Abstract
The genetic sexing strain (GSS) of the Mediterranean fruit fly (Ceratitis capitata (Wiedemann)) Vienna 8D53− is based on a male-linked translocation system and uses two selectable markers for male-only production, the white pupae (wp) and the temperature sensitivity lethal (tsl) genes. In this GSS, males emerge from brown pupae and are resistant to high temperatures while females emerge from white pupae, are sensitive to high temperatures. However, double homozygous females (wp tsl/wp tsl) exhibit a slower development rate compared to heterozygous males (wp+tsl+/wp tsl) during the larval stage, which was attributed to the pleiotropic effects of the tsl gene. We present the first evidence that this slower development is due to a different gene, here namely slow development (sd), which is closely linked to the tsl gene. Taking advantage of recombination phenomena between the two loci, we report the isolation of a novel temperature sensitivity lethal strain using the wp mutation as a morphological marker, which showed faster development (wp tsl FD) during the larval stage and increased in its temperature sensitivity compared with the normal tsl strain. Moreover, the introgression of this novel wp tsl FD combined trait into the Vienna 8D53− GSS, resulted in a novel Vienna 8D53− FD GSS, where females showed differences in the thermal sensibility, larval development speed, and productivity profiles. The modification of these traits and their impact on the mass rearing of the GSS for sterile insect technique applications are discussed.
Keywords: Medfly, mass rearing, insect pest control, mutation
The Mediterranean fruit fly (Medfly), Ceratitis capitata (Wiedemann), is a major agricultural pest that causes direct damage to more than 400 fruit crops worldwide (De Meyer et al. 2002). The sterile insect technique (SIT) is successful tool for the areawide integrated pest management (IPM) programs of Medfly (Dyck et al. 2006). In SIT, mass-reared males are sterilized by irradiation and released into the field to mate with wild females, inseminating the wild females with sterile sperm resulting in fertility reduction and driving fly population densities below economic thresholds, or even local eradication (Dyck et al. 2006). SIT success relies on the mating efficiency of sterile males, which increases by threefold when only males are released (McInnis et al. 1994, Rendón et al. 2004).
Several genetic sexing strains (GSS) were developed that allow male-only releases, to improve C. capitata SIT efficiency (Franz 2005). These GSS are based on a reciprocal translocation between the Y-chromosome and the region of chromosome (autosome) 5 which includes the genes used as selectable markers, the white pupae (wp) and the temperature sensitivity lethal (tsl), both located on chromosome 5 (Robinson 1999, Franz 2005). In the GSS, only the white pupae (wp) marker (GSS-wp) was used, allowing the separation of white pupae females from brown (wild-type color) pupae males using a sorting machine (Robinson and Van Heemert 1982). Subsequently, the temperature sensitivity lethal (tsl) marker, closely linked to the wp gene, was isolated and used to construct a second GSS in which the males were heterozygous for the wild-type alleles of both the wp and tsl gene markers (Vienna 8D53−) emerged from brown pupae and were resistant to elevated temperatures while the females were homozygous for the recessive alleles of the wp and tsl gene markers, emerged from white pupae and were sensitive to high temperatures (Kerremans and Franz 1994, Franz 2005). Based on these traits, a protocol was developed for male-only production, which allowed the elimination of the double homozygous mutant females (wp tsl/wp tsl) by incubating 24-h-old embryos (eggs) at 34°C for 24 h while allowing the heterozygous males to survive.
The slow larval development phenotype was previously reported in C. capitata (sw) as well as in Anastrepha ludens (sl) (Diptera: Tephritidae) (Delprat et al. 2002, Meza et al. 2019). In both cases, the genes responsible for the slow larval development phenotype were mapped on chromosome 2. The slow larval development observed in Vienna 8D53− females has been so far considered as a pleiotropic effect of the tsl gene, which resides on the right arm of chromosome 5 (Caceres 2002, Franz 2005). However, this genetic correlation of thermal sensitivity and slow larval development could also arise from a tight, independent linkage between two different genes controlling the respective traits. In the case of pleiotropy, the correlation between traits is inherent, while a linkage can be broken by genetic recombination (Paaby and Rockman 2013).
Genetic recombination phenomena have been observed in the Vienna 8D53− GSS. For example, type-1 male recombination in Vienna 8D53− can occur either between the translocation breakpoint and the wp gene producing wp tsl male and wp+tsl+ female recombinants (type-1a) or between the wp gene and the tsl gene producing wp+tsl male and wp tsl+ female recombinants (type-1b) (Franz 2002, 2005). To contain the threat of recombination to the genetic integrity of the GSS, a filter rearing system (FRS) consisting of the establishment of a small mother colony, the only colony contributing biological material to the mass-rearing system, systematically eliminating the recombinant individuals to avoid its massive accumulation (Fisher and Caceres 2000). An additional approach was the development of inversions, which are known recombination suppressors, such as the chromosomal inversion D53 used for the construction of the Vienna 8D53+ GSS (Franz 2002, Augustinos et al. 2017, Zacharopoulou et al. 2017).
In the mass-rearing facilities, six consecutive daily larval collections are usually obtained during the rearing of the Vienna 8D53− strain. Brown pupae (males) are collected mainly during the first 2 d, while white pupae (females) are collected in the last 3 d (Caceres 2002). The slow larval development in females potentially can be used as a sexing mechanism alone or in combination with other traits like the pupal color morphological markers. However, in Medfly, the main sexing system is based on the temperature-sensitive lethal gene with the white pupae mutation acting as a selectable morphological marker. The slow development of female larvae is considered a negative trait because: 1) females may be forced to complete their development on a poor-quality larval diet since important nutrients may have been consumed by the fast developing males and 2) larval trays must be kept in the respective rearing area for two to three additional days with significant accompanying costs in terms of space, labor, and energy needed to maintain the necessary environmental rearing conditions (Caceres 2002, Meza et al. 2018).
In the present study, we report the construction of a novel tsl-based GSS, the Vienna 8D53− FD GSS, in which the females have faster development (FD) than in the conventional tsl strain. The new strain was developed using classical genetic approaches and exploiting genetic recombination phenomena between the two closely linked loci, tsl and slow development (sd), and has novel biological characteristics that can be used for the improvement of the mass-rearing efficiency as well as the overall enhancement and cost-effectiveness of SIT applications.
Materials and Methods
Study Site and Strains
The study was carried out at the Insect Pest Control Laboratory (IPCL) of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Seibersdorf, Austria, using the pure wp, wp tsl double mutant, and the FiM1 from the IPCL stock. The heterozygous lethal FiM1 balancer strain, which carries the wp recessive allele in repulsion and has the Sergeant dominant mutation (Sr2) as morphological marker (Gourzi et al. 2000), was used to avoid recombination during the isolation of the sd+ wild-type allele from an exceptional wp tsl sd+ female, resulting in a temperature-sensitive lethal strain marked with white pupae and fast development during the larval stage (wp tsl FD). We also used the Vienna 8D53− strain, which is a GSS generally used for mass rearing and SIT applications almost worldwide, from two different sources (although both strains had a common origin): 1) Valencia, Spain, refreshed in 2013 using flies from Valencia (J. Garcia, personal communication) and 2) El Pino, Guatemala, refreshed in 2012 with wild flies from Guatemala (E. Ramirez, personal communication). In a preliminary test, the Vienna 8D53− strain from Spain showed a higher number of white pupae in the first collection compared to the one from Guatemala and was therefore used to screen the first larval collection for the presence of wp, while the genetic background of Vienna 8D53− males from Guatemala were used for the construction of the novel GSS. During the comparative analysis of the novel GSS, the Vienna 8D53− from Guatemala was used as a positive tsl reference. In addition, a GSS-wp strain was constructed by backcrossing Guatemala Vienna 8D53− males to pure wp females and was used as a negative tsl reference (absence of tsl allele). All colonies were kept at 25 ± 1°C, 65% RH, and a photoperiod of 14:10 (L:D) h, with a photophase starting at 0800 hours.
Isolation of a Fast Developing Strain and the Construction of a Novel GSS (Vienna 8D53− FD)
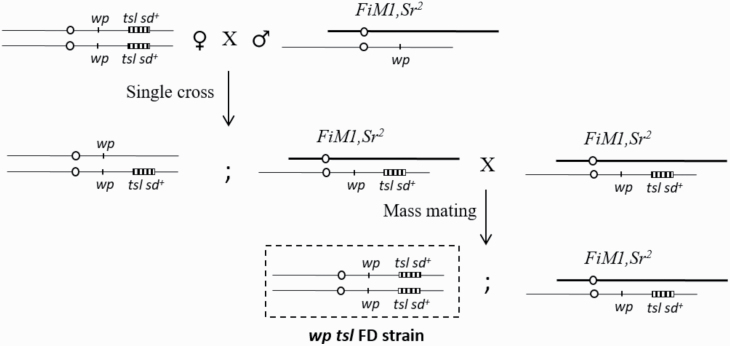
In an extensive screening of approximately 250,000 pupae Vienna 8D53− from Spain, 50 exceptional wp females were collected from the first larval collection and were crossed in single pairs with males from the FiM1 balancer strain. From the F1 offspring of every single cross (family), the wp mutants were discarded, and the Sr2 mutants that emerged from the brown pupae were interbred (FiM1, Sr2/wp tsl) en masse (Fig. 1). The larval development rate (larvae were collected daily; Supp. Table S1 [online only]) was recorded in the F2 offspring of each family. From families in which the white pupae exhibited similar larval development time as the brown pupae, all white pupae individuals with the fast development phenotype were selected and interbred, leading to the isolation of wp tsl FD lines (fast developing strains).
Fig. 1.
Crossing scheme used to recover the fast developing strain carrying the white pupae marker, from a single wp tsl sd+ homozygous female (circle = centromere).
The thermal sensitivity pattern of the fast developing strains was determined at six different heat treatments (31, 32, 33, 34, 35, and 36°C), using the wp and wp tsl mutant strains as a negative and positive reference, respectively. Then, the fast developing strains were selected and were used to construct the novel Vienna 8D53− FD GSS by crossing and backcrossing the Vienna 8D53− males from the Guatemala strain with the previously selected females from the fast developing strains.
Egg Heat Treatment
Immediately after collection, the eggs were aerated in bubbling water for 24 h at 24°C and then subjected to the heat treatment (31– 36°C) for another 24 h. Egg hatch was used as a response variable to measure thermal sensitivity.
Thermal Sensitivity
To measure the thermal sensitivity of the novel Vienna 8D53− FD, 1,000 eggs from each strain were exposed to four different heat treatments (24, 34, 35, and 36°C), with each treatment being replicated 10 times. After 24 h of incubation at 24°C, eggs were subject to heat treatment and then were placed onto carrot diet for eclosion and larval development (Tanaka et al. 1970). Eggs kept at 24°C during the entire incubation period were used as control. The number of white and wild-type (brown) pupae was recorded. The GSS-wp and Vienna 8D53− from Guatemala were used as negative and positive tsl references.
Larval Developmental Duration and Pupal Production Pattern
One thousand eggs from each one of the following strains GSS-wp, Vienna 8D53−, Vienna 8D53− FD_28, and Vienna 8D53− FD_37 were incubated for 2 d (25 ± 1°C, 65% RH) on moist filter paper placed in Petri dishes. After incubation, mature embryos were transferred into carrot diet (26°C, 50% RH) in a Petri dish (20 cm diameter × 2 cm) located inside a plastic rearing try (30 cm length × 30 cm width × 8 cm height) with the bottom covered by sawdust (Augustinos et al. 2017). This provided ventilation and space for mature larvae that leave the diet for pupation. Mature larvae and prepupae were collected daily, and upon the completion of ecdysis, the number of white and wild-type (brown) pupae was daily recorded. The pupae of each group (brown and white) were transferred to Petri dishes where adult emergence was also recorded daily. This procedure was replicated five times per strain.
Fecundity Following Mass-Rearing Procedures
Thirty single pair crosses were set up for each of the following GSS, GSS-wp, Vienna 8D53−, Vienna 8D53− FD_28, and Vienna 8D53− FD_37 strains in a transparent plastic cages (0.4-liter capacity) using freshly emerged adults. Each cage was provided with a standard diet of enzymatic yeast hydrolysate and sugar (1:3) with water supplied ad libitum. An oviposition device filled with water and covered with mesh was located on the cage side. Stress-related mortality (e.g., transfer) was extremely low (<1% mortality during the first 48 h postcapture). Survival of both sexes was monitored daily, and the number of eggs laid by individual females was recorded for 20 d postemergence.
Statistical Analyses
All data were tested for normality (Shapiro–Wilks test) and homogeneity of variance (Levene’s test). For the isolation of fast developing strain and the construction of a novel Vienna 8D53−FD GSS experiment, the thermal sensitivity and pupal production patterns were analyzed for differences among the strains using a two-way analysis of variance (ANOVA) followed by post hoc multiple comparisons and Tukey–Kramer tests. The response variable was egg hatch and the factors were the strains and temperature (thermal treatment). For the experiment of thermal sensitivity, the response variable was the number of white pupae and brown pupae, with thermal treatment as an independent variable. A two-way ANOVA was used to analyze the differences in pupae production among the strains, while one-way ANOVAs were used to independently analyze the production of white and brown pupae. For the experiment larval developmental duration and pupal production pattern, we used ANOVA to identify differences in the time required for larval development until pupation among the GSS. For the experiment fecundity following mass-rearing procedures, we used a generalized linear model (GLM) with Poisson distribution to test for differences in fecundity (number of eggs per female) among the strains, with strains and time (days) as factors. All analyses were performed using JMP-Pro version 13.1.0.
Results
Establishment of Fast Developing Strains and Construction of a Novel Vienna 8D53− FD GSS
Establishment of fast developing strains
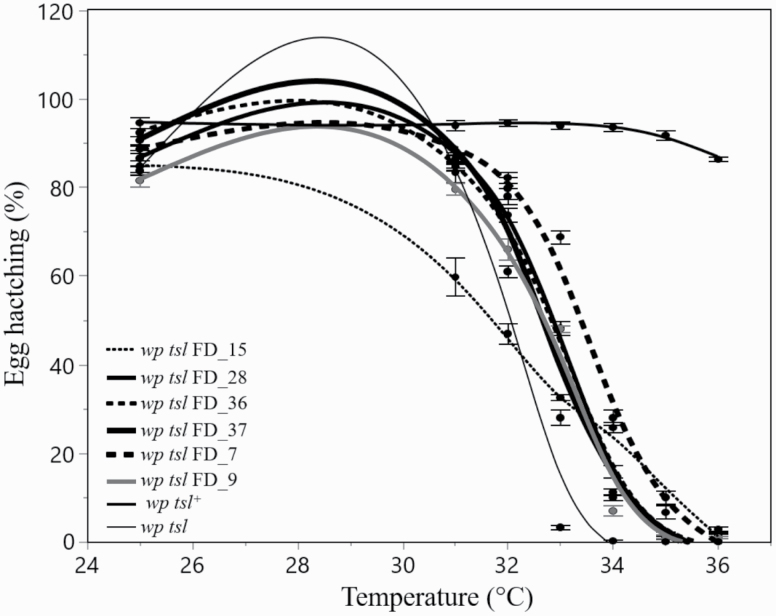
After applying the appropriate crossing scheme illustrated in Fig. 1, fast development was observed in 6 out of 50 wp families where the first larval collection resulted in the production of white pupae (Supp Table S1 [online only]). The egg hatch of these six wp strains was greatly affected by temperature treatments (wp tsl FD) (two-way ANOVA: F15,263 = 54.42, P < 0.0001; Supp Tables S2 and S3 [online only]; Fig. 2). The strain, temperature, and their interaction significantly affected egg hatching. The wp tsl FD_7, _9, _15, _28, _36, and _37 strains had different thermal sensitivity patterns compared to the normal tsl strain. Specifically, the wp tsl FD_9, _28, _36, and _37 lines presented 100% lethality at 35°C, while the wp tsl FD_7 and _15 strains demonstrated, complete lethality at 36°C.
Fig. 2.
Thermal sensitivity pattern of the six selected fast developing strains compared to the wp and wp tsl mutant strains. Note that the thermal sensitivity pattern of the wp tsl FD_15 and wp tsl FD_7 strains suggested that the complete lethality for these two lines is achieved at 2°C higher than the other strains (mean ± SE; N = 10).
Vienna 8D53− FD construction
Due to their higher productivity (recovery of pupae and adults per given number of embryos; data not shown) and complete lethality at 35°C, the wp tsl FD_28 and _37 strains were selected for the construction of two novel GSS. This was achieved by crossing the first-emerging female from the wp tsl FD strain with Vienna 8D53− Guatemala males and backcrossing the F1 males with the respective females of each wp tsl FD strain, thus resulting in the Vienna 8D53− FD_28 and Vienna 8D53− FD_37 GSS.
Thermal Sensitivity
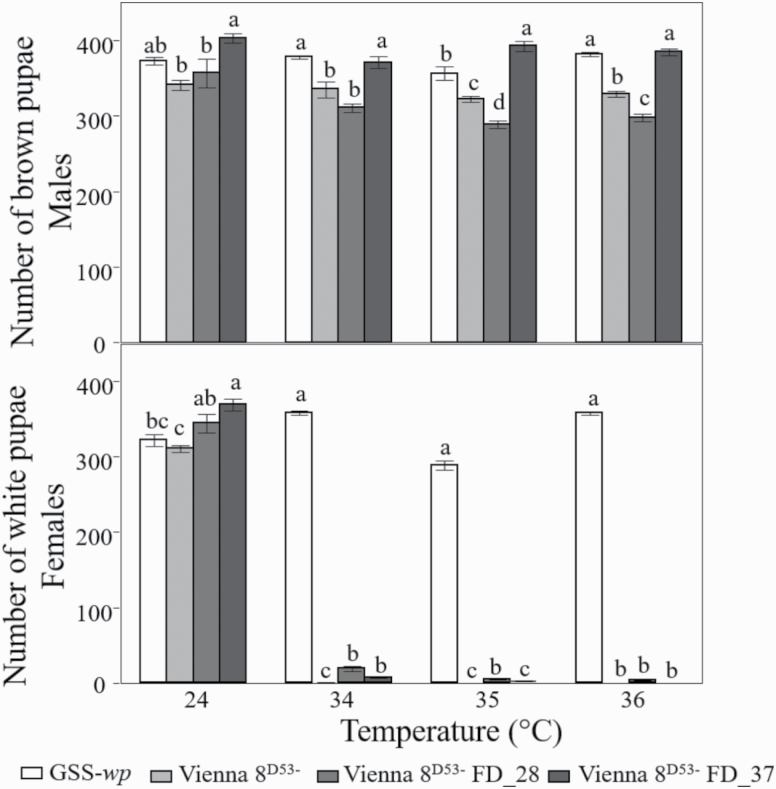
The pupal productivity of the novel Vienna 8D53− FD_28, Vienna 8D53− FD_37 strains as well as the existing Vienna 8D53− and GSS-wp strains was tested under normal rearing conditions (24°C), and their thermal sensitivity was determined. At 24°C, the Vienna 8D53− FD_37 strain was the most productive, followed by the GSS-wp. After heat treatment, white pupae were only produced by the GSS-wp, the strain that did not carry the tsl gene. The number of brown pupae significantly varied among the strains (two-way ANOVA: F7,152 = 41.02, P < 0.0001; Supp Tables S4 and S5 [online only]; Fig. 3). At 34, 35, and 36°C, the number of brown pupae produced depended on the temperature (F1,152 = 25.34, P < 0.0001), the strain (F3,152 = 79.34, P < 0.0001), and their interaction (F1,152 = 7.92, P < 0.0001), but the Vienna 8D53− FD_37 strain kept their productivity level in all tested temperatures. Similarly, the number of white pupae produced depended on the temperature, strain, and their interaction of both factors (F1,152 = 10.08, P = 0.0018; F3,152 = 119.75, P < 0.0001; F1,152 = 6.60, P = 0.01; Fig. 3).
Fig. 3.
Number of white pupae and brown pupae (wild-type) recovered from 1,000 eggs exposed to four different thermal treatments (24, 34, 35, 35°C). Note that the number of brown pupae of Vienna 8D53−/FD_37 is higher than that of the other strains (mean ± SE; N = 10).
Larval Developmental Duration and Pupal Production Pattern
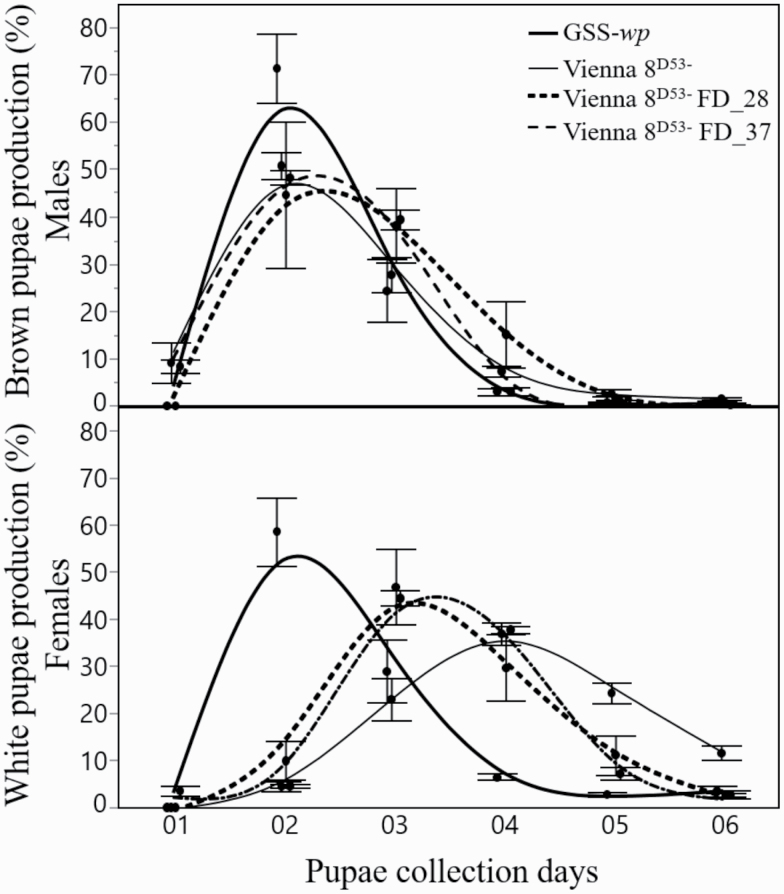
As shown in Fig. 4, larval development required significantly less time for the GSS-wp compared to all other strains, which were all carrying the tsl gene (Vienna 8D53− FD_28, Vienna 8D53− FD_37, and Vienna 8D53−), with no evidence of sex separation (F3,16 = 15.16, P < 0.0001). The production pattern of brown pupae was significantly different among the four strains studied, but only males from GSS-wp were statistically faster than Vienna 8D53−, Vienna 8D53− FD_28 and _37 (F3,16 = 10.90, P = 0.0004). The wp production pattern of both novel GSS (Vienna 8D53− FD_28 and _37) proved to be faster than Vienna 8D53− but slower than GSS-wp, where the tsl is absent.
Fig. 4.
Daily records of the larval developmental duration for females (white pupae) and males (brown pupae), as well as daily records of the number of pupae produced. Note that the GSS-wp and Vienna 8D53− FD_37 strains have a similar pattern of brown pupae production, and the females of the Vienna 8D53− FD_37 and Vienna 8D53− FD_28 are faster than Vienna 8D53− (mean ± SE; N = 10).
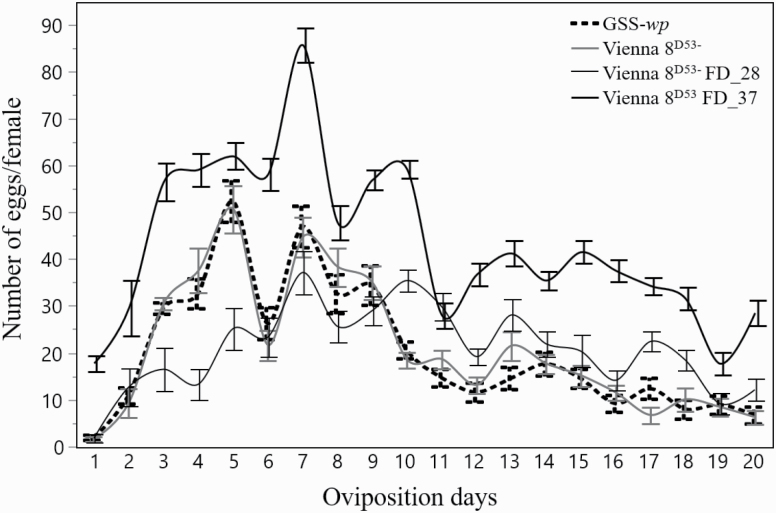
Fecundity Following Mass-Rearing Procedures
The fecundity of Vienna 8D53− FD_28, Vienna 8D53− FD_37, Vienna 8D53−, and GSS-wp strains was significantly greater in the Vienna 8D53− FD_37 compared to the other three strains (F79,1318 = 32.28, P < 0.0001; Supp Tables S6 and S7 [online only]; Fig. 5); the Vienna 8D53− FD_37 females laid 85% more eggs than the females of the other strains. The first oviposition was asynchronous, with the Vienna 8D53− FD_37 females laying eggs a day earlier than the other three strains. As clearly shown in Fig. 5, the most productive period for all four strains was between the 4th and the 10th day after emergence, while a significant reduction in egg laying was observed after the 14th day (Fig. 5).
Fig. 5.
Oviposition pattern of GSS-wp, Vienna 8D53−, Vienna 8D53− FD_28, and Vienna 8D53− FD_37 strains (mean ± SE; N = 30).
Discussion
The present study presents clear evidence that the tsl genetic region of C. capitata does directly control the slow larva development. Through the use of classical genetic approaches and the exploitation of genetic recombination phenomena between the two closely linked loci, tsl and sd, we were able to isolate a new version of the tsl strain in which the larvae develop faster, almost like wild-type strains (Fig. 4). This new wp tsl FD phenotype was then used for the construction of novel GSS (Vienna 8D53− FD), which shortens the larval developmental time of females, exhibits differences in their pupal production pattern, and also affects the thermosensitivity profile, properties which are expected to significantly improve mass rearing, reduce costs, and enhance SIT applications.
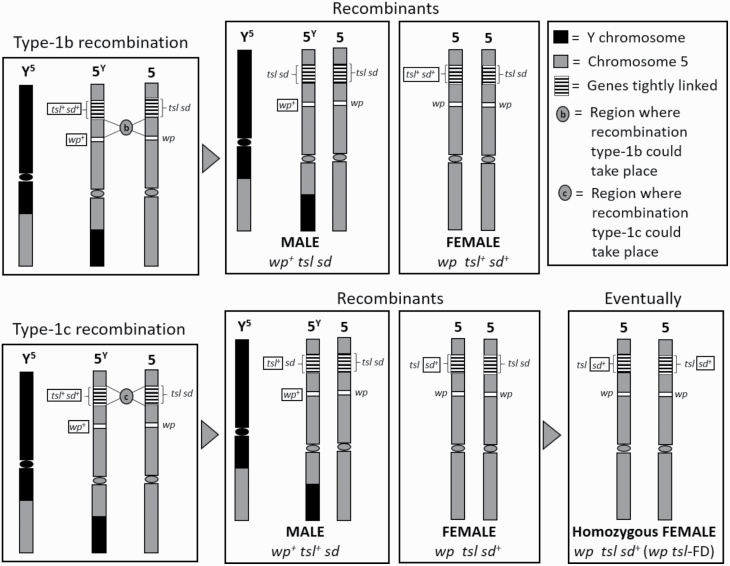
Traditionally, during the mass rearing of Vienna 8D53−, white pupae females have been detected during the first day of larvae collection. In most cases, these white pupae females were resistant to elevated temperatures, and this trait was attributed to the type-1b recombination phenomena, where the tsl allele is lost (Fig. 6; type-1b recombination). However, we showed that some of these white pupae females are still sensitive to high temperatures, which is probably due to a different type of recombination (Fig. 6; type-1c recombination), resulting in the modification of the phenotypic properties like developmental time and fitness of the normal tsl strain.
Fig. 6.
Schematic representation of type-1b and the possible type-1c recombination outcomes. The sd+ allele results in different phenotypic traits that can be obtained by type-1c recombination and can only be evident in an eventual homozygosis. Type-1b recombination results in the production of heterozygous tsl sd females, which are resistant to elevated temperatures, thus disrupting the sexing mechanism. Type-1c recombination results in the production of homozygous tsl females but heterozygous for sd, which are sensitive to elevated temperatures and develop faster than the original Vienna 8D53−.
The isolation of recombinants between the tsl and sd genetic loci allowed the selection of the proper alleles of these two selectable markers for the construction of a novel GSS using the genetic background of Vienna 8D53−, namely Vienna 8D53− FD, which displayed differences in thermal sensitivity, pupal production pattern, and the fecundity profile compared to the parental strain. Although the Vienna 8D53− FD showed a faster female developmental time than that of the Vienna 8D53− females, it was not as fast as that of the wild-type male (wp+tsl+). Furthermore, it was necessary to increase the temperature during the heat treatment from 34 to 35 or even 36°C to induce the 100% female lethality required to allow the production of male-only pupae. Indeed, in the case of the Vienna 8D53− FD_28 and Vienna 8D53− FD_37 strains, complete lethality was achieved at 35°C without affecting fecundity and male survival. It is worth noting that the Vienna 8D53− FD_37 GSS was shown to be the most productive in terms of fecundity and egg to pupa survival. Along with variations in the rearing practices, the phenotypic traits of the new wp tsl FD strain may explain the differences observed in the Vienna GSS, all of common origin, used in the Medfly mass-rearing facilities worldwide (Augustinos et al. 2017).
Optimization and cost reduction of the mass-rearing process has the potential to improve the cost-benefit ratio of current operational SIT programs and facilitate the implementation of new ones. Therefore, the development of new Medfly GSS with better biological performance, such as faster development of females during the larval stage and females with higher fecundity (Vienna 8D53− FD_37) and viability, suggesting that the Vienna 8D53− FD has real potential to improve the mass-rearing process, efficiency, and cost-effectiveness. The thermotolerance observed in the Vienna 8D53− FD will benefit the effectiveness of the strain because the broad thermal range tolerated by females may permit more flexibility in the rearing temperatures (Caceres 2002). The potential use of this novel GSS for SIT applications will first require the development of novel protocols for mass rearing as well as its thorough evaluation as concerns the rearing efficiency, genetic stability, and biological quality, including male mating competitiveness.
Supplementary Material
Acknowledgments
We thank Jaime Garcia and Edwin Ramirez for kindly providing strains used in the present study. We also thank the technicians of the Plant Pests and Genetics and Molecular Biology groups of the Insect Pest Control Laboratory for their excellent technical support in the frame of this study.
References Cited
- Augustinos A, Targovska A, Cancio-Martinez E, Schorn E, Franz G, Cáceres C, Zacharopoulou A, and Bourtzis K. 2017. Ceratitis capitata genetic sexing strains: laboratory evaluation of strains from mass-rearing facilities worldwide. Entomol. Exp. Appl. 164: 305–317. [Google Scholar]
- Caceres C. 2002. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica. 116: 107–116. [DOI] [PubMed] [Google Scholar]
- Delprat M A, Stolar C E, Manso F C, and Cladera J L. 2002. Genetic stability of sexing strains based on the locus sw of Ceratitis capitata. Genetica. 116: 85–95. [DOI] [PubMed] [Google Scholar]
- De Meyer M, Copeland R S, Wharton R A, McPheron B A, and Barnes B N. 2002. On the geographic origin of the Medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). pp. 45-53. In Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance, Stellenbosch, 6-10 May, 2002, Isteg Scientific Publications, Irene, South Africa. [Google Scholar]
- Dyck V A, Hendrichs J, and Robinson A S. 2006. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Fisher K, and Caceres C. 2000. A filter rearing system for mass reared genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae), pp. 543–550. InArea-wide control of fruit flies and other insect pests. Joint proceedings of the international conference on area-wide control of insect pests, 28 May-2 June, 1998 and the Fifth International Symposium on Fruit Flies of Economic Importance, Penang, Malaysia, 1–5 June 1998. Penerbit Universiti Sains Malaysia, Pengan, Malaysia. [Google Scholar]
- Franz G. 2002. Recombination between homologous autosomes in Medfly (Ceratitis capitata) males: type-1 recombination and the implications for the stability of genetic sexing strains. Genetica. 116: 73–84. [DOI] [PubMed] [Google Scholar]
- Franz G. 2005. Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the sterile insect technique, pp. 427–451. In V. A. Dyck, J. Hendrichs, and A. S. Robinson (eds.), Sterile insect technique principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Gourzi P, Gubb D, Livadaras Y, Caceres C, Franz G, Savakis C, and Zacharopoulou A. 2000. The construction of the first balancer chromosome for the Mediterranean fruit fly, Ceratitis capitata. Mol. Gen. Genet. 264: 127–136. [DOI] [PubMed] [Google Scholar]
- Kerremans P, and Franz G. 1994. Cytogenetic analysis of chromosome 5 from the Mediterranean fruit fly, Ceratitis capitata. Chromosoma. 103: 142–146. [DOI] [PubMed] [Google Scholar]
- McInnis D, Tam S, Grace C, and Miyashita D. 1994. Population suppression and sterility rates induced by variable sex ratio, sterile insect releases of Ceratitis capitata (Diptera: Tephritidae) in Hawaii. Ann. Entomol. Soc. Am. 87: 231–240. [Google Scholar]
- Meza J S, Ul Haq I, Vreysen M J B, Bourtzis K, Kyritsis G A, and Cáceres C. 2018. Comparison of classical and transgenic genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS One. 13: e0208880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza J S, Cáceres C, and Bourtzis K. 2019. Slow larvae mutant and its potential to improve the pupal color-based genetic sexing system in Mexican fruit fly, (Diptera: Tephritidae). J. Econ. Entomol. 112: 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaby A B, and Rockman M V. 2013. The many faces of pleiotropy. Trends Genet. 29: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón P, McInnis D, Lance D, and Stewart J. 2004. Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 97: 1547–1553. [DOI] [PubMed] [Google Scholar]
- Robinson A. 1999. Genetic sexing strains in the Medfly, Ceratitis capitata: development, mass rearing and field application. Trends Entomol. 2: 81–104. [Google Scholar]
- Robinson A, and Van Heemert C. 1982. Ceratitis capitata—a suitable case for genetic sexing. Genetica. 58: 229–237. [Google Scholar]
- Tanaka N, Okamoto R, and Chambers D. 1970. Methods of mass rearing the Mediterranean fruit fly currently used by the US Department of Agriculture, pp. 19–23. In The Proceedings on the Sterile Male Techniques for Control of Fruit Flies. International Atomic Energy Agency, 1–5 September 1969, Vienna, Austria. [Google Scholar]
- Zacharopoulou A, Augustinos A, Drosopoulou E, Tsoumani K, Gariou-Papalexiou A, Franz G, Mathiopoulos K, Bourtzis K, and Mavragani-Tsipidou P. 2017. A review of more than 30 years of cytogenetic studies of Tephritidae in support of sterile insect technique and global trade. Entomol. Exp. Appl. 164: 204–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.