Abstract
As the incidence of acute kidney injury (AKI) increases, prevention strategies are needed across the health care continuum, which begins in the community. Recognizing this knowledge gap, the 22nd Acute Disease Quality Initiative (ADQI) was tasked to discuss the evidence for quality-of-care measurement and care processes to prevent AKI and its consequences in the community. Using a modified Delphi process, an international and interdisciplinary group provided a framework to identify and monitor patients with AKI in the community. The recommendations propose that risk stratification involve both susceptibilities (eg, chronic kidney disease) and exposures (eg, coronary angiography), with the latter triggering a Kidney Health Assessment. This assessment should include blood pressure, serum creatinine, and urine dipstick, followed by a Kidney Health Response to prevent AKI that encompasses cessation of unnecessary medications, minimization of nephrotoxins, patient education, and ongoing monitoring until the exposure resolves. These recommendations give community health care providers and health systems a starting point for quality improvement initiatives to prevent AKI and its consequences in the community.
Keywords: Acute kidney injury (AKI), Quality improvement, Prevention
INTRODUCTION
The complete report and summary of recommendations from the 22nd Acute Disease Quality Initiative (ADQI) Consensus Conference have previously been published in the Clinical Journal of the American Society of Nephrology.1
Acute kidney injury (AKI) is a rapid decline in kidney function, which has substantial effects on health care outcomes and costs across all care settings.2–6 The incidence of AKI ranges from 4% to 17% of all hospital admissions,7,8 with in-hospital mortality approaching 20%–25%2 and the incremental health care costs attributed to AKI exceeding $10 billion per year.3,4 These consequences of AKI extend even after discharge back to the community,9 where over 1 in 4 survivors of AKI die within 1 year of hospital discharge.10
Closer inspection has determined that AKI can originate in the community or the hospital, with community-acquired AKI accounting for up to two-thirds of all patients with AKI being admitted to the hospital.8 However, the true incidence and causes of community-acquired AKI remains uncertain because most reports only capture patients admitted to hospital. In a worldwide, cross-sectional study of AKI, common etiologies of patients with community-acquired AKI admitted to the hospital included sepsis, nephrotoxic drugs, and low blood pressure in high-income countries and dehydration, infection, and shock in low-income countries.11,12 Strategies to prevent community-acquired AKI and its consequences have been limited until more recently with the introduction of electronic AKI alerts in primary care; these notices reduced both the response time to severe AKI and 30-day all-cause mortality.13 Response time was further reduced when the electronic alerts were combined with an educational outreach program. Whether similar efforts can prevent the onset of AKI is unclear, but these results reinforce that providing high-quality care for patients with AKI starts in the community with more work needed to test different strategies of identifying, monitoring, and managing patients at risk for AKI.
One approach to motivate improvement efforts is the creation of quality indicators that can serve as benchmarks from which to measure care gaps, guide quality improvement efforts, and generate evidence-based solutions. How prepared an organization and individuals are to improve care is always dependent on the local environment in which the work occurs.14 Therefore, some health care systems and community providers will possess the infrastructure and resources to begin quality improvement efforts immediately (eg, using the electronic medical record to pilot a care pathway for high-risk patients undergoing coronary angiography), whereas others may instead focus on developing the structures and mechanisms necessary to identify and monitor patients before improvement work can occur (eg, development of a system to record baseline serum creatinine prior to coronary angiography). In both instances, steps have been taken to decrease the risk of AKI and its consequences in the community.
On this background, the steering committee of the 22nd ADQI conference on “Quality Improvement for AKI” dedicated 1 of 5 workgroups with the task of creating quality standards at the community level to mitigate the risk of AKI. The consensus statements from all 5 groups have been previously summarized,1 and the meeting followed the established ADQI modified Delphi process (Supplementary Appendix,15). The workgroup addressed 3 questions related to AKI preventive measures in the community, with the results summarized in Table 1:
Table 1.
Summary of Consensus Statements and Sample Quality Indicators to Prevent AKI and Its Consequences in the Community
| Consensus Statements | Sample Quality Indicators |
|---|---|
| 1A: Health care professionals and systems should be able to identify patients and populations in community settings who are at high risk for AKI. 1B: We suggest that a minimum set of susceptibilities and exposures be considered for AKI risk stratification. |
|
| 2A: We suggest patients at risk for AKI have a KHA periodically, and at least every 12 months, to define and modify their AKI risk profile. 2B: We suggest that high-risk patients have another KHA at least 30 days before and again 2–3 days after a planned exposure that carries AKI risk. The KHA should be tailored to the clinical context and clinician judgment. 2C: The KHA should occur as soon as an unplanned exposure is recognized that carries AKI risk. |
|
| 3A: The KHR should precede an acute exposure that carries AKI risk or occur as soon as an unplanned acute exposure is recognized. 3B: We suggest high-risk patients and their caregivers receive formal education on their baseline kidney function and exposures for AKI. 3C: We suggest coordination between all stakeholders to address socioeconomic-cultural and environmental factors that increase the risk of AKI. |
|
AKI = acute kidney injury; CKD = chronic kidney disease; KHA = Kidney Health Assessment; KHR = Kidney Health Response.
Question 1: What are the roles and responsibilities of clinicians and health care systems to identify community-dwelling patients at risk for AKI?
Question 2: How should community-dwelling patients who are at high risk for AKI be monitored?
Question 3: How should AKI preventive strategies be implemented for community-dwelling high-risk patients?
RATIONALE FOR THE RECOMMENDATIONS
Question 1: Identification and Risk Stratification
The high volume of potentially high-risk patients in the community and primary care setting means that an initial step toward quality improvement involves the identification of high-risk populations for which monitoring and preventative strategies can be targeted.16 These risks can be categorized into 5 dimensions of inherent and nonmodifiable (eg, age), exposure-based (eg, nephrotoxic medications), processes of care (eg, electronic health records alerts), socioeconomic-cultural (eg, health care access and nutrition), and environmental (eg, water quality) risk factors (Figure 1). Each community’s definition of high risk for AKI should vary by the local prevalence of different risk factors, with a focus on elements that are common or modifiable. Some risk factor categories to consider include common patient comorbidities (eg, prior AKI,17 chronic kidney disease18), medication use (eg, diuretics,19 renin-angiotensin-system inhibitors20), planned medical procedures (eg, angiography,21 cardiac surgery22), and unplanned illnesses (eg, infection, diarrhea, Table 2).16 The combination of several risk factors from different dimensions may further increase risk, as is the case when combining nonsteroidal anti-inflammatory drugs (NSAIDs) with diuretics and reninangiotensin-system inhibitors in patients >75 years of age with chronic kidney disease (CKD).20 These patient-level risks will be of more interest to clinicians who may be able to modify these, whereas governmental systems should focus on socioeconomic-cultural and environmental risks.
Figure 1.
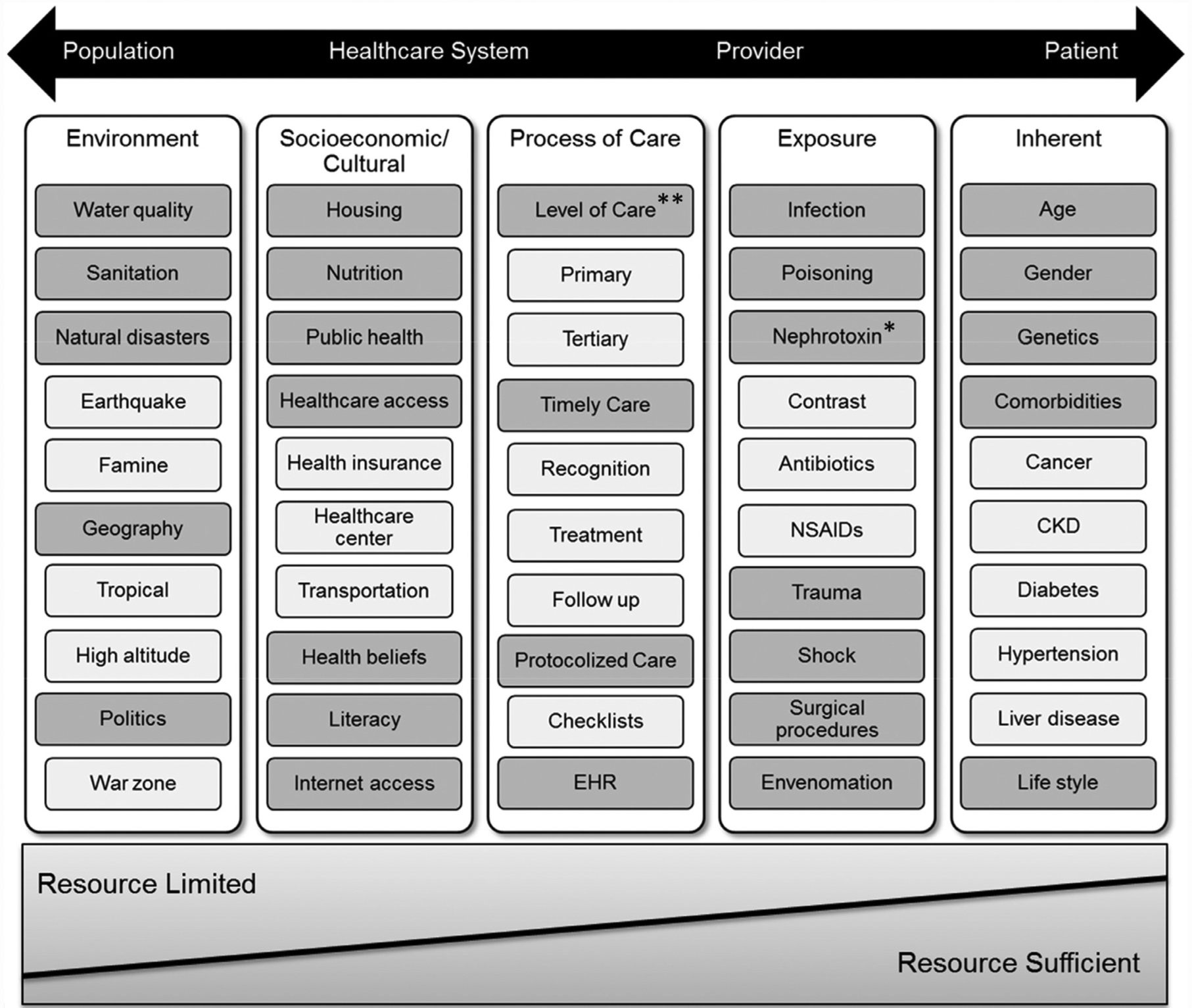
Risk dimensions and risk factors for Acute Kidney Injury (AKI). Nonexhaustive list of risk factors within each risk dimension (from population to patient level) to highlight differences in their impact on the overall risk of AKI, based on resource availabilities. Additional factors may exist for each category, and the factors listed may also span multiple dimensions.
*Although three important classes of nephrotoxins are named here, there are other medications that are renally eliminated or have nephrotoxic characteristics that also may need to be considered.
**Availability of experts and specialists (ie, physicians, nurses, pharmacists) is one of the important factors in the determination of the processes of care provided.
CKD = chronic kidney disease; EHR = electronic health record; NSAIDs =nonsteroidal anti-inflammatory drugs. Source: Reprinted from Acute Disease Quality Initiative (ADQI) with permission.
Table 2.
Common Risk Factors for Acute Kidney Injury
| Susceptibilities | Exposures | ||
|---|---|---|---|
| Non-modifiable | Modifiable | Planned | Unplanned |
| Old age | Renin-angiotensin-system i nhibitors | Iodinated contrast | Infection/antibiotics |
| Previous acute kidney injury | Diuretics | Cardiac/vascular surgery | Diarrhea |
| Chronic kidney disease | Calcineurin inhibitors | Major abdominal surgery | Intravascular volume depletion |
| Diabetes | Nonsteroidal anti-inflammatory drugs/Cox-2 inhibitors | Prolonged physical work in unhealthy environments | Hypotension |
| Chronic heart disease | Chemotherapy | Trauma | |
| Liver disease | Low socioeconomic status | Public health event | |
| Cancer | Poor health literacy | ||
| Tropical locale | Malnourished | ||
| High altitude | Inadequate housing/water quality/sanitation | ||
Question 2: Monitoring Patients for AKI—The Kidney Health Assessment
There is limited literature about how to monitor for AKI in the community and primary care setting.23 For example, despite being common practice, there is a lack of evidence that checking electrolytes and serum creatinine after initiation of a renin-angiotensin-system inhibitor prevents AKI. Nonetheless, it seems reasonable in high-risk patients to perform basic elements relevant to all patients with kidney disease (ie, AKI and CKD) that should be available to health care providers in the event of an episode of AKI, which we coin the Kidney Health Assessment (KHA; Figure 2). The components of the KHA include documentation of prior AKI, Blood pressure measurement, serum Creatinine measurement, Drug review for nephrotoxins, and Dipstick assessment for hematuria/proteinuria (acronym ABCD). These recommendations also correspond with the UK National Institute for Health and Care Excellence (NICE) AKI quality standards to assess for AKI and perform urine dipstick testing on the suspicion of AKI.24 We envision the KHA to be analogous to cardiac risk assessment before major surgery and reviewed by health care professionals who are commonly associated with acute exposures for AKI (eg, primary care, interventional cardiology, anesthesiology).25 The optimal timing of the KHA for high-risk patients remains to be determined, and it should be adjusted to the clinical scenario and based on the judgment of the health care provider. However, the KHA should be performed with enough time to allow for implementation of risk-mitigation strategies (described herein). For example, a patient with CKD on a diuretic medicine who undergoes a coronary angiogram may need his or her KHA completed sooner than an elderly patient with diabetes newly started on a renin-angiotensin-system inhibitor. Furthermore, an unplanned acute exposure (eg, infection, diarrhea, hypotension) in a high-risk patient should immediately trigger completion of the KHA. In this way, the KHA serves as a living document to monitor patients at high risk for AKI.
Figure 2.
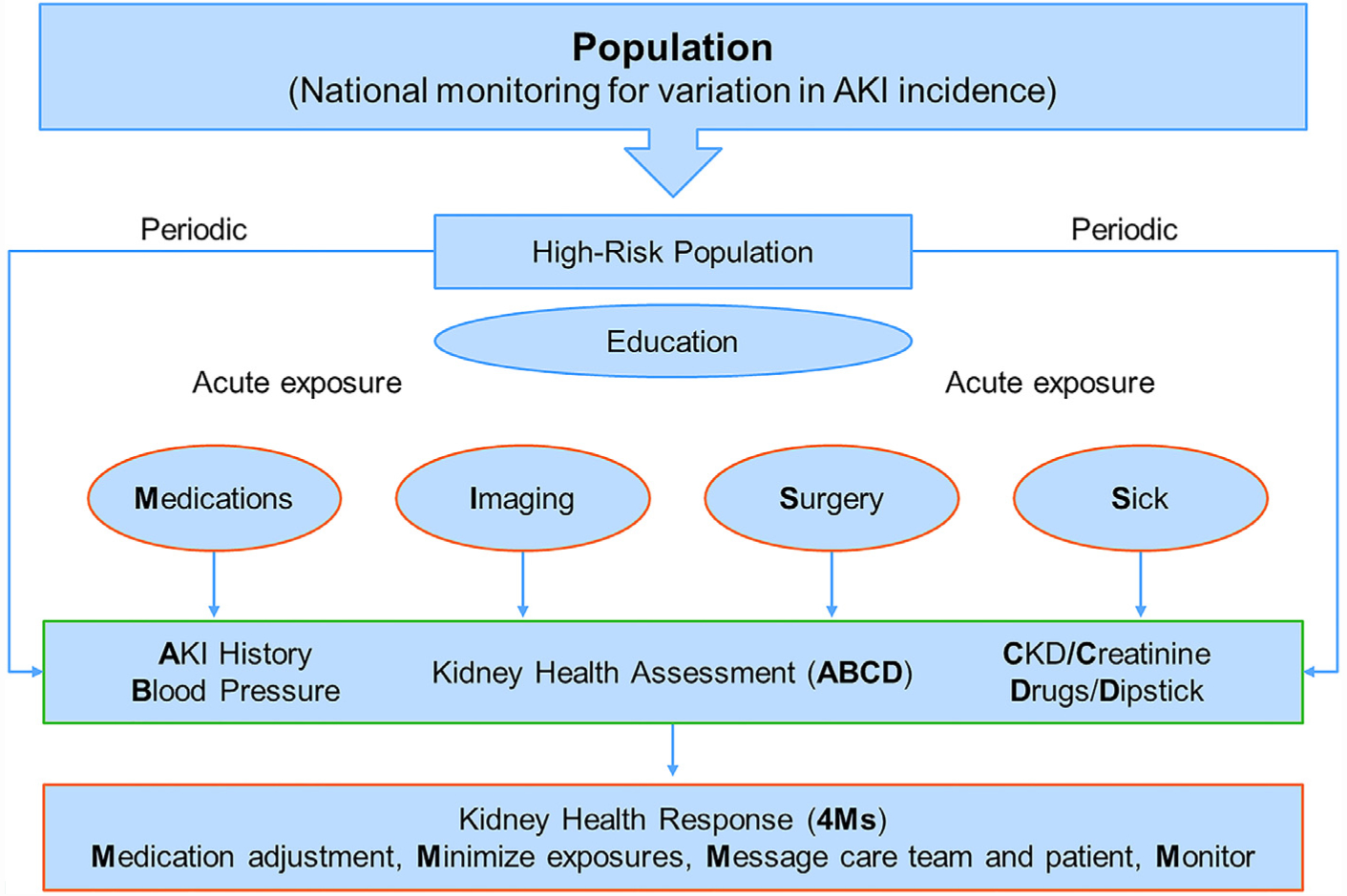
Kidney Health Assessment and Response. Strategies to identify, monitor, and prevent acute kidney injury (AKI) in the community setting, which can be modified to develop suitable quality indicators. The government and health system help with the identification and education of high-risk patients. The Kidney Health Assessment monitors these patients for AKI and chronic kidney disease (CKD) at least yearly and, more frequently, at the time of a high-risk exposure (eg, nephrotoxic medications, imaging, surgery, sickness). The Kidney Health Assessment includes AKI history, Blood pressure, serum Creatinine level, Drug list, and urine Dipstick (ABCD). This assessment should trigger a Kidney Health Response (the 4Ms) to actively prevent AKI, which encompasses cessation of unnecessary medications (eg, nonsteroidal anti-inflammatory drugs], the minimization of nephrotoxic exposures (eg, intravenous contrast), messaging the health care team and patient to alert the high-risk of AKI, and monitoring for AKI and its consequences (eg, serum creatinine and potassium).
Source: Reprinted from Acute Disease Quality Initiative (ADQI) with permission.
Question 3: Implementing Risk-Mitigation Strategies—The Kidney Health Response
There is limited literature about how to prevent AKI in the community and primary care settings. For instance, most pharmacologic measures involve preoperative statins or preprocedure N-acetyl-cysteine (NAC), whose evidence is at best mixed.26–28 The Kidney Disease Improving Global Outcomes (KDIGO) AKI bundle, which comprises optimization of blood volume status and hemodynamics, avoidance of nephrotoxic drugs, and prevention of hyperglycemia, reduced the absolute risk of AKI by 17% in patients undergoing cardiac surgery.29 Similar “do no harm” principles guided components of the Kidney Health Response (KHR), which lists the therapeutic actions health care providers should take after the KHA to prevent AKI. The KHR uses the 4M acronym to encompass the cessation of unnecessary medications (eg, NSAIDs), the minimization of nephrotoxic exposures (eg, intravenous contrast, aminoglycosides), messaging the health care team and patient to communicate the high risk of AKI, and monitoring for AKI and its consequences (Figure 2). Much like the KHA, the KHR is intended to occur before a planned exposure for AKI in high-risk patients (Table 2 and Figure 1), but it would also be reasonable to complete the KHR immediately after recognition of an unplanned high-risk exposure (eg, a patient with CKD on a diuretic who is febrile with diarrhea).
More research is needed to determine the utility of “sick day” medication guidance and education as an AKI preventive tool, which directs patients to withhold certain medications when ill and resume them when well (eg, diuretics, NSAIDs, renin-angiotensin-system inhibitors). In a recent systematic review, discontinuation of renin-angiotensin-system inhibitors prior to coronary angiography or cardiac surgery slightly reduced the risk of AKI (relative risk reduction of 17%).30 However, study quality was low, and the results were no longer significant when limited to randomized controlled trials. There was also no evidence pertaining to discontinuation of diuretics or the effect of drug cessation during an intercurrent illness in the community. When combined with implementation challenges in primary care and patient errors during usability testing,31,32 health care provider time may be better spent on other educational endeavors until the benefit (and lack of harm) of “sick day” medication guidance is clarified. Instead, we emphasize that patient education should focus on baseline kidney function and exposures for AKI (Table 2), which complements the NICE AKI quality standards to make individuals who are at risk of AKI aware of its potential causes.24 Our rationale is that qualitative research suggests patients and health care providers view AKI as a complex condition with organizational challenges that disrupt the coordination and communication of information.33,34 Therefore, it is possible many high-risk patients for AKI will not be recognized as such at the time of a high-risk exposure.35 Although patients and caregivers should not be the first line of defense against AKI, their knowledge of their baseline kidney function and common AKI exposures may serve as a safety net to alert others of their high-risk status and trigger the appropriate KHA and KHR.
CASE EXAMPLE OF RECOMMENDATIONS IN ACTION
The following case illustrates the principles of the KHA and KHR applied to patient care, with the medical encounters and corresponding risk of AKI displayed visually in Figure 3.
Figure 3.
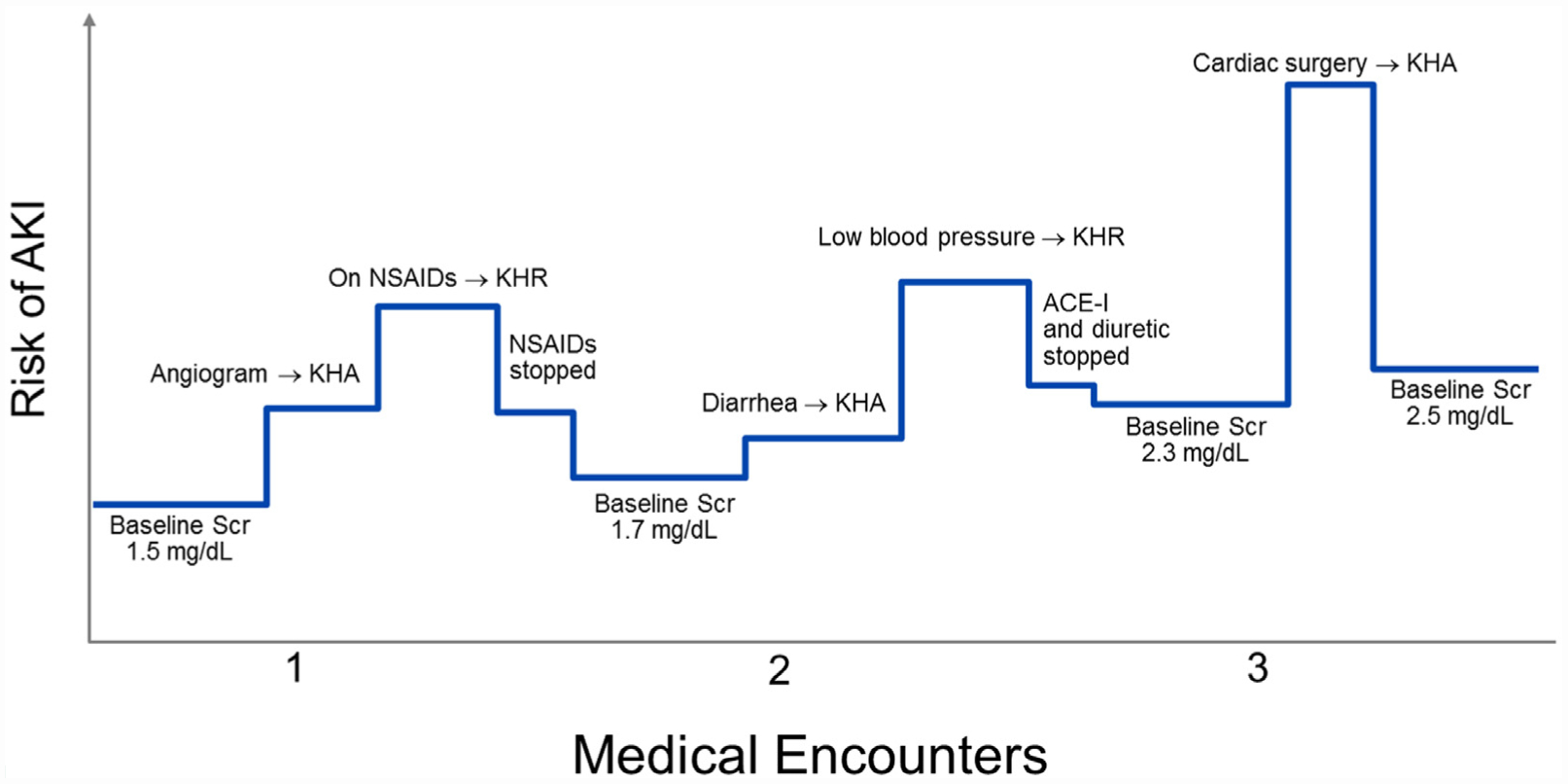
Patient example of the Kidney Health Assessment and Response. The following case illustrates how the Kidney Health Assessment (KHA) and Response (KHR) may be applied to patient care. The risk of acute kidney injury (AKI) is displayed on the y-axis, which changes at each medical encounter on the x-axis, based on the patient’s susceptibility for AKI and acute exposures (both axes are not drawn to scale). In some instances, application of the KHA and KHR are able to decrease the risk of AKI (eg, detection of hypotension in a patient with diarrhea and subsequent temporary cessation of diuretics).
ACE-I = angiotensin-converting-enzyme inhibitor; NSAIDs = nonsteroidal anti-inflammatory drugs; Scr = serum creatinine.
A 70-year-old female with diabetes mellitus type 2 and stage 3 CKD (serum creatinine of 1.5 mg/dL) presents to her primary care provider with angina. She is subsequently scheduled to undergo a coronary angiogram in 2 weeks. Her medication list includes an angiotensin-converting-enzyme inhibitor (ACE-I) and a diuretic.
Her physician notes that she has several susceptibilities for AKI, with the coronary angiogram acting as a planned acute exposure.
The physician checks the patient’s medical record for the components of the KHA, which was completed 9 months ago. Because an angiogram is a high-risk exposure, her physician updates the KHA to ensure her risk for AKI has not increased.
The updated KHA reveals that the patient’s serum creatinine is still 1.5 mg/dL and urine albumin to creatinine ratio 25 mg/g, but she is taking NSAIDs several times per week for back pain. Her physician completes the KHR, asking the patient to stop the NSAIDs and communicates the risk of AKI to the patient and her cardiologist. The patient is also asked to complete bloodwork 2–3 days after the angiogram to monitor her kidney function.
The patient presents to her primary care provider 1 year later with diarrhea and low appetite for 1 week. She is still taking the ACE-I and diuretic, but she no longer uses NSAIDs for pain. Her baseline creatinine is now 1.7 mg/dL and urine albumin-to-creatinine ratio 80 mg/g (so her risk of AKI has increased).
Her physician notes that diarrhea and low appetite are unplanned acute exposures for AKI.
Because this is an unplanned exposure, the physician completes the KHA immediately. Her creatinine is 1.7 mg/dL, but her blood pressure is 100/60 mm Hg when it is usually 130/80 mm Hg based on last year’s KHA.
Her physician completes the KHR, asking the patient to hold the ACE-I and diuretic and communicates the increased risk of AKI to the patient (including education on other AKI exposures and NSAIDs avoidance). The patient is also asked to complete bloodwork in the next week to monitor her kidney function.
Three years later, the patient’s CKD has progressed to where her baseline serum creatinine is 2.3 mg/dL and urine albumin-to-creatinine ratio is 150m g/g. She is admitted to hospital with acute myocardial infarction and undergoes cardiac bypass surgery. She is visiting with her primary care physician 2 weeks after hospital discharge.
Her physician notes that cardiac bypass surgery is an acute exposure for AKI.
Because this exposure was unexpected by the primary care physician and the patient’s discharge summary does not include mention of her discharge kidney function, the physician completes the KHA immediately. Her creatinine has increased to 2.5 mg/dL, and there have been no changes to her medication list.
Her physician completes the KHR, educating the patient about acute exposures for AKI and arranging for bloodwork in the next 4–8 weeks to ensure her kidney function remains stable.
This case illustrates that the KHA and KHR should be dynamic and flexible, responsive to the patient’s susceptibility for AKI and recent medical events both of which may change over time and modify the risk of AKI.
SAMPLE QUALITY IMPROVEMENT PROJECT BASED ON PROPOSED QUALITY INDICATORS
The following example illustrates how health care providers can use the recommendations summarized in Table 1 to support frontline quality improvement initiatives. The improvement activities follow the Six Sigma framework (Supplementary Figure 1),36 but it would be appropriate to use any framework with which the improvement team is familiar.
Step 1—Identifying target project and prioritization:
On the management of a patient with diabetes and vascular disease, you review the components of the KHA but note that the patient does not have any recently measured serum creatinine available. You highlight to the director of your out-patient clinic the importance of access to baseline serum creatinine, specifically that ADQI suggests “patients at risk for AKI have a KHA periodically, and at least every 12 months, to define and modify their AKI risk profile,” which includes baseline serum creatinine (Table 1 and Figure 2). In further evaluation of local practices, you note that only 18% of patients with diabetes and vascular disease have available baseline serum creatinine measured in the past 12 months. Following additional conversations among colleagues, you identify a quality improvement project to increase the availability of baseline serum creatinine to have a high priority (ie, a project with high impact and minimal effort, Supplementary Figure 2).
Step 2—Best practices information
In the review of literature, you notice baseline serum creatinine in the past 12 months is available for 40%−50% of the general population.37,38 You decide to adopt the ADQI quality indicator (Table 1) of “the proportion of patients at high risk for AKI with a serum creatinine measurement in the past 12 months,” with the aim to increase the proportion of patients with diabetes and vascular disease who have a serum creatinine available in the past 12 months from 18% to 40% by December 31, 2020.
Step 3—Baseline practice information:
You meet with stakeholders to determine the reasons for the low availability of baseline serum creatinine. Based on these meetings, you use fishbone analysis and a Pareto Chart (Supplementary Figures 3A and B) that help identify that the 2 main reasons for the low availability of baseline serum creatinine are: 1) lack of awareness regarding the importance of this test in patients with diabetes and vascular disease; and 2) lack of inclusion of serum creatinine measurement in the routine care protocols for this patient population.
Steps 4 and 5—Strategize improvement and implement:
You decide to conduct 2 Plan-Do-Study-Act projects to address the most common reasons behind the quality-of-care problem (Supplementary Figure 4). In the first project, you schedule a conference over lunchtime to provide education on the importance of baseline serum creatinine levels for patients at high risk of AKI. For the second project, you integrate serum creatinine measurement with preexisting protocols for patients with diabetes.
Step 6—Assess process and clinical impact:
Following the education session and new protocol, you notice the measurement of baseline serum creatinine increases from 18% to 45%. To ensure there are no unintended consequences of these changes, your clinic director measures the added cost of creatinine measurement along any additional laboratory staff time. These data will help you determine if these changes can be spread to other patient populations at high-risk for AKI (Table 2 and Figure 1).
CONCLUSIONS
The health and economic consequences of AKI suggest it is becoming an increasingly important public health concern. These consensus statements and potential quality indicators emphasize that high-quality care for patients with AKI begins in the community by identifying and monitoring patients at risk for AKI (Table 1). More research is clearly needed to improve risk stratification and simplify monitoring pathways, as has been done in the United Kingdom for in-hospital AKI.39 One possibility includes system integration with other existing disease states such as diabetes mellitus,40 heart failure,41 and hypertension,42 which was illustrated in the quality improvement example. Such implementation work is vital to provide the busy clinician with the tools to review risk, flag high-risk patients, and act to reduce risk as detailed. In the meantime, the ADQI recommendations equip community health care providers and governments with a starting point to measure the quality of care provided to patients at risk of AKI, which is a necessary first step to begin quality improvement initiatives for this vulnerable population.
Supplementary Material
CLINICAL SIGNIFICANCE.
Risk stratification for acute kidney injury involves consideration of both susceptibilities (eg, chronic kidney disease) and exposures (eg, coronary angiography).
An exposure should trigger a Kidney Health Assessment (including blood pressure, serum creatinine, and urine dipstick) followed by a Kidney Health Response (encompassing cessation of unnecessary medications, patient education, and ongoing monitoring).
More research is needed to simplify risk stratification and monitoring, which should be the focus of ongoing quality improvement initiatives.
ACKNOWLEDGMENTS
ADQI 22 consensus group contributors: Alexander Zarbock, Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Münster, Münster, Germany; Marlies Ostermann, King’s College London, Guy’s & St Thomas’ Hospital, London, United Kingdom; Sandra L. Kane-Gill, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, Pittsburgh, PA; Xiaoqiang Ding, Department of Nephrology, Shanghai Institute for Kidney Disease and Dialysis, Shanghai Medical Center for Kidney Disease, Zhongshan Hospital, Fudan University, Shanghai, China; Peter Pickkers, Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, The Netherlands; Azra Bihorac, Precision and Intelligent Systems in Medicine (PRISMAP), Division of Nephrology, Hypertension & Renal Transplantation, Department of Medicine, University of Florida, Gainesville, FL; Edward D. Siew, Division of Nephrology and Hypertension, Department of Medicine, Vanderbilt University Medical Center; Vanderbilt Center for Kidney Disease (VCKD) and Integrated Program for AKI Research (VIP-AKI), Nashville, TN Tennessee Valley Healthcare System (TVHS), Veterans Administration (VA) Medical Center, Veteran’s Health Administration; Erin F. Barreto, Department of Pharmacy; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN; Etienne Macedo, University of California San Diego, Department of Medicine, Division of Nephrology, San Diego, CA; Paul M. Palevsky, Renal Section, Medical Service, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania; and Renal-Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA; Ashita Tolwani, Division of Nephrology, University of Alabama at Birmingham, Birmingham, Alabama, USA; Luis A. Juncos, Division of Nephrology, Central Arkansas Veterans’ Healthcare System, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA; Oleksa G. Rewa, Department of Critical Care Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada; Sean M. Bagshaw, Department of Critical Care Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada; Theresa A. Mottes, Department of Pediatrics, Texas Children’s Hospital, Houston, Texas, USA; Jay L. Koyner, Section of Nephrology, Department of Medicine, University of Chicago, IL; Kathleen D. Liu, Divisions of Nephrology and Critical Care, Departments of Medicine and Anesthesia, University of California, San Francisco, CA; Lui G. Forni, Department of Clinical & Experimental Medicine, University of Surrey and Royal Surrey County Hospital NHS Foundation Trust, Guildford, United Kingdom; Michael Heung, Division of Nephrology, Department of Medicine, University of Michigan, Ann Arbor, MI; Vin-Cent Wu, Division of Nephrology, Department of Internal Medicin, National Taiwan University Hospital, Taipei, Taiwan.
Funding: The Acute Disease Quality Initiative (ADQI) consensus meeting received unrestricted grants from Baxter International Inc., La Jolla Pharmaceutical Company, Astute Medical Inc., MediBeacon Inc., AMPharma B.V., and AbbVie Inc. Corporate sponsors could attend all meeting sessions as observers but did not participate in the consensus process. These funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest: SAS is supported by a Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award (co-funded by the Kidney Foundation of Canada, Canadian Society of Nephrology, and Canadian Institutes of Health Research) and has received speaker fees from Baxter. JAK is a consultant for Baxter, NxStage, and Astute Inc and has received grant support from Baxter and Astute Inc. RLM is a consultant for Baxter, AM Pharma, CSL-Behring, Astute Inc, Regulus, Intercept, Quark, La Jolla Pharma, and Durect and has received grant support from Relypsa, Fresenius, and Grifols. CR is a consultant for ODC, Astute Inc, ASAHI Medica, Jafron, and Baxter and has received speaker fees from Estor, FMC, Medtronic, Cytosorbents, Toray, and GE. MHR acted as a consultant for Baxter Corporation and sat on the data safety monitoring committee for Retrophin, Abbvie. MH received speaker fees from Abbott, Alere, Astute Inc, Baxter, Novartis, Roche, and Siemens. MKN, DJO, FPW, KK, AJPL report none.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2019.10.038.
References
- 1.Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 2019;14:941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med 2017;12:70–6. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 5.Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014;29:1362–8. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int 2007;72:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans RD, Hemmila U, Craik A, et al. Incidence, aetiology and outcome of community-acquired acute kidney injury in medical admissions in Malawi. BMC Nephrol 2017;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014;9:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver SA, Harel Z, McArthur E, et al. Causes of death after a hospitalization with AKI. J Am Soc Nephrol 2018;29:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Burdmann EA, Cerda J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 2016;387:2017–25. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015;385:2616–43. [DOI] [PubMed] [Google Scholar]
- 13.Tollitt J, Flanagan E, McCorkindale S, et al. Improved management of acute kidney injury in primary care using e-alerts and an educational outreach programme. Fam Pract 2018;35:684–9. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf 2012;21:13–20. [DOI] [PubMed] [Google Scholar]
- 15.Kellum JA, Bellomo R, Ronco C. Acute Dialysis Quality Initiative (ADQI): methodology. Int J Artif Organs 2008;31:90–3. [DOI] [PubMed] [Google Scholar]
- 16.Kashani K, Macedo E, Burdmann EA, et al. Acute kidney injury risk assessment: differences and similarities between resource-limited and resource-rich countries. Kidney Int Rep 2017;2:519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siew ED, Parr SK, Abdel-Kader K, et al. Predictors of recurrent AKI. J Am Soc Nephrol 2016;27:1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015;66:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ 2013;346:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreischulte T, Morales DR, Bell S, Guthrie B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 2015;88:396–403. [DOI] [PubMed] [Google Scholar]
- 21.Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ 2015;351:h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huen SC, Parikh CR. Predicting acute kidney injury after cardiac surgery: a systematic review. Ann Thorac Surg 2012;93:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emmett L, Tollitt J, McCorkindale S, Sinha S, Poulikakos D. The evidence of acute kidney injury in the community and for primary care interventions. Nephron 2017;136:202–10. [DOI] [PubMed] [Google Scholar]
- 24.Ftouh S, Thomas M. Acute kidney injury: summary of NICE guidance. BMJ 2013;347:f4930. [DOI] [PubMed] [Google Scholar]
- 25.Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17–32. [DOI] [PubMed] [Google Scholar]
- 26.Navarese EP, Gurbel PA, Andreotti F, et al. Prevention of contrast-induced acute kidney injury in patients undergoing cardiovascular procedures—a systematic review and network meta-analysis. PLoS One 2017;12:e0168726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J of Med 2018;378:603–14. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Xie X, Liu L, et al. Comparative effectiveness of 12 treatment strategies for preventing contrast-induced acute kidney injury: a systematic review and bayesian network meta-analysis. Am J Kidney Dis 2017;69:69–77. [DOI] [PubMed] [Google Scholar]
- 29.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiting P, Morden A, Tomlinson LA, et al. What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open 2017;7:e012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martindale AM, Elvey R, Howard SJ, McCorkindale S, Sinha S, Blakeman T. Understanding the implementation of ‘sick day guidance’ to prevent acute kidney injury across a primary care setting in England: a qualitative evaluation. BMJ Open 2017;7:e017241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerfler RM, Diamantidis CJ, Wagner LA, et al. Usability testing of a sick-day protocol in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver SA, Saragosa M, Adhikari NK, et al. What insights do patients and caregivers have on acute kidney injury and posthospitalisation care? A single-centre qualitative study from Toronto, Canada. BMJ Open 2018;8:e021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps DL, Morris RL, Blakeman T, Ashcroft DM. What is involved in medicines management across care boundaries? A qualitative study of healthcare practitioners’ experiences in the case of acute kidney injury. BMJ Open 2017;7:e011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meersch M, Schmidt C, Zarbock A. Perioperative acute kidney injury: an under-recognized problem. Anesth Analg 2017;125:1223–32. [DOI] [PubMed] [Google Scholar]
- 36.Pande PS, Neuman RP, Cavanagh RR. The Six Sigma way: how GE, Motorola, and other top companies are honing their performance. New York: McGraw-Hill; 2000. [Google Scholar]
- 37.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 2010;77:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acedillo RR, Wald R, McArthur E, et al. Characteristics and outcomes of patients discharged home from an emergency department with AKI. Clin J Am Soc Nephrol 2017;12:1215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selby NM, Casula A, Lamming L, et al. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol 2019:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–61. [DOI] [PubMed] [Google Scholar]
- 41.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart 2005;91:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ 2002;325:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


