Fig. 4.
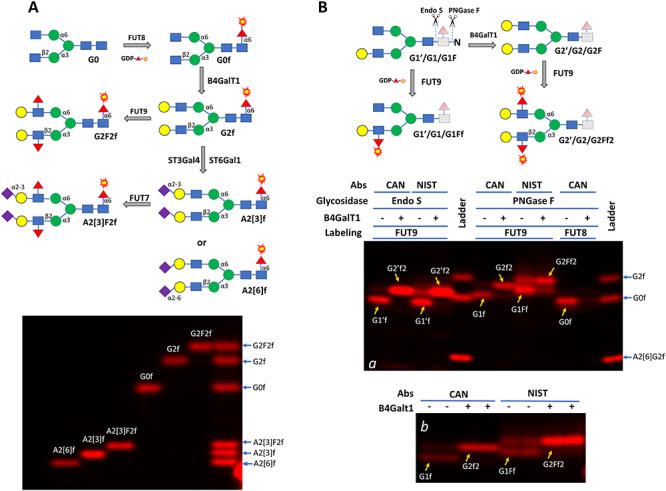
Establishing reference glycan standards (A) and characterizing glycans on Cantuzumab (CAN) and NIST mAb (NIST) (B). In (A), G0 was first labeled by FUT8 and then enzymatically converted to five other glycans, including G2F2 that carries Lewis X structure and A2[3]F2 that carries sialyl Lewis X structure. The labeled glycans were then separated on a 17% SDS–PAGE (about 0.25 ng each of the labeled glycan was loaded in each lane). In (B), glycans on Cantuzumab and NIST mAb (2.5 μg per sample) were released by either Endo S or PNGase F and then labeled by FUT9 or FUT8 directly or after galactosylation by B4GalT1. The labeled glycans were separated on a 17% SDS–PAGE together with some of the glycan standards generated in (A) (panel a). Endo S released glycans lack the core GlcNAc residue at the reducing ends (with dashed lines and light shades) and are indicated with prime symbols. For better viewing the glycan separation, labeling on PNGase F released glycans by FUT9 was repeated in panel b. Nomenclature follows the same rules of Figure 3. The galactose residue in the monogalactosylated glycans in (B) can be on either arm but only one is presented.
