Abstract
Optoelectronic devices based on lead halide perovskites are processed in facile ways, yet are remarkably efficient. There are extensive research efforts investigating lead-free perovskite and perovskite-related compounds, yet there are challenges to synthesize these materials in forms that can be directly integrated into thin film devices rather than as bulk powders. Here, we report on the colloidal synthesis and characterization of lead-free, antifluorite Cs2ZrX6 (X = Cl, Br) nanocrystals that are readily processed into thin films. We use transmission electron microscopy and powder X-ray diffraction measurements to determine their size and structural properties, and solid-state nuclear magnetic resonance measurements reveal the presence of oleate ligand, together with a disordered distribution of Cs surface sites. Density functional theory calculations reveal the band structure and fundamental band gaps of 5.06 and 3.91 eV for Cs2ZrCl6 and Cs2ZrBr6, respectively, consistent with experimental values. Finally, we demonstrate that the Cs2ZrCl6 and Cs2ZrBr6 nanocrystal thin films exhibit tunable, broad white photoluminescence with quantum yields of 45% for the latter, with respective peaks in the blue and green spectral regions and mixed systems exhibiting properties between them. Our work represents a critical step toward the application of lead-free Cs2ZrX6 nanocrystal thin films into next-generation light-emitting applications.
Metal halide perovskite materials with the general formula ABX3 (where A = cation, B = metal cation (typically Pb2+), and X = halide anion) have shown excellent performance in optoelectronic devices.1 Their facile fabrication renders them attractive for scaled-up production; however, limited operational lifetimes of perovskite-based devices, as well as the toxicity of lead, remain major issues,2,3 particularly for consumer electronics applications. Consequently, there is motivation to find viable lead replacements for these materials. The two mainstream approaches for lead replacement rely on incorporation of either homovalent ions (such as Ge2+ or Sn2+) or heterovalent ions (such as Sb3+ or Bi3+).4 The latter approaches require ion-splitting or the occurrence of ordered vacancies to maintain charge neutrality, thereby creating halide perovskites or perovskite derivatives with altered chemical formulas, such as AB(Ch,X)3, A2B1+B3+X6, A3□B3+2X9, and A2□B4+X6 (with Ch denoting a chalcogen and the open box symbol (□) indicating a vacancy).4
Many of these A2BX6 materials, often loosely referenced as vacancy-ordered double perovskites,5 adopt the K2PtCl6 (antifluorite) crystal structure, where isolated [BX6]2– octahedra form a face-centered cubic lattice with the A-site cations occupying all tetrahedral sites. The isolated nature of the octahedra and subsequent opportunity for localizing excitations renders these A2BX6 materials particularly promising for light-emitting applications. The latest works on lead-free perovskite alternatives, particularly A2BX6 materials, often report the synthesis and characterization of single crystals or bulk powders,6,7 which are not compatible with facile thin film integration required for device applications. So far, thin films of A2BX6 materials, for example, have been fabricated for Cs2PdBr6,8 Cs2TiBr6,9 and Cs2SnI6,10 but this is only a small subset of all experimentally known A2BX6 materials; this small sample space limits us from exploiting the full family of these materials for optoelectronic device application. One approach to fabricate thin films with excellent optoelectronic properties is through deposition of colloidal suspensions of nanocrystals (NCs), yet only Sn4+- or Pd4+-based A2BX6 NC systems11,12 have been reported to date.
Here, we report the novel synthesis and characterization of colloidal, antifluorite cesium hexachlorozirconate(IV) and cesium hexabromozirconate(IV) NCs, thereby opening up the range of tunable antifluorite NC compositions. Powder X-ray diffraction (XRD) measurements show that the Cs2ZrX6 (X = Cl, Br) NCs crystallize into the antifluorite structure. Solid-state nuclear magnetic resonance (NMR) experiments validate that the surfaces of Cs2ZrCl6 NCs are capped with oleate ligands and a disordered distribution of Cs sites. Using density functional theory (DFT), we calculate fundamental band gaps of 5.06 eV for Cs2ZrCl6 and 3.91 eV for Cs2ZrBr6, which is consistent with the experimentally measured values and band structure. Thin films of Cs2ZrCl6 and Cs2ZrBr6 NCs show broad photoluminescence (PL) peaking at ∼450 nm (blue-white) and ∼528 nm (green-white), respectively, with the bromide system exhibiting a photoluminescence quantum yield (PLQY) of 45%. By mixing the colloidal suspensions of Cs2ZrCl6 and Cs2ZrBr6 NCs and depositing films, we demonstrate tunable white emission, which is not limited by anion exchange reactions, as is otherwise the case for lead-based CsPbX3 NC systems.13,14 These results reveal that halide engineering is a viable strategy for tuning the optical properties of Cs2ZrX6 NC thin films, and that they will be particularly interesting for light-emission applications requiring optically homogeneous thin films such as light-emitting diodes and scintillators.15,16
Using strict air-free Schlenk line techniques, in conjunction with a hot-injection approach, we fabricate colloidal suspensions of Cs2ZrCl6 NCs. Specifically, we rapidly inject benzoyl chloride diluted in dried 1-octadecene (ODE) into a solution of stoichiometric quantities of cesium and zirconium precursors in a mixture of oleylamine (OLA) and oleic acid (OA) in ODE at 185 °C, then isolate and redisperse the NCs in toluene, following previous reports on the synthesis of halide perovskite NCs17,18 (see Supporting Information (SI) for details). We also fabricated Cs2ZrBr6 NCs using a similar method but without addition of OLA; we found that omission of OLA also leads to more reliable and reproducible syntheses of the Cs2ZrCl6 NCs (see the SI and NMR section below). The synthesis of bulk Cs2ZrCl6 and Cs2ZrBr6 reference powders follows a published method with slight modifications19 (see the SI for details).
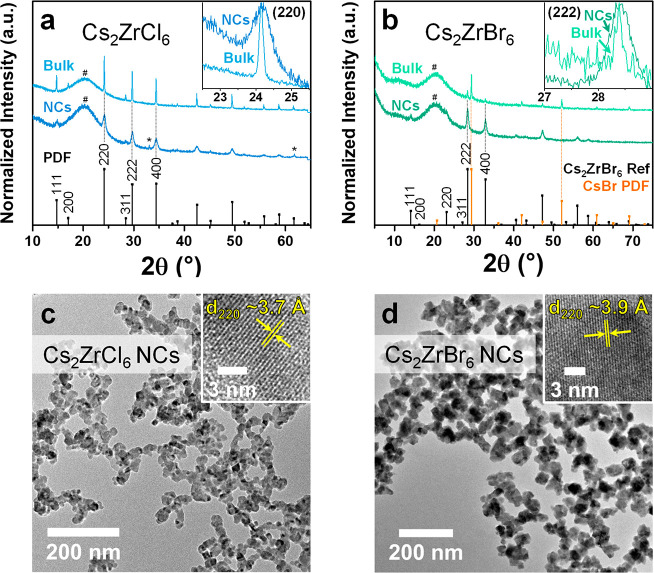
Figures 1a and 1b show the XRD patterns of bulk powder and NC samples of Cs2ZrCl6 and Cs2ZrBr6, respectively, measured under air-free conditions using an air-free sample holder. Here, we prepared the NC thin film samples by depositing the colloidal suspensions of Cs2ZrCl6 NCs on a Si substrate and of Cs2ZrBr6 NCs on a glass substrate. The patterns in Figure 1a match the reference XRD pattern of Cs2ZrCl6 (Joint Committee on Powder Diffraction Standards (JCPDS) File No. 01-074-1001, space group Fm3m, a = 10.407 Å),20 suggesting that the samples are phase-pure with any phase impurities at concentrations below the detection limit or amorphous in nature. The corresponding pure Cs2ZrBr6 reference XRD pattern is not available from the literature; therefore, we calculated a crystallographic information file (CIF) for Cs2ZrBr6, by replacing Cl with Br in the structure of Cs2ZrCl6 and performed a full hybrid DFT geometry optimization, allowing all lattice parameters to change (see the SI for details and CIF file). We then used the program VESTA21 to simulate the reference XRD pattern for Cs2ZrBr6, as shown in Figure 1b, with the computed unit-cell parameter reduced from 11.16 Å to 10.89 Å, to better match the experimental XRD data. From these XRD measurements (Figure 1b), we find that the NC thin film sample is also phase-pure Cs2ZrBr6, although the bulk powder contains CsBr impurities (see CsBr reference JCPDS File No. 00-005-0588, shown in orange in figure).22 We note that extended exposure of the Cl- and Br-containing compositions to ambient air conditions can lead to the formation of various impurity phases including (but not limited to) CsCl and CsBr, respectively, in both NC thin film and bulk powder form (see Figure S1 in the SI for examples), which we attribute to the hygroscopic nature of these compounds.23−25 The insets of Figure 1a and 1b compare XRD peaks of the Cs2ZrCl6 (220) and Cs2ZrBr6 (222) bulk powder and NC thin film, respectively. The peak widths are narrower for the bulk powder, which implies larger crystallite sizes, compared to those in the NC thin film samples.
Figure 1.
XRD and TEM characterization of solid-state Cs2ZrX6 samples. (a) XRD patterns of Cs2ZrCl6 bulk powder (light blue) and a Cs2ZrCl6 NC thin film (blue), as well as the reference XRD pattern of Cs2ZrCl6 (JCPDS File No. 01-074-1001, shown in black).20 The diffraction patterns are normalized to the respective maximum intensity of the scans and are vertically offset for clarity. The air-free sample holder introduces a broad background and a peak at ∼20° (marked with a hash symbol (#)); the asterisks (*) mark peaks that originate from the Si substrate. The inset shows an expanded comparison of the (220) XRD peaks of Cs2ZrCl6 bulk powder with the Cs2ZrCl6 NC thin film. The patterns are normalized to the maximum peak intensity and scaled between 0 and 1 along the ordinate, respectively. (b) XRD patterns of Cs2ZrBr6 bulk powder (light green; including CsBr phase impurity) and a Cs2ZrBr6 NC thin film (green) as well as the reference XRD pattern of Cs2ZrBr6 (shown in black; see the SI for details on calculation) and CsBr (JCPDS File No. 00-005-0588, shown in orange).22 The diffraction patterns are normalized to the respective maximum intensity of the scans and vertically offset for clarity. The air-free sample holder introduces a broad background and peak at ∼20° (marked with a hash symbol (#)). The inset shows an expanded view comparing the (222) XRD peaks of Cs2ZrBr6 bulk powder with the Cs2ZrBr6 NC thin film. The patterns are normalized to the maximum peak intensity and scaled between 0 and 1 along the ordinate, respectively. For both Cs2ZrCl6 and Cs2ZrBr6 reference XRD patterns, we index the first six diffraction peaks to the respective (hkl) planes, according to the powder diffraction file (PDF) for Cs2ZrCl6 and the simulated reference XRD pattern for Cs2ZrBr6. (c) TEM image of Cs2ZrCl6 NCs. The inset shows a HR-TEM image (see Figure S2 in the SI for a larger area) of a Cs2ZrCl6 NC, showing (220) lattice fringes separated by ∼3.7 Å. (d) TEM image of Cs2ZrBr6 NCs. The inset shows an HR-TEM image (see Figure S3 in the Supporting Information for a larger area) of a Cs2ZrBr6 NC, showing (220) lattice fringes separated by ∼3.9 Å.
Figures 1c and 1d show transmission electron microscopy (TEM) images for Cs2ZrCl6 and Cs2ZrBr6 NCs, respectively. In the high-resolution TEM (HR-TEM) image of a Cs2ZrCl6 NC (inset of Figure 1c), we observe lattice fringes with ∼3.7 Å separation, which we index as the (220) plane of Cs2ZrCl6. We corroborate this assignment by performing a fast Fourier transform (FFT) analysis of the HR-TEM image (see Figure S2), which confirms the structure of Cs2ZrCl6 and reveals that the analyzed Cs2ZrCl6 NC is oriented along the [1̅14̅] zone axis in the real-space image. In the HR-TEM image of a Cs2ZrBr6 NC (inset of Figure 1d), we observe lattice fringes with ∼3.9 Å separation that index as the (220) plane of Cs2ZrBr6. FFT analysis of the HR-TEM image (see Figure S3) confirms the structure of Cs2ZrBr6 and shows that the analyzed Cs2ZrBr6 NC is oriented along the [1̅11] zone axis. We note that the NCs of both halide compositions appear slightly aggregated and, therefore, do not exhibit well-defined shapes, even when washed multiple times (see Figure S4 in the SI), although we attribute this in part to the drop-casting deposition method employed that leads to heterogeneous drying of the solvent on the TEM grid; the NCs are colloidally stable in suspension with slow precipitation occurring over a period of weeks to months that can be easily redispersed by agitating the suspension. Further work would be required to achieve well-dispersed particles upon deposition.
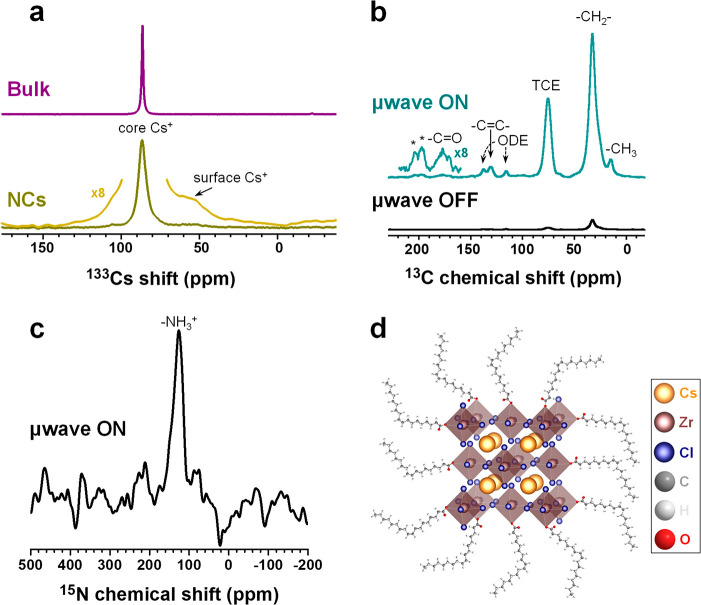
To study the atomic-level microstructure of Cs2ZrCl6 bulk powders and dried Cs2ZrCl6 NCs, as well as the surface ligands of the dried NCs, we use solid-state magic angle spinning (MAS) NMR spectroscopy and surface-enhanced dynamic nuclear polarization (DNP SENS) solid-state MAS NMR. DNP SENS has emerged as a powerful extension of solid-state NMR for studying dilute surface species and nanoparticles by providing signal enhancements that, compared to conventional NMR, can reach a factor of 660 for protons.26−30Figure 2a shows the 133Cs MAS NMR spectrum of Cs2ZrCl6 bulk powder and dried Cs2ZrCl6 NCs (see the SI for details). The spectrum of Cs2ZrCl6 bulk powder has a single peak (shift δ = 86 ppm, full width at half maximum (FWHM) = 106 ± 1 Hz), consistent with the presence of a single Cs site in the crystal structure of Cs2ZrCl6. The spectrum of dried Cs2ZrCl6 NCs yields a signal at the same 133Cs shift (δ = 86 ppm) but is considerably broader (FWHM = 474 ± 3 Hz), because of the presence of a wider distribution of local cesium environments in the NCs than in the bulk powder sample. In addition, we observe a very broad signal spanning ∼80 ppm visible on the vertical zoom (×8) of the NC signal, which we attribute to the presence of a particularly large distribution of environments for the surface layer of Cs+ ions, because of the intrinsically disordered nature of surfaces,29 which are dominant in NC samples (because of the large surface-area-to-volume ratios).
Figure 2.
Solid-state MAS NMR characterization of Cs2ZrCl6 samples. (a) 133Cs echo-detected spectrum of Cs2ZrCl6 bulk powders (at 10 kHz MAS) and a Bloch decay (single pulse) spectrum of Cs2ZrCl6 NCs (at 12 kHz MAS) at 16.4 T and RT. The spectra are vertically offset for clarity. The arrow indicates a broad signal corresponding to surface Cs+ sites of the Cs2ZrCl6 NCs. (b) 1H–13C CP spectrum of the carbon-containing species in a Cs2ZrCl6 NC sample recorded under DNP SENS conditions. The asterisks (*) indicate spinning sidebands. (c) 1H–15N CP spectrum recorded under DNP SENS conditions evidencing the presence of highly disordered −NH3+ environments in a Cs2ZrCl6 NC sample. (d) Schematic of an oleate-capped Cs2ZrCl6 NC.
For DNP SENS NMR experiments (see the SI for details), we mix the colloidal suspension of Cs2ZrCl6 NCs with hexagonal boron nitride (h-BN), dry the mixture at ∼50 °C in the glovebox, dope it with a stable binitroxide radical (here, TEKPol)31 in an inert solvent (1,1,2,2-tetrachloroethane, TCE) and irradiate with continuous wave high power and high-frequency microwaves, which drive the polarization transfer from the radicals to the solvent protons.32 This subsequently transfers the increased Boltzmann polarization of the solvent protons to the protons in the surface ligands of the Cs2ZrCl6 NCs through spin diffusion and can transfer to 133Cs of Cs2ZrCl6 through 1H–133Cs cross-polarization (CP).33,34 The purpose of mixing the Cs2ZrCl6 NCs with microcrystalline dielectric particles (here, h-BN) is to further boost the DNP enhancement by improving the homogeneity of the microwave field inside the sample.35,36
The DNP surface-enhanced 1H–133Cs spectrum of Cs2ZrCl6 NCs (see Figure S5 in the SI, top) shows a broad distribution of shifts, which is comparable to that recorded using conventional MAS NMR at room temperature (RT; cf. Figure 2a) and further corroborates our earlier assignment of surface Cs+ environments. Despite its large line width, the DNP-enhanced 133Cs spectrum does resolve at least two distinct local surface Cs environments (at 104 and 124 ppm). While their unambiguous assignment to specific surface sites is beyond the scope of the present work, we note that similar complexity of surface signals has been previously seen in semiconducting ZnSe NCs of various shapes and surface-to-volume ratios,37 as well as in CsPbBr3 nanocuboids38 and nanoplatelets.39 We also detect the NC core signal with an echo sequence without microwave irradiation (see Figure S5, bottom). The signal is broader than at RT (δ = 104 ppm, FWHM = 874 ± 18 Hz), suggesting that the crystal structure undergoes slight lattice parameter changes upon going from RT to 100 K. Both the core and surface signals are slightly shifted to higher frequencies at 100 K, with respect to RT, which we also attribute to temperature-induced structural changes.
In turn, we use DNP SENS to probe the surface ligands of Cs2ZrCl6 NCs. The 1H–13C spectrum (Figure 2b) yields enhancement factors of 42 and 15 for the TCE solvent and −CH2– signals, respectively, and evidences the presence of aliphatic chains, −C=C– moieties, as well as carbonyl (C=O) groups, consistent with the presence of oleate (OA+) as a surface ligand. In particular, the C=O signal spans 20 ppm and has a non-Gaussian envelope, which is indicative of highly disordered C=O groups that may originate from OA+ species bound to the surface of the NCs. The 1H–15N CP spectrum recorded under DNP SENS conditions (Figure 2c) contains a single peak (δ = 126 ppm, FWHM = 1343 ± 28 Hz), which we attribute to the −NH3+ group of oleylammonium (OLA+). The large FWHM of this peak suggests the presence of highly disordered OLA+ environments that may arise from surface-bound molecules. The presence of OA+ and OLA+ as capping agents for these systems is analogous to those seen in lead halide perovskite NCs.40−46 We note that the 1H–13C spectrum (Figure 2b) also contains −C=C– moieties belonging to ODE used as solvent in the synthesis. This suggests that the post-synthetic workup used here yields a material that may contain traces of the unreacted precursors, which we may have not detected with XRD if they are amorphous in nature or their concentrations are below the XRD detection limit. While the large FWHM of the peak in the 1H–15N spectrum suggests the presence of OLA+ on the surface, we cannot exclude that it originates from free residual OLA+ in the frozen sample. Nevertheless, we show that OLA is not necessarily required for the formation of phase-pure Cs2ZrCl6 NCs by performing a control experiment in which we use OA as the sole ligand precursor. The colloidal stability of these OA-only NCs suggests successful OA+-capping and, as previously mentioned, this synthesis approach shows even better reproducibility than the method that also involves OLA. Figure S4 shows the TEM and HR-TEM images of OA+-capped Cs2ZrCl6 NCs, which are very similar in size and shape to the NCs synthesized using both OLA and OA. Hence, we conclude that OA+ is the predominant ligand on Cs2ZrCl6 NCs, regardless of the presence/absence of OLA during synthesis. Figure 2d summarizes our conclusions on the form of the NC surfaces from our combined conventional MAS and DNP SENS NMR studies.
We now assess the optical properties of the samples. Figure 3a shows the ultraviolet–visible (UV-Vis) absorption and PL spectra of Cs2ZrCl6 and Cs2ZrBr6 NC thin films deposited by drop-casting the colloidal suspensions on fused silica substrates and encapsulated by a fused silica substrate in an argon glovebox. We observe a strong absorption onset at ∼260 nm for Cs2ZrCl6, consistent with photoluminescence excitation (PLE) measurements (Figure S7 in the SI), which we attribute to excitation across the band gap. The spectrum at wavelengths below this absorption onset is structured, and we tentatively assign the peaks at ∼203 and ∼225 nm to the Cl(t1u(π + σ)) → Zr(t2g(d)) and Cl(t2u(π)) → Zr(t2g(d)) transitions, respectively, because they are in similar positions to the absorption bands reported by Brisdon et al. for [(C2H5)2NH2)]2ZrCl6 salts.47 We provisionally attribute the weak sub-bandgap absorption with an onset at ∼380 nm to defects or impurities (e.g., ligands or unreacted precursors), although further work will be required to better understand the nature of these states. For the Cs2ZrBr6 NC thin film, we find an absorption onset at ∼330 nm (similar for the NC suspension; see Figure S6 in the SI) and absorption peaks between this onset and ∼225 nm, which may also be related to transitions similar to those observed for the Cl samples but now with Br.
Figure 3.
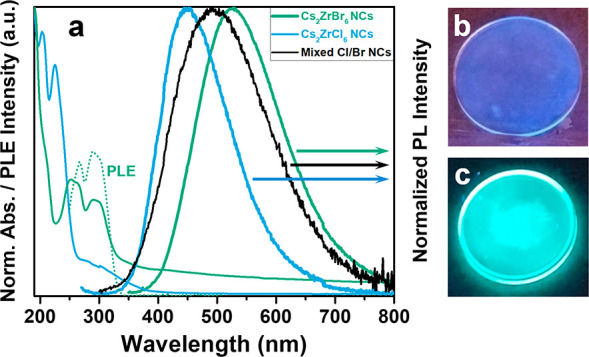
Optical characterization of Cs2ZrX6 NC thin films. (a) Blue curves represent UV-Vis absorption and PL spectrum (excitation: 250 nm) of encapsulated Cs2ZrCl6 NC thin film. Green curves represent UV-Vis absorption, PLE (dotted line; emission: 519 nm) and PL spectrum (excitation: 300 nm) of encapsulated Cs2ZrBr6 NC thin film. The black curve represents PL spectrum (excitation: 250 nm) of encapsulated, mixed Cs2ZrCl6 and Cs2ZrBr6 NC thin film. The UV-Vis absorption and PL spectra are normalized to the respective maximum intensity of the scan. The UV-Vis absorption spectrum of the Cs2ZrBr6 NC thin film shows a broad background that we attribute to scattering. (b, c) Photographs of emission from a Cs2ZrCl6 NC thin film (panel (b)) and a Cs2ZrBr6 NC thin film (panel (c)) on fused silica substrates, respectively, irradiated with 254 nm light from a UV lamp. The PL arises from the NC thin films.
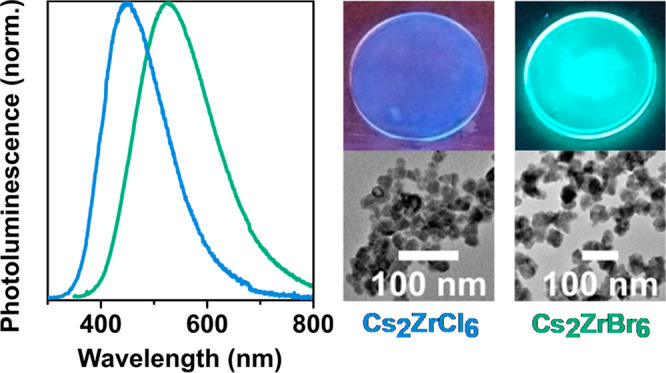
Figure 3a shows the PL spectra of encapsulated Cs2ZrCl6 and Cs2ZrBr6 NC thin films upon photo-excitation at 250 and 300 nm, respectively. The spectra show broad, asymmetric peaks with maxima at ∼450 nm (blue-white) and ∼528 nm (green-white) for Cs2ZrCl6 and Cs2ZrBr6 NC thin films, respectively. The PL spectra of the NC thin films and suspensions are similar to the spectra of the respective bulk powders (see Figure S6), and are in good agreement with reports on PL emission of Cs2ZrCl6 bulk powders48 and Cs2ZrBr6 crystals.15Figures 3b and 3c show photographs of the PL from thin films of Cs2ZrCl6 NCs and Cs2ZrBr6 NCs, respectively, under 254 nm UV-light excitation, clearly showing the difference in emission color (see Figure S6 in the SI for a photograph of the NC suspension in toluene). We determine a large Stokes shift of ∼225 nm for the Cs2ZrCl6 NC thin film sample, defined as the difference between the peak at ∼225 nm in the absorption spectrum and the peak emission at ∼450 nm in the PL spectrum, and a similar magnitude (∼230 nm) for the Cs2ZrBr6 NC thin film sample. The progressive shift of the absorption/PL between the Cl and Br samples demonstrates that the optical properties are also tunable in NC thin film form through halide engineering. We demonstrate this further by depositing films from precursor solutions comprised of mixed colloidal suspensions of Cs2ZrCl6 and Cs2ZrBr6 NCs, observing a PL spectrum intermediate between the two original PL peaks of pure Cs2ZrCl6 and Cs2ZrBr6 NC thin films (Figure 3a); selective PLE measurements confirm the contributions to the emission from both Cs2ZrCl6 (higher energy emission) and Cs2ZrBr6 (lower energy emission) in the mixed film (see Figure S7). These observations suggest the simultaneous presence of Cs2ZrCl6 and Cs2ZrBr6 NCs in the mixed sample, allowing us to tune the broad, white emission without being limited by fast anion exchange reactions that hinder such tunability in conventional lead-based CsPbX3 NCs.13,14 Although further work will be required to ascertain the exact reason for the stability of the mixed-anion Cs2ZrX6 NC sample in comparison to the lead-based mixed-anion perovskites, we speculate that it could be due to a lack of interstitial sites in the crystal structure of Cs2ZrX6, because of its structurally isolated ZrX62– octahedra.
Time-resolved PL measurements of an encapsulated Cs2ZrBr6 NC thin film (see Figure S8 in the SI) reveal a long-lived component that we fit with a double-exponential decay yielding decay constants of τ1 = 780 ± 40 ns and τ2 = 4500 ± 100 ns of comparable amplitude contribution, in good agreement with the values extracted from scintillation decay measurements on Cs2ZrBr6 bulk crystals.15 Furthermore, the PLQY of the Cs2ZrBr6 NC thin film is 45% without considering emitted photons lost to thin film waveguiding (see the Characterization section in the SI), with the PLE spectrum closely matching the absorption spectrum (Figure 3a). These results match well with the PLQY of the Cs2ZrBr6 NC suspension (∼40%) and powder (∼44%), demonstrating that we can effectively translate the solution or powder properties to thin film forms required for device applications. Such PLQY values are obtained even after depositing films from solutions stored under inert glovebox conditions for several months, indicating the encouraging stability of these materials. We note that instrumental limitations inhibit similar quantitative or time-resolved analyses of the Cs2ZrCl6 NCs, which requires deeper UV excitation. The combination of large Stokes shifts and broad, asymmetric PL features suggests radiative recombination may originate from self-trapped excitons,49 which has been reported for similar compounds such as Cs2HfCl6.50−52 The lack of subgap contribution to the PLE spectrum of the Cs2ZrBr6 NC thin films also indicates that radiative recombination is not due to permanent defect states.53 The similar PLQY values of the bulk powder, NC suspensions, and NC thin films support the hypothesis that (i) the self-trapped exciton emission is intrinsic, related to the bulk, and (ii) the emission, consequently, is not strongly dependent on the crystallite size for the sizes studied here nor on its surface/volume ratio. Although further work will be required to elucidate all of the recombination processes in these Cs2ZrX6 NC thin films, these results reveal very efficient and tunable radiative recombination.
In order to support our collective experimental findings, we perform electronic structure and optical property calculations on Cs2ZrCl6. We performed all periodic DFT calculations within the Vienna Ab Initio Simulation Package (VASP);54−57 further computational details are found in the SI. Relaxation of the crystal structure of Cs2ZrCl6 with HSE06 (the Heyd–Scuseria–Ernzerhof exchange-correlation functional) results in an optimized lattice parameter of 10.67 Å, overestimated by ∼3%, with respect to the RT experimental lattice parameter of 10.407 Å (cf. JCPDS File No. 01-074-1001).20
The band structure of Cs2ZrCl6 (Figure 4a), calculated using HSE06 with the addition of spin-orbit coupling (SOC), demonstrates a localized nature of both valence and conduction bands, with very low dispersion in both. The structure is similar to that previously established for Cs2HfCl6 by hybrid DFT.50 The fundamental band gap of Cs2ZrCl6 at 5.06 eV is indirect, with the valence band maximum (VBM) occurring at Γ, and the conduction band minimum (CBM) at X. However, the minimal band dispersion means that the lowest available direct transition is only 20 meV higher, at 5.08 eV (244 nm), aligning well with the experimental onset of absorbance seen at ∼260 nm (4.77 eV) in the Cs2ZrCl6 NC thin film sample. The HSE06+SOC band structure of Cs2ZrBr6 (see Figure S9 in the SI) is almost identical in shape to that of Cs2ZrCl6 with VBM and CBM at Γ and X, respectively, but with a gap of reduced magnitude, because of the substitution of Cl with Br; the separation between the indirect fundamental gap (3.91 eV) and the lowest direct transition at Γ (3.93 eV) is also similar (∼20 meV). This magnitude of gap is also in reasonable agreement with the experimental absorbance onset at ∼330 nm (3.76 eV) for the Cs2ZrBr6 NC thin film sample. Calculation of the high-frequency dielectric function and absorption coefficient of Cs2ZrCl6 demonstrates that the absorption onset at the direct gap is strong, albeit localized in the 5–6 eV region, because of the narrow bandwidth of the conduction band (Figure 4b); the calculated optical absorption of Cs2ZrBr6 is again similar to that of Cs2ZrCl6, with the onset of absorption at lower energy, concomitant with the lower fundamental gap (see Figure S9).
Figure 4.
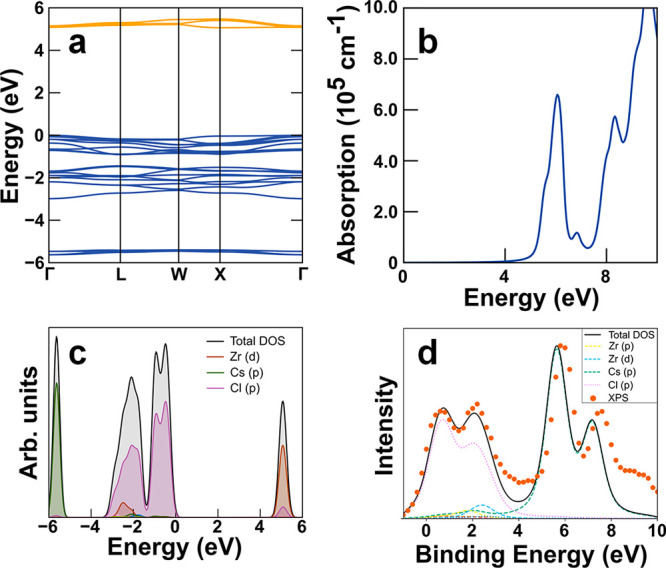
Electronic structure and optical property calculations within DFT-HSE on Cs2ZrCl6. (a) Calculated band structure for Cs2ZrCl6, showing the indirect 5.06 eV fundamental band gap (Γ–X) and the direct band gap at 5.08 eV (Γ–Γ). The valence and conduction bands are marked in blue and orange, respectively. The VBM is set to 0 eV. (b) Calculated absorption coefficient spectrum of Cs2ZrCl6. (c) Total and partial DOS of Cs2ZrCl6. For reasons of clarity, the legend omits labels for individual elements and corresponding orbitals that provide only minor contributions to the DOS in the regions around valence and conduction bands. (d) XPS valence band spectrum of a Cs2ZrCl6 NC sample (orange dots), shifted by ∼4.5 eV toward lower binding energies, in comparison to the photoionization cross-section weighted DOS broadened by a convolution of Gaussian and Lorentzian curves of suitable width (black curve).
The electronic density of states (DOS, cf. Figure 4c) calculated using HSE06+SOC, demonstrates that the valence electronic states of both Cs2ZrCl6 and Cs2ZrBr6 are highly localized: the upper valence band is fully dominated by halide p states, while the conduction band primarily consists of the empty Zr d states mixed with some halide p, with the corresponding occupied states lying lower in the valence band (−3 eV).
After weighting each orbital by its photoionization cross section and convolving with Gaussian and Lorentzian curves of suitable width to simulate experimental broadening,58 we compare the calculated electronic DOS directly with X-ray photoelectron spectroscopy (XPS) valence band spectra measured on a Cs2ZrCl6 NC thin film sample (Figure 4d, orange dots). Here, for Cs2ZrCl6, the weighted HSE06 DOS accurately reproduces the primary features of the valence band determined from XPS measurements and, other than a shift of the Cs p, split by SOC, toward lower binding energies by 0.2 eV, presents strong agreement with experiment. Such agreement between peak positions and intensities predicted by hybrid DFT with XPS is similar to previous reports in a variety of d-block materials in the absence of plasmon features.59−61 In addition, we detect Cs, Zr, and Cl in the XPS survey scan (see Figure S10 and the SI for details), which is consistent with the expected presence of these elements for the Cs2ZrCl6 NC thin film sample.
In summary, we have reported the colloidal synthesis of antifluorite Cs2ZrCl6 and Cs2ZrBr6 NC samples that can be processed into thin films. We used XRD and conventional and surface-enhanced solid-state NMR measurements to confirm the structural and compositional properties of the products, validating that the surfaces are primarily capped by oleate ligands and a distribution of disordered Cs configurations. Thin films of the Cs2ZrCl6 and Cs2ZrBr6 NCs exhibit broad blue-white and green-white PL, respectively, with a large Stokes shift. In addition, we measured a PLQY of 45% for a Cs2ZrBr6 NC thin film. The white emission color is tunable by modular mixing of the Cs2ZrCl6 and Cs2ZrBr6 NC components, revealing that anion exchange does not limit color tunability in the same way as that observed for lead halide perovskites. We present band structure calculations, which, in turn, provide strong support for our chemical and optical experimental results. These results provide an important step toward opening up a new family of tunable lead-free emitting materials for emerging thin film optoelectronic applications, including use as light-converting phosphors and electroluminescent light-emitting diodes.
Acknowledgments
S.D.S. acknowledges the Royal Society and Tata Group (UF150033). J.S. and S.D.S. acknowledge the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (HYPERION, Grant Agreement No. 756962). This work has received funding from the European Union’s Horizon 2020 Research and Innovation Program, under Marie Skłodowska-Curie Grant Agreement No. 841136. A.A. acknowledges the Royal Society for funding. We are grateful to the UK Materials and Molecular Modelling Hub for computational resources, which is partially funded by EPSRC (No. EP/P020194/1) and to UCL for the provision of the Legion (Legion@UCL), Myriad (Myriad@UCL) and Grace (Grace@UCL) computing clusters. Computational work was also performed on the ARCHER UK National Supercomputing Service, via our membership of the UK’s HEC Materials Chemistry Consortium, funded by EPSRC (Nos. EP/L000202 and EP/R029431). J.X. thanks the EPSRC Cambridge NanoDTC (No. EP/L015978/1). W.-W.L. and J.L.M.-D. acknowledge support from EPSRC Grant Nos. EP/L011700/1 and EP/N004272/1, and the Isaac Newton Trust (Minute 13.38(k)). J.L.M.-D. acknowledges support from the Royal Academy of Engineering, through Grant No. CiET1819_24. This work was supported through the Cambridge Royce Facilities (Grant No. EP/P024947/1) and Sir Henry Royce Institute (recurrent Grant No. EP/R00661X/1). S.M. acknowledges an EPSRC studentship. K.G. appreciates support from the Polish Ministry of Science and Higher Education within the Mobilnosc Plus program (Grant No. 1603/MOB/V/2017/0). The DNP MAS NMR experiments were performed at the Nottingham DNP MAS NMR Facility, which is funded by the University of Nottingham and EPSRC (Nos. EP/L022524/1 and EP/R042853/1). We thank Adam Brown for support with XPS measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmaterialslett.0c00393.
Additional experimental details; details on sample characterization with XRD, TEM, NMR spectroscopy, UV-Vis absorption, PL, PLE and time-resolved PL spectroscopy, PLQY measurements, and XPS; computational details on DFT calculations; XRD data on sample degradation in air; FFT analysis on HR-TEM images of Cs2ZrX6 NCs; additional NMR, TEM, UV-Vis absorption, PL, PL decay, PLE and XPS data; results on electronic and optical property calculations on Cs2ZrBr6) (PDF)
CIF of Cs2ZrBr6 (CIF)
The authors declare no competing financial interest.
Notes
Additionally, the data of this publication is available under https://doi.org/10.17863/CAM.59283.
Supplementary Material
References
- Stranks S. D. Nonradiative Losses in Metal Halide Perovskites. ACS Energy Lett. 2017, 2, 1515–1525. 10.1021/acsenergylett.7b00239. [DOI] [Google Scholar]
- Giustino F.; Snaith H. J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. 10.1021/acsenergylett.6b00499. [DOI] [Google Scholar]
- Li J.; Cao H.-L.; Jiao W.-B.; Wang Q.; Wei M.; Cantone I.; Lü J.; Abate A. Biological Impact of Lead from Halide Perovskites Reveals the Risk of Introducing a Safe Threshold. Nat. Commun. 2020, 11, 310. 10.1038/s41467-019-13910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z.; Song Z.; Yan Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. 10.1002/adma.201803792. [DOI] [PubMed] [Google Scholar]
- Zhao X.-G.; Yang D.; Ren J.-C.; Sun Y.; Xiao Z.; Zhang L. Rational Design of Halide Double Perovskites for Optoelectronic Applications. Joule 2018, 2, 1662–1673. 10.1016/j.joule.2018.06.017. [DOI] [Google Scholar]
- Ju M.-G.; Chen M.; Zhou Y.; Garces H. F.; Dai J.; Ma L.; Padture N. P.; Zeng X. C. Earth-Abundant Nontoxic Titanium(IV)-Based Vacancy-Ordered Double Perovskite Halides with Tunable 1.0 to 1.8 eV Bandgaps for Photovoltaic Applications. ACS Energy Lett. 2018, 3, 297–304. 10.1021/acsenergylett.7b01167. [DOI] [Google Scholar]
- Evans H. A.; Fabini D. H.; Andrews J. L.; Koerner M.; Preefer M. B.; Wu G.; Wudl F.; Cheetham A. K.; Seshadri R. Hydrogen Bonding Controls the Structural Evolution in Perovskite-Related Hybrid Platinum(IV) Iodides. Inorg. Chem. 2018, 57, 10375–10382. 10.1021/acs.inorgchem.8b01597. [DOI] [PubMed] [Google Scholar]
- Sakai N.; Haghighirad A. A.; Filip M. R.; Nayak P. K.; Nayak S.; Ramadan A.; Wang Z.; Giustino F.; Snaith H. J. Solution-Processed Cesium Hexabromopalladate(IV), Cs2PdBr6, for Optoelectronic Applications. J. Am. Chem. Soc. 2017, 139, 6030–6033. 10.1021/jacs.6b13258. [DOI] [PubMed] [Google Scholar]
- Chen M.; Ju M.-G.; Carl A. D.; Zong Y.; Grimm R. L.; Gu J.; Zeng X. C.; Zhou Y.; Padture N. P. Cesium Titanium(IV) Bromide Thin Films Based Stable Lead-Free Perovskite Solar Cells. Joule 2018, 2, 558–570. 10.1016/j.joule.2018.01.009. [DOI] [Google Scholar]
- Saparov B.; Sun J.-P.; Meng W.; Xiao Z.; Duan H.-S.; Gunawan O.; Shin D.; Hill I. G.; Yan Y.; Mitzi D. B. Thin-Film Deposition and Characterization of a Sn-Deficient Perovskite Derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. 10.1021/acs.chemmater.6b00433. [DOI] [Google Scholar]
- Zhou L.; Liao J.-F.; Huang Z.-G.; Wang X.-D.; Xu Y.-F.; Chen H.-Y.; Kuang D.-B.; Su C.-Y. All-Inorganic Lead-Free Cs2PdX6 (X = Br, I) Perovskite Nanocrystals with Single Unit Cell Thickness and High Stability. ACS Energy Lett. 2018, 3, 2613–2619. 10.1021/acsenergylett.8b01770. [DOI] [Google Scholar]
- Wang A.; Yan X.; Zhang M.; Sun S.; Yang M.; Shen W.; Pan X.; Wang P.; Deng Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. 10.1021/acs.chemmater.6b01329. [DOI] [Google Scholar]
- Nedelcu G.; Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Grotevent M. J.; Kovalenko M. V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. 10.1021/acs.nanolett.5b02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman Q. A.; D’Innocenzo V.; Accornero S.; Scarpellini A.; Petrozza A.; Prato M.; Manna L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. 10.1021/jacs.5b05602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K.; Fujimoto Y.; Koshimizu M.; Nakauchi D.; Tanaka H.; Yanagida T.; Asai K. Luminescence and Scintillation Properties of Cs2HfBr6 and Cs2ZrBr6 Crystals. Jpn. J. Appl. Phys. 2018, 57, 030310. 10.7567/JJAP.57.030310. [DOI] [Google Scholar]
- Saeki K.; Fujimoto Y.; Koshimizu M.; Yanagida T.; Asai K. Comparative Study of Scintillation Properties of Cs2HfCl6 and Cs2ZrCl6. Appl. Phys. Express 2016, 9, 042602. 10.7567/APEX.9.042602. [DOI] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M.; Caligiuri V.; Wang M.; Goldoni L.; Prato M.; Krahne R.; De Trizio L.; Manna L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. 10.1021/jacs.7b13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummen P. J. H.; Donker H.; Smit W. M. A.; Blasse G. Jahn-Teller Distortion in the Excited State of Tellurium(IV) in Cs2MCl6 (M=Zr, Sn). Chem. Phys. Lett. 1988, 144, 460–462. 10.1016/0009-2614(88)87296-8. [DOI] [Google Scholar]
- Engel G. Die Kristallstrukturen Einiger Hexachlorokomplexsalze. Z. Kristallogr. - Cryst. Mater. 1935, 90, 341–373. 10.1524/zkri.1935.90.1.341. [DOI] [Google Scholar]
- Momma K.; Izumi F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. 10.1107/S0021889811038970. [DOI] [Google Scholar]
- Swanson H. E.; Fuyat R. K.; Ugrinic G. M. Standard X-Ray Diffraction Powder Patterns. Natl. Bur. Stand. (U.S.) 1954, 3, 49.(Circular 539). [Google Scholar]
- Metcalf D. H.; Dai S.; Del Cul G. D.; Toth L. M. Luminescence Spectra of Single Crystals of Cs2ZrCl6:UO2Cl42- at Low Temperatures. Vibronic Structure of UO22+ Doped in a Cubic Host. Inorg. Chem. 1995, 34, 5573–5577. 10.1021/ic00126a030. [DOI] [Google Scholar]
- Xu W.; Dai S.; Toth L. M.; Peterson J. R. Blue Up-Conversion Emission from U4+-Ion Doped into Cs2ZrCl6 Single Crystal under Green Light (19436 cm-1) Excitation. Chem. Phys. 1995, 193, 339–344. 10.1016/0301-0104(94)00393-O. [DOI] [Google Scholar]
- Aebischer A.; Salley G. M.; Güdel H. U. Near Infrared to Visible Photon Upconversion in Re4+ Doped Cs2ZrBr6. J. Chem. Phys. 2002, 117, 8515–8522. 10.1063/1.1513455. [DOI] [Google Scholar]
- Rossini A. J.; Zagdoun A.; Lelli M.; Gajan D.; Rascón F.; Rosay M.; Maas W. E.; Copéret C.; Lesage A.; Emsley L. One Hundred Fold Overall Sensitivity Enhancements for Silicon-29 NMR Spectroscopy of Surfaces by Dynamic Nuclear Polarization with CPMG Acquisition. Chem. Sci. 2012, 3, 108–115. 10.1039/C1SC00550B. [DOI] [Google Scholar]
- Rossini A. J.; Zagdoun A.; Lelli M.; Lesage A.; Copéret C.; Emsley L. Dynamic Nuclear Polarization Surface Enhanced NMR Spectroscopy. Acc. Chem. Res. 2013, 46, 1942–1951. 10.1021/ar300322x. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Rossini A. J.; Kriegner D.; Valla M.; de Kergommeaux A.; Walter M.; Kravchyk K. V.; Nachtegaal M.; Stangl J.; Malaman B.; Reiss P.; Lesage A.; Emsley L.; Copéret C.; Kovalenko M. V. Unraveling the Core-Shell Structure of Ligand-Capped Sn/SnOx Nanoparticles by Surface-Enhanced Nuclear Magnetic Resonance, Mössbauer, and X-Ray Absorption Spectroscopies. ACS Nano 2014, 8, 2639–2648. 10.1021/nn406344n. [DOI] [PubMed] [Google Scholar]
- Piveteau L.; Ong T.-C.; Rossini A. J.; Emsley L.; Copéret C.; Kovalenko M. V. Structure of Colloidal Quantum Dots from Dynamic Nuclear Polarization Surface Enhanced NMR Spectroscopy. J. Am. Chem. Soc. 2015, 137, 13964–13971. 10.1021/jacs.5b09248. [DOI] [PubMed] [Google Scholar]
- Walder B. J.; Berk C.; Liao W.-C.; Rossini A. J.; Schwarzwälder M.; Pradere U.; Hall J.; Lesage A.; Copéret C.; Emsley L. One- and Two-Dimensional High-Resolution NMR from Flat Surfaces. ACS Cent. Sci. 2019, 5, 515–523. 10.1021/acscentsci.8b00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagdoun A.; Casano G.; Ouari O.; Lapadula G.; Rossini A. J.; Lelli M.; Baffert M.; Gajan D.; Veyre L.; Maas W. E.; Rosay M.; Weber R. T.; Thieuleux C.; Coperet C.; Lesage A.; Tordo P.; Emsley L. A Slowly Relaxing Rigid Biradical for Efficient Dynamic Nuclear Polarization Surface-Enhanced NMR Spectroscopy: Expeditious Characterization of Functional Group Manipulation in Hybrid Materials. J. Am. Chem. Soc. 2012, 134, 2284–2291. 10.1021/ja210177v. [DOI] [PubMed] [Google Scholar]
- Maly T.; Miller A.-F.; Griffin R. G. In Situ High-Field Dynamic Nuclear Polarization—Direct and Indirect Polarization of 13C Nuclei. ChemPhysChem 2010, 11, 999–1001. 10.1002/cphc.200900908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abragam A.The Principles of Nuclear Magnetism; International Series of Monographs on Physics; Oxford University Press: Oxford, U.K., 1961. [Google Scholar]
- Pines A.; Gibby M. G.; Waugh J. S. Proton-Enhanced Nuclear Induction Spectroscopy 13C Chemical Shielding Anisotropy in Some Organic Solids. Chem. Phys. Lett. 1972, 15, 373–376. 10.1016/0009-2614(72)80191-X. [DOI] [Google Scholar]
- Kubicki D. J.; Rossini A. J.; Purea A.; Zagdoun A.; Ouari O.; Tordo P.; Engelke F.; Lesage A.; Emsley L. Amplifying Dynamic Nuclear Polarization of Frozen Solutions by Incorporating Dielectric Particles. J. Am. Chem. Soc. 2014, 136, 15711–15718. 10.1021/ja5088453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan M. P.; Chen Y.; Blome-Fernández R.; Stein J. L.; Pach G. F.; Adamson M. A. S.; Neale N. R.; Cossairt B. M.; Vela J.; Rossini A. J. Probing the Surface Structure of Semiconductor Nanoparticles by DNP SENS with Dielectric Support Materials. J. Am. Chem. Soc. 2019, 141, 15532–15546. 10.1021/jacs.9b05509. [DOI] [PubMed] [Google Scholar]
- Cadars S.; Smith B. J.; Epping J. D.; Acharya S.; Belman N.; Golan Y.; Chmelka B. F. Atomic Positional Versus Electronic Order in Semiconducting ZnSe Nanoparticles. Phys. Rev. Lett. 2009, 103, 136802. 10.1103/PhysRevLett.103.136802. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Smock S. R.; Flintgruber A. H.; Perras F. A.; Brutchey R. L.; Rossini A. J. Surface Termination of CsPbBr3 Perovskite Quantum Dots Determined by Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2020, 142, 6117–6127. 10.1021/jacs.9b13396. [DOI] [PubMed] [Google Scholar]
- Shamsi J.; Kubicki D.; Anaya M.; Liu Y.; Ji K.; Frohna K.; Grey C. P.; Friend R. H.; Stranks S. D. Stable Hexylphosphonate-Capped Blue-Emitting Quantum-Confined CsPbBr3 Nanoplatelets. ACS Energy Lett. 2020, 5, 1900–1907. 10.1021/acsenergylett.0c00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M.; Ijaz P.; Baranov D.; Goldoni L.; Petralanda U.; Akkerman Q.; Abdelhady A. L.; Prato M.; Bianchini P.; Infante I.; Manna L. Shape-Pure, Nearly Monodispersed CsPbBr3 Nanocubes Prepared Using Secondary Aliphatic Amines. Nano Lett. 2018, 18, 7822–7831. 10.1021/acs.nanolett.8b03598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassitepe E.; Yang Z.; Voznyy O.; Kim Y.; Walters G.; Castañeda J. A.; Kanjanaboos P.; Yuan M.; Gong X.; Fan F.; Pan J.; Hoogland S.; Comin R.; Bakr O. M.; Padilha L. A.; Nogueira A. F.; Sargent E. H. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. 10.1002/adfm.201604580. [DOI] [Google Scholar]
- Krieg F.; Ochsenbein S. T.; Yakunin S.; Ten Brinck S.; Aellen P.; Süess A.; Clerc B.; Guggisberg D.; Nazarenko O.; Shynkarenko Y.; Kumar S.; Shih C.-J.; Infante I.; Kovalenko M. V. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. 10.1021/acsenergylett.8b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo J.; Ibáñez M.; Geiregat P.; Nedelcu G.; Walravens W.; Maes J.; Martins J. C.; Van Driessche I.; Kovalenko M. V.; Hens Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. 10.1021/acsnano.5b06295. [DOI] [PubMed] [Google Scholar]
- Ravi V. K.; Santra P. K.; Joshi N.; Chugh J.; Singh S. K.; Rensmo H.; Ghosh P.; Nag A. Origin of the Substitution Mechanism for the Binding of Organic Ligands on the Surface of CsPbBr3 Perovskite Nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. 10.1021/acs.jpclett.7b02192. [DOI] [PubMed] [Google Scholar]
- Bodnarchuk M. I.; Boehme S. C.; Ten Brinck S.; Bernasconi C.; Shynkarenko Y.; Krieg F.; Widmer R.; Aeschlimann B.; Günther D.; Kovalenko M. V.; Infante I. Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals. ACS Energy Lett. 2019, 4, 63–74. 10.1021/acsenergylett.8b01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M.; Ijaz P.; Goldoni L.; Maggioni D.; Petralanda U.; Prato M.; Almeida G.; Infante I.; Manna L. Simultaneous Cationic and Anionic Ligand Exchange For Colloidally Stable CsPbBr3 Nanocrystals. ACS Energy Lett. 2019, 4, 819–824. 10.1021/acsenergylett.9b00140. [DOI] [Google Scholar]
- Brisdon B. J.; Lester T. E.; Walton R. A. Complex Halides of Transition Metals—III Electronic Absorption Spectra of Hexahalotitanates(IV), Vanadates(IV), and Zirconates(IV). Spectrochim. Acta, Part A 1967, 23, 1969–1976. 10.1016/0584-8539(67)80083-7. [DOI] [Google Scholar]
- Bryan P. S.; Ferranti S. A. Luminescence of Cs2ZrCl6 and Cs2HfCl6. J. Lumin. 1984, 31-32, 117–119. 10.1016/0022-2313(84)90220-5. [DOI] [Google Scholar]
- Li S.; Luo J.; Liu J.; Tang J. Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. 10.1021/acs.jpclett.8b03604. [DOI] [PubMed] [Google Scholar]
- Kang By; Biswas K. Carrier Self-Trapping and Luminescence in Intrinsically Activated Scintillator: Cesium Hafnium Chloride (Cs2HfCl6). J. Phys. Chem. C 2016, 120, 12187–12195. 10.1021/acs.jpcc.6b02496. [DOI] [Google Scholar]
- Buryi M.; Král R.; Babin V.; Páterek J.; Vaněček V.; Veverka P.; Kohoutková M.; Laguta V.; Fasoli M.; Villa I.; Cova F.; Vedda A.; Nikl M. Trapping and Recombination Centers in Cesium Hafnium Chloride Single Crystals: EPR and TSL Study. J. Phys. Chem. C 2019, 123, 19402–19411. 10.1021/acs.jpcc.9b05760. [DOI] [Google Scholar]
- Král R.; Babin V.; Mihóková E.; Buryi M.; Laguta V. V.; Nitsch K.; Nikl M. Luminescence and Charge Trapping in Cs2HfCl6 Single Crystals: Optical and Magnetic Resonance Spectroscopy Study. J. Phys. Chem. C 2017, 121, 12375–12382. 10.1021/acs.jpcc.7b02327. [DOI] [Google Scholar]
- Smith M. D.; Karunadasa H. I. White-Light Emission from Layered Halide Perovskites. Acc. Chem. Res. 2018, 51, 619–627. 10.1021/acs.accounts.7b00433. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B: Condens. Matter Mater. Phys. 1993, 47, 558–561. 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal--Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 49, 14251–14269. 10.1103/PhysRevB.49.14251. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 11169–11186. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Jackson A. J.; Ganose A. M.; Regoutz A.; Egdell R. G.; Scanlon D. O. Galore: Broadening and Weighting for Simulation of Photoelectron Spectroscopy. JOSS 2018, 3, 773. 10.21105/joss.00773. [DOI] [Google Scholar]
- Savory C. N.; Ganose A. M.; Travis W.; Atri R. S.; Palgrave R. G.; Scanlon D. O. An Assessment of Silver Copper Sulfides for Photovoltaic Applications: Theoretical and Experimental Insights. J. Mater. Chem. A 2016, 4, 12648–12657. 10.1039/C6TA03376H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathasivam S.; Williamson B. A. D.; Althabaiti S. A.; Obaid A. Y.; Basahel S. N.; Mokhtar M.; Scanlon D. O.; Carmalt C. J.; Parkin I. P. Chemical Vapor Deposition Synthesis and Optical Properties of Nb2O5 Thin Films with Hybrid Functional Theoretical Insight into the Band Structure and Band Gaps. ACS Appl. Mater. Interfaces 2017, 9, 18031–18038. 10.1021/acsami.7b00907. [DOI] [PubMed] [Google Scholar]
- Regoutz A.; Ganose A. M.; Blumenthal L.; Schlueter C.; Lee T.-L.; Kieslich G.; Cheetham A. K.; Kerherve G.; Huang Y.-S.; Chen R.-S.; Vinai G.; Pincelli T.; Panaccione G.; Zhang K. H. L.; Egdell R. G.; Lischner J.; Scanlon D. O.; Payne D. J. Insights into the Electronic Structure of OsO2 Using Soft and Hard X-Ray Photoelectron Spectroscopy in Combination with Density Functional Theory. Phys. Rev. Materials 2019, 3, 025001. 10.1103/PhysRevMaterials.3.025001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.