Abstract
Purpose of review
The medical management of Inflammatory Bowel Disease (IBD) remains problematic with a pressing need for innovation in drug development as well as delivery of personalized therapies. Both the disease’s inherent pathophysiologic complexity and its etiological heterogeneity conspire in making it difficult to accurately model for either the purposes of basic research or drug development. Multiple attempts at creating meaningful experimental models have fallen short of adequately recapitulating the disease and most do not capture any aspect of the cause or the effects of patient heterogeneity that underlays most of the difficulties facing physicians and their patients. In vivo animal models, tissue culture systems, and more recent synthetic biology approaches are all too simplistically reductionist for the task. However, ex vivo culture platforms utilizing patient biopsies offer a system that more closely mimics end stage disease processes that can be studied in detail and subjected to experimental manipulations.
Recent findings
Recent studies describe further optimization of mucosal explant cultures in order to increase tissue viability and maintain a polarized epithelial layer. Current applications of the platform include studies of the interplay between the epithelial, immune and stromal compartment of the intestinal tissue, investigation of host-microbial interactions, pre-clinical evaluation of candidate drugs and uncovering mechanisms of action of established or emerging treatments for IBD.
Summary
Patient explant-based assays offer an advanced biological system in IBD that recapitulates disease complexity and reflects the heterogeneity of the patient population. In its current stage of development, the system can be can be utilized for drug testing prior to the costlier and time consuming evaluation by clinical trials. Further refinement of the technology and establishment of assay readouts that correlate with therapeutic outcomes will yield a powerful tool for personalized medicine approaches in which individual patient responses to available treatments are assessed a priori, thus reducing the need for trial and error within the clinical setting.
Keywords: Organ culture, tissue explants, ex-vivo culture, colonic explant, ex-vivo drug testing, mucosal biopsies, inflammatory bowel disease, therapeutic predictors
Introduction
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD) which differ from each other in anatomical distribution, disease drivers and susceptibility factors [1, 2]. There have been many attempts to develop disease models (animal, ex-vivo, in-vitro) to understand disease mechanisms of pathophysiology and test potential therapies. However, because of the underlying complexity, patient responses to treatment vary and historically popular experimental systems fall short of being able to predict efficacy of nascent therapies. Most commonly used animal models of experimental colitis utilize dextran sodium sulfate, TNBS and CD45RB+ T cell transfer or genetic susceptibility (IL10 deficiency) to drive different types of intestinal inflammatory processes that likely only mimic aspects of IBD pathobiology [3, 4]. Furthermore, these models vary significantly from each other in biological responses depending on genetic background and housing environment that affects their gut microbiome [5]. Most importantly, there are notable differences between murine and human immune systems [6–8]. One other obvious issue is that and inbreed laboratory mice fail to capture the genetic heterogeneity of the disease state existing in diverse patient populations [8–11].
According to a report from the Centre for Medicines Research, the phase II success rate for new molecular entities is less than 20%. Part of the problem, and specifically for diseases like IBD, obesity and cancer, is that efficacy data gathered from testing in animal models do not necessarily translate to new therapies [3, 12]. On the other hand, cases have been described in which drugs had shown little or no efficacy in animal models of IBD, but turned out to be successful for the treatment of human disease [12]. Thus testing patient-derived material in predictive assays might prove pivotal in minimizing the risk of drug failure [11, 13]. To attempt to address these concerns and the need for producing systems that are tractable and allow for sufficient experimental control, assays using human material, often patient-derived, have been developed. For instance, VanDussen et al. have isolated a large panel of human gastrointestinal epithelial cell lines from patient biopsies (N=65) to study host-microbial interactions and the ultimate goal to develop patient-based assays [14]; Pedersen developed an intestinal epithelium model for metabolic and immunological functional analyses [15]; West et al. discovered high level Oncostatin M expression by non-hematopoietic mucosal stromal cells in IBD that correlated both with severity of disease and resistance to anti-TNFα therapy [16]; and Dahlen et al used T-cells derived from UC patients to interrogate underlying mechanisms of anti-TNF effects [17].
In more recent years, such studies are supported by an explosion of synthetic biology and bioengineering methodologies including organoids derived from stem cells residing in the intestinal crypts or inducd from pluripotent stem cells, and variations of a “gut on a chip” platforms [18–20]. Each one of these approaches has advantages and disadvantages compared to the others and represent an excellent test bed for exploring basic biology and intestinal (patho)-physiology pertinent to constituent cells primarily in the areas of gut development, epithelial barrier functions and epithelial-microbial interactions [21]. From a drug development perspective, they can be used to study absorption of various drug formulations and to a lesser extent drug toxicity. One major advantage is that they offer a suitable platform for high throughput testing that can facilitate the drug discovery process. Current efforts focus on the introduction of more than one cell type in the above platforms along with components of the microbiome in an attempt to mimic the complexity of the system in-vivo [21]. However, in their current stage of development, such platforms do not fully recapitulate the full multicellular gut architecture and the intimate functional interactions between microflora, epithelial, immune and mucosal stromal elements, as well as a constant blood flow that are necessary for physiologic intestinal function; the same ones that have been pathologically perturbed in the disease state.
Biological and Technical Considerations for the utility of Patient-derived Mucosal Explants
In an attempt to address the above-mentioned concerns of current research methodologies and in particular the need for producing appropriate systems for preclinical drug testing and proof-of-principle studies, experimental assays using ex-vivo culture and manipulations of human tissues have been introduced for the gastrointestinal system almost 50 years ago [22] and have been recently summarized by Russo et al. [23••] and Randal et al [24]. These approaches are described in the literature as “gut explants”, “ex-vivo culture of colon or ileum” and “human intestinal organ culture”. Samples can be derived from mucosal biopsies of terminal ileum or colon obtained during an endoscopic procedure or from discarded surgical specimens. In the latter, the mucosa layer is stripped off the muscle layers; nevertheless, in rare reports aiming to prolonged culture or answer a specific research question, epithelial cells are also depleted from the organ culture [25, 26].
Typical mucosal explants consist of intestinal epithelial cells, endothelium, stromal cells and constituent immune cells that in the case of IBD possess an activation state often driven by individual patient pathology. Particularly when the culture material is obtained from the affected areas of the mucosa, immune activation and cytokine secretion GM-CSF, TNFα, IFNγ, IL-1b, IL-10, IL17, IL18 is spontaneous and in proportion to the degree of inflammation [27••–31]. Furthermore, we and others have found that biopsies originating from the ileum secrete higher cytokine levels compared to colon [31]. In one study, muscle layer explants from strictured ileum was investigated, IL-1b, TGFβ1 and collagen were found to be upregulated [29]. Spontaneous cytokine release constitutes a fundamental advantage of mucosal explants for drug testing compared to cultures of patient-derived peripheral blood cells, a more feasible approach but with the drawback that external cell stimulation is required for cytokine release [32]. It is well recognized now that there is a high degree of variability in immune responses among different individuals to various stimuli and such system manipulations introduce stimulus-dependent bias in patient responses to various drug treatments that might have nothing to do with their IBD [17, 19].
Often investigators use for IBD-related research explant culture biopsy material derived from control subjects that subsequently are activated ex-vivo after incubation with various proinflammatory cytokines, alone or in combination (TNFα, IFNγ, IL1b, IL17A) [33, 34•, 35]. Others used stimulation (anti-CD3, pokweed mitogen, LPS, bacterial antigens) of IBD-derived mucosal explants [27••, 28]. While such approaches are useful in illuminating specific signaling pathways in purely mechanistic studies, their contribution to drug testing is limited in terms of relevance to disease underlying mechanisms and patient heterogeneity.
Typically, mucosal biopsies when kept on ice immediately after harvesting and placed in culture within 1 hour of their procurement retain a good viability up to 24 hours, particularly under high oxygenation conditions [23••, 27••, 36•, 37, 38]. Several investigators prefer to use tissue support systems which allow polarized cultures and selective exposure of the luminal surface of the biopsy to the various experimental manipulations. This technique has been pioneered by Tsilingini et al. [39, 40] and has become the method of preference for the study of gut microbiome effects in IBD [41, 42•]. For instance, Llopis et al. used mucosal explants derived from patients with CD to demonstrate that treatment with the probiotic Lactobacillus casei decreased TNFα, IFNγ, IL-2, IL-6, IL-8 and CXCCL1 secretion in the culture supernatant but did not modify expression of IL-12, IL-23 and IL-17F [43]. Using a unique polarized explant culture system in which a cylinder is adhered to the apical surface of the intestinal biopsy specimen in order to limit bacterial exposure to the luminal surface, as is the case in-vivo, Tsilingiri et al. made the striking observation that treatment of biopsies from patients with IBD with three probiotic strains, one previously shown by the same group to be protective in a mouse model of experimental colitis resulted in worsening of inflammation and pronounced tissue degradation [44, 45], further underscoring the fact that studies in mice often do not translate to humans. Such findings suggest that while probiotics might benefit IBD patients in remission they may not be beneficial and can even harm the patient during the acute phase of the disease. The same study demonstrated that healthy mucosal explant exposure to supernatant of Lactococcus paracasei could not only counteract the deleterious effects of Salmonella infection, but also was capable to downregulate spontaneous cytokine secretion (CCL2, CCL4, TNFα, IFNγ, IL23p40, but not IL10) and NF-κB activity in IBD mucosal explants introducing thus the concept of post-biotics to IBD therapeutics. Further support for the role of pre-biotics in IBD is provided by the study of Terciolo et al. showing that treatment of colonic IBD explants with Saccharomyces boulardii culture supernatant restored epithelial barrier function [42•]. Along the same lines, Llopis et al. by using inflamed mucosal explants from CD patients in bacterial co-cultures showed that the presence of Lactobacillus casei could result in attenuation of commensal E.coli-induced upregulation of various pro-inflammatory cytokines (IL6, IL23p19, IL17F, IL8, CXCL1, CXCL2) [43].
The role of mucosal explants in understanding IBD pathophysiology
In spite of the introduction of various anti-TNFs almost a decade ago and more recently of vedolizumab (anti- integrin α4β7), ustekinumab (anti-p40, the common subunit of IL12 and IL23) and tofacitinib (Janus kinase inhibitor), the medical management of IBD remains sub-optimal [46]. This is due in part because the mechanisms of disease pathogenesis have not been completely elucidated [1, 41, 47]. There are several ways to address this knowledge gap particularly nowadays because of the rapid expansion of highly sophisticated technologies like whole genome sequencing, single cell RNAseq, and mass cytometry (CyTOF-Cytometry by Time-of-Flight) [48] that can be used to analyze human samples in increasing detail. In our opinion, the study of tissue explants can complement other high throughput approaches by offering a platform supporting functional interactions that can be used for testing hypotheses generated by other methodologies. So far, the power of such testing has not fully materialized, but there are several important studies elucidating important aspects of T-cell and non-T-cell mediated inflammatory cross-talk in IBD that have identified key cell types and soluble mediators. The pioneering studies by Sabatino et al. and Jarry et al. utilized mucosal explants from control patients to investigate how the breakdown of immune tolerance to the components of the gut microbiota can lead to immune hyper-reactivity as seen in CD [49, 50]. Depletion of TGFβ or IL10 from the explants elicited a pronounced Th1 response, upregulation of IFNγ, TNFα, IL2, IL8 and IL17 and concomitant epithelial barrier disruption. Building upon those findings, a follow-up study by Jarry et al. further investigated the potential mechanisms of pathogenesis in CD and found macrophage/epithelial cell-secreted IL18 and IL12 as distinct drivers of IFNγ surge upon IL-10 or TGFβ neutralization, respectively [51]. Notably the study by Huff et al. uncovered an innate regulatory function of the intestinal stromal compartment that is compromised in CD resulting in expansion of Th1 and Th17 cells via secretion of IL-6 and IL1β [52].
Another interesting concept that has emerged from the study of a non-reductionistic system like the mucosal explant culture is the bi-directional communication between various cell types (epithelial, immune, stroma) that maintains intestinal homeostasis. For example, blocking IL21 reduced the production of MIP-3α, a chemokine produced by epithelial cells that drives T cell gut homing [53] while exogenous administration of the colitogenic T-cell expressed IL17 or IFNα in normal colonic mucosa caused alterations in epithelial cell morphology, crypt disorganization, and upregulation of matrix metalloprotease (MMP) activity [54, 55•]. More relevant to IBD pathogenesis, two different studies showed that in healthy mucosal explants, loss of the epithelial layer resulted in a pronounced inflammatory response characterized by cytokine upregulation (IL1β, IL6, IL8, IL23, TNFα, MIP2α) and induction of co-stimulatory molecules and pattern recognition receptors by resident lamina propria cells [25, 26]. Taken together, the above studies demonstrate the utility for investigating the distinct molecular pathways that have been found to be compromised in IBD using explant cultures which resemble the most the situation in vivo.
Pre-clinical drug evaluation in IBD using mucosal explants
Proof-of-principle drug testing studies using patient-derived material are pivotal in minimizing the risk of drug failure during clinical evaluation [11, 13, 56]. Therapeutic antibodies directed against CD3 (otelixizumab) [56]; CB2R agonists [35]; a selective mu-opioid receptor agonist [57]; a peptide derived from the kiwi fruit [58] and a SMAD7 antisense oligonucleotide (morgensen) that restores TGFβ1 signaling [59] have been shown to be effective in producing changes in explant cultures consistent with potential positive clinical outcomes. The read-out of explant cultures treated with candidate drugs can be quite versatile. One common approach is to measure the effect of treatment on the various disease-related cytokines secreted in culture supernatant (i.e. (IL1β, IL2, IL10, IL12p70, IL13, IL17A, GM-CSF, IFNγ and TNFα,) often using a multiplex assay or cytokine array [27••, 56]. In Figure 1 we show a schematic representation of the set-up of explant cultures derived from the inflamed mucosa of an IBD patient in an experiment aiming to evaluate a candidate drug. Our group uses a panel of 16 cytokines as the assay read-out and for each patient the results are presented as % of cytokine inhibition in the presence of drug vs vehicle. In addition to measuring secretion of various cytokines in the culture supernatant, the explant tissue at the end of the experiment can be used for Western Blot, gene expression or immunohistochemistry analysis, the latter of which that can go beyond the inflammatory response and evaluate aspects of cellular architecture and epithelial barrier integrity [33, 36•, 39, 42•, 44, 50, 55•, 60], apoptotic pathways [59] or intracellular signaling [56]. However, investigators should keep in mind that when the analysis is done at the end of the culture period, results most likely represent secondary drug effects and perhaps earlier time points are need to be studied to address immediate drug targets. Furthermore, culturing conditions per se present the complication of artefactual alterations in the expression of certain genes, particularly in epithelial cells [37].
Figure 1:
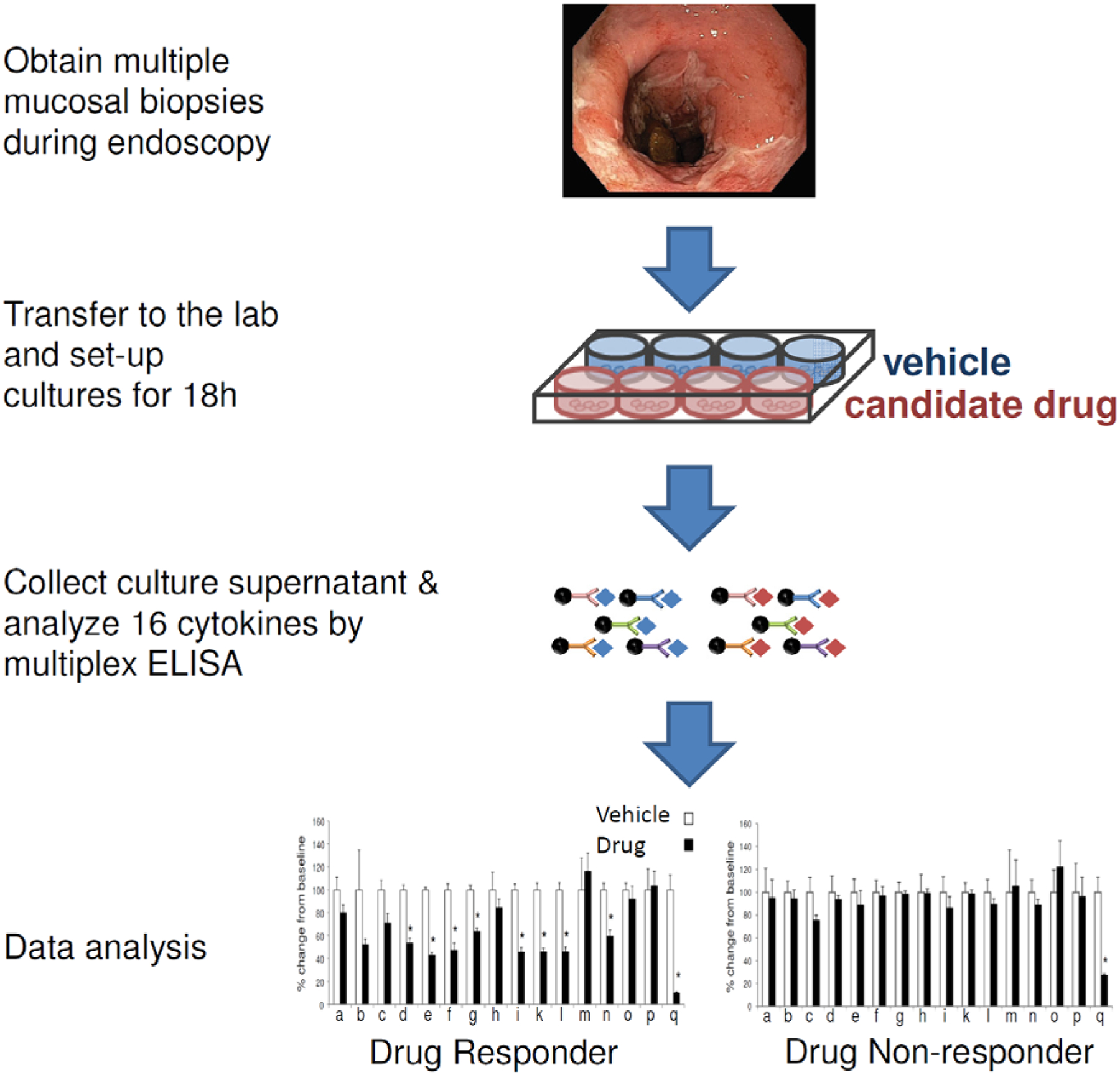
Schematic representation of an assay for predicting therapeutic responses in IBD based on treatment of mucosal explants
a-q: cytokines used for the assay read-out
q: cytokine targeted by the candidate drug
Besides providing a platform for testing drug efficacy using human samples, explant cultures present the opportunity to investigate mechanisms of action of partially characterized IBD therapeutics or illuminate unexpected effects on pathways modulated by the more established treatments. For example, Skikszai et al. using an intestinal organ culture model demonstrated that dexamethasone inhibits migration and activation of lamina propria cells and cytokine secretion (IL6, IL8, TNFα) [26]. Vossenkamper et al. treated 20 CD and 14 UC colonic mucosal explants with otelixizumab, an antibody against CD3 and showed significant inhibition of IFNγ and IL-17A release in culture supernatant along with significant reductions in their intracellular transcriptional regulators T-bet and RORγT, respectively. Targeted cytokine analysis was extended in a fraction of patients (6UC and 6CD) patients with a cytokine array analysis which identified 29 potential targets for CD treated samples and 22 for UC. In the same study, normalization of kinase activity in IBD explants was achieved after treatment with otelixizumab. Mechanistically, the beneficial anti-inflammatory effects of the drug were attributed at least in part to IL-10 upregulation. To our knowledge, more often IL-10 is observed to decrease, along with the down regulation of its proinflammatory counterparts, with the exception of upregulation in response to probiotics [44]. Based on our own explant-based studies, this is also true for treatment with infliximab. Most importantly, by combining analysis of explant culture and of isolated immune cells, the otelixizumab study clearly demonstrated that this particular anti-CD3 antibody did not induce T-cell death, in contrast to a previous ant-CD3 drug candidate, visilizumab, suggesting distinct mechanisms of action of two different drugs targeting the same molecule [11]. This might have significant translational implications not only for drug efficacy but also for drug safety. In a previous clinical study, treatment of UC patients with visilizumab was not only found to be ineffective, but caused increased cytokine release and appeared to increase disease complications such as colectomy [61].
Marafini et al. provided another example in which mucosal explants of patients with CD were used to demonstrate that MIP3α (CCL20) is a downstream target of mongersen, a SMAD7 anti-sense oligonucleotide [62•]. MIP3α was subsequently demonstrated to be an effective serum biomarker for monitoring therapeutic responses in patients treated with mongersen and potentially other drugs targeting the same pathway [62•].
Regarding additional mechanisms of action of anti-TNFα [63], of importance is the link between TNFα and IL25 (IL17E), a rather counter-regulatory protein the synthesis of which is defective in IBD and can be restored upon treatment with infliximab ex-vivo [64]. At a different level of regulation provided by tissue remodeling, Meijer et al. showed modulation of MMP activity in IBD explant cultures by infliximab [38]. Petito et al. demonstrated that infliximab treatment of UC mucosal explants affected more than one cell type, including increased E-cadherin expression and ERK1/2 phosphorylation in epithelial cells; induction of apoptosis in immune cells (CD3+, CD68+); and heterologous downregulation of various pro-inflammatory cytokines (IFNγ, IL1β, IL6, IL8); observations that were subsequently confirmed using isolated cell types [60]. Our own studies of mucosal explants from 45 patients with IBD (29CD/16UC) also confirm the broader effects of infliximab treatment on spontaneous cytokine secretion.
Mucosal explants and precision Medicine
Vadstrup et al. noticed heterogeneity in downregulation of certain cytokines (IL1β, TNFα, GM-CSF) in response to treatment with infliximab among the different patient explants tested [27••]. In our experience as well, treatment with infliximab resulted in differences in cytokine downregulation across patient samples. Interestingly, another study reported variability in patient responses to a caspase inhibitor, which was reflected at the level of pathway-specific cytokines (IL18, IFNγ, but not IL17A) [31]. Taken together, the above findings confirm at the molecular level disease and patient heterogeneity in IBD that result in differential responses to current and emerging therapies [65, 66]. Indeed, after more than 10 years of experience with anti-TNFα treatments it is well accepted now that “one size does not fit all” in IBD therapeutics [67, 68]. It is estimated that over 30 novel compounds are in development for IBD, and explant-based assays as the ones described in this review can offer an invaluable tool for delivering in the future personalized therapies [69–72]. Standardization of the explant treatment methodology in formats for widespread use such as “biopsy on a chip” [36•] and their prospective validation as predictors of therapeutic responses using FDA approved drugs, along with integration of newest OMICs technologies, including RNAseq and metabolomics into the assay read-out hold great promise for informing treatment decisions for each and every patient and for developing precision medicine biomarkers. Moreover, by comparing the mechanisms of action of different drugs, new drug combinations might emerge, with improved efficacy and fewer side effects.
Limitations of explant-based assays
In contrast to the significant progress in incorporating highly sophisticated bio-engineering technologies to support the development of organoid culture systems (i.e. 3-D support systems and special media) and “organ on a chip” related platforms, tissue explant based systems still suffer from the lack of technological advancements that will optimize culture conditions, improve tissue survival and reduce experimental variability among different labs and efforts towards this direction has just started to emerge [36•]. There will eventually be a need for assay standardization within this space to enable results to be compared between groups.
One of the major drawbacks in performing explant culture assays is the access and availability of patient biopsy samples, constraints that reduce the capacity for a high throughput assay and its broader applicability unless appropriate tissue cryopreservation techniques are implemented. Since IBD is heterogeneous in both its etiology and pathophysiology, for preclinical drug evaluation a sufficiently large number of patients need to be tested to account for interpatient variability, typically 15–20 patients. Inherent to this paradigm is also the fact that the process of creating the explant culture produces confounding wound healing responses due to tissue injury and disruption. Excising the biopsy material also removes the tissues from their blood flow and interrupts neuron-mediated interactions and the flux of immune cells to and from the inflamed tissue. Together, these factors may influence the sensitivity or veracity of the assay and should be considered for their potential as confounding variables when interpreting the test results. Along the same lines, mucosal explant cultures cannot be used for testing drugs that target cell trafficking processes involving migration of cells into sites of inflammation, as is the case of vedolizumab. On the other hand, testing such classes of drugs using explant-based platforms might reveal additional and previously unappreciated mechanisms of drug action.
Conclusion
Overall, the likelihood of explant culture systems more faithfully recapitulating disease pathobiology, capturing patient heterogeneity and avoiding systematic biases is greater than other existing systems at three different levels of patient care. i.e. drug development, drug testing and drug selection for personalized treatments. The ultimate utility of tissue explant based assays will depend on the refinement of culture conditions, standardization of the testing platform and development of metrics that more accurately define responses that mimic therapeutic efficacy.
Key points.
Patient-derived tissue explants afford several advantages over approaches such as isolated cells or co-cultures of different types of cells, organoids and gut-on-chip technologies in terms of approximation of the tissue complexity in vivo and of capturing patient heterogeneity
Explants derived from the inflamed areas of the mucosa are in an activation state and spontaneously secrete significant amounts of cytokines that can be used to assess the efficacy of candidate drugs prior to phase II clinical testing
Mucosal explant cultures offer a tractable platform for evaluating individual patient responses to various treatments and selecting tailored therapies for IBD
Tissue explants represent a non-reductionistic system that can be easily manipulated in order to answer fundamental mechanistic questions related to IBD pathogenesis and mode of action of various IBD therapeutics
Acknowledgements
Financial support and sponsorship: This work was supported by the Crohn’s and Colitis Foundation (senior research award to EK), by the National Center for Advancing Translational Studies (TR00175301 to EK). Conflicts of interest: EK is currently receiving grant support from Bristol-Myers Squibb and Pfizer
References
- [1].de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- [2].Uniken Venema WT, Voskuil MD, Dijkstra G, Weersma RK, Festen EA. The genetic background of inflammatory bowel disease: from correlation to causality. J Pathol. 2017;241:146–58. [DOI] [PubMed] [Google Scholar]
- [3].Valatas V, Vakas M, Kolios G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2013;305:G763–85. [DOI] [PubMed] [Google Scholar]
- [4].Bamias G, Arseneau KO, Cominelli F. Mouse models of inflammatory bowel disease for investigating mucosal immunity in the intestine. Curr Opin Gastroenterol. 2017;33:411–6. [DOI] [PubMed] [Google Scholar]
- [5].Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RR. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hayday AC, Peakman M. The habitual, diverse and surmountable obstacles to human immunology research. Nat Immunol. 2008;9:575–80. [DOI] [PubMed] [Google Scholar]
- [8].Masopust D, Sivula CP, Jameson SC. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. J Immunol. 2017;199:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. [DOI] [PubMed] [Google Scholar]
- [10].Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang JT, Sandborn WJ, Ernst PB. Studies in human intestinal tissues: is it time to reemphasize research in human immunology? Gastroenterology. 2014;147:26–30. [DOI] [PubMed] [Google Scholar]
- [12].Koboziev I, Karlsson F, Zhang S, Grisham MB. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis. 2011;17:1229–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Edwards AM, Arrowsmith CH, Bountra C, Bunnage ME, Feldmann M, Knight JC, et al. Preclinical target validation using patient-derived cells. Nat Rev Drug Discov. 2015;14:149–50. [DOI] [PubMed] [Google Scholar]
- [14].VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pedersen G. Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium. Dan Med J. 2015;62:B4973. [PubMed] [Google Scholar]
- [16].West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nature medicine. 2017;23:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dahlen R, Strid H, Lundgren A, Isaksson S, Raghavan S, Magnusson MK, et al. Infliximab inhibits activation and effector functions of peripheral blood T cells in vitro from patients with clinically active ulcerative colitis. Scand J Immunol. 2013;78:275–84. [DOI] [PubMed] [Google Scholar]
- [18].Clevers H Modeling Development and Disease with Organoids. Cell. 2016;165:1586–97. [DOI] [PubMed] [Google Scholar]
- [19].Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113:E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, et al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol Gastroenterol Hepatol. 2018;5:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yissachar N, Zhou Y, Ung L, Lai NY, Mohan JF, Ehrlicher A, et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell. 2017;168:1135–48 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Browning TH, Trier JS. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969;48:1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]••.Russo I, Zeppa P, Iovino P, Del Giorno C, Zingone F, Bucci C, et al. The culture of gut explants: A model to study the mucosal response. J Immunol Methods. 2016;438:1–10. [DOI] [PubMed] [Google Scholar]; This excellent review discusses fundamental aspects of explant culture conditions and provides a comprehensive list of studies that have used this experimental model in the past along. For each study, key experimental details and major findings are included.
- [24].Randall KJ, Turton J, Foster JR. Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol Toxicol. 2011;27:267–84. [DOI] [PubMed] [Google Scholar]
- [25].Schroder-Braunstein J, Gras J, Brors B, Schwarz S, Szikszai T, Lasitschka F, et al. Initiation of an inflammatory response in resident intestinal lamina propria cells -use of a human organ culture model. PLoS One. 2014;9:e97780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szikszai T, Lasitschka F, Giese T, Greulich M, Lee YS, Ilse J, et al. Standardization of a human organ culture model of intestinal inflammation and its application for drug testing. J Immunol Methods. 2015;421:96–103. [DOI] [PubMed] [Google Scholar]
- [27]••.Vadstrup K, Galsgaard ED, Gerwien J, Vester-Andersen MK, Pedersen JS, Rasmussen J, et al. Validation and Optimization of an Ex Vivo Assay of Intestinal Mucosal Biopsies in Crohn’s Disease: Reflects Inflammation and Drug Effects. PLoS One. 2016;11:e0155335. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper offers a comprehensive description of the explant culture model, including optimized experimental conditions, as well as evaluation of the assay performance using treatment of CD explants with infliximab. The authors have developed a cytokine-based assay readout and demonstrate spontaneous cytokine release in proportion to the severity of inflammation. Most importantly, based on heterogeneity of patient responses to the ex-vivo treatment, they suggest that the assay can be used to select personalized treatments
- [28].Dionne S, Laberge S, Deslandres C, Seidman EG. Modulation of cytokine release from colonic explants by bacterial antigens in inflammatory bowel disease. Clin Exp Immunol. 2003;133:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Biancheri P, Di Sabatino A, Ammoscato F, Facciotti F, Caprioli F, Curciarello R, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44:370–85. [DOI] [PubMed] [Google Scholar]
- [30].Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–36. [DOI] [PubMed] [Google Scholar]
- [31].Jarry A, Bossard C, Droy-Dupre L, Volteau C, Bourreille A, Meurette G, et al. Heterogeneity of subordination of the IL-18/IFN-gamma axis to caspase-1 among patients with Crohn’s disease. Lab Invest. 2015;95:1207–17. [DOI] [PubMed] [Google Scholar]
- [32].Magnusson MK, Strid H, Isaksson S, Bajor A, Lasson A, Ung KA, et al. Cultured blood T-cell responses predict anti-TNF therapy response in patients with ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1149–61. [DOI] [PubMed] [Google Scholar]
- [33].Nicotra LL, Vu M, Harvey BS, Smid SD. Prostaglandin ethanolamides attenuate damage in a human explant colitis model. Prostaglandins Other Lipid Mediat. 2013;100–101:22–9. [DOI] [PubMed] [Google Scholar]
- [34]•.Couch DG, Tasker C, Theophilidou E, Lund JN, O’Sullivan SE. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci (Lond). 2017;131:2611–26. [DOI] [PubMed] [Google Scholar]; This paper demonstrates anti-inflammatory effects of two cannabinoid drugs in IBD mucosal explants, an effect that was not apparent when cultured colonic epithelial cells were used
- [35].Harvey BS, Nicotra LL, Vu M, Smid SD. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine. 2013;63:209–17. [DOI] [PubMed] [Google Scholar]
- [36]•.Costa MO, Nosach R, Harding JCS. Development of a 3D printed device to support long term intestinal culture as an alternative to hyperoxic chamber methods. 3D Print Med. 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first description of a 3D-printed device that supports the culture of polarized mucosal explant. The design includes a separate gas mixture supply to their luminal surface that allows co-culture with anaerobic bacteria.
- [37].Drew JE, Farquharson AJ, Vase H, Carey FA, Steele RJ, Ross RA, et al. Molecular Profiling of Multiplexed Gene Markers to Assess Viability of Ex Vivo Human Colon Explant Cultures. Biores Open Access. 2015;4:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meijer MJ, Mieremet-Ooms MA, van Duijn W, van der Zon AM, Hanemaaijer R, Verheijen JH, et al. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:200–10. [DOI] [PubMed] [Google Scholar]
- [39].Tsilingiri K, Rescigno M. Should probiotics be tested on ex vivo organ culture models? Gut Microbes. 2012;3:442–8. [DOI] [PubMed] [Google Scholar]
- [40].Tsilingiri K, Sonzogni A, Caprioli F, Rescigno M. A novel method for the culture and polarized stimulation of human intestinal mucosa explants. J Vis Exp. 2013:e4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ahluwalia B, Moraes L, Magnusson MK, Ohman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53:379–89. [DOI] [PubMed] [Google Scholar]
- [42]•.Terciolo C, Dobric A, Ouaissi M, Siret C, Breuzard G, Silvy F, et al. Saccharomyces boulardii CNCM I-745 Restores intestinal Barrier Integrity by Regulation of E-cadherin Recycling. J Crohns Colitis. 2017;11:999–1010. [DOI] [PubMed] [Google Scholar]; Colonic explants from IBD patients were used to demonstrate beneficial effects of S. boulardii culture supernatant (post-biotic) on epithelial barrier function
- [43].Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, et al. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15:275–83. [DOI] [PubMed] [Google Scholar]
- [44].Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut. 2012;61:1007–15. [DOI] [PubMed] [Google Scholar]
- [45].Wildenberg ME, van den Brink GR. A major advance in ex vivo intestinal organ culture. Gut. 2012;61:961–2. [DOI] [PubMed] [Google Scholar]
- [46].Holleran G, Lopetuso L, Petito V, Graziani C, Ianiro G, McNamara D, et al. The Innate and Adaptive Immune System as Targets for Biologic Therapies in Inflammatory Bowel Disease. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, et al. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods. 2014;415:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Di Sabatino A, Pickard KM, Rampton D, Kruidenier L, Rovedatti L, Leakey NA, et al. Blockade of transforming growth factor beta upregulates T-box transcription factor T-bet, and increases T helper cell type 1 cytokine and matrix metalloproteinase-3 production in the human gut mucosa. Gut. 2008;57:605–12. [DOI] [PubMed] [Google Scholar]
- [50].Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jarry A, Bossard C, Sarrabayrouse G, Mosnier JF, Laboisse CL. Loss of interleukin-10 or transforming growth factor beta signaling in the human colon initiates a T-helper 1 response via distinct pathways. Gastroenterology. 2011;141:1887–96 e1–2. [DOI] [PubMed] [Google Scholar]
- [52].Huff KR, Akhtar LN, Fox AL, Cannon JA, Smith PD, Smythies LE. Extracellular matrix-associated cytokines regulate CD4+ effector T-cell responses in the human intestinal mucosa. Mucosal Immunol. 2011;4:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology. 2007;132:166–75. [DOI] [PubMed] [Google Scholar]
- [54].Harvey BS, Sia TC, Wattchow DA, Smid SD. Interleukin 17A evoked mucosal damage is attenuated by cannabidiol and anandamide in a human colonic explant model. Cytokine. 2014;65:236–44. [DOI] [PubMed] [Google Scholar]
- [55]•.Jarry A, Malard F, Bou-Hanna C, Meurette G, Mohty M, Mosnier JF, et al. Interferon-Alpha Promotes Th1 Response and Epithelial Apoptosis via Inflammasome Activation in Human Intestinal Mucosa. Cell Mol Gastroenterol Hepatol. 2017;3:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the advantage of an integrated human colonic mucosa explant model is used to decipher the sequence of interactions taking place at the tissue level following treatment with IFNα. Specifically, by using selective pathway inhibitors and anti-cytokine antibodies, the authors demonstrate that an innate cytokine can activate an adaptive immune response which in turn becomes the driver of epithelial barrier dysfunction.
- [56].Vossenkamper A, Hundsrucker C, Page K, van Maurik A, Sanders TJ, Stagg AJ, et al. A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease. Gastroenterology. 2014;147:172–83. [DOI] [PubMed] [Google Scholar]
- [57].Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K, et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ciacci C, Russo I, Bucci C, Iovino P, Pellegrini L, Giangrieco I, et al. The kiwi fruit peptide kissper displays anti-inflammatory and anti-oxidant effects in in-vitro and ex-vivo human intestinal models. Clin Exp Immunol. 2014;175:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Petito V, Lopetuso LR, Arena V, Stigliano E, Boninsegna A, Bibbo S, et al. Direct effect of infliximab on intestinal mucosa sustains mucosal healing: exploring new mechanisms of action. Dig Liver Dis. 2016;48:391–8. [DOI] [PubMed] [Google Scholar]
- [61].Sandborn WJ, Colombel JF, Frankel M, Hommes D, Lowder JN, Mayer L, et al. Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis. Gut. 2010;59:1485–92. [DOI] [PubMed] [Google Scholar]
- [62]•.Marafini I, Monteleone I, Dinallo V, Di Fusco D, De Simone V, Laudisi F, et al. CCL20 Is Negatively Regulated by TGF-beta1 in Intestinal Epithelial Cells and Reduced in Crohn’s Disease Patients With a Successful Response to Mongersen, a Smad7 Antisense Oligonucleotide. J Crohns Colitis. 2017;11:603–9. [DOI] [PubMed] [Google Scholar]; By treating mucosal explants with mongersen, a SMAD7 antisense oligonucleotide, the authors identified CCL20 as the drug’s downstream target and demonstrated that CCL20 can be used as a non-invasive biomarker of therapeutic responses to mongersen.
- [63].Danese S Mechanisms of action of infliximab in inflammatory bowel disease: an anti-inflammatory multitasker. Dig Liver Dis. 2008;40 Suppl 2:S225–8. [DOI] [PubMed] [Google Scholar]
- [64].Fina D, Franze E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, et al. Interleukin-25 production is differently regulated by TNF-alpha and TGF-beta1 in the human gut. Mucosal Immunol. 2011;4:239–44. [DOI] [PubMed] [Google Scholar]
- [65].Yamamoto-Furusho JK. Inflammatory bowel disease therapy: blockade of cytokines and cytokine signaling pathways. Curr Opin Gastroenterol. 2018;34:187–93. [DOI] [PubMed] [Google Scholar]
- [66].Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–78. [DOI] [PubMed] [Google Scholar]
- [67].Gerich ME, McGovern DP. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol. 2014;11:287–99. [DOI] [PubMed] [Google Scholar]
- [68].Lopetuso LR, Gerardi V, Papa V, Scaldaferri F, Rapaccini GL, Gasbarrini A, et al. Can We Predict the Efficacy of Anti-TNF-alpha Agents? Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chang S, Hanauer S. Optimizing pharmacologic management of inflammatory bowel disease. Expert Rev Clin Pharmacol. 2017;10:595–607. [DOI] [PubMed] [Google Scholar]
- [70].Hindryckx P, Vande Casteele N, Novak G, Khanna R, D’Haens G, Sandborn WJ, et al. The Expanding Therapeutic Armamentarium for Inflammatory Bowel Disease: How to Choose the Right Drug[s] for Our Patients? J Crohns Colitis. 2018;12:105–19. [DOI] [PubMed] [Google Scholar]
- [71].Ma C, Panaccione R, Fedorak RN, Parker CE, Khanna R, Levesque BG, et al. Development of a core outcome set for clinical trials in inflammatory bowel disease: study protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. BMJ Open. 2017;7:e016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Flamant M, Roblin X. Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol. 2018;11:1756283X17745029. [DOI] [PMC free article] [PubMed] [Google Scholar]


