (Extended Data Fig. 2). This gene encodes for phosphodiesterase 4A, which has been shown to reduce the levels of cAMP, and thus influence T cell activity to module immune responses24.
In summary, several severe COVID-19-risk variants show cell-type-restriction of their effects on gene expression, and thus have the potential to impact the function of diverse immune cell types and gene pathways. Our analysis of eQTLs and cis-interaction maps in multiple immune cell types enabled a precise definition of the cell types and genes that drive genetic susceptibility to severe COVID-19 illness, potentially contributing to the different clinical outcomes. Our study also highlights how information about common genetic polymorphisms can be used to define molecular pathways and cell types that play a role in disease pathogenesis.
METHODS
The Institutional Review Board (IRB) of the La Jolla Institute for Allergy and Immunology (LJI; IRB protocol no. SGE-121–0714) approved the study. For the DICE study, a total of 91 healthy volunteers were recruited in the San Diego area, who provided leukapheresis samples at the San Diego Blood Bank (SDBB) after written informed consent. Details of gene expression and eQTL analysis in 13 immune cell types and 2 activation conditions have been reported for the DICE project (recalculated to incorporate 4 previously missing RNA-seq samples)8, H3K27ac HiChIP data in 5 common immune cell types has been previously reported for the DICE cis-interactome project9. Genetic variants associated with COVID-19 were downloaded from the COVID-19 Host Genetics Initiative (release 4 from 20 October 2020). Lead genetic variants with GWAS association P value < 5×10−8 were utilized for downstream analysis. Linkage disequilibrium (LD) for lead COVID-19-risk-variants was calculated using PLINK v1.90b3w25 for continental ‘super populations’ (AFR, AMR, EAS, EUR, SAS) based on data from the phase 3 of the 1,000 Genomes Project26. SNPs in tight genetic linkage with GWAS lead SNPs (LD threshold R2 > 0.8) in any of the five super-populations were retrieved along with the SNP information (e.g., genomic location, allelic variant, allele frequencies). Utilizing this data set, GWAS SNPs (lead SNPs and SNPs in LD) were analyzed for overlap with eQTLs in the DICE database (raw P value < 0.0001, adj. association P (FDR) < 0.05, TPM > 1.0) separately for each cell type to identify COVID-19-risk variants that were associated with gene expression in immune cell types.
Extended Data
Extended data Figure 1.
Gene and cell types most susceptible to severe COVID-19-risk associated GWAS SNPs. (a) GWAS SNP datasets defined by the COVID-19 Host Genetics Initiative (see Online Methods), number of cases and controls in each study (release 4 from 20 October 2020), retrieved GWAS lead SNPs (GWAS association P value < 5×10−8) and number of GWAS haploblocks. (b) For each separate GWAS SNP dataset (as defined by the COVID-19 Host Genetics Initiative), the adj. association P value for the peak GWAS cis-eQTL associated with the indicated eGenes in each cell type and activation condition is shown.
Extended data Figure 2.
Genes and cell types most susceptible to severe COVID-19-risk associated GWAS variants. Mean expression levels (TPM) of PDE4A, a severe COVID-19-risk associated GWAS eGene (GWAS association P value < 5×10−8), in the indicated cell types from subjects (n=91) categorized based on the genotype at the indicated peak GWAS cis-eQTL; each symbol represents an individual subject, * adj. association P value < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH grants R24-AI108564 (P.V., F.A., C.H.O.), the William K. Bowes Jr Foundation (P.V.), and R35-GM128938 (F.A.).
Footnotes
DATA AND SOFTWARE AVAILABILITY
The DICE project provides anonymized data for public access at http://dice-database.org. Individual-specific RNA-sequencing, genotype, HiChIP and ChIP-seq data are available from the database of Genotypes and Phenotypes (dbGaP Accession number: phs001703.v3.p1).
CODE AVAILABILITY
The code used for the analyses performed in this study is available upon request. The codes used for HiChIP data analysis is available on GitHub at https://github.com/ay-lab/pieQTL_NG.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Tay M.Z., Poh C.M., Renia L., MacAry P.A. & Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vabret N. et al. Immunology of COVID-19: Current State of the Science. Immunity 52, 910–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meckiff B.J. et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Initiative C.-H.G. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet 28, 715–718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Severe Covid G.G. et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med 383, 1522–1534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts G.H.L. et al. AncestryDNA COVID-19 Host Genetic Study Identifies Three Novel Loci. medRxiv, 2020.10.06.20205864 (2020). [Google Scholar]
- 7.Pairo-Castineira E. et al. Genetic mechanisms of critical illness in Covid-19. medRxiv, 2020.09.24.20200048 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Schmiedel B.J. et al. Impact of Genetic Polymorphisms on Human Immune Cell Gene Expression. Cell 175, 1701–1715 e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra V. et al. Promoter-interacting expression quantitative trait loci are enriched for functional genetic variants. Nature Genetics (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeberg H. & Paabo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature (2020). [DOI] [PubMed] [Google Scholar]
- 11.Szabo P.A. et al. Analysis of respiratory and systemic immune responses in COVID-19 reveals mechanisms of disease pathogenesis. medRxiv, 2020.10.15.20208041 (2020). [Google Scholar]
- 12.Arunachalam P.S. et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastard P. et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science, eabd4585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjadj J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science, eabd4570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang W., Rutz S., Crellin N.K., Valdez P.A. & Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29, 71–109 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Sadler A.J. & Williams B.R. Interferon-inducible antiviral effectors. Nat Rev Immunol 8, 559–68 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Rivera A., Santiago-Algarra D., Puthier D. & Spicuglia S. Widespread Enhancer Activity from Core Promoters. Trends Biochem Sci 43, 452–468 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Izon D.J. et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity 16, 231–43 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Hsiao H.W. et al. Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity 31, 72–83 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Best J.A. et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol 14, 404–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z.H. et al. TCF19 contributes to cell proliferation of non-small cell lung cancer by inhibiting FOXO1. Cell Biol Int (2019). [DOI] [PubMed] [Google Scholar]
- 23.Krautkramer K.A. et al. Tcf19 is a novel islet factor necessary for proliferation and survival in the INS-1 beta-cell line. Am J Physiol Endocrinol Metab 305, E600–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehbi V.L. & Tasken K. Molecular Mechanisms for cAMP-Mediated Immunoregulation in T cells - Role of Anchored Protein Kinase A Signaling Units. Front Immunol 7, 222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559–75 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genomes Project C. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong A. et al. A high-resolution recombination map of the human genome. Nat Genet 31, 241–7 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Kong A. et al. Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467, 1099–103 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.