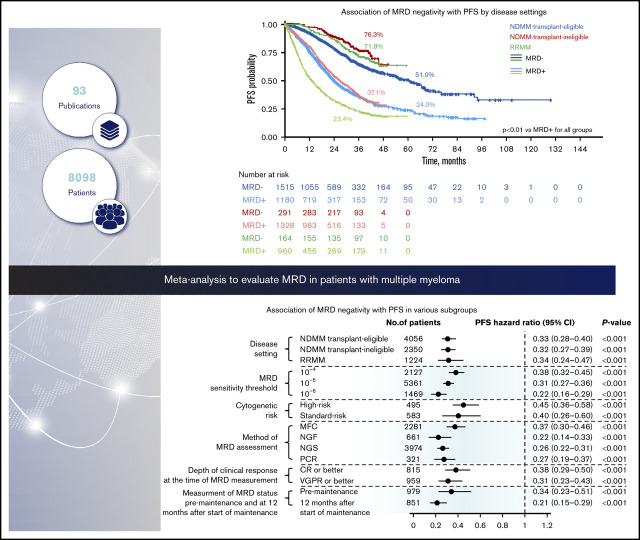
Key Points
This meta-analysis establishes the role of MRD negativity in improving long-term survival in a heterogeneous cohort of patients with MM.
The strong prognostic value of MRD negativity sets the stage to adopt MRD as a clinically valid surrogate biomarker for PFS and OS in MM.
Abstract
The prognostic value of minimal residual disease (MRD) for progression-free survival (PFS) and overall survival (OS) was evaluated in a large cohort of patients with multiple myeloma (MM) using a systematic literature review and meta-analysis. Medline and EMBASE databases were searched for articles published up to 8 June 2019, with no date limit on the indexed database. Clinical end points stratified by MRD status (positive or negative) were extracted, including hazard ratios (HRs) on PFS and OS, P values, and confidence intervals (CIs). HRs were estimated based on reconstructed patient-level data from published Kaplan-Meier curves. Forty-four eligible studies with PFS data from 8098 patients, and 23 studies with OS data from 4297 patients were identified to assess the association between MRD status and survival outcomes. Compared with MRD positivity, achieving MRD negativity improved PFS (HR, 0.33; 95% CI, 0.29-0.37; P < .001) and OS (HR, 0.45; 95% CI, 0.39-0.51; P < .001). MRD negativity was associated with significantly improved survival outcomes regardless of disease setting (newly diagnosed or relapsed/refractory MM), MRD sensitivity thresholds, cytogenetic risk, method of MRD assessment, depth of clinical response at the time of MRD measurement, and MRD assessment premaintenance and 12 months after start of maintenance therapy. The strong prognostic value of MRD negativity and its association with favorable outcomes in various disease and treatment settings sets the stage to adopt MRD as a treatment end point, including development of therapeutic strategies. This large meta-analysis confirms the utility of MRD as a relevant surrogate for PFS and OS in MM.
Visual Abstract
Introduction
New treatment options for patients with multiple myeloma (MM) have expanded rapidly in the past decade, leading to deeper responses. Complete responses (CR) have been observed in >50% of transplant-eligible and transplant-ineligible patients with newly diagnosed MM (NDMM).1 With an increase in the proportion of patients achieving CR, various methods have been developed to detect even deeper responses, including stringent CR,2 and, in recent years, minimal residual disease (MRD) assessment. Sophisticated methods such as next-generation flow (NGF) and next-generation sequencing (NGS) have been able to detect MRD sensitivity thresholds up to 10−5 and 10−6. Using these sensitive methods, superior progression-free survival (PFS) and overall survival (OS) outcomes were observed in patients achieving MRD negativity,3-5 leading to wider use of MRD assessment.6-11 Collectively, these studies have established the clinical utility of MRD assessment as an important prognostic marker of survival.
Although PFS and OS are considered appropriate end points by regulatory agencies for approval of treatments for MM,12,13 a long duration of PFS can delay the timely approval and access to effective therapeutic intervention in patients with MM. Given the growing consensus for the use of MRD as an early clinical end point to compare different therapies, a robust quantitative MRD analysis of treatment efficacy is needed in large multicenter trials in a broad spectrum of patients with MM. Although previous studies have shown improved PFS and OS outcomes in patients who achieved MRD-negative status, they were mainly in patients with NDMM as a group. The clinical utility of MRD is important to evaluate in patients with MM in different disease settings, including those with relapsed/refractory MM (RRMM), and in various subgroups such as patients on and after maintenance therapy as well as cytogenetic subgroups. Currently, MRD assessment data are limited in these patients. This study evaluates the prognostic value of MRD for PFS and OS in a large cohort of patients with MM, including those with RRMM, using a systematic literature review and meta-analysis. The utility of MRD as a surrogate marker was evaluated in various subgroups, including those stratified by MRD-negativity achievement at different disease settings, MRD sensitivity thresholds, cytogenetic risk, method of MRD assessment, depth of clinical response at the time of MRD assessment, and MRD assessment before or after start of maintenance therapy.
Methods
Literature search and study selection
The literature review was conducted in adherence with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Medline and EMBASE databases were searched for articles published in English up to 8 June 2019; there was no date limit on the indexed database searches. Details of the search strategy, including a full list of the search terms, are shown in the supplement.
Two independent investigators selected the articles for potential inclusion; disagreements were resolved by a third investigator. Randomized controlled trials and observational studies that reported PFS or OS rates stratified by MRD status in patients with MM following therapy were eligible for inclusion (supplemental Table 1). Methodological quality of the studies was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting recommendations.14 A manual search was conducted to identify any updated publications on selected studies.
Data extraction and preparation
Key study characteristics including year/date of publication, study type, study population, study treatment, and MRD assessment details were extracted (supplemental Table 1). Data on clinical end points stratified by MRD status (positive or negative) were extracted, including hazard ratios (HRs) on PFS and OS, P values, and confidence intervals (CIs). If HRs were not reported, they were estimated based on reconstructed individual patient-level data (IPD) from published Kaplan-Meier (KM) curves.15
In studies with multiple HR estimates on the same population, only 1 estimate was chosen to avoid duplicates. For example, in studies with MRD assessments using different techniques on the same group of patients, the HR estimate using the most common technique was included in the base case analysis. All patients from all eligible studies were included in the main analysis (base case). HRs for PFS and OS, stratified by MRD status, were estimated for subgroups by (1) disease settings, (2) MRD sensitivity thresholds, (3) cytogenetic risk, (4) method of MRD assessment, (5) depth of clinical response at the time of MRD measurement, and (6) measurement of MRD status premaintenance and at 12 months after start of maintenance therapy.
Meta-analysis
To obtain a pooled effect estimate of HR for MRD negativity, a meta-analysis was performed by fitting a random effects model. Pooled KM curves were generated using reconstructed IPD from studies where available. Survival curves were first digitized, and IPD were reconstructed using an algorithm endorsed by health technology assessment agencies15; for curves with missing at-risk patient numbers, individual data were manually reconstructed.
Heterogeneity in design and population among the studies eligible for meta-analysis was assessed with multiple estimators.16 Differences within subgroups were tested for statistical significance with the (2-sided) log-rank test, and HRs with 2-sided 95% CIs were estimated with a Cox regression model. Statistical analyses were performed using the “metafor” R package, version 2, for meta-analyses17; statistical significance level was set at P < .05.
Results
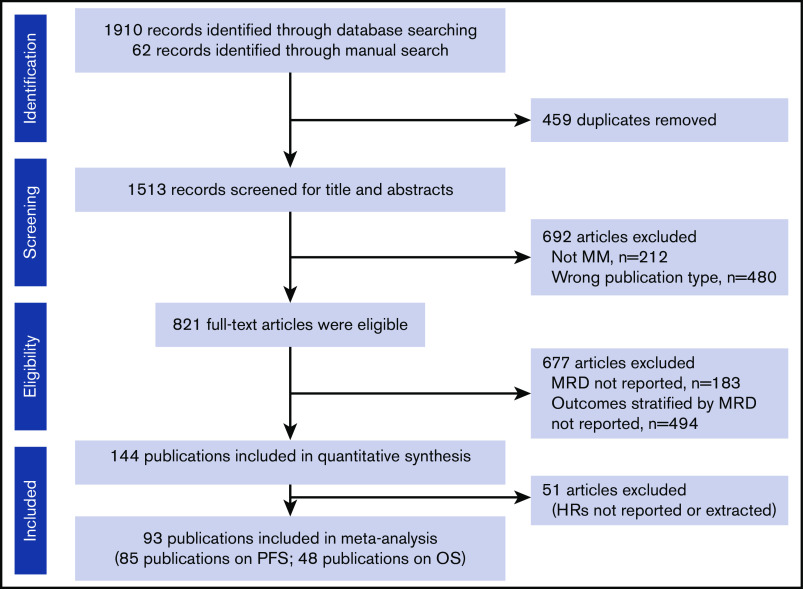
From the initial database search, 144 eligible publications were identified for quantitative analysis (Figure 1); 93 publications reported HRs from 45 studies and were included in the meta-analysis (full dataset). Subgroup analyses were conducted to adjust for heterogeneity in the dataset and to adjust for effect modifiers (ie, variables that differentially modify the observed MRD effect on PFS/OS outcomes) that were selected based on available qualitative evidence from the extracted publications.
Figure 1.
PRISMA flowchart of the systematic literature review search strategy and article selection.
MRD and survival outcomes (base case analysis)
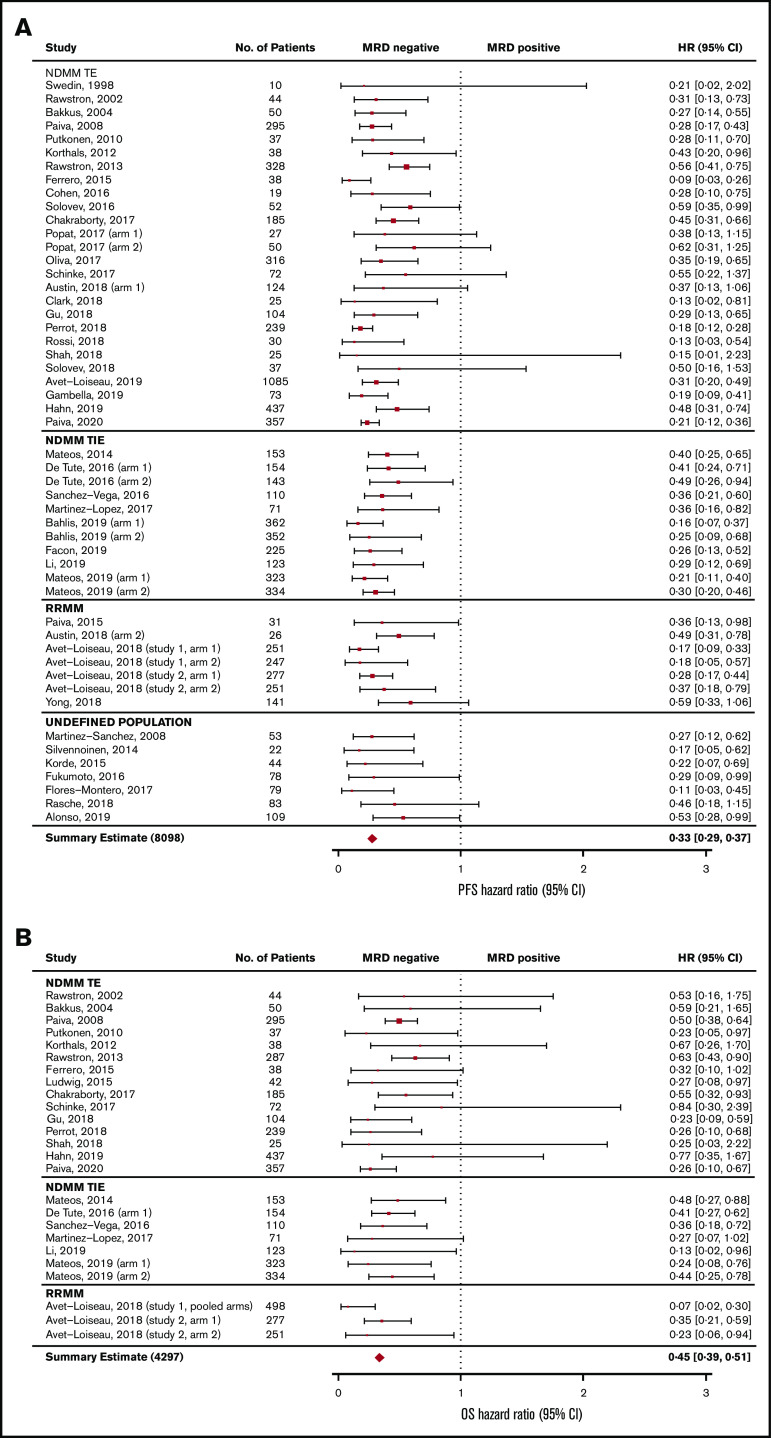
The association between MRD status and survival outcomes was assessed in 44 studies with PFS data from 8098 patients (3111 MRD negative, 4987 MRD positive),4,6,7,10,11,18-55 and 23 studies with OS data from 4297 patients (1605 MRD negative, 2692 MRD positive) (Figure 2).6,10,11,19-24,27,30,33,35,39-44,47,49,56 Compared with MRD positivity, achieving MRD negativity was associated with significantly improved PFS (HR, 0.33; 95% CI, 0.29-0.37; P < .001; Figure 2A) and OS (HR, 0.45; 95% CI, 0.39-0.51; P < .001; Figure 2B).
Figure 2.
Base-case analysis of association of MRD negativity. PFS (A) and OS (B). No., number; TE, treatment eligible; TIE, treatment ineligible.
By MM disease setting.
Survival outcomes were reported in 25 studies in transplant-eligible patients with NDMM,6,10,18-40 8 studies in transplant-ineligible patients with NDMM,11,41-47 and 5 studies in patients with RRMM.31,48-50 Seven studies reported PFS/OS outcomes in patients in whom the disease setting was undefined.4,7,51-55
PFS outcomes.
PFS was significantly improved with MRD negativity vs MRD positivity across all the disease settings (Figure 3; supplemental Figure 1A). In the transplant-eligible NDMM subgroup, MRD negativity was associated with significantly improved PFS (HR, 0.33; 95% CI, 0.28-0.40; P < .001) (Figure 3A). Median PFS was 61.0 months (95% CI, 54.5-67.1) for patients who are MRD negative and 24.1 months (95% CI, 22.6-26.9) for patients who were MRD positive. Five-year PFS rates were 51.0% and 24.0% for patients who were MRD negative and MRD positive, respectively (P < .001; Figure 3B).
Figure 3.
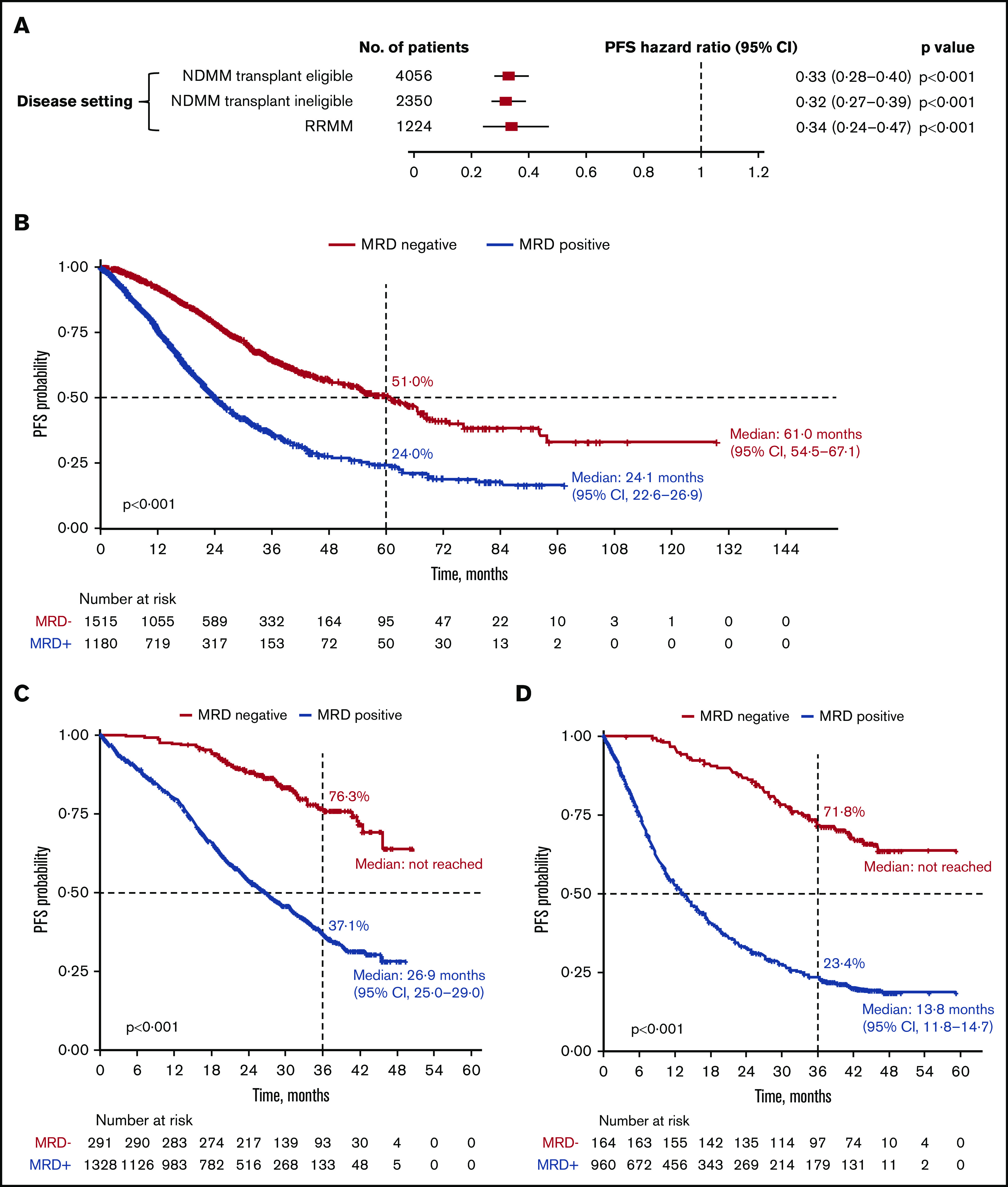
PFS outcomes. (A) Association of MRD negativity with PFS outcomes in patients by disease setting. (B-D) KM estimates of PFS in patients with NDMM who were transplant eligible (B), NDMM who were transplant ineligible (C), and RRMM (D).
In the transplant-ineligible NDMM subgroup, PFS was significantly improved with MRD negativity (HR, 0.32; 95% CI, 0.27-0.39; P < .001) (Figure 3A). Median PFS was not reached for MRD-negative patients and was 26.9 months (95% CI, 25.0-29.0) for MRD-positive patients. Three-year PFS rates were 76.3% and 37.1% for MRD-negative and MRD-positive patients, respectively (P < .001; Figure 3C).
In patients with RRMM, MRD negativity was associated with a significantly improved PFS (HR, 0.34; 95% CI, 0.24-0.47; P < .001; Figure 3A). Median PFS was not reached for MRD-negative patients and was 13.8 months (95% CI, 11.8-14.7) for MRD-positive patients. Three-year PFS rates were 71.8% and 23.4% for patients who were MRD negative and MRD positive, respectively (P < .001; Figure 3D).
OS outcomes.
MRD negativity was associated with significantly improved OS across disease settings (Figure 4; supplemental Figure 1B). In the transplant-eligible patients with NDMM, OS was significantly improved with MRD negativity (HR, 0.50; 95% CI, 0.42-0.59; P < .001) (Figure 4A). Median OS was not reached for MRD-negative patients and was 60.9 months (95% CI, 52.3-68.1) for patients who were MRD-positive. Five-year OS rates were 70.9% and 50.5% in patients who were MRD negative and MRD positive, respectively (P < .001; Figure 4B).
Figure 4.
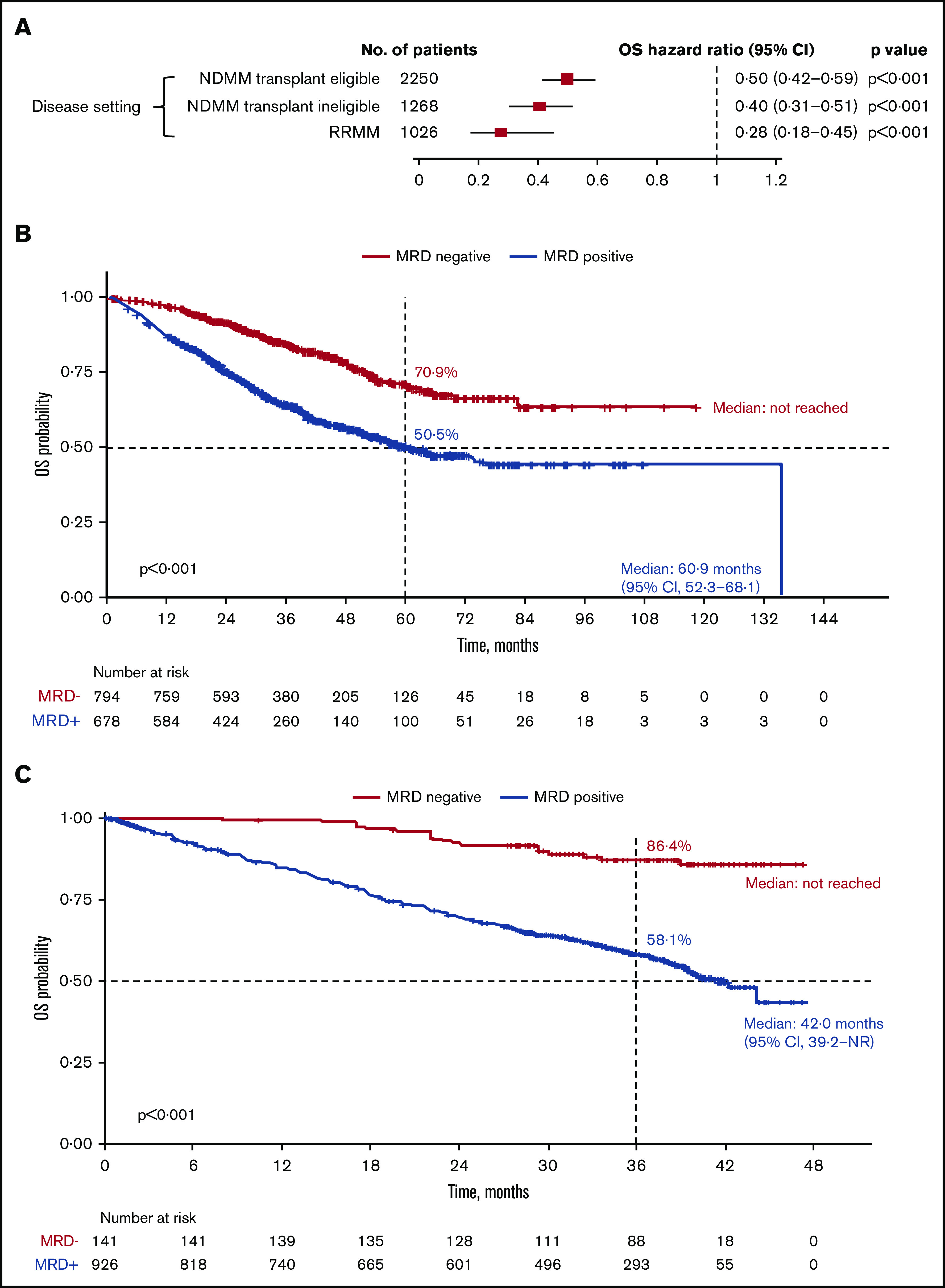
OS outcomes. (A) Association of MRD negativity with OS outcomes in patients by disease setting. KM estimates of OS in patients with NDMM who were transplant eligible (B) and RRMM (C).
Similarly, in the transplant-ineligible NDMM subgroup, MRD negativity was associated with significantly improved OS (HR, 0.40; 95% CI, 0.31-0.51; P < .001) (Figure 4A). A pooled KM curve for OS by MRD status was not generated because it was reported only in 1 study.43
In patients with RRMM, MRD negativity was associated with significantly improved OS (HR, 0.28; 95% CI, 0.18-0.45; P < .001) (Figure 4A). Median OS was not reached for MRD-negative patients and was 42.0 months (95% CI, 39.2-not reached) for MRD-positive patients. OS rates at 3 years were 86.4% in patients who were MRD negative and 58.1% in patients who were MRD positive (P < .001; Figure 4C).
By MRD sensitivity threshold.
Twenty studies reported survival outcomes at the 10−4 MRD sensitivity threshold,6,11,19-25,27,28,31,32,34,38,42,48,50,53,55 18 studies at the 10−5 threshold,4,29,30,33,36-41,43-45,47,49,52,54 and 5 studies at the 10−6 threshold.7,10,40,46,57 MRD negativity was associated with improved survival across all sensitivity thresholds (P < .001 for all subgroups vs MRD positivity; Figure 5; supplemental Figure 2A-B). MRD negativity was associated with significantly improved PFS at both 10−4 (HR, 0.38; 95% CI, 0.32-0.45; P < .001; Figure 5A) and 10−5 sensitivity thresholds (HR, 0.31; 95% CI, 0.27-0.36; P < .001; Figure 5A). Improvements in PFS outcomes were associated with increasingly stringent sensitivity thresholds; PFS was most improved with MRD negativity at the sensitivity threshold of 10−6 (HR, 0.22; 95% CI, 0.16-0.29; P < .001; Figure 5A). Similarly, MRD negativity was associated with OS improvements at increasing sensitivity thresholds (Figure 5B).
Figure 5.
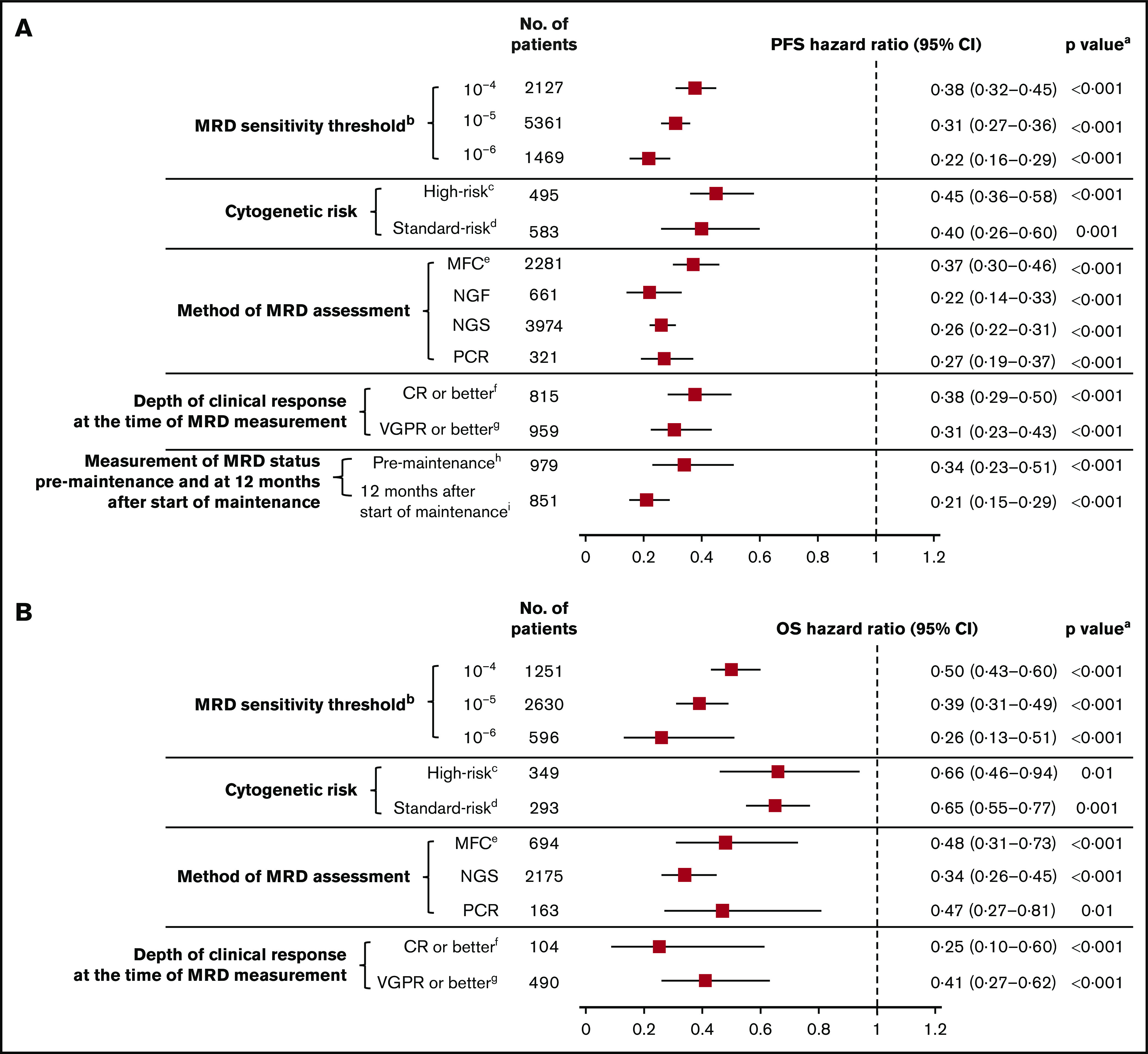
MRD sensitivity threshold. Association of MRD negativity with PFS (A) and OS (B) outcomes in various subgroups of patients with MM. aP vs MRD positive. bMRD sensitivity thresholds at 10−4, 10−5, and 10−6 were defined as 1 MM cell per 10 000, 100 000, and 1 000 000 nucleated cells, respectively. cGenetic abnormalities reported in high-risk patients in this meta-analysis were predominantly defined as the presence of t(4,14), t(14,16), and/or del(17p). dStandard risk was defined as the absence of genetic abnormalities seen in high-risk patients. eOnly includes studies with MRD sensitivity thresholds at 10−5 and 10−6; in studies including 10−4, 10−5, and 10−6 MRD sensitivity thresholds, the HR estimates for PFS and OS were 0.41 (95% CI, 0.36-0.46) and 0.49 (95% CI, 0.42-0.57), respectively. fIncludes studies that reported immunophenotypic CR, stringent CR, or near CR. gDoes not overlap with CR. hMRD assessed at 100 days post-ASCT. iMRD assessed at 12 months after start of maintenance therapy.
By cytogenetic risk.
Six studies reported survival outcomes in patients with high-risk cytogenetics,10,11,23,27,38,58 (supplemental Table 2) and 5 studies reported outcomes in those with standard-risk cytogenetics.10,11,23,38,58 Compared with MRD positivity, benefit of MRD negativity was observed for PFS in both the high-risk (HR, 0.45; 95% CI, 0.36-0.58; P < .001) and standard-risk groups (HR, 0.40; 95% CI, 0.26-0.60; P = .001; Figure 5A; supplemental Figure 3A). Similarly, MRD negativity was associated with significant OS improvement in high-risk and standard-risk groups (Figure 5B; supplemental Figure 3B).
By method of MRD assessment.
A total of 9 studies reported survival outcomes in patients whose MRD status was assessed by the multiparameter flow cytometry (MFC) method.4,29,33,36,37,39,41,52,54 Studies that reported assessment of MRD by the MFC method at the 10−4 sensitivity threshold were mostly older and excluded from this analysis to ensure results without bias. Three studies reported survival outcomes in patients whose MRD status was assessed by the NGF method.7,40,46 Nine studies reported outcomes by the NGS method,10,30,37,43-45,47,49 and 8 studies assessed outcomes by the polymerase chain reaction (PCR) method.18,20-22,24,38,51,52
Survival outcomes were favorable in MRD-negative patients regardless of the method of MRD assessment (Figure 5; supplemental Figure 4A-B). HR for PFS was 0.37 (95% CI, 0.30-0.46) in the MFC studies, 0.22 (95% CI, 0.14-0.33) in the NGF studies, 0.26 (95% CI, 0.22-0.31) in the NGS studies, and 0.27 (95% CI, 0.19-0.37) in the PCR studies (P < 0.001 vs MRD positivity for all 4 subgroups; Figure 5A). Similarly, HR estimates for OS were favorably associated with MRD negativity assessed by MFC, NGS, and PCR methods (Figure 5B). OS was not available for meta-analysis by the NGF method because it was reported only in 1 study.
By depth of clinical response at the time of MRD measurement.
Thirteen studies reported survival outcomes in patients whose MRD status was assessed after the achievement of CR or better,18,21,35,36,46,52,53,55,59 and 9 studies reported outcomes in patients whose MRD status was assessed after the achievement of very good partial response (VGPR) or better.11,24,28-30,33,34,38,41 Compared with MRD positivity, MRD negativity was associated with favorable PFS outcomes in patients whose MRD status was assessed after achievement of CR or better (HR, 0.38; 95% CI, 0.29-0.50; P < .001) or VGPR or better (HR, 0.31; 95% CI, 0.23-0.43; P < .001; Figure 5A; supplemental Figure 5A). Similarly, MRD negativity was associated with significant OS improvement in both groups (Figure 5B; supplemental Figure 5B).
By measurement of MRD status premaintenance and at 12 months after start of maintenance therapy.
Four studies reported PFS outcomes in patients whose MRD status was assessed before maintenance therapy and at 12 months after start of maintenance therapy.10,30,33,39 Compared with MRD positivity, MRD negativity was associated with significantly improved PFS in patients before they received maintenance therapy (HR, 0.34; 95% CI, 0.23-0.51; P < .001) and 12 months after the start of maintenance therapy (HR, 0.21; 95% CI, 0.15-0.29; P < .001; Figure 5A; supplemental Figure 6). PFS estimates were more favorable in patients who achieved MRD negativity at 12 months after the start of maintenance than those with MRD negativity before maintenance therapy. HR estimates for OS were not possible because of limited data.
Discussion
Recent studies have demonstrated the utility of MRD as a prognostic biomarker for PFS and OS outcomes, wherein achievement of MRD-negative status was associated with a significant improvement in both PFS and OS.6,9,23,60,61 However, these studies were relatively small and included primarily transplant-eligible patients with NDMM. Thus, the applicability of these findings to various subgroups of patients with MM remains unclear.
This large meta-analysis, which includes PFS data from 8098 patients and OS data from 4297 patients, further establishes the role of MRD negativity in improving long-term survival outcomes in a broad and heterogeneous MM patient population, regardless of the types of treatment used or method of MRD assessment. These results are consistent with the HR estimates reported previously in a large meta-analysis (PFS HR, 0.41; 95% CI, 0.36-0.48; and OS HR, 0.57; 95% CI, 0.46-0.71).9 The improvement in HR estimates between the 2 analyses could be attributed to the large number of additional studies, including those with transplant-ineligible NDMM and RRMM patient populations, significant increase in sample sizes, and use of more sensitive MRD assessment methods.
The favorable impact of MRD negativity across the disease settings highlights the applicability of MRD as a deeper response criterion in a broader context. Importantly, the achievement of MRD negativity and associated OS improvement in the RRMM population underscores the utility of MRD as a surrogate marker for assessing long-term improvements even among those exposed to several lines of therapy.
This meta-analysis also confirms an association between survival outcomes for MRD-negative patients and increasing MRD sensitivity thresholds up to 10−6. As expected, the number of patients achieving MRD-negative status decreased with increasing MRD sensitivity thresholds. Importantly, every study that examined the depth of response supported the use of 10−6 as the optimal threshold for MRD negativity. However, achieving this threshold may not always be feasible because of limited access to sensitive assays, low bone marrow aspirate volume, or hemodilution of the aspirate resulting in suboptimal cellularity. For MRD to be used as a surrogate biomarker, it must be universally evaluable and applicable, and, hence, a minimum sensitivity of 10−5 threshold is required to achieve that goal.
MRD negativity was associated with improved survival in both high- and standard-risk patients with MM. However, the definition of high-risk cytogenetics varied between the studies, and included patients with del(1p) or gain(1q).11,23,27,58 Despite this variability, MRD negativity was associated with PFS/OS improvements regardless of cytogenetic risk. Furthermore, the magnitude of improvement was largely similar in standard- and high-risk patients. This is particularly relevant in high-risk patients with MM in whom earlier assessment of long-term outcomes by MRD assessment would allow timely intervention with effective therapies and reduce the risk of disease progression and/or death.
In our meta-analysis, MRD negativity was associated with a beneficial effect on survival outcomes regardless of the method used to detect MRD. Based on growing evidence in support of using MRD in routine clinical care, MRD testing is recommended in all patients who achieve CR according to the International Myeloma Working Group 2016 response criteria.62 This meta-analysis included 9 studies in which MRD was assessed after achieving VGPR or better.11,24,28-30,33,34,38,41 Each of these studies confirmed that achieving MRD negativity in patients who had only achieved VGPR or better was associated with superior outcomes. This may be due to the longer half-life of the paraproteins, extravascular deposits of the protein, and, in some cases, extramedullary disease.
MRD negativity before maintenance or 12 months after start of maintenance therapy was associated with improved PFS outcomes. This improvement was greater among MRD-negative patients at 12 months after start of maintenance therapy. These findings highlight its prognostic value as a surrogate marker even at later stages of the treatment algorithm, and are consistent with previous studies showing significantly improved long-term survival outcomes (after autologous stem cell transplant [ASCT]) in patients who achieved MRD negativity before ASCT.6,23,63 Improved outcomes were also observed in patients who achieved post-ASCT MRD negativity while on maintenance therapy.64-66 MRD negativity that is sustained over the long term can potentially influence treatment decisions in daily clinical practice, including the intensity and duration of maintenance therapy. Data on sustained MRD and survival outcomes are not widely reported, and hence a meta-analysis was not performed. Future trials with sequential measurements of MRD status will provide further guidance on the clinical management of patients with MM who achieve long-term MRD negativity.
A key strength of this meta-analysis is its large sample size, which allowed for MRD assessment in various subgroups of patients with MM. Significant heterogeneity between studies is inherent to this meta-analysis. Standardized MRD techniques are lacking, particularly in older studies, and differences in eligibility for MRD assessment may contribute to variability in MRD detection across the studies. The timing of MRD assessment (analyzed only in transplant-eligible patients with NDMM) varied across the included studies, and may have contributed to the heterogeneity in this dataset. Of the trials that reported survival outcomes by timing of MRD assessment, few assessed MRD status in patients on or after maintenance therapy. However, overall, the consistent association of MRD negativity with improved PFS and OS outcomes in a large number of studies conducted at multiple centers in patients receiving different treatment regimens, and MRD assessment using different methods could also be considered a strength of this analysis.
There are limitations associated with assessment of MRD itself. Some of them include the patchy quality of the bone marrow samples, hemodilution of bone marrow aspirates that affect the sample cellularity, differences in sensitivity of the MRD detection methods used, and a risk of false-negative results because of the presence of extramedullary disease. These limitations will remain an obstacle to MRD assessment until alternative technologies are developed to measure cell-free tumor DNA or circulating tumor cell DNA. Another limitation of this meta-analysis is the potential risk of publication bias because of the lack of IPD. In studies in which HRs were not reported, they were estimated based on the simulated IPD from digitized KM curves and may be associated with information bias (in instances in which the true censor was unknown). To minimize measurement bias with reconstructed IPD from digitized KM curves resulting from the poor quality of the initial input and the level of information available in published studies, we have scrutinized the reconstructed data to ensure that all P values, CIs, HRs, numbers of events/deaths, median survival times, and durations of patient follow-up matched those reported in the original publications. Nevertheless, the quality, number of included studies, and the robustness of the analysis demonstrates the association of MRD negativity with improved survival outcomes, supporting the use of MRD as a surrogate.
In summary, achieving MRD-negative status is emerging as an important treatment goal in patients with MM because it has the potential to predict their survival outcomes. This meta-analysis showed that MRD negativity was associated with significant improvements in PFS and OS outcomes in a large cohort of patients with MM including both transplant-eligible and transplant-ineligible patients with NDMM and those with RRMM. Thus, MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in MM, including superseding the prognostic value of CR, demonstrating broad applicability across disease settings, and yielding consistent results regardless of the method of MRD assessment.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Charlene Lee of Janssen Global Services, LLC, for her review of the manuscript.
This study was funded by Janssen-Cilag GmbH. Editorial and medical writing support was provided by Jaya Kolipaka of Eloquent Scientific Solutions, and funded by Janssen Global Services, LLC. This work was partly supported by National Institutes of Health Research Program Project grants PO1-155258 and P50-100707 (N.C.M., H.A.-L., M.S., and K.C.A.) and the VA Healthcare System (5I01BX00158) (N.C.M.).
Footnotes
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access Project site at http://yoda.yale.edu.
Authorship
Contribution: S.C., A.L., N.C.M., M.S., and J.U. contributed to the conception and design and data analysis and interpretation; M.H., B.H., and M.K. contributed to the collection and assembly of data and data analysis and interpretation; and all authors drafted and reviewed the manuscript, approved the final version for submission, and vouch for data accuracy and completeness.
Conflict--interest disclosure: N.C.M. has received consulting fees from AbbVie, Adaptive, Amgen, Celgene, Janssen, Legend, Oncopep, and Takeda. H.A.-L. has received consulting fees, research funding, and travel and lecture fees from AbbVie, Amgen, BMS/Celgene, Janssen, Sanofi, and Takeda. K.C.A. has received consulting fees from Bristol-Myers Squibb, Celgene, Gilead, Janssen, Precision Biosciences, Sanofi-Aventis, Takeda, and Tolero. P.N. has received consulting fees, honoraria, and research funding from Amgen, Celgene, and Janssen. B.P. has received consulting fees from Celgene, Janssen, and Sanofi; was on the advisory boards and received honoraria for lectures from Adaptive, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, Sanofi, and Takeda; and received unrestricted grants from Celgene, EngMab, Sanofi, and Takeda. M.D. has received consulting fees, honoraria, and research funding from Amgen, Celgene, Janssen, and Takeda and participated in advisory boards for Sanofi-Aventis. M.K., M.H., and B.H. are employees of Ingress-Health. A.L., J.H., J.U., J.V., and S.C. are employees of Janssen. N.B. has received consulting fees and honoraria from AbbVie, Celgene, Janssen, Karyopharm, and Takeda. M.S. declares no competing financial interests.
Correspondence: Nikhil C. Munshi, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana B106, Boston, MA 02215; e-mail: nikhil_munshi@dfci.harvard.edu.
References
- 1.Anderson KC, Alsina M, Atanackovic D, et al. ; National Comprehensive Cancer Network . Multiple myeloma, version 2.2016: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2015;13(11):1398-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills JR, Barnidge DR, Dispenzieri A, Murray DL. High sensitivity blood-based M-protein detection in sCR patients with multiple myeloma. Blood Cancer J. 2017;7(8):e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(15 suppl):8002. [Google Scholar]
- 4.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoroidaka T, Takamatsu H, Yamashita T, et al. Minimal residual disease assessment using Euroflow-NGF in patients with multiple myeloma treated with a combination of carfilzomib, lenalidomide, and dexamethasone (KRD). Blood. 2019;134(suppl 1):3130. [Google Scholar]
- 6.Paiva B, Vidriales MB, Cerveró J, et al. ; GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Groups . Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paiva B, Gutiérrez NC, Rosiñol L, et al. ; PETHEMA/GEM (Programa para el Estudio de la Terapéutica en Hemopatías Malignas/Grupo Español de Mieloma) Cooperative Study Groups . High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687-691. [DOI] [PubMed] [Google Scholar]
- 9.Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3(1):28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Li F, Zhou X, et al. Achieving minimal residual disease-negative by multiparameter flow cytometry may ameliorate a poor prognosis in MM patients with high-risk cytogenetics: a retrospective single-center analysis. Ann Hematol. 2019;98(5):1185-1195. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Regulatory considerations for use of minimal residual disease in development of drug and biological products for treatment guidance for industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/hematologic-malignancies-regulatory-considerations-use-minimal-residual-disease-development-drug-and. Accessed 11 November 2020.
- 13.European Medicines Agency Guideline on the use of minimal residual disease as a 4 clinical endpoint in multiple myeloma studies. Available at: https://www.ema.europa.eu/en/guideline-use-minimal-residual-disease-clinical-endpoint-multiple-myeloma-studies. Accessed 11 November 2020.
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 15.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83-98. [DOI] [PubMed] [Google Scholar]
- 17.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. [Google Scholar]
- 18.Swedin A, Lenhoff S, Olofsson T, Thuresson B, Westin J. Clinical utility of immunoglobulin heavy chain gene rearrangement identification for tumour cell detection in multiple myeloma. Br J Haematol. 1998;103(4):1145-1151. [DOI] [PubMed] [Google Scholar]
- 19.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100(9):3095-3100. [DOI] [PubMed] [Google Scholar]
- 20.Bakkus MH, Bouko Y, Samson D, et al. Post-transplantation tumour load in bone marrow, as assessed by quantitative ASO-PCR, is a prognostic parameter in multiple myeloma. Br J Haematol. 2004;126(5):665-674. [DOI] [PubMed] [Google Scholar]
- 21.Putkonen M, Kairisto V, Juvonen V, et al. Depth of response assessed by quantitative ASO-PCR predicts the outcome after stem cell transplantation in multiple myeloma. Eur J Haematol. 2010;85(5):416-423. [DOI] [PubMed] [Google Scholar]
- 22.Korthals M, Sehnke N, Kronenwett R, et al. The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high-dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biol Blood Marrow Transplant. 2012;18(3):423-431. [DOI] [PubMed] [Google Scholar]
- 23.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540-2547. [DOI] [PubMed] [Google Scholar]
- 24.Ferrero S, Ladetto M, Drandi D, et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia. 2015;29(3):689-695. [DOI] [PubMed] [Google Scholar]
- 25.Cohen OC, Rabin N, Counsell N, et al. Bortezomib consolidation following upfront ASCT for multiple myeloma deepens disease response and MRD-negative rate without compromising response to subsequent bortezomib salvage: results of a phase II study. Blood. 2016;128(22):4508. [Google Scholar]
- 26.Solovev M, Mendeleeva L, Pokrovskaya O, et al. Maintenance therapy with bortezomib in patients with multiple myeloma (MM) after ASCT and minimal residual disease (MRD). Haematologica. 2017;102(2):E1295. [Google Scholar]
- 27.Chakraborty R, Muchtar E, Kumar SK, et al. Impact of post-transplant response and minimal residual disease on survival in myeloma with high-risk cytogenetics. Biol Blood Marrow Transplant. 2017;23(4):598-605. [DOI] [PubMed] [Google Scholar]
- 28.Popat R, De Tute R, Counsell N, et al. Outcomes of stratification to ASCT or not based on depth of response: results of a phase 2 trial assessing the impact of minimal residual disease (MRD) in multiple myeloma patients with deferred ASCT (PADIMAC). Blood. 2017;130(1):1864. [Google Scholar]
- 29.Oliva S, op Bruinink D, ŘÍhová L, et al. Minimal residual disease by multiparameter cytometry in transplant eligible patients with newly diagnosed multiple myeloma: results from the EMN02/HO95 phase 3 trial. Haematologica. 2017;102(2):S102. [Google Scholar]
- 30.Schinke C, Hoering A, Wang H, et al. The prognostic value of the depth of response in multiple myeloma depends on the time of assessment, risk status and molecular subtype. Haematologica. 2017;102(8):e313-e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin M, Pawlyn C, Woods H, et al. Sustained MRD negativity at 12 months post-ASCT predicts outcomes for myeloma patients: a real world study. EHA Library. 2018;215626:2018. [Google Scholar]
- 32.Clark CA, Mosse CA, Chen H, et al. Prospective trial of minimal residual disease assessment by multiparametric flow cytometry for multiple myeloma in the era of bortezomib-based chemotherapy. Bone Marrow Transplant. 2018;53(12):1589-1592. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Liu J, Chen M, Huang B, Li J. Longitudinal flow cytometry identified “minimal residual disease” (MRD) evolution patterns for predicting the prognosis of patients with transplant-eligible multiple myeloma. Biol Blood Marrow Transplant. 2018;24(12):2568-2574. [DOI] [PubMed] [Google Scholar]
- 34.Rossi G, Falcone AP, Minervini MM, et al. Minimal residual disease and log-reduction of plasma cells are associated with superior response after double autologous stem cell transplant in younger patients with multiple myeloma. Cytometry B Clin Cytom. 2019;96(3):195-200. [DOI] [PubMed] [Google Scholar]
- 35.Shah GL, Seier K, Devlin S, et al. Depth of response and outcomes in patients with multiple myeloma undergoing autologous stem cell transplantation. Blood. 2018;132(suppl 1):4619. [Google Scholar]
- 36.Solovev MV, Mendeleeva L, Firsova M, et al. Efficacy of maintenance therapy following auto-HSCT depending on MRD status in patients with multiple myeloma. Blood. 2018;132(suppl 1):3432. [Google Scholar]
- 37.Avet-Loiseau H, Moreau P, Attal M, et al. Efficacy of daratumumab (DARA) + bortezomib/thalidomide/dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) based on minimal residual disease (MRD) status: analysis of the CASSIOPEIA trial [abstract]. J Clin Oncol 2020;37(15 suppl). Abstract 8017. [Google Scholar]
- 38.Gambella M, Omedé P, Spada S, et al. Minimal residual disease by flow cytometry and allelic-specific oligonucleotide real-time quantitative polymerase chain reaction in patients with myeloma receiving lenalidomide maintenance: a pooled analysis. Cancer. 2019;125(5):750-760. [DOI] [PubMed] [Google Scholar]
- 39.Hahn TE, Wallace P, Fraser R, et al. Minimal residual disease (MRD) assessment before and after autologous hematopoietic cell transplantation (AutoHCT) and maintenance for multiple myeloma (MM): results of the Prognostic Immunophenotyping for Myeloma Response (PRIMeR) study. Biol Blood Marrow Transplant. 2019;25(3):S4-S6. [Google Scholar]
- 40.Paiva B, Puig N, Cedena MT, et al. ; GEM (Grupo Español de Mieloma)/PETHEMA (Programa Para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group . Measurable residual disease by next-generation flow cytometry in multiple myeloma. J Clin Oncol. 2020;38(8):784-792. [DOI] [PubMed] [Google Scholar]
- 41.Mateos MV, Oriol A, Martínez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887-1893. [DOI] [PubMed] [Google Scholar]
- 42.de Tute R, Rawstron A, Cairns D, et al. Impact of minimal residual disease in transplant ineligible myeloma patients: results from the UK NCRI Myeloma XI trial. Blood. 2016;128(22):245. [Google Scholar]
- 43.Sanchez-Vega B, Alonso R, Cuenca I, et al. Prognostic impact of molecular response assessed by next-generation sequencing in a large cohort of multiple myeloma patients. Blood. 2016;128(22):3283. [Google Scholar]
- 44.Martinez-Lopez J, Sanchez-Vega B, Barrio S, et al. Analytical and clinical validation of a novel in-house deep-sequencing method for minimal residual disease monitoring in a phase II trial for multiple myeloma. Leukemia. 2017;31(6):1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahlis N, Facon T, Usmani S, et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant: updated analysis of MAIA. Blood. 2019;134(suppl 1):1875. [Google Scholar]
- 46.Facon T, Lee JH, Moreau P, et al. Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2019;133(18):1953-1963. [DOI] [PubMed] [Google Scholar]
- 47.Mateos MV, Dimopoulos MA, Cavo M, et al. ; ALCYONE Trial Investigators . Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518-528. [DOI] [PubMed] [Google Scholar]
- 48.Paiva B, Chandia M, Puig N, et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica. 2015;100(2):e53-e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avet-Loiseau H, San-Miguel J, Casneuf T, et al. Evaluation of sustained minimal residual disease (MRD) negativity in relapsed/refractory multiple myeloma (RRMM) patients (Pts) treated with daratumumab in combination with lenalidomide plus dexamethasone (D-Rd) or bortezomib plus dexamethasone (D-Vd): analysis of pollux and castor. Blood. 2018;132(suppl 1):3272. [Google Scholar]
- 50.Yong K, Hinsley S, De Tute R, et al. Maintenance with carfilzomib following carfilzomib, cyclophosphamide and dexamethasone at first relapse or primary refractory multiple myeloma (MM) on the phase 2 Muk Five Study: effect on minimal residual disease. Blood. 2018;132(suppl 1):802. [Google Scholar]
- 51.Martínez-Sánchez P, Montejano L, Sarasquete ME, et al. Evaluation of minimal residual disease in multiple myeloma patients by fluorescent-polymerase chain reaction: the prognostic impact of achieving molecular response. Br J Haematol. 2008;142(5):766-774. [DOI] [PubMed] [Google Scholar]
- 52.Silvennoinen R, Lundan T, Kairisto V, et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood Cancer J. 2014;4(10):e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukumoto K, Fujisawa M, Suehara Y, et al. Prognostic impact of immunophenotypic complete response in patients with multiple myeloma achieving better than complete response. Leuk Lymphoma. 2016;57(8):1786-1792. [DOI] [PubMed] [Google Scholar]
- 54.Rasche L, Alapat D, Kumar M, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia. 2019;33(7):1713-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alonso R, Cedena MT, Gómez-Grande A, et al. Imaging and bone marrow assessments improve minimal residual disease prediction in multiple myeloma. Am J Hematol. 2019;94(8):853-861. [DOI] [PubMed] [Google Scholar]
- 56.Ludwig H, Greil R, Masszi T, et al. Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with previously untreated multiple myeloma: 5-year follow-up. Br J Haematol. 2015;171(3):344-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu B, Thall P, Milton DR, et al. High-risk myeloma and minimal residual disease postautologous-HSCT predict worse outcomes. Leuk Lymphoma. 2019;60(2):442-452. [DOI] [PubMed] [Google Scholar]
- 59.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125(20):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51(12):1565-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson KC, Auclair D, Kelloff GJ, et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: current value and future applications. Clin Cancer Res. 2017;23(15):3980-3993. [DOI] [PubMed] [Google Scholar]
- 62.Landgren O, Rustad E. Meeting report: advances in minimal residual disease testing in multiple myeloma 2018. Adv Cell Gene Ther. 2019;2(1):e26. [Google Scholar]
- 63.Holstein SA, Avet-Loiseau H, Hahn T, et al. BMT CTN Myeloma Intergroup Workshop on minimal residual disease and immune profiling: summary and recommendations from the Organizing Committee. Biol Blood Marrow Transplant. 2018;24(4):641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel DA, Gopalakrishnan R, Engelhardt BG, et al. Minimal residual disease negativity and lenalidomide maintenance therapy are associated with superior survival outcomes in multiple myeloma. Bone Marrow Transplant. 2020;55(6):1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson GH, Davies FE, Pawlyn C, et al. ; UK NCRI Haemato-oncology Clinical Studies Group . Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.