Abstract
Background:
Cancer cachexia (CC) is a significant contributor to mortality and morbidity in patients with gastrointestinal (GI) cancer. Treatment options to prevent or halt the progression of CC are limited. Targeted acupuncture (TA) was used in GI patients with CC to evaluate for a potential gender effect.
Patients and methods:
Participants (n=30) were recruited from two outpatient clinics in the northern central part of Florida. All participants were diagnosed with CC and GI cancers. A randomized, single-blind, placebo-controlled clinical trial was used to compare TA to non-targeted acupuncture (NTA) over the course of eight weeks. Primary endpoints were weight and body composition changes measured by bioelectrical impedance analysis (BIA) and biomarker analysis (tumor necrosis factor (TNF)-α and leptin). Gender differences across and within TA and NTA groups were additionally examined as part of a secondary analysis.
Results:
A significant (p=0.026) interaction between weight and gender was noted, which manifested in a non-significant increase in the male intervention (MI) group while TNF-α levels significantly increased by gender (p=0.028) and group (p=0.006) over the course of the study. All other groups either lost or did not change weight. The extracellular-to-intracellular water (ECW/ICW) ratio was significantly elevated for the TA group (p=0.02) and for males (p=0.009) at completion of the study. TNF-α and leptin levels were positively correlated within the MI group at the end of the study.
Conclusions:
A decrease in leptin in the MI group corresponded to higher appetite and weight gain. The elevated ECW/ICW ratio indicates an inflammatory response in the MI group. This gender-specific response may be based on hormone-specific regulation of food intake. Further studies with larger sample sizes are required to support the results.
Keywords: acupuncture, gender, cancer cachexia, gastrointestinal
INTRODUCTION
Cancer cachexia (CC) is a multi-factorial syndrome disorder characterized by progressive unintentional weight loss, decreased lean muscle mass and derailed physiological functioning affecting appetite, immune system response, quality of life and gastrointestinal (GI) motility.1 Patients in advanced cancer stages are more likely to develop CC that can exacerbate their condition, shorten their lifespan, and negatively impact their therapy plan.2 The multi-factorial nature and variable symptom presentation of CC often leads to a delayed diagnosis and treatment.3 The most reliable clinical predictor for the development and progression of CC to date has been weight loss, which is used as the determining diagnostic factor. Other correlations have been attempted to allow earlier diagnosis of CC and to explain the underlying pathophysiology and progressive nature of the disorder.4 Current pharmacotherapeutic interventions to increase protein synthesis for appetite stimulation5,6 using corticosteroids, megestrol acetate and cannabis have demonstrated short-term limited benefits on improvement of appetite and weight gain with various side effects without preventing or halting the progression of CC.
Leptin is a hormone that regulates hunger and appetite with increased blood levels being linked to satiety in healthy individuals and reduced appetite in patients with CC.7 This further contributes to weight loss and leptin may serve as a biological marker of progression of CC. Opposing the effects of leptin is the hormone ghrelin, which stimulates appetite and can suppress systemic inflammation.8 An often referenced indicator of systemic inflammation is tumor necrosis factor α (TNF-α), which has been used as a surrogate marker for inflammation related to cancer cachexia, together with interleukins.9,10 Since systemic inflammation is another contributor to the progression of cachexia, a shift from intracellular (ICW) to extracellular water (ECW) is indicative of an inflammatory process and potential loss of cell integrity relative to total body water (TBW).11 Hence bioelectrical impedance analysis (BIA) can be utilized to evaluate the overall health status of a patient and relate body composition to potential other biomarkers of CC.12 Acupuncture has been evaluated in clinical studies and has been shown to benefit cancer patients by improving GI symptoms and physiological functions, and modulating leptin and inflammatory mediators.13 A recent study indicated that gender differences may impact the development and progression of GI cancers and the related CC.14 It is, however, not known whether the acupuncture intervention may affect patients differently based on gender. This study investigated gender differences via a secondary analysis of a trial comparing a targeted acupuncture (TA) intervention group and non-targeted acupuncture (NTA) control group in CC.
Acupuncture can be defined in two ways; one is based on acupuncture needling procedure and another is based on traditional acupuncture point selection.15 Definition of an acupuncture protocol for this study was insertion of acupuncture needles at defined acupuncture points. Targeted acupuncture intervention included pre-determined traditional acupuncture points that were selected based on the core symptoms of CC such as systemic inflammation, loss of appetite and loss of muscle mass.16,17 The main hypothesis of this study was that there would be no gender differences in biomarkers within the TA or NTA group. Gender differences in body composition, particularly in body water, were expected but not within the same gender in the TA or NTA group.
METHODS
Study design and sample
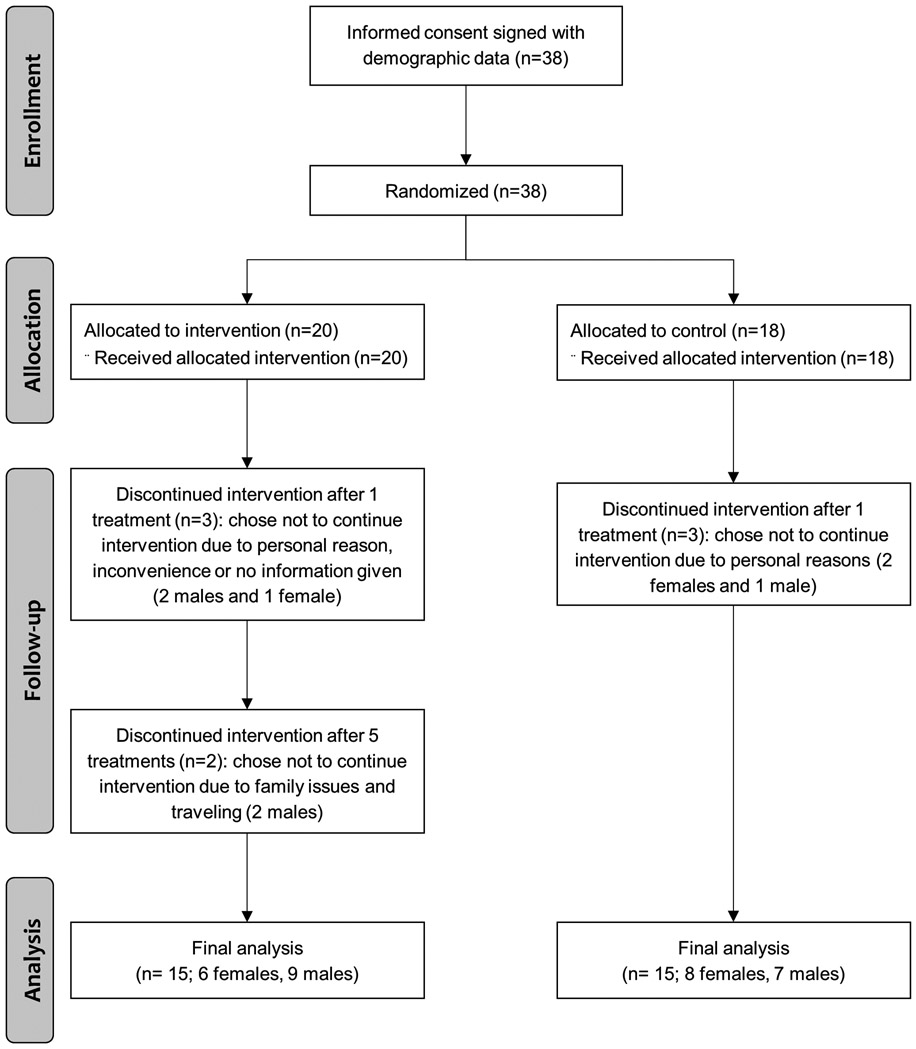
This single-blinded, randomized pilot study was registered at ClinicalTrials.gov (NCT02148159) and approved by the local institutional review board (IRB201400340). Written informed consent was obtained from all subjects prior to initiation of the study. A total of 38 subjects received at least one intervention, and 30 of those 38 subjects completed the entire eight sessions of weekly acupuncture intervention between 10/6/2014 and 4/5/2016. In both the intervention and control groups, three subjects in each group chose not to continue for various reasons after receiving only one treatment. Two subjects in the intervention group withdrew from the study after completing five treatments, which left each 15 subjects in the TA and NTA groups (Figure 1). Demographic variables and tumor staging of the subjects have already been published.17 Inclusion criteria were adults who: (1) were 21 years and older; (2) were able to communicate in English; (3) agreed to follow the research protocol; (4) had received a medical diagnosis of gastrointestinal cancer (gastric, biliary, small intestine or colorectal; (5) were starting or continuing chemotherapy at the time of screening patients; and (6) had 5% or more weight loss over the preceding six-month period. All patients were receiving standard of care, which could include measures to stimulate appetite such as steroids or appetite-promoting medications. Exclusion criteria were: (1) planned surgical procedures at the time of recruitment; (2) scheduled radiation therapy alone or in addition to chemotherapy during the study period; (3) surgery during the study or in the month prior to the study without scheduled chemotherapy post-surgery; (4) comorbidities that could affect the interpretation of the study findings (e.g. HIV, AIDS, hepatitis, Alzheimer’s disease, movement disorder, or acute myocardial infarction within the last 3 months); (5) open burn sites or infected wounds; (6) esophageal cancer diagnosis with swallowing difficulty of a mechanical nature; (7) uncorrected, mechanical digestive obstruction or inability to tolerate enteral nutrition; (8) diagnosis of pancreatic adenocarcinoma; and/or (9) life expectancy of less than 6 months as assessed by an attending physician.
Figure 1.
CONSORT flowchart of study enrollment, allocation, follow-up and analysis.
Randomization
Randomization was based on numbers pre-assigned by the statistician in the order of the date of signature of informed consent. The project coordinator informed only the acupuncturist of the randomization assignment before implementation of the first acupuncture treatment for both control and intervention groups. The research staff assessed outcomes and were blinded to the group assignment, as was the statistician analyzing the data. By design, it was not possible to blind the acupuncturist to group assignment.
Acupuncture protocol
A detailed description of the acupuncture protocol for this study has been published elsewhere.18 Briefly, the targeted traditional acupuncture points were specifically related to the underlying mechanisms of cachexia based on auriculotherapy19 and Traditional Chinese Medicine.20 The patients were fully dressed except exposure of upper and lower extremities for needling purpose and needled in a supine position with single-use, sterile stainless steel, disposable needles measuring 0.20 x 15 mm or 0.20 x 30 mm. Each acupuncture session lasted approximately 45-50 minutes with the needles being retained for 15-20 minutes. Needling (de qi) sensation or needle grasp was obtained with the manual stimulation even rotation method. All subjects in the TA group received acupuncture at the same pre-determined 23 traditional auricular and body acupuncture points while all subjects in the control group received NTA, which consisted of acupuncture at five traditional acupuncture points that were not specific to the mechanisms of cachexia. The control group received eight acupuncture sessions with NTA for CC while the intervention group followed a TA protocol for the eight sessions. Both groups were given the same amount of attention in the same environment and similar time for conversation during their interactions with the acupuncturist.
Measurements
Measurements included changes in body weight and body composition, and blood levels of leptin and other biological markers at baseline and week 8. Demographic data were collected.
Weight
We weighed subjects each week to evaluate weight change using a stand-on scale with ramp (Health-O-Meter®). The scale was calibrated annually and more frequently, as needed. Subjects were asked to remove their shoes, hats, outer clothing (e.g., jackets) and personal items, which could potentially interfer with an accurate measurement, from their pockets before stepping on the scale. Height (cm) was measured to the nearest millimeter once at baseline using a stadiometer (Holtain Limited®) mounted on the wall. The stadiometer was calibrated annually and checked daily. Height measure was used as a reference value for calculation of body mass index (BMI) and other body composition calculations.
Body composition
Body composition including total body water (TBW), intracellular water (ICW) and extracellular water (ECW) was measured at 50 kHz using the ImpediMed Imp™ SFB7, (ImpediMed Ltd., Eight Mile Plains, QLD, Australia). BIA was measured every week prior to providing NTA or TA.
Biomarkers
TNF-α, leptin and ghrelin blood levels were measured in batch using the EMD Millipore (now Merck Millipore) at the end of the study to prevent lot-to-lot variability. All blood samples were transported immediately to the laboratory in a prepared ice-packed container and then centrifuged and stored at −80°C until analysis.
Statistical analysis
All numerical values were standardized by adjusting to the respective baseline (week 1) values as follows: (value_week_x/value_week_1)*100 to obtain percent change. Statistical analysis for initial comparison of means was conducted using a two-way ANOVA to test for interactions between changes over time in weight, TBW and ECW/ICW ratio with gender (female/male) and group (NTA/TA) as fixed factors. Only if a main effect was significant was a one-way ANOVA with post-hoc Bonferroni’s test conducted. Bivariate Pearson correlation analysis was conducted for absolute pre- and post-intervention values of weight, TBW, ECW/ICW ratio, leptin, TNF-α, gender (female, male) and group (NTA, TA). The significance level was set to p<0.05.
RESULTS
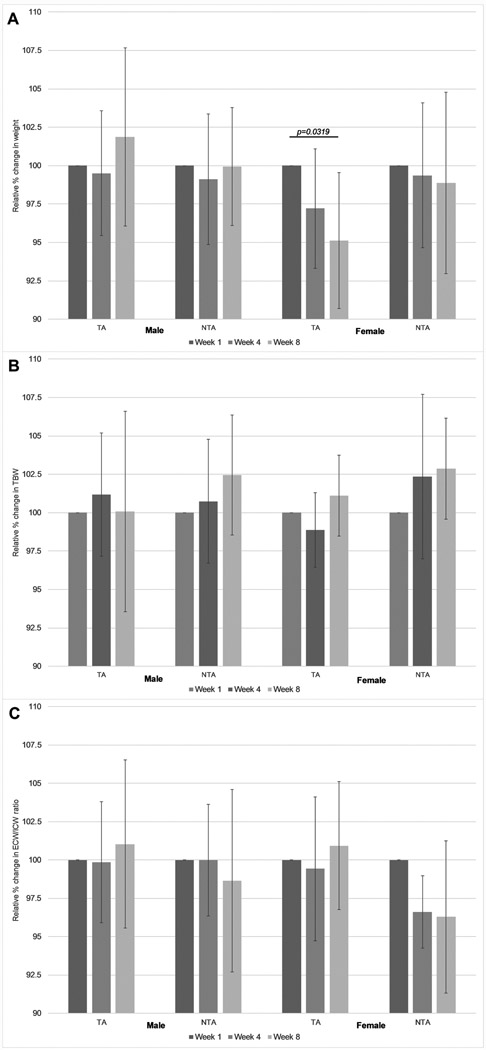
Two-way ANOVA revealed a significant interaction between body weight and gender (p=0.026). Relative body weight only decreased significantly (p=0.032) in the female intervention (FI) group whereas both genders maintained stable weight in the control groups (Figure 2). The only group with increased weight post-intervention was the male intervention (MI) group although the weight gain was not significant compared to baseline (Figure 2A).
Figure 2.
(A) Relative weight in percent change from week 1 for non-targeted (control, NTA) and targeted (intervention, TA) acupuncture groups by gender. (B) Relative total body water (TBW) for non-targeted (control, NTA) and targeted (intervention, TA) acupuncture groups by gender. (C) Relative extracellular water (ECW) / intracellular water (ICW) ratio for non-targeted (control, NTA) and targeted (intervention, TA) acupuncture groups by gender. Bar graphs show mean and standard deviation at baseline (week 1), week 4, and post-intervention (week 8). All significances are p<0.05 calculated using Bonferroni’s post-hoc test.
While absolute TBW was significantly different based on gender at baseline (as expected since fat mass is higher and therefore TBW is lower in women), there were no significant interactions observed in the two-way ANOVA for both the relative TBW change over time and the relative ECW/ICW ratios between the control and intervention groups (Figure 2).
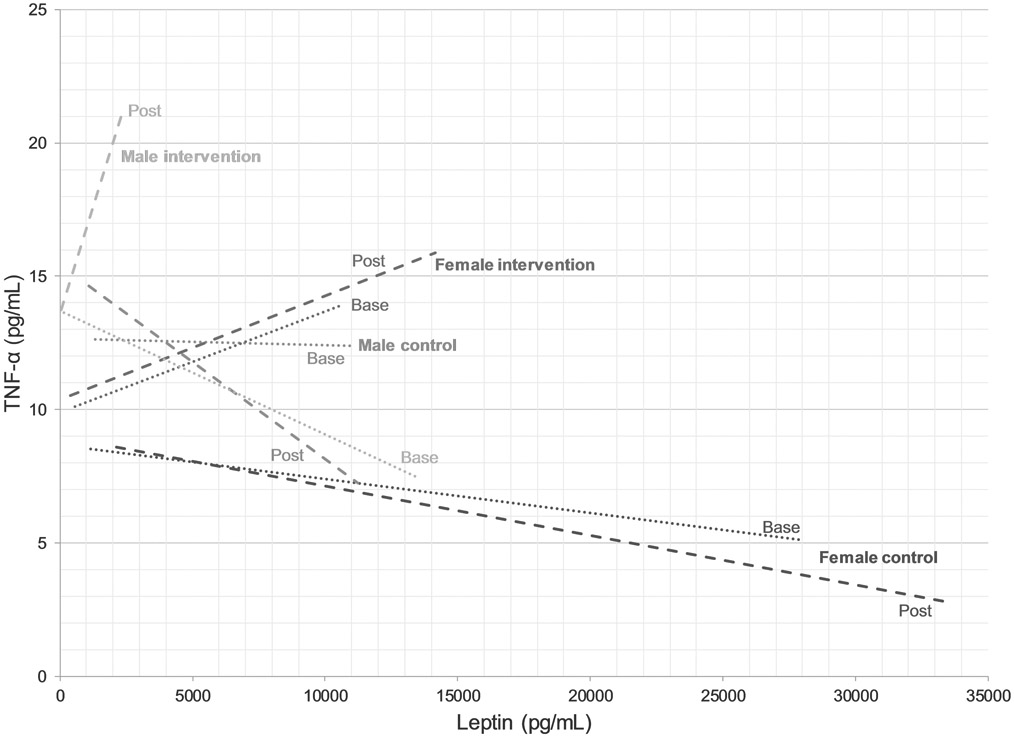
The interactions between relative leptin blood levels, gender and group assignment between baseline and post-intervention were not significant in the two-way ANOVA. Relative leptin blood levels increased for all groups post-intervention and appeared to trend higher in the MI group compared to the other three groups (Figure 3). Relative TNF-α blood levels were significant (p=0.009) for an interaction with group assignment between baseline and post-intervention in the two-way ANOVA. The increase in TNF-α levels can be attributed to the MI group, which was significantly different (p=0.047) compared to the male NTA group post-intervention. Levels in the FI also increased whereas they decreased for both genders in the control group (Figure 3).
Figure 3.
Correlation between blood tumor necrosis factor (TNF)-α and leptin levels for female and male control and female and male intervention groups. Dotted and dashed lines are represent baseline and post-intervention correlations, respectively.
Bivariate Pearson correlation using gender (female/male) and group assignment (NTA/TA) supported the former findings as gender was different both at baseline (p<0.001) and post-intervention (p<0.001) for this parameter (Table 1). Similarly, the ECW/ICW ratio was significantly different between genders at the beginning (p=0.022) and the end (p=0.009) of the trial (Table 1). There was no difference in ECW/ICW ratio between NTA and TA groups at baseline but there was at the end of the intervention (p=0.02). For leptin and TNF-α blood levels, both gender and group assignment were not significantly different at baseline, but group assignment correlated negatively with leptin levels post-intervention (p=0.012) while TNF-α levels were positively correlated with group assignment (p=0.006) and also with gender (p=0.028). This is in agreement with the relative changes in leptin and TNF-α levels shown in Figure 3.
TABLE 1.
Bivariate Pearson correlation and significance for absolute values of weight, total body water (TBW), extracellular water (ECW) / intracellular water (ICW) ratio, leptin and tumor necrosis factor (TNF)-α blood levels pre- and post-intervention by gender (female/male) and group (NTA/TA).
| Gender | Group | Weight | TBW | ECW/ICW | Leptin | TNF-α | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 8 | Week 1 | Week 8 | Week 1 | Week 8 | Week 1 | Week 8 | Week 1 | Week 8 | |||
| Gender | 1 | 0.134 (p=0.481) |
0.146 (p=0.440) |
0.246 (p=0.190) |
0.698 (p<0.001) |
0.657 (p<0.001) |
0.416 (p=0.022) |
0.471 (p=0.009) |
−0.253 (p=0.177) |
−0.339 (p=0.067) |
0.306 (p=0.099) |
0.401 (p=0.028) |
| Group | 0.134 (p=0.481) |
1 | −0.251 (p=0.181) |
−0.241 (p=0.200) |
0.406 (p=0.026) |
0.332 (p=0.073) |
0.284 (p=0.128) |
0.423 (p=0.020) |
−0.346 (p=0.061) |
−0.455 (p=0.012) |
0.189 (p=0.318) |
0.492 (p=0.006) |
Subjects in the NTA and TA groups exhibited no adverse effects related to the acupuncture treatment as evaluated by both the acupuncture interventionist and the study staff.18 Use of appetite stimulants and other medications were collected based on self-report. Only four subjects reported use of megestrol (two each in the intervention and control groups) and one subject in the control group used dronabinol (Marinol®). Dexamethasone was used pre- or post-chemotherapy regimen during the weeks of chemotherapy. Of the 38 subjects at baseline, 21 subjects (55%) were undergoing the first or second cycle of chemotherapy when they enrolled in the study, while 14 subjects (37%) were between their 3rd and 12th chemotherapy cycles; information about chemotherapy cycle was missing for three subjects. Of the eight subjects who withdrew from the study, four subjects were within their first two chemotherapy cycles, three subjects in various chemotherapy cycles from the 3rd to 12th, and one subject had missing information regarding chemotherapy cycle.
DISCUSSION
Weight loss in CC is the result of a complex metabolic derangement involving inflammatory processes and hormonal imbalances. All patients entering the study had lost at least 5% body weight over the past 6 months. This change in body composition alone often has a profound impact on the immune system and metabolic processes, hence resulting in wide variability of responses to adjust to the changes.1 Patients with a low BMI are more prone to develop CC and also have lower survival rates.21 The results from this study indicate that blood levels of the satiety hormone leptin differ widely among patients with GI cancers but appear to be higher in women than in men.22 In response to TA, decreased leptin levels in male subjects suggested that appetite overall increased, which may have led to the observed weight gain. However, MI patients also presented with increased systemic inflammation, as seen by a significant increase in TNF-α, that may be an initial response of the body to activate its immune system.23 This differential response to acupuncture may indicate a hormone-specific metabolic change between genders that needs to be considered in customizing acupuncture treatment. The slightly increased ECW/ICW ratio in the MI group is indicative of an inflammatory process, which is mirrored by the higher % TBW. Naturally men have a higher % TBW than women, who have a higher % fat mass.24 The ECW/ICW ratio decreased for women in the control group indicating a reduction in inflammation that was confirmed by a lower TNF-α to leptin correlation. In the FI group, the correlation between TNF-α and leptin remained the same throughout the trial while it reversed in the MI group, indicating an impact of the acupuncture intervention. It remains unclear if the FI group benefited from the acupuncture intervention since the correlation was already positive at baseline.
Leptin is also related to the number of adipocytes and amount of fat tissue in the body and hence women would naturally have a higher amount of circulating leptin.25 Leptin levels are affected by megestrol administration and correlate positively with fat mass according to one study with a small sample size in GI cancer patients.26 That study did not address the different gender responses and concluded that the proportion of free and total leptin were not different between an age- and gender-matched healthy control group and advanced GI cancer patients. If not differentiated by gender, this is in agreement with the findings from this study. Use of megestrol acetate and medroxyprogesterone as corticosteroids in the treatment of CC has also been shown to decrease blood levels of TNF-α blood among other pro-inflammatory cytokines.27 The results from this study indicate that gender differences do exist that may point to higher TNF-α levels and a distinct response to acupuncture between female and male patients. It is not clear which patients were consistently using a corticosteroid concomitantly with the acupuncture sessions during the study period.
There are several limitations of the current pilot study to be addressed. Given this was a pilot study, both the NTA and TA groups were limited in their respective sample sizes. However, the sample size did meet the minimum number needed to adequately conduct statistical analysis, although generalization of the results should be attempted only after repetition with a larger sample size given that this was a secondary analysis by gender sub-classification. Subjects were not asked if acupuncture was objectively or subjectively benefitting them prior to entering the study or at the completion of the treatment. At the completion of the acupuncture treatments subjects were not asked about their suspected treatment allocation, which is another limitation of the study. A few subjects mentioned that the acupuncture interventions were helpful; however, this information was anecdotal, not part of the approved IRB protocol and not documented or reported. Another limitation is the absence of determination of food intake for subjects, which would have reduced the allocation of subjects to even smaller sub-groups. Lastly, self-reported appetite stimulant use among subjects was much less than was expected. This may have been due to lack of understanding significance of unintentional weight loss and appetite loss related to disease process and treatment effects. Thus, patients either fail to report these issues to the healthcare providers or healthcare providers do not initiate the appropriate treatment in a timely manner. Despite these drawbacks, the demographics and tumor staging have been reported for this trial elsewhere.17,18
The results of this pilot study may serve as indicators that TA should be adjusted by gender to address complex metabolic responses in GI patients with CC. Further studies are necessary to confirm these findings.
ACKNOWLEDGMENTS
The authors would like to express deep appreciation to the patients who participated in the study and the research staff who assisted during all stages of the study.
FINANCIAL DISCLOSURE
The study was funded by the University of Florida Opportunity Research Fund, partly by the Clinical Translational Science Institute (CTSI), the National Center for Advancing Translational Sciences of the National Institute of Health (UL1TR001427) and the UF PRICE-UF Health Cancer Center Seed Grant Program (Dr. Yoon).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- 1.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. February 22 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. December 2014;15(12):866–872. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte G, van de Wiel M, Wouters K, et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol. 2016;3(1):e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. July 2018;127:91–104. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz Garcia V, Lopez-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;3:Cd004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuca A, Jimenez-Fonseca P, Gascon P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol. December 2013;88(3):625–636. [DOI] [PubMed] [Google Scholar]
- 7.Mondello P, Lacquaniti A, Mondello S, et al. Emerging markers of cachexia predict survival in cancer patients. BMC Cancer. November 16 2014;14:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBoer MD. Ghrelin and cachexia: will treatment with GHSR-1a agonists make a difference for patients suffering from chronic wasting syndromes? Mol Cell Endocrinol. June 20 2011;340(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel HJ, Patel BM. TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. February 01 2017;170:56–63. [DOI] [PubMed] [Google Scholar]
- 10.Molfino A, Iannace A, Colaiacomo MC, et al. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle. February 2017;8(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malbrain ML, Huygh J, Dabrowski W, De Waele JJ, Staelens A, Wauters J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther. Nov-Dec 2014;46(5):381–391. [DOI] [PubMed] [Google Scholar]
- 12.Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients--a comprehensive review. Eur J Clin Nutr. December 2015;69(12):1290–1297. [DOI] [PubMed] [Google Scholar]
- 13.Firouzjaei A, Li GC, Wang N, Liu WX, Zhu BM. Comparative evaluation of the therapeutic effect of metformin monotherapy with metformin and acupuncture combined therapy on weight loss and insulin sensitivity in diabetic patients. Nutr Diabetes. May 2 2016;6:e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson LJ, Liu H, Garcia JM. Sex Differences in Muscle Wasting. Adv Exp Med Biol. 2017;1043:153–197. [DOI] [PubMed] [Google Scholar]
- 15.NIH/NCCIH. Translating Fundamental Science of Acupuncture into Clinical Practice for Cancer Symptom Management, Pain, and Substance Abuse. 2019, 2019.
- 16.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. May 2011;12(5):489–495. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SL, Grundmann O, Williams JJ, Gordan L, George TJ Jr. Body composition changes differ by gender in stomach, colorectal, and biliary cancer patients with cachexia: Results from a pilot study. Cancer Med. August 2018;7(8):3695–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundmann O, Yoon SL, Williams JJ, Gordan L, George TJ Jr. Augmentation of Cancer Cachexia Components With Targeted Acupuncture in Patients With Gastrointestinal Cancers: A Randomized Controlled Pilot Study. Integr Cancer Ther. Jan-Dec 2019;18:1534735418823269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strittmatter B Ear Acupuncture: A Precise Pocket Atlas Based on the Works of Nogier/Bahr. New York: Thieme; 2001. [Google Scholar]
- 20.Xinnong C Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1999. [Google Scholar]
- 21.Yoon SL, Kim JA, Kelly DL, Lyon D, George TJ Jr. Predicting unintentional weight loss in patients with gastrointestinal cancer. J Cachexia Sarcopenia Muscle. March 4 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf I, Sadetzki S, Kanety H, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. February 15 2006;106(4):966–973. [DOI] [PubMed] [Google Scholar]
- 23.Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. December 1 2017;3(12):e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel). June 19 2014;14(6):10895–10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung OK, Cheng AS. Gender Differences in Adipocyte Metabolism and Liver Cancer Progression. Front Genet. 2016;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace AM, Kelly A, Sattar N, McArdle CS, McMillan DC. Circulating concentrations of “free” leptin in relation to fat mass and appetite in gastrointestinal cancer patients. Nutr Cancer. 2002;44(2):157–160. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog. 1998;9(2):99–106. [DOI] [PubMed] [Google Scholar]