Abstract
XiaoLuoWan (XLW) is a classical formula in traditional Chinese medicine (TCM) that has satisfactory therapeutic effects for uterine fibroids (UFs). However, its underlying mechanisms remain unclear. To elucidate the pharmacological actions of XLW in treating UFs, an ingredient–target–disease framework was proposed based on network pharmacology strategies. The active ingredients in XLW and their putative targets were obtained from the TCM systems pharmacology database and analysis platform (TCMSP) and Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM) platforms. The known therapeutic targets of UFs were acquired from the DigSee and DrugBank databases. Then, the links between putative XLW targets and therapeutic UF targets were identified to establish interaction networks by Cytoscape. Finally, Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of overlapping gene targets were performed in the STRING database and visualized in R software. In total, 9 active compounds were obtained from 74 ingredients, with 71 curative targets predicted in XLW. Moreover, 321 known therapeutic targets were closely related to UFs, with 29 targets overlapping with XLW and considered interacting genes. Pathway enrichment revealed that the calcium signaling pathway was significantly enriched and the mitogen-activated protein kinase (MAPK) signaling pathway, cAMP signaling pathway, cancer and vascular smooth muscle contraction pathways, cGMP-PKG signaling pathway, and AGE-RAGE signaling pathway were closely associated with XLW intervention for UFs. In conclusion, the network pharmacology detection identified 9 available chemicals as the active ingredients in XLW that may relieve UFs by regulating 29 target genes involved in the calcium signaling pathway, MAPK pathway and cAMP pathway. Network pharmacology analyses may provide more convincing evidence for the investigation of classical TCM prescriptions, such as XLW.
Keywords: calcium signaling pathway, network pharmacology, Traditional Chinese medicine (TCM), uterine fibroids (UFs), XiaoLuoWan (XLW)
Introduction
Uterine fibroids (UFs), which are also known as leiomyomas or myomas, are the most common benign pelvic tumors and affect 50–60% of women of child-bearing age [1] and 70% of women up to 50 years of age [2]. Most fibroids are asymptomatic, whereas 30–40% of patients may suffer from symptoms, such as pelvic pain, infertility [3] and obstetric complications [4], with 30% of such cases leading to morbidity due to excessive uterine bleeding and pelvic pressure [5]. Current mainstream treatments include surgical procedures and medical therapies [6]. However, associated surgeries, such as hysterectomy or myomectomy, may not increase pregnancy rates [7] or reverse pregnancy loss [8] and may actually increase the risk of mortality [9,10]. Nonextirpative procedures for UFs including uterine artery embolization, focused ultrasound, laparoscopic radiofrequency ablation were reported to show similar outcomes to myomectomy [11]. Drug-based therapy, such as gonadotropin-releasing hormone (GnRH) agonists, which is the only agent for short-term management of UFs approved by Food and Drug Administration (FDA) [12], may cause symptoms associated with false menopause and dormant fertility after long-term intervention [13]. Therefore, novel effective alternatives with fewer side effects must be developed.
Traditional Chinese medicine (TCM), a group of complementary and alternative medicine remedies, has attracted increasing attention in the treatment of UFs [14]. A nationwide population-based study of 35786 newly diagnosed UF patients found that 87.1% of patients had visited TCM clinics and that 61.8% of patients used Chinese herbal remedies [15]. A systematic review revealed that TCM seems to contribute to a decrease in the development of fibroids [16] and a reduction in myoma-related surgeries [17,18]. XiaoLuoWan (XLW), which includes Radix Scrophulariae (XuanShen), Concha Ostreae (ShengMuLi), and Bulbus Fritillariae ferganensis (ZheBeiMu), was first recorded in Medical Enlightenment from Heart during the Qing dynasty and is one of the classical and standard TCM formulas for relieving leiomyoma in TCM [19].
Radix Scrophulariae (XuanShen) is derived from the roots of Scrophularia ningpoensis Hemsl and demonstrates excellent pharmacological activities, such as regulation of immune response and uric acid metabolism as well as protection of heart, liver and neuron functions [20], potentially by affecting mitogen-activated protein kinase (MAPK) [21] and NF-κB pathways [22]. Bulbus Fritillariae ferganensis (ZheBeiMu) is a well-known herb that can eliminate phlegm, detoxify carbuncles, and relieve swelling. Modern pharmacological research has found that verticine and peiminine derived from ZheBeiMu can reverse the multidrug resistance of tumor cells, induce cell cycle arrest and apoptosis in malignant cells [23], and regulate inflammatory cytokine the secretion in macrophages via the MAPK pathway [24]. Concha Ostreae (ShengMuLi), the crushed shell of Ostrea gigas Thunberg, Ostrea talienwhanensis Crosse, or Ostrea rivularis Gould, could resolve hard lumps, soothe the nerves, and reinforce body fluids according to TCM terminology, and this multimineral and multivitamin marine TCM was found to exert anti-tumor effects, including anti-UF effects via specific medication rules [25].
XLW is now frequently used and has satisfactory therapeutic effects in patients suffering from UFs. Our unpublished study showed that XLW treatment could effectively relieve the symptoms of menorrhagia and pelvic pain caused by UF and produced less obvious side effects after short-term application for 3 months in UF patients. Moreover, a drug serum of XLW may suppress cell proliferation and promote cell apoptosis in cultured human uterine leiomyoma cells in vitro according to our unpublished experiment. However, the underlying mechanisms remain unclear. Thus, the pharmacological mechanisms of XLW must be precisely characterized before it can be widely administered to UF patients.
Network pharmacology has recently been developed as a novel strategy to elucidate complex pharmacological problems in new drug discovery [26] because it can provide a holistic view of the mechanisms induced by multiple ingredients [27]. Most TCM prescriptions have numerous ingredients and multiple targets, and the active compounds and potential pathways of some of the TCM remedies that have roles in gynecological diseases have been demonstrated by network pharmacology, including Guizhi Fuling Wan on UFs [28], Erxian Decoction on polycystic ovary syndrome [29], and Cangfu Daotan Decoction for the treatment of polycystic ovary syndrome [30], thus highlighting the pharmacological potential of TCM for women. Here, we used the network pharmacology approach to predict the active ingredients and potential targets of XLW for application to UFs and obtain insights into the mechanisms of XLW in treating UFs.
Materials and methods
Database and platform
We identified the chemical ingredients in XLW, gene targets of active ingredients in XLW, and known therapeutic targets of UFs by using online databases and platforms as follows (Table 1).
Table 1. Databases and platforms used for the network pharmacology analysis.
| Database | Full name of database | Sources |
|---|---|---|
| TCMSP | TCM systems pharmacology database and analysis platform | https://tcmspw.com/tcmsp.php |
| BATMAN | Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine | http://bionet.ncpsb.org/batman-tcm |
| ChemSpider | ChemSpider database | http://www.chemspider.com |
| UnitProt | UnitProt knowledgebase | https://www.uniprot.org/uniprot |
| DrugBank | DrugBank database | https://www.drugbank.ca |
| DigSee | Disease Gene Search Engine with Evidence Sentences | http://gcancer.org/digsee |
| STRING | STRING: functional protein association networks | https://string-db.org/ |
| Cytoscape | Cytoscape (Version 3.7.1) | https://www.cytoscape.org |
| Bioinformatics | Bioinformatics database | http://www.bioinformatics.com.cn |
| R software | R Project for Statistical Computing (Version 3.6.3) | https://www.r-project.org |
Chemical ingredients of each material in XLW
The chemical ingredients in XLW were screened via the TCM systems pharmacology database and analysis platform (TCMSP), Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM) platform and ChemSpider database and updated on 1 February 2020. The TCMSP database is designed for all 499 Chinese herbs registered in the Chinese pharmacopeia, and each herb contains 12 pharmacokinetic characteristics. This database captures the relationships between herbs, ingredients, diseases, and targets based on identified drug–target networks and drug–disease networks [31]. The BATMAN-TCM platform mainly contributes to understanding the therapeutic mechanisms of TCM by providing valuable clues about gene targets and performing functional analyses of TCM ingredients [32]. The ChemSpider database contains over 26 million entries from hundreds of data sources, and it is valuable in the identification of compounds in natural product samples [33].
Pharmacokinetic features of ingredients in XLW
The pharmacokinetic properties of active ingredients in XLW were demonstrated in the TCMSP and filtered on the basis of oral bioavailability (OB), Caco-2 permeability (Caco-2), and drug-likeness (DL), which are the three most key indicators of pharmacology. Specifically, the ingredients with OB ≥ 30%, Caco-2 ≥ 0.4, and DL ≥ 0.18 were chosen as candidate ingredients in XLW for further analysis. Concha Ostreae (ShengMuLi) is not a herbal medicine that can be found in TCMSP; therefore, its pharmacokinetic information was obtained from the BATMAN-TCM database, with a score cutoff ≥ 20 and an adjusted P-value <0.05. The candidate ingredients in XLW were further identified in the ChemSpider database for detailed pharmaceutical information.
Target exploration of ingredients in XLW
The TCMSP database and BATMAN-TCM platform were used for target exploration of active chemical ingredients in XLW. Components that met the pharmacokinetic criteria above were analyzed online to predict their potential targets, and only predicted targets that had interactions with active ingredients in XLW were selected. The protein names of these selected targets were converted into official gene names via UniProtKB, a popular protein sequence database that consists of information curated by biologists, provides crosslinks to approximately 100 external associated databases, and demonstrates comprehensive protein annotations [34]. Here, we searched UniProtKB with the organism limited to Homo sapiens (humans).
Known therapeutic targets in treating UFs
The known therapeutic targets of UFs were acquired from the DigSee and DrugBank databases. The DigSee database was developed to describe genes that are involved in the development of biological events based on searches of MEDLINE abstracts for evidence-containing sentences [35]. The DrugBank database is one of the most widely used drug resources worldwide and contains detailed drug, drug–target, drug action, and drug interaction information for almost all the FDA-approved drugs [36,37]. As a protocol method, we searched the DigSee database with the keywords ‘uterine fibroids’ and ‘uterine leiomyoma’ to obtain reported gene targets of UFs. In addition, based on the ‘drug–target’ interactions in DrugBank, only drugs that were approved to treat UFs were selected, and their targets were considered therapeutic targets of UFs. After integrating the targets from both the DigSee and DrugBank databases, all the referred therapeutic targets for the treatment of UFs were reserved for further network analysis.
Protein–protein interaction
To clarify the connection of target proteins involved with both XLW and UFs, we intersected the potential targets of XLW and UF-related therapeutic targets, and the overlapping targets were considered XLW-treated targets of UFs. A protein–protein interaction (PPI) network analysis of these overlapping targets was performed using the STRING database, a functional protein association networks platform [38]. The organism type was selected as Homo sapiens (humans), the minimum required interaction score was set with a medium confidence = 0.4, and the default setting was retained for other parameters. The ‘string_interactions.tsv’ file was downloaded for network visualization and the ‘enrichment.Component.tsv’, ‘enrichment.Function.tsv,’ ‘enrichment.Process.tsv,’ and ‘enrichment.KEGG.tsv’ files were exported as the results of Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses.
Network visualization and enrichment analysis
Network visualization was performed using Cytoscape software (version 3.7.1) [39], and the interactions of potential XLW targets and therapeutic targets of UFs were constructed using the links between them. Data from the STRING platform were imported into Cytoscape to visualize the PPI network. In the network, nodes represent the target proteins and edges represent the interaction between proteins. There were four important properties in the Cytoscape network that were quantified to screen the putative targets for topological importance: ‘degree,’ ‘betweenness,’ ‘closeness,’ and ‘coreness.’ GO enrichment and KEGG pathway analyses are important methods used to annotate the functional characteristics of target genes. We performed the enrichment analysis in the STRING platform and visualized the results by R software (version 3.6.3) [40,41].
Results
Active ingredients in XLW
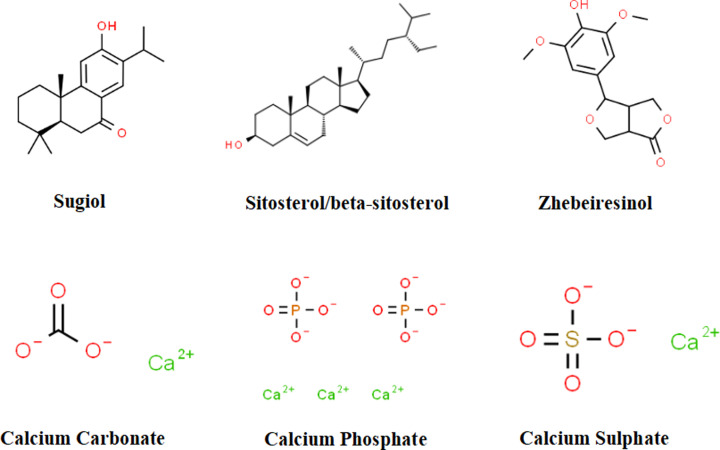
A total of 74 chemical ingredients in XLW were retrieved from the TCMSP and BATMAN-TCM databases: 47 in Radix Scrophulariae (XuanShen), 17 in Bulbus Fritillariae ferganensis (ZheBeiMu), and 10 in Concha Ostreae (ShengMuLi). After eliminating the repeated and invalid ingredients with unqualified pharmacokinetic conditions, 9 of these chemicals were identified as candidate components (Table 2): 3 in Radix Scrophulariae (XuanShen), 2 in Bulbus Fritillariae ferganensis (ZheBeiMu), and 5 in Concha Ostreae (ShengMuLi). The chemical information for the identified ingredients was recorded from the ChemSpider database (Table 2 and Figure 1).
Table 2. Chemical information for the active ingredients in XLW.
| TCM materials | Active ingredients | Chemical information | |||
|---|---|---|---|---|---|
| Latin names | Chinese names | ID | Mass (Da) | Formula | |
| Radix Scrophulariae | XuanShen | Sugiol | 84979 | 300.435 | C20H28O2 |
| β-Sitosterol | 192962 | 414.386 | C29H50O | ||
| Sitosterol | 192962 | 414.386 | C29H50O | ||
| Bulbus Fritillariae Ferganensis | ZheBeiMu | β-Sitosterol | 192962 | 414.386 | C29H50O |
| Zhebeiresinol | 4474746 | 280.273 | C14H16O6 | ||
| Concha Ostreae | |||||
| ShengMuLi | Aluminum | 4514248 | 26.982 | Al | |
| Calcium carbonate | 9708 | 100.087 | CCaO3 | ||
| Calcium phosphate | 22864 | 310.177 | Ca3O8P2 | ||
| Calcium sulphate | 22905 | 136.141 | CaO4S | ||
| Silicon | 4574465 | 28.086 | Si | ||
Figure 1. Chemical structural formulas of active ingredients in XLW.
Aluminum and silicon are not shown in this figure because they are chemical elements.
Target exploration of active ingredients in XLW
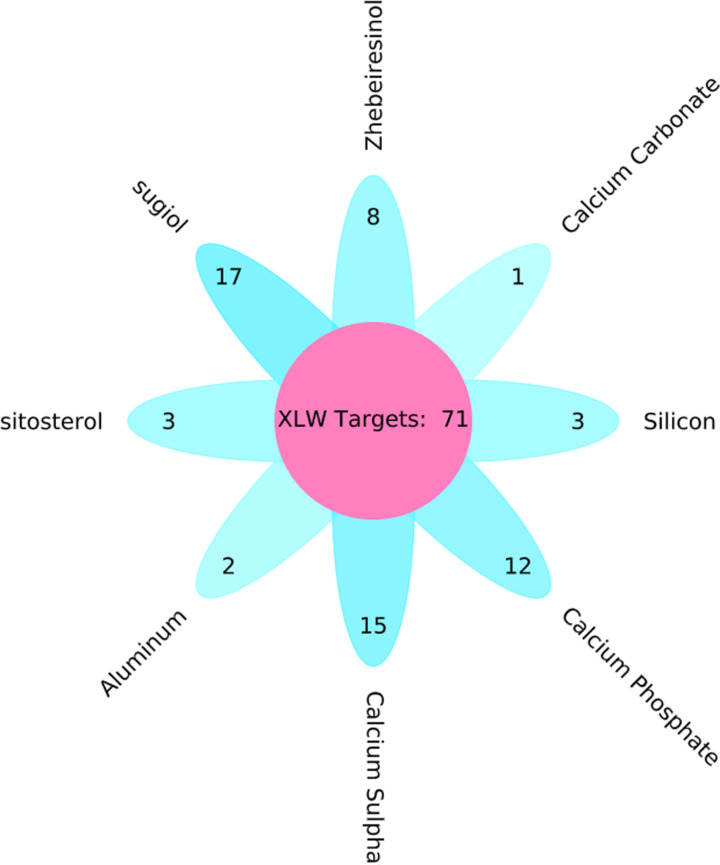
A total of 131 potential targets from the active ingredients in XLW were retrieved from the TCMSP and BATMAN-TCM databases: 58 targets of Radix Scrophulariae (XuanShen), 46 targets of Bulbus Fritillariae ferganensis (ZheBeiMu), and 27 targets of Concha Ostreae (ShengMuLi). After elimination of overlapping targets, 71 targets were obtained (Figure 2) and the protein and gene names were identified in UniProtKB, in which the organism was limited to H. sapiens (humans). Detailed information on the active ingredients and putative targets of XLW is provided in Supplementary Tables S1 and S2.
Figure 2. Number of potential targets for each identified active ingredient in XLW shown by a flower plot.
The petals show the number of target genes found in each identified ingredients of XLW, and the center shows the total gene number of XLW. By the way, sitosterol and β-sitosterol have the same gene targets.
UF-related target network
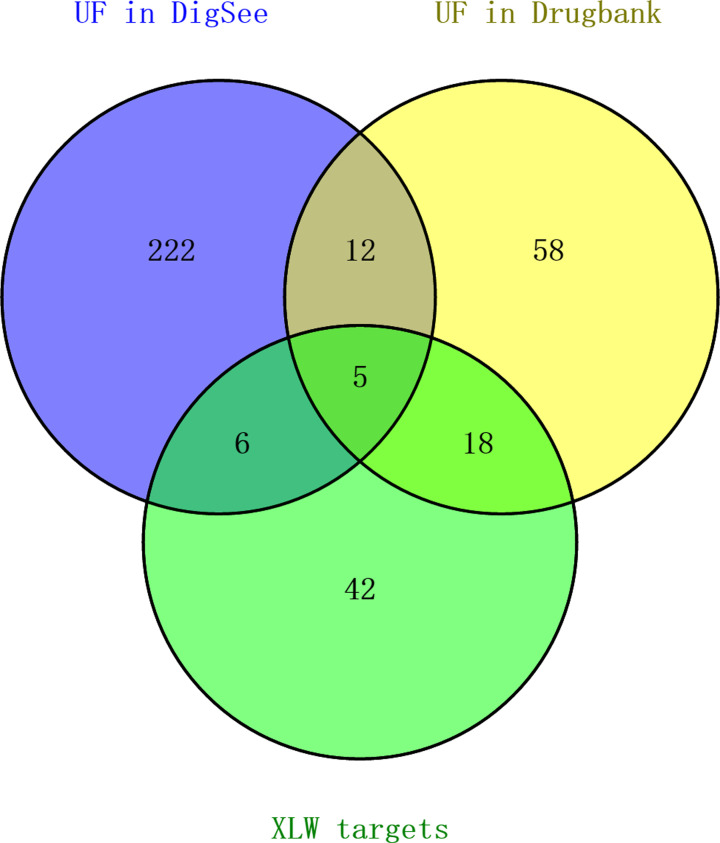
The DigSee and DrugBank databases were searched using the keywords ‘uterine fibroids’ and ‘uterine leiomyoma’ as the keywords to explore the target genes of UFs. In the DigSee database, 245 known therapeutic genes were finally included after eliminating the duplicates. In the DrugBank database, 69 approved drugs with 146 targets were included and 93 targets were chosen for treating UFs after eliminating duplicates. Finally, after integrating the two databases, 321 known therapeutic targets for UFs were used for further data analysis. Detailed information on all the identified known therapeutic targets of UFs is provided in Supplementary Table S3. After comparing the 321 UF-associated targets with the 71 XLW-related targets, 29 common targets (approximately 9.03% of disease targets) were considered effective targets for XLW intervention in UFs (Figure 3).
Figure 3. Number of XLW-targeted UF genes shown by a Venn diagram.
The blue and yellow circles represent UF targets found in the DigSee database and DrugBank database, respectively. The green circle represents the identified XLW targets.
PPI network analysis
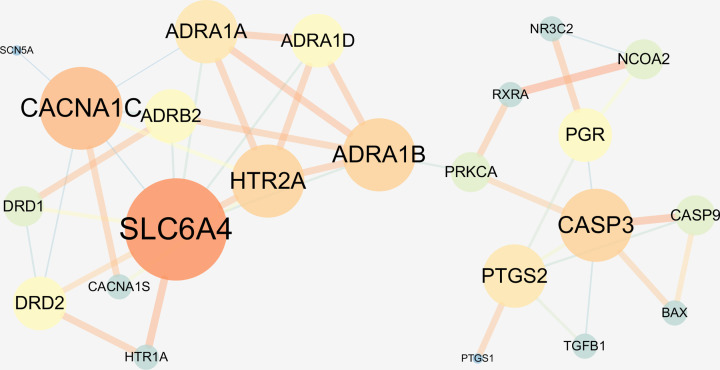
The 29 putative XLW-treated UF targets were imported into the STRING database to obtain the interaction analysis of these proteins and then imported into Cytoscape (version 3.7.2) to perform and visualize the PPI network (Figure 4). There were 23 hub nodes and 86 connected edges, the average node degree was 3.74, the average shortest path length was 3.13, and the average clustering coefficient was 0.53. The degrees of freedom increase from yellow to blue, larger nodes suggest a higher degree, and thicker edges suggest stronger interactions. Detailed information on the topological features of these hubs is provided in Supplementary Table S4.
Figure 4. PPI network of targets for XLW in treating UFs.
The freedom increases from yellow to blue, larger nodes suggest a higher degree, and thicker edges suggest stronger interactions.
Network construction of XLW action in treating UFs
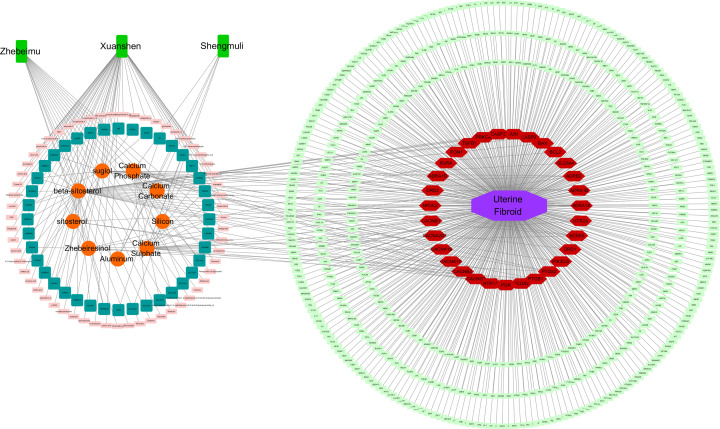
Finally, Cytoscape (version 3.7.1) software was used to combine the XLW–UF target network and the PPI network and visualize the ‘XLW–ingredient–target–UF’ interaction network (Figure 5). The results showed that the 3 TCM materials in XLW with 9 active ingredients had 29 putative targets in treating UFs, with 23 targets presenting interactions with other targets, including SLC6A4, CACNA1C, CASP3, ADRA1B, HTR2A, ADRA1A, PTGS2, ADRA1D, DRD2, PGR, ADRB2, NCOA2, CASP9, DRD1, PRKCA, RXRA, HTR1A, NR3C2, CACNA1S, BAX, TGFB1, PTGS1, and SCN5A.
Figure 5. Network connection of ‘XLW–ingredients–targets–UFs’ in terms of the therapeutic mechanisms involving XLW treatment of UFs.
The grass-green rectangle, orange ellipse, pink rectangle, and dark-green rectangle nodes represent TCM materials in XLW, active ingredients in XLW, inactive ingredients in XLW, and XLW-related targets, respectively (left). The purple octagon, light green rectangle, and red hexagon nodes represent disease, UF-related targets, and the common targets of XLW–UFs, respectively (right).
GO enrichment and KEGG pathway analyses
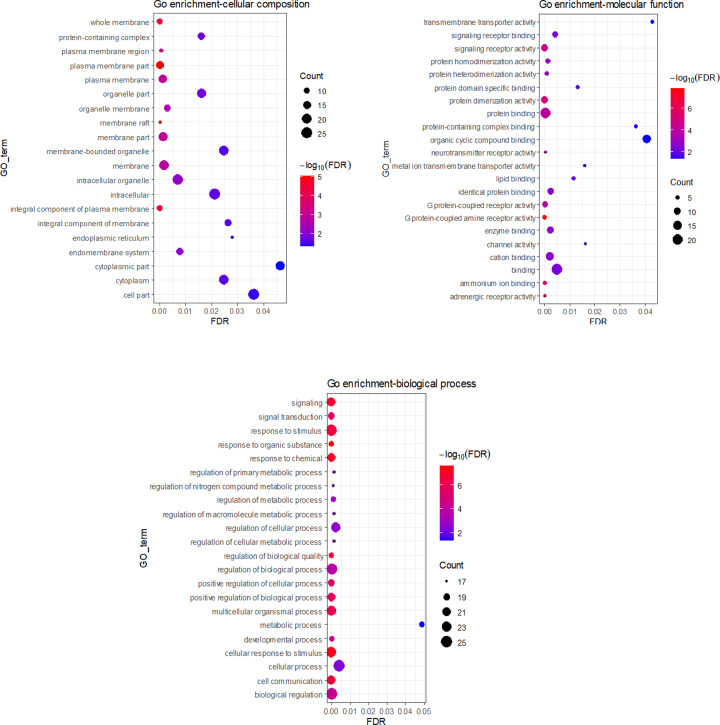
To further elucidate the biological effects of the UF treatment with XLW, we performed GO enrichment and KEGG pathway analyses of the 29 UF-related potential therapeutic target genes in the STRING database. Detailed information on the results of the enrichment analysis is provided in Supplementary Table S5. The GO annotation and enrichment were conducted from three aspects: cellular composition (C), molecular function (F), and biological process (P). The most enriched terms were compiled as bubble diagrams by R software (Figure 6).
Figure 6. GO enrichment analysis of therapeutic targets of XLW in treating UFs.
The negative FDR value of the enrichment analysis increases from blue to red, the gene count enriched in GO term increases from small to large, that is larger nodes suggest more enriched genes. Abbreviation: FDR, false discovery rate.
Specifically, the top 10 enriched terms in GO-cellular composition were cell parts (Count = 25, false discovery rate (FDR) = 0.0363), intracellular parts (Count = 24, FDR = 0.0212), intracellular organelle (Count = 23, FDR = 0.0069), cytoplasm (Count = 21, FDR = 0.0247), membrane-bounded organelle (Count = 21, FDR = 0.0247), membrane (Count = 20, FDR = 0.0017), organelle parts (Count = 19, FDR = 0.0159), membrane parts (Count = 18, FDR = 0.0011), cytoplasmic parts (Count = 18, FDR = 0.0464), and plasma membrane (Count = 16, FDR = 0.0011).
The top 10 enriched terms in GO-molecular function were binding (Count = 23, FDR = 0.0048), protein binding (Count = 20, FDR < 0.0001), cation binding (Count = 14, FDR = 0.0020), organic cyclic compound binding (Count = 13, FDR = 0.0407), protein dimerization activity (Count = 11, FDR < 0.0001), signaling receptor activity (Count = 11, FDR < 0.0001), enzyme binding (Count = 10, FDR = 0.0022), identical protein binding (Count = 9, FDR = 0.0022), G protein-coupled receptor activity (Count = 8, FDR = 0.0002), and signaling receptor binding (Count = 8, FDR = 0.0041).
The top 10 enriched terms in GO-biological process were biological regulation (Count = 25, FDR < 0.0001), cellular process (Count = 25, FDR = 0.0042), response to stimulus (Count = 24, FDR < 0.0001), regulation of biological process (Count = 24, FDR = 0.0002), cellular response to stimulus (Count = 23, FDR < 0.0001), multicellular organismal process (Count = 22, FDR < 0.0001), regulation of cellular process (Count = 22, FDR = 0.0021), signaling (Count = 21, FDR < 0.0001), cell communication (Count = 21, FDR < 0.0001), response to chemical (Count = 20, FDR < 0.0001), and positive regulation of biological process (Count = 20, FDR < 0.0001).
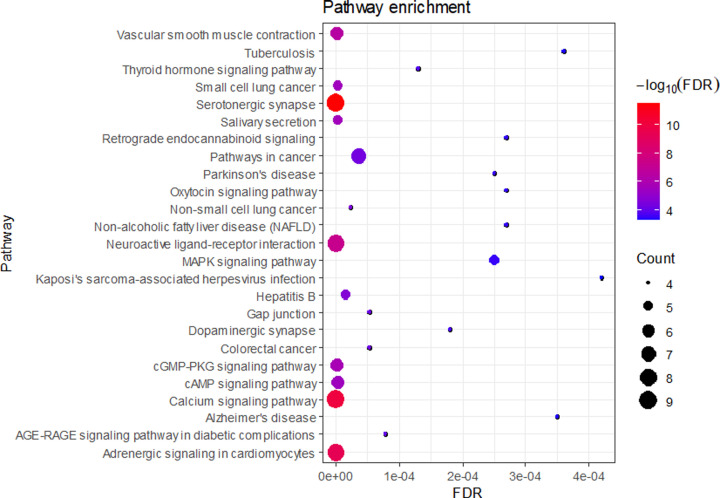
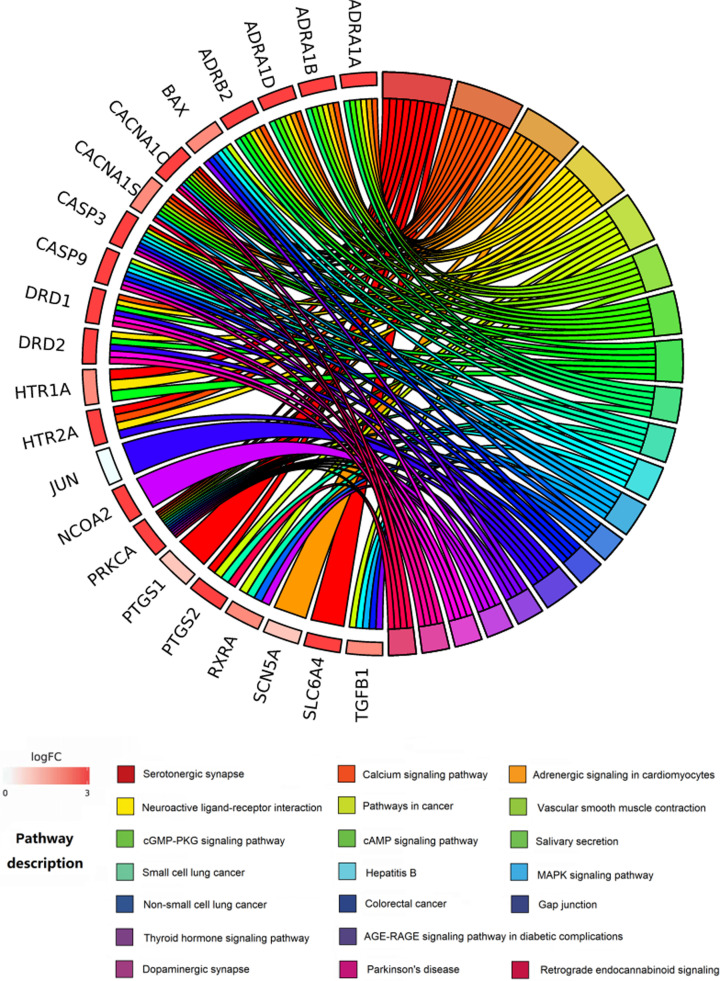
In total, 85 signaling pathways were significantly enriched through the KEGG pathway enrichment analysis. In the bubble diagram of the top 25 pathways (Figure 7), the size and color of the nodes were determined based on the counts and FDRs of the related pathways. The chordal graph was constructed according to the connection between target genes and enriched pathways (Figure 8). The top 15 terms in the KEGG pathway were enriched in serotonergic synapse (Count = 9, FDR < 0.0001), calcium signaling pathway (Count = 9, FDR < 0.0001), adrenergic signaling in cardiomyocytes (Count = 8, FDR < 0.0001), neuroactive ligand–receptor interaction (Count = 8, FDR < 0.0001), pathways in cancer (Count = 7, FDR < 0.0001), vascular smooth muscle contraction (Count = 6, FDR < 0.0001), cGMP-PKG signaling pathway (Count = 6, FDR < 0.0001), cAMP signaling pathway (Count = 6, FDR < 0.0001), salivary secretion (Count = 5, FDR < 0.0001), small cell lung cancer (Count = 5, FDR < 0.0001), hepatitis B (Count = 5, FDR < 0.0001), MAPK signaling pathway (Count = 5, FDR < 0.0001), non-small cell lung cancer (Count = 4, FDR < 0.0001), colorectal cancer (Count = 4, FDR < 0.0001), and gap junction (Count = 4, FDR < 0.0001).
Figure 7. KEGG pathway analysis of the therapeutic targets of XLW in treating UFs.
The negative FDR value of the enrichment analyse increases from blue to red, the gene count enriched in KEGG pathway increases from small to large, that is larger nodes suggest more enriched genes.
Figure 8. Chordal graph of the KEGG pathway analysis of the therapeutic targets of XLW in treating UFs.
The different shades of red rectangle nodes around the chordal graph represent the therapeutic targets, the varicolored square nodes around the chordal graph represent the KEGG pathways, and the chordal lines represent the ownership of targets and pathways.
Discussion
TCM products have been widely used for UFs in China for thousands of years. Moreover, the pharmacological actions of many TCM herbs or pairs in myomagenesis have been confirmed recently. The Cinnamon Twig and Poria Pill (GuiZhi FuLing Wan) formula was the most frequently prescribed for UFs in TCM clinics, and it has beneficial effects on both fibroid characteristics and sex hormones in vivo [42] and can efficiently inhibit the survival of leiomyoma cells in vitro [43]. XLW, including Radix Scrophulariae (XuanShen), Concha Ostreae (ShengMuLi), and Bulbus Fritillariae ferganensis (ZheBeiMu), a classical and standard formula for relieving scrofula in TCM, is now frequently used in UFs and has satisfactory therapeutic effects in the clinic. However, the efficacy of XLW has not been confirmed in standard clinical trials and the underlying mechanisms are not entirely clear, thus leaving obstacles to its popularization and delaying benefits to more UF patients.
Recently, a network pharmacology approach has provided a new paradigm for driving TCM from experience-based medicine to evidence-based medicine [44], which could provide more convincing clues to TCM pharmacological research. This approach is effective for establishing the interaction network of chemical compounds, proteins or genes, and specific diseases [45]. Moreover, network pharmacology reveals the regulation of small molecules in a high-throughput manner and would thus be efficient for the analysis of multidrug combinations, especially for TCM preparations and worldwide ethnomedicine, whose therapeutic effects occur via targeting of multiple molecules in the human body [46]. In this study, we elucidated the potential mechanisms of XLW in treating UFs via network pharmacology strategies and found that the calcium signaling pathway is significantly enriched and the MAPK signaling pathway, cAMP signaling pathway, pathways in cancer and vascular smooth muscle contraction, cGMP-PKG signaling pathway, and AGE-RAGE signaling pathways are closely associated in XLW intervention for UFs.
The calcium signaling pathway has been highlighted in XLW-treated UFs, and it involves six calcium voltage-gated channel genes: CACNA1C, CACNA1S, CACNA2D1, CACNB1, CACNB2, and CACNG1. The relationship between calcium channels and UF pathogenesis has been reported in previous studies. An assessment of cell membrane calcium channel proteins in UF tissues showed that the expression patterns of the calcium channel proteins TRPC1 and TRPM7 in UF tissues were different from that in adjacent smooth muscle tissues and the in vitro modification of TRPC1 and TRPM7 expression significantly affected the proliferation of uterine leiomyoma cells [47]. Inhibition of store-operated Ca2+ channels was demonstrated to induce the second sustained [Ca2+]i and suppress cell proliferation in uterine leiomyoma cells [48]. Voltage-gated calcium channels are also involved in the proliferation of uterine leiomyoma cells via their participation in apoptotic calcium release [49]. XLW may affect the expression of calcium channel proteins, influence calcium homeostasis in uterine leiomyoma cells, and consequently regulate cell survival.
The MAPK pathways are well known for their important roles in myomagenesis, and they have targets in estrogen-dependent benign gynecological disorders, such as UFs [50]. The binding of estrogen to receptors initiates a cascade of molecular events, including MAPK activation, Ras/Raf activation, and MEK phosphorylation [51], which lead to the transcription of target genes associated with cell proliferation, survival, and apoptosis. Moreover, UFs are characterized by increased levels of extracellular matrix (ECM), including collagens, fibronectin, laminins and proteoglycans, in the interstitial uterine tissue [52]. Pathological factors can induce the activation of the MAPK-regulated integrin-Rho/ERK pathway [53], resulting in cellular responses and ECM deposition that are involved in altered bidirectional signaling between leiomyoma cells and the ECM. XLW may intervene with UFs via regulation of the MAPK cascade, leading to anti-fibroid effects involving cell proliferation, apoptosis, and ECM production.
cAMP is a vital second messenger that regulates various cellular functions, including inflammation, lipid metabolism, and cell differentiation, by affecting the expression and functions of important genes or proteins [54]. The cAMP pathway was proven to be related to psychological stress, which can activate the neuroendocrine system and induce the secretion of catecholamines (CAs) [55], which promote emotion-related illness by the adrenal receptor (AR)-regulated cAMP-PKA signaling pathway. In cultured uterine leiomyoma cells, AR agonists were demonstrated to increase the expression levels of estrogen receptor (ER), progesterone receptor (PR), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) via cAMP-dependent signaling pathways to influence uterine myomagenesis [56]. Therefore, XLW may relieve mental stress-induced UF development by regulating cAMP pathways.
As complex biological processes and complicated signaling are involved in UFs [57], therapeutic strategies that have effects on multiple targets and pathways could be regarded as alternative approaches to managing UFs, such as TCM prescriptions with combined materials. Although XLW only contains three TCM materials, it shown to have anti-fibroid effects through multiple targets and multiple pathways after the network pharmacologic analysis in this study. Further research is urgently needed to verify our hypothesis that the anti-fibroid effects of XLW were related to its regulation of the calcium signaling pathway, MAPK pathway and cAMP pathways. The strong and vigorous potential of XLW in treating UFs may promote pharmacological investigations of simple herbal formulas that are more efficient and have fewer side effects when used to treat intractable human diseases.
Supplementary Material
Abbreviations
- AR
adrenal receptor
- BATMAN-TCM
Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine
- CA
catecholamine
- DL
drug-likeness
- ECM
extracellular matrix
- ER
estrogen receptor
- FDA
Food and Drug Administration
- FDR
false discovery rate
- FGF
fibroblast growth factor
- GnRH
gonadotropin-releasing hormone
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MAPK
mitogen-activated protein kinase
- OB
oral bioavailability
- PPI
protein–protein interaction
- PR
progesterone receptor
- TCM
traditional Chinese medicine
- TCMSP
TCM systems pharmacology database and analysis platform
- UF
uterine fibroid
- VEGF
vascular endothelial growth factor
- XLW
XiaoLuoWan
Data Availability
The data used to support the findings of the present study are included within the Supplementary Materials.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Inheritance Studio of Traditional Chinese Medicine Master Prof. Xu Runsan, founded by the Beijing Administration of Traditional Chinese Medicine [grant number 1001018-1-02].
Author Contribution
The work presented here was carried out via collaboration among all authors. Hong Liu defined the theme and designed the study. Yonghui Yu and Fang Yang performed the analysis, interpreted the results, and drafted the manuscript. Hong Liu revised and finally confirmed the manuscript. All authors approved the final manuscript and are responsible for the accuracy and integrity of the work.
References
- 1.Drayer S.M. and Catherino W.H. (2015) Prevalence, morbidity, and current medical management of uterine fibroidss. Int. J. Gynaecol. Obstet. 131, 117–122 10.1016/j.ijgo.2015.04.051 [DOI] [PubMed] [Google Scholar]
- 2.Baird D.D., Dunson D.B., Hill M.C., Cousins D. and Schectman J.M. (2003) High cumulative incidence of uterine fibroids in black and white women: ultrasound evidence. Am. J. Obstet. Gynecol. 188, 100–107 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 3.Whynott R.M., Vaught K.C.C. and Segars J.H. (2017) The effect of uterine fibroids on infertility: a systematic review. Semin. Reprod. Med. 35, 523–532 [DOI] [PubMed] [Google Scholar]
- 4.Zhao R., Wang X., Zou L., Li G., Chen Y., Li C. et al. (2017) Adverse obstetric outcomes in pregnant women with uterine fibroids in China: a multicenter survey involving 112,403 deliveries. PLoS ONE 12, e0187821 10.1371/journal.pone.0187821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson A.L. and Ritchie J.J. (2015) Severe anemia from heavy menstrual bleeding requires heightened attention. Am. J. Obstet. Gynecol. 213, 97.e1–97.e6 10.1016/j.ajog.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 6.Bulun S.E. (2013) Uterine fibroids. N. Engl. J. Med. 369, 1344–1355 10.1056/NEJMra1209993 [DOI] [PubMed] [Google Scholar]
- 7.Metwally M., Raybould G., Cheong Y.C. and Horne A.W. (2020) Surgical treatment of fibroids for subfertility. Cochrane Database Syst. Rev. 1, CD003857, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady P.C., Stanic A.K. and Styer A.K. (2013) Uterine fibroids and subfertility: an update on the role of myomectomy. Curr. Opin. Obstet. Gynecol. 25, 255–259 10.1097/GCO.0b013e3283612188 [DOI] [PubMed] [Google Scholar]
- 9.Boyd L.R., Novetsky A.P. and Curtin J.P. (2010) Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet. Gynecol. 116, 909–915 10.1097/AOG.0b013e3181f395d9 [DOI] [PubMed] [Google Scholar]
- 10.Vitale S.G., Padula F. and Gulino F.A. (2015) Management of uterine fibroids in pregnancy: recent trends. Curr. Opin. Obstet. Gynecol. 27, 432–437 10.1097/GCO.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 11.Cope A.G., Young R.J. and Stewart E.A. (2020) Nonextirpative treatments for uterine fibroids: measuring success. J. Minim. Invasive Gynecol. S1553-4650(20)30396-4 10.1016/j.jmig.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 12.Lethaby A., Vollenhoven B. and Sowter M. (2001) Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst. Rev. 2, CD000547 10.1002/14651858.CD000547 [DOI] [PubMed] [Google Scholar]
- 13.Lewis T.D., Malik M., Britten J., San Pablo A.M. and Catherino W.H. (2018) A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res. Int. 2018, 2414609 10.1155/2018/2414609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J.P., Yang H., Xia Y. and Cardini F. (2013) Herbal preparations for uterine fibroids. Cochrane Database Syst. Rev. 4, CD005292 10.1002/14651858.CD005292.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen H.R., Chen Y.Y., Huang T.P., Chang T.T., Tsao J.Y., Chen B.C. et al. (2015) Prescription patterns of Chinese herbal products for patients with uterine fibroid in Taiwan: a nationwide population-based study. J. Ethnopharmacol. 171, 223–230 10.1016/j.jep.2015.05.038 [DOI] [PubMed] [Google Scholar]
- 16.Chen N.N., Han M., Yang H., Yang G.Y., Wang Y.Y., Wu X.K. et al. (2014) Chinese herbal medicine Guizhi Fuling Formula for treatment of uterine fibroids: a systematic review of randomized clinical trials. BMC Complement. Altern. Med. 98, e18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su S.Y., Muo C.H. and Morisky D.E. (2012) Use of chinese medicine and subsequent surgery in women with uterine fibroid: a retrospective cohort study. Evid. Based Complement. Alternat. Med. 2012, 617918 10.1155/2012/617918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu W.C., Tsai Y.T., Hou Y.C. and Lai J.N. (2019) Prescription of Chinese herbal products is associated with a decreased risk of uterine fibroids: a population-based cohort study. Medicine (Baltimore) 98, e18195 10.1097/MD.0000000000018195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang J.H., Bai W.P., Chen C.L. et al. (2017) Chinese expert consensus expert group on the diagnosis and treatment of uterine fibroids. Chin. J. Obstet. Gynecol. 52, 793–800 [Google Scholar]
- 20.You-Hua C., Jin Q., Jing H. and Bo-Yang Y. (2014) Structural characterization and identification of major constituents in radix scrophulariae by HPLC coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 12, 47–54 [DOI] [PubMed] [Google Scholar]
- 21.Meng X., Xie W., Xu Q. et al. (2018) Neuroprotective effects of Radix Scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules 23, 2401 10.3390/molecules23092401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X., Eichhorn T., Greten H.J. and Efferth T. (2012) Effects of Scrophularia ningpoensis Hemsl. on inhibition of proliferation, apoptosis induction and NF-κB signaling of immortalized and ancer cell lines. Pharmaceuticals (Basel) 5, 189–208 10.3390/ph5020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun Y.G., Jeon B.H., Lee J.H. et al. (2008) Verticinone induces cell cycle arrest and apoptosis in immortalized and malignant human oral keratinocytes. Phytother. Res. 22, 416–423 10.1002/ptr.2345 [DOI] [PubMed] [Google Scholar]
- 24.Yi P.F., Wu Y.C., Dong H.B. et al. (2013) Peimine impairs pro-inflammatory cytokine secretion through the inhibition of the activation of NF-κB and MAPK in LPS-induced RAW264.7 macrophages. Immunopharmacol. Immunotoxicol. 35, 567–572 [DOI] [PubMed] [Google Scholar]
- 25.Wei M.C., Lin J., Yi J.C. et al. (2018) Medication rules of anti-tumor formulas containing marine Chinese medicinals. J. Beijing Univ. TCM 41, 253–258 10.3109/08923973.2013.822508 [DOI] [PubMed] [Google Scholar]
- 26.Zhang R., Zhu X., Bai H. and Ning K. (2019) Network pharmacology databases for traditional Chinese medicine: review and assessment. Front. Pharmacol. 10, 123 10.3389/fphar.2019.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kibble M., Saarinen N., Tang J., Wennerberg K., Mäkelä S. and Aittokallio T. (2015) Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 32, 1249–1266 10.1039/C5NP00005J [DOI] [PubMed] [Google Scholar]
- 28.Zeng L., Yang K., Liu H. and Zhang G. (2017) A network pharmacology approach to investigate the pharmacological effects of Guizhi Fuling Wan on uterine fibroids. Exp. Ther. Med. 14, 4697–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Du B., Zhang H., Guo X., Zhou Z., Xiu A. et al. (2018) A network pharmacology approach to explore the mechanisms of Erxian decoction in polycystic ovary syndrome. Chin. Med. 13, 46 10.1186/s13020-018-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W.T., Tang M.Y., Wang J.H. and Wang L.H. (2020) Identification of the active constituents and significant pathways of Cangfu Daotan Decoction for the treatment of PCOS based on network pharmacology. Evid. Based Complement. Alternat. Med. 2020, 4086864 10.1155/2020/4086864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ru J., Li P., Wang J., Zhou W., Li B., Huang C. et al. (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H. et al. (2016) BATMAN-TCM: a Bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine. Sci. Rep. 6, 21146 10.1038/srep21146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little J.L., Williams A.J., Pshenichnov A. and Tkachenko V. (2012) Identification of “known unknowns” utilizing accurate mass data and ChemSpider. J. Am. Soc. Mass Spectrom. 23, 179–185 10.1007/s13361-011-0265-y [DOI] [PubMed] [Google Scholar]
- 34.Breuza L., Poux S., Estreicher A., Famiglietti M.L., Magrane M., Tognolli M. et al. (2016) The UniProtKB guide to the human proteome. Database (Oxford) 2016, pii: bav120 10.1093/database/bav120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J., So S., Lee H.J., Park J.C., Kim J.J. and Lee H. (2013) DigSee: Disease gene search engine with evidence sentences (version cancer). Nucleic Acids Res. 41, W510–W517 10.1093/nar/gkt531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wishart D.S., Knox C., Guo A.C., Cheng D., Shrivastava S., Tzur D. et al. (2008) DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 36, D901–D906 10.1093/nar/gkm958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R. et al. (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J. et al. (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu G.C., Wang L.G., YanY Han and He Q.Y. (2012) ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter W., Sánchez-Cabo F. and Ricote M. (2015) GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics 31, 2912–2914 10.1093/bioinformatics/btv300 [DOI] [PubMed] [Google Scholar]
- 42.Li M., Hung A. and Yang A.W.H. (2019) Guizhi Fuling Wan for uterine fibroids: a systematic review of in vivo studies. J. Ethnopharmacol. 245, 112177 10.1016/j.jep.2019.112177 [DOI] [PubMed] [Google Scholar]
- 43.Shen Q., Ye W., Hu X., Zhao C., Zhou L. and Zhu X. (2016) The effects of Guizhi Fuling Capsule drug serum on uterine leiomyoma cells and its mechanism. Evid. Based Complement. Alternat. Med 2016, 2393640 10.1155/2016/2393640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S. and Zhang B. (2013) Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin. J. Nat. Med. 11, 110–120 10.3724/SP.J.1009.2013.00110 [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z., Chen B., Chen S., Lin M., Chen Y., Jin S. et al. (2020) Applications of network pharmacology in traditional Chinese medicine research. Evid. Based Complement Alternat. Med. 2020, 1646905 10.1155/2020/1646905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao D.C. and Xiao P.G. (2014) Network pharmacology: a rosetta stone for traditional Chinese medicine. Drug Dev. Res. 75, 299–312 10.1002/ddr.21214 [DOI] [PubMed] [Google Scholar]
- 47.Ke X., Cheng Z., Qu X., Dai H., Zhang W. and Chen Z.J. (2014) High expression of calcium channel subtypes in uterine fibroid of patients. Int. J. Clin. Exp. Med. 7, 1324–1330 [PMC free article] [PubMed] [Google Scholar]
- 478.Kim B.Y., Cho C.H., Song D.K., Mun K.C., Suh S.I., Kim S.P. et al. (2005) Ciglitizone inhibits cell proliferation in human uterine leiomyoma via activation of store-operated Ca2+ channels. Am. J. Physiol. Cell Physiol. 288, C389–C395 10.1152/ajpcell.00154.2004 [DOI] [PubMed] [Google Scholar]
- 49.Borahay M.A., Kilic G.S., Yallampalli C., Snyder R.R., Hankins G.D., Al-Hendy A. et al. (2014) Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J. Biol. Chem. 289, 35075–35086 10.1074/jbc.M114.583575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makker A., Goel M.M., Das V. and Agarwal A. (2012) PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol. Endocrinol. 28, 175–181 10.3109/09513590.2011.583955 [DOI] [PubMed] [Google Scholar]
- 51.Borahay M.A., Asoglu M.R., Mas A., Adam S., Kilic G.S. and Al-Hendy A. (2017) Estrogen receptors and signaling in fibroids: role in pathobiology and therapeutic implications. Reprod. Sci. 24, 1235–1244 10.1177/1933719116678686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islam M.S., Ciavattini A., Petraglia F., Castellucci M. and Ciarmela P. (2018) Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum. Reprod. Update 24, 59–85 10.1093/humupd/dmx032 [DOI] [PubMed] [Google Scholar]
- 52.Chen H.M., Lin Y.H., Cheng Y.M., Wing L.Y.C. and Tsai S.J. (2013) Overexpression of integrin-β1 in leiomyoma promotes cell spreading and proliferation. J. Clin. Endocrinol. Metab. 5, E837–E846 10.1210/jc.2012-3647 [DOI] [PubMed] [Google Scholar]
- 54.Wahlang B., McClain C., Barve S. and Gobejishvili L. (2018) Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 49, 105–115 10.1016/j.cellsig.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin K.J., Lee Y.J., Yang Y.R., Park S., Suh P.G., Follo M.Y. et al. (2016) Molecular mechanisms underlying psychological stress and cancer. Curr. Pharm. Des. 22, 2389–2402 10.2174/1381612822666160226144025 [DOI] [PubMed] [Google Scholar]
- 56.Xia T., Li S., Ma R., Guan S., Li J., Li H. et al. (2017) Effects of liver depression and psychological stress on human uterine leiomyoma cells by an AR-cAMP- PKA signal transduction pathway. Taiwan J. Obstet. Gynecol. 56, 291–301 10.1016/j.tjog.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 57.Donnez J. and Dolmans M.M. (2016) Uterine fibroid management: from the present to the future. Hum. Reprod. Update 22, 665–686 10.1093/humupd/dmw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of the present study are included within the Supplementary Materials.