Key Points
No previous studies have reported the impact of G6PD deficiency on a broad range of childhood diseases in Africa.
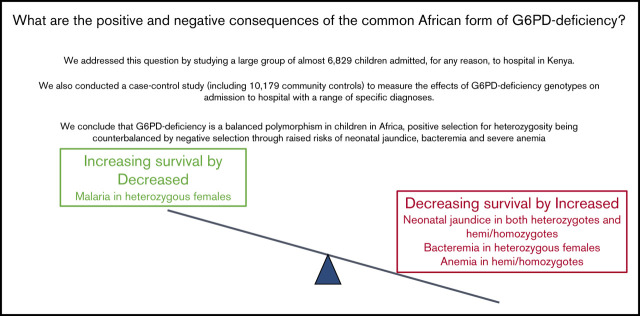
Positive selection for heterozygosity by malaria is balanced by negative selection from severe neonatal jaundice, anemia, and bacteremia.
Abstract
Few previous studies have reported the effects of glucose-6-phosphate dehydrogenase (G6PD)–deficiency on child health in Africa. We conducted a case-control study in which cases (n = 6829) were children admitted, for any reason, to Kilifi County Hospital, Kenya, while controls (n = 10 179) were recruited from the surrounding community. Cases were subclassified based on their clinical and laboratory findings at admission. We calculated the prevalence of specific diseases by G6PD c.202 genotype, the only significant cause of G6PD-deficiency in this area, then estimated the association between genotype and admission with specific conditions using logistic regression. Among neonates, the prevalence of jaundice was higher in both G6PD c.202T heterozygotes (40/88; 45.5%; P = .004) and homo/hemizygotes (81/134; 60.5%; P < .0001) than in wild-type homozygotes (157/526; 29.9%). Median bilirubin levels also increased across the groups, being highest (239 mmol/L; interquartile range 96-390 mmol/L) in G6PD c.202T homo/hemizygotes. No differences were seen in admission hemoglobin concentrations or the prevalence of anemia or severe anemia by G6PD c.202 genotype. On case control analysis, G6PD heterozygosity was negatively associated with all-cause hospital admission (odds ratio 0.81; 95% confidence interval 0.73-0.90; P < .0001) and, specifically, admission with either pneumonia or Plasmodium falciparum parasitemia; while, conversely, it was positively associated with Gram-positive bacteremia. G6PD c.202T homo/heterozygosity was positively associated with neonatal jaundice, severe pneumonia, the receipt of a transfusion, and in-patient death. Our study supports the conclusion that G6PD c.202T is a balanced polymorphism in which a selective advantage afforded to heterozygous females against malaria is counterbalanced by increased risks of neonatal jaundice, invasive bacterial infections, and anemia.
Visual Abstract
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) plays an important role in the defense against oxidant damage, particularly in red blood cells.1 G6PD is an enzyme that is encoded by an X-linked gene (G6PD) that is among the most polymorphic loci of humans. Approximately 200 allelic variants have been reported to date of which some 40 have reached high frequencies in multiple populations.2 G6PD deficiency is most common in Africa, Asia, the Mediterranean, and the Middle East, which almost certainly reflects a survival advantage against malaria.3 G6PD deficiency is categorized by the World Health Organization (WHO) into 4 classes according to the residual enzyme activity in hemizygous vs normal males.1 Clinically, the most common consequences of G6PD deficiency are neonatal jaundice and acute hemolytic anemia, although the frequency and severity of these complications vary substantially depending on the degree to which enzyme activity is impaired.2 The G6PD c.202T allele, which results in the A-haplotype, is the most common cause of G6PD-deficiency in Africa and affects >30% of males in some populations.3 G6PD c.202T is a WHO class III mutation in which hemizygotes retain ∼12% of normal G6PD activity. As a consequence, it is often considered to have a mild phenotype, although this assumption has recently been challenged.4 Despite being so common, few epidemiological studies have been conducted in Africa that have investigated the broad impacts of G6PD c.202T deficiency on children’s health. In the current study, we have investigated this question in an area where the G6PD c.202T allele is the only significant cause of G6PD deficiency.5
Methods
Study design
First, we undertook a descriptive analysis of the clinical features and outcome of hospital-admitted patients according to their G6PD c.202 genotypes before then conducting a series of case-control studies in which we investigated the association between G6PD c.202 genotypes and admission to hospital overall and with a range of specific diagnoses. Cases were children aged <14 years who were residents of the area served by the Kilifi Health and Demographic Surveillance System in Kenya5 and who were admitted to the wards of Kilifi County Hospital (KCH) for any reason between 1 January 2003 and 31 December 2004. Children aged 3 to 12 months who were born within the same study area as cases between August 2006 and September 2010, and who were recruited to a cohort study primarily designed to study the childhood epidemiology of sickle cell disease,6 were used as controls. As such, controls were not matched to cases but were recruited from the same catchment area. The latter were recruits to an ongoing study, designed to investigate genetic susceptibility to a range of childhood diseases.6 Cases were further classified based on their clinical phenotype at the time of admission, derived from data from a long-running surveillance system of pediatric admissions to KCH.7 The following clinical syndromes were included in the analysis. Neonatal conditions were defined as admission to hospital within the first 28 days of life. Anemia and severe anemia were defined as a hemoglobin (Hb) level of <110 g/L and <80 g/L, respectively, in neonates8 and as an Hb level of <110 g/L and <50 g/L beyond the neonatal period. The latter was based on a modification of the WHO definitions9 in which definitions for severe malaria range between <60 g/L and <40 g/L depending on underlying conditions and the presence of specific clinical complications. Clinical malaria was defined as a fever in the presence of Plasmodium falciparum parasitemia at any density in children <1 year old or at a density of >2500 parasites per microliter in older children, and severe malaria was defined as malaria in association with a range of specific complications, which included prostration, coma (cerebral malaria), respiratory distress, an Hb level of <50 g/L (severe malaria anemia), and other syndromes as defined by the WHO.10 Severe and very severe pneumonia were defined using standard methods,11 whereas meningitis/encephalitis was defined by the presence of neck stiffness, a bulging fontanelle, prostration, or coma (defined as a Blantyre Coma Score of 3 to <5 and <3, respectively). Severe malnutrition was defined on the basis of a mid-upper-arm circumference of ≤7.5 cm in children <6 months of age or of ≤11.5 in children ≥6 months of age.9,12 Gastroenteritis was defined as diarrhea (≥3 loose watery stools per day) with or without vomiting (≥3 episodes per day), and jaundice was defined clinically by its recognition by the admitting clinician. Severe anemia was defined as an Hb level of <50 g/L irrespective of the presence of malaria parasites. Finally, in order to identify episodes of acute intravascular hemolysis, a specific potential complication of G6PD deficiency, we searched both the admission and the discharge diagnoses together with the free-text admission notes of our electronic database for the terms “hemolysis,” “hemolytic,” “hemoglobinuria,” “dark (urine),” “G6PD,” and “favism,” allowing for permutations including the use of US spellings. Because of its association with multiple health consequences and a raised incidence of premature mortality,13 for the purpose of the current analysis, we excluded children with sickle cell anemia (HbSS) from both the case and the control groups.
Laboratory procedures
Hematological, biochemical, and malaria parasite data were derived by standard methods,14 whereas blood cultures were processed in BACTEC Peds Plus bottles using a BACTEC 9050 automated blood-culture instrument (Becton Dickinson, UK). Positive samples were subcultured on standard media by routine microbiological techniques.7 Quality assurance for all laboratory tests was provided by the UK National External Quality Assessment Service (www.ukneqas.org.uk). Genomic DNA was extracted for genotyping from fresh or frozen samples of whole blood using proprietary methods (either ABI PRISM [Applied Biosystems, CA] or Qiagen DNA Blood Mini Kit [Qiagen, West Sussex, United Kingdom]). We typed the G6PD c.202T allele, the only significant cause of G6PD deficiency in our study population, by amplification refractory system polymerase chain reaction as described in detail previously.15 All samples were also typed for the confounder HbS as previously described.14,16 Children typing positive for HbSS were excluded from both case and control sets for the purpose of the current analysis.
Statistical analysis
We compared the clinical, laboratory, and demographic characteristics of children who were admitted to hospital during the study period (cases), defined according to G6PD c.202T genotype, using parametric or nonparametric tests as appropriate, whereas proportions were compared using the χ2 test. For the purpose of our analyses, we categorized study participants into the 3 most physiologically meaningful G6PD categories based on G6PD c.202T genotypes: (i) biochemically normal individuals, girls or boys who were either homozygous or hemizygous, respectively, for the wild-type allele at position 202 of the G6PD gene; (ii) G6PD c.202T heterozygous girls, who have intermediate levels of G6PD activity5; and (iii) G6PD c.202T homozygous girls and hemizygous boys, both of whom have G6PD deficiency. The latter group is physiologically identical with regard to their G6PD enzyme activity, each manifesting ∼12% of normal levels.5,15 In our case-control analysis, we investigated the association between G6PD c.202 genotype and a range of specific diagnoses by logistic regression, adjusting for the confounders administrative division of residence, sex, and HbS genotype. All analyses were conducted using Stata v14.2 (StataCorp, Timberlake).
Code availability
The code associated with the statistical analysis of the primary data, written in Stata v14.2, has been deposited on the data repository for the KEMRI–Wellcome Trust Research Program in Kilifi and is available, along with appropriately anonymized data, by application through mmunene@kemri-wellcome.org.
Ethics
Informed written consent was obtained from all study participants, their parents, or guardians. Ethical permission for the study was granted by the KEMRI/National Ethics Research Committee in Nairobi, Kenya.
Results
Study population
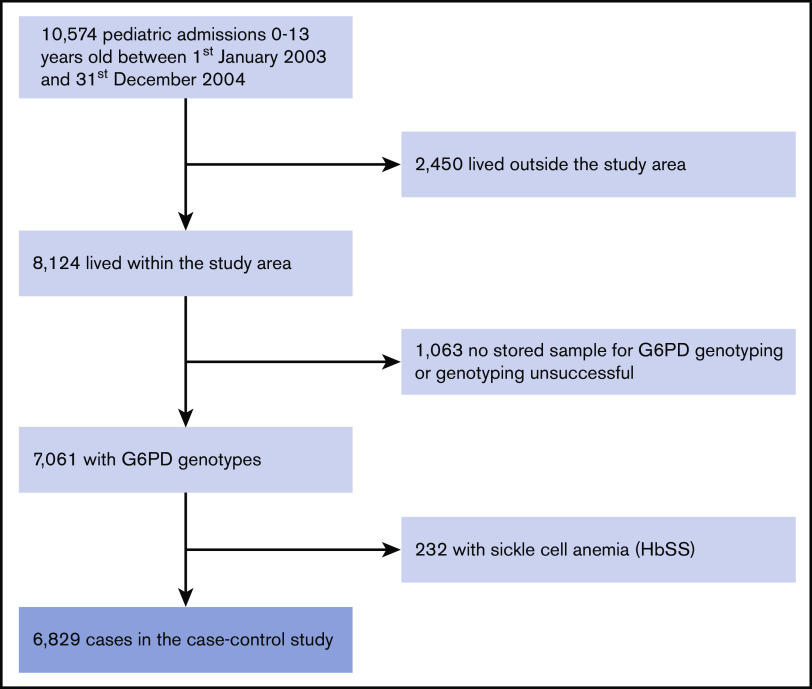
The study included 8124 children <14 years of age who were admitted to KCH from within the study area covered by the Kilifi Health and Demographic Surveillance System during the 2-year period from 1 January 2003 to 31 December 2004. Of these potential cases, 6829 (84.1%) had samples available for analysis, were genotyped successfully at the G6PD c.202T locus, did not have HbSS, and were included in the current analysis (Figure 1). The study flow for controls was as described in Uyoga et al.6 The baseline clinical and hematological characteristics of these children, stratified by G6PD c.202T category, are summarized in Table 1. The groups were similar with the exceptions that G6PD c.202T–deficient homo/hemizygotes were younger and mean cell volumes were higher within both deficiency groups, while female heterozygotes were significantly less stunted than G6PD c.202T wild-type homo/hemizygotes.
Figure 1.
Study flow.
Table 1.
Clinical and laboratory characteristics of case patients
| Characteristic | G6PD normal boys and girls | G6PD c.202T heterozygous girls | P | G6PD c.202T homozygous girls and hemizygous boys | P |
|---|---|---|---|---|---|
| All admissions, n (%) | 5130 (75.1) | 809 (11.9) | n/a | 890 (13.0) | n/a |
| Median age (IQR), mo | 17.2 (6.9-35.8) | 16.0 (6.9-32.5) | .13 | 14.6 (5.0-33.5) | .0008 |
| Mean WAZ (95% CI) | −1.65 (−1.70 to 1.62) | −1.65 (−1.76 to 1.55) | .93 | −1.73 (−1.83 to 1.63) | .18 |
| Mean HAZ (95% CI) | −1.38 (−1.42 to 1.33) | −1.24 (−1.35 to 1.13) | .026 | −1.43 (−1.53 to 1.33) | .37 |
| Mean Hb level (95% CI), g/L | 94.3 (93.4-95.1) | 91.5 (89.3-93.6) | .017 | 93.6 (91.6-95.6) | .53 |
| Mean MCV (95% CI), fL | 74.6 (74.3-75.0) | 76.6 (75.7-77.5) | .0001 | 77.7 (76.8-78.7) | <.0001 |
| Median WBC (IQR), ×109/L | 12.1 (8.8-17.0) | 12.6 (8.7-17.8) | .18 | 12.7 (9.0-17.8) | .86 |
| Median platelets (IQR), ×109/L | 308 (152-464) | 293 (151-459) | .18 | 314 (180-490) | .049 |
| Median bilirubin* (IQR), μmol/L | 19 (9-69) | 136 (27-223) | .26 | 110 (37-383) | .48 |
| Median parasite density† (IQR), per μL | 49 720 (3 599-224 000) | 28 399 (3 383-176 400) | .10 | 33 800 (3 770-180 120) | .24 |
Percentages reflect column percentages with the exception of all admissions. P values estimated by Student t, χ2, or Mann-Whitney U tests as appropriate in comparison with the G6PD normal group.
HAZ, height-for-age Z-score; IQR, interquartile range; MCV, mean cell volume; n/a, not applicable; WAZ, weight-for-age Z-score; WBC, white blood cell count.
Bilirubin levels among those with detectable jaundice.
Parasite densities among the fraction with positive blood films. No independent association was seen between sex and any parameter individually.
G6PD deficiency among children admitted with a range of clinical diagnoses
The clinical phenotypes and outcome of hospital-admitted neonatal (≤28 days of age) and postneonatal (>28 days of age) cases are summarized in Tables 2 and 3, respectively. Compared with G6PD c.202T wild-type homo/hemizygotes, the prevalence of clinical jaundice was higher in both G6PD c.202T deficiency groups. The prevalence was highest (60.5%) in homo/hemizygous children admitted during the neonatal period. Although the prevalence of jaundice was also higher in G6PD c.202T homo/hemizygous–deficient than G6PD c.202T homozygous wild-type children admitted during the postneonatal period, it was rare overall and was seen in only 2.4% of those who were homo/hemizygous deficient. In children with detectable jaundice during the neonatal period, median bilirubin levels were higher in G6PD c.202T heterozygous or homo/hemizygous children than among wild-type homozygotes (Tables 2 and 3). Although admission Hb levels were significantly lower among deficient homo/hemizygotes overall, this was not true in the subgroup with clinical jaundice, suggesting that jaundice was not related predominantly to hemolysis. Severe anemia was rare and was no more frequent among children in the G6PD c.202 deficiency groups than in wild-type homo/hemizygotes (Table 2). We detected only 6 occurrences in 5 individuals of terms suggestive of intravascular hemolysis. “Dark urine” was mentioned in 4 admissions, all associated with acute malaria, whereas “hemolysis” was mentioned during 3 admissions, 2 of which were in children with a suspected diagnosis of sickle cell disease, although genotyping results from our current study showed that neither were actually affected. Two episodes were among G6PD c.202 wild-type homo/hemizygotes, whereas 1 episode was seen among each of the deficiency groups.
Table 2.
Distribution of clinical syndromes and outcomes among case patients admitted during the neonatal period
| Syndrome | G6PD normal boys and girls (n = 526) | G6PD c.202T heterozygous girls (n = 88) | P | G6PD c.202T homozygous girls and hemizygous boys (n = 134) | P |
|---|---|---|---|---|---|
| Clinical, laboratory, and demographic features | |||||
| Clinical jaundice, n (%) | 157 (29.9) | 40 (45.5) | .004 | 81 (60.5) | <.0001 |
| Bacteremia,* n (%) | 34 (6.5) | 9 (10.2) | .20 | 7 (5.2) | .59 |
| Mean Hb level (95% CI), g/L | 146 (143-149) | 143 (136-150) | .49 | 138 (133-143) | .0086 |
| Anemia (Hb <110 g/L), n/N (%) | 63/512 (12.3) | 13/85 (15.3) | .44 | 19/127 (15.0) | .42 |
| Severe anemia (Hb <80 g/L), n/N (%) | 14/512 (2.7) | 1/85 (1.2) | .40 | 2/127 (1.6) | .45 |
| Median bilirubin† (IQR), μmol/L | 82.5 (37-191) | 223 (66-319) | .0001 | 239 (96-390) | <.0001 |
| Mean Hb level in those with jaundice (95% CI), g/L | 148 (142-153) | 149 (139-159) | .82 | 141 (134-147) | .12 |
| Median weight overall (IQR), kg | 2.7 (2.1-3.2) | 2.8 (2.2-3.1) | .80 | 2.7 (2.0-3.2) | .92 |
| Median weight in those with jaundice (IQR), kg | 2.7 (2.2-3.1) | 2.7 (3.4-3.0) | .91 | 2.8 (2.4-3.2) | .12 |
| Median age (IQR), d | 2 (0-9) | 4 (0-9) | .23 | 4 (1-7) | .60 |
| Median age in those with jaundice (IQR), d | 5 (2-8) | 4.5 (3-7.5) | .71 | 5 (3-7) | .97 |
| Outcome | |||||
| Median duration of admission (IQR), d | 5 (3-8) | 6 (3-8.5) | .08 | 6 (3-10) | .036 |
| Transfused, n (%) | 25 (4.8) | 11 (12.5) | .004 | 28 (20.9) | <.0001 |
| Died, n (%) | 118 (22.4) | 15 (17.1) | .26 | 32 (23.9) | .72 |
Bacteremia was caused by the following organisms (n): Acinetobacter spp (4), Aeromonas spp (1), Enterobacter spp (8), Escherichia coli (6), Haemophilus influenzae (1), Klebsiella pneumoniae (2), Pseudomonas aeruginosa (3), Salmonella spp (3), Staphylococcus aureus (5), β-hemolytic Streptococcus (7), Streptococcus pneumoniae (7), Vibrio cholera (3). No independent association was seen between sex and any parameter individually.
Plasma bilirubin levels at admission in children with visible jaundice. Denominators are shown in cases where data were missing.
Table 3.
Distribution of clinical syndromes and outcomes among post-neonatal case patients
| Syndrome | G6PD normal boys and girls (n = 4604) | G6PD c.202T heterozygous girls (n = 721) | P | G6PD c.202T homozygous girls and hemizygous boys (n = 756) | P |
|---|---|---|---|---|---|
| Clinical syndromes,* n (%) | |||||
| Malaria | 1422 (30.9) | 231 (32.0) | .53 | 228 (30.2) | .69 |
| Severe malaria | 280 (6.1) | 53 (7.4) | .19 | 36 (4.8) | .15 |
| Severe pneumonia | 96 (2.1) | 15 (2.1) | .99 | 26 (3.4) | .021 |
| Very severe pneumonia | 2593 (56.3) | 406 (56.3) | .99 | 427 (56.5) | .93 |
| Meningitis/encephalitis | 843 (18.3) | 147 (20.4) | .18 | 137 (18.1) | .90 |
| Severe malnutrition | 408 (8.9) | 104 (14.4) | <.0001 | 54 (7.1) | .20 |
| Gastroenteritis | 1161 (25.2) | 173 (24.0) | .48 | 193 (25.5) | .86 |
| Jaundice | 49 (1.1) | 9 (1.3) | .66 | 18 (2.4) | .003 |
| Other | 550 (12.0) | 72 (10.0) | .12 | 79 (8.9) | .097 |
| Laboratory-based syndromes | |||||
| Bacteremia,† n (%) | 201 (4.4) | 46 (6.4) | .017 | 38 (5.0) | .41 |
| Severe anemia, n (%) | 333 (7.2) | 62 (8.6) | .19 | 59 (7.8) | .58 |
| Mean Hb level in children with jaundice (95% CI), g/L | 72.7 (63.5-81.9) | 79.4 (67.9-91.0) | .54 | 84.6 (74.2-94.9) | .15 |
| Malaria blood film positive, n (%) | 1793 (38.9) | 298 (41.3) | .22 | 281 (37.2) | .35 |
| Outcome | |||||
| Median duration of admission (IQR), d | 3 (2-5) | 3 (2-5) | .30 | 3 (2-5) | .33 |
| Transfused, n (%) | 340 (7.4) | 55 (7.6) | .87 | 67 (8.9) | .16 |
| Died, n (%) | 201 (4.4) | 35 (4.9) | .55 | 35 (4.6) | .74 |
Some children displayed >1 clinical feature and therefore appear more than once.
Bacteremia was caused by the following organisms (n): Acinetobacter spp (32), Enterobacter spp (1), Escherichia coli (23), Haemophilus influenzae (18), Klebsiella spp (8), Neisseria spp (2), Proteus mirabilis (1), Pseudomonas aeruginosa (6), other Pseudomonas spp (5), Salmonella spp (30), Serratia spp (1), Staphylococcus aureus (21), β-hemolytic Streptococcus (30), S pneumoniae (107). No independent association was seen between sex and any parameter individually with the exception of severe malnutrition, which was significantly more common in girls overall (see text).
The independent association between sex with a range of clinical diagnoses
Because G6PD deficiency is sex linked, we checked our data for any independent association between sex and our specific outcomes of interest. None were significantly associated except for one, a significantly higher proportion of girls (325/2900; 11.2%) than boys (258/3363; 7.7%) >28 days of age were admitted with severe malnutrition (P < .0001).
Odds ratios for G6PD c.202T genotypes among children admitted with various conditions
The overall frequencies of G6PD deficiency genotypes among the 6829 genotyped cases were G6PD c.202 wild-type homo/hemizygotes, 5130 (75.1%), G6PD c.202T heterozygous girls, 809 (11.9%), and G6PD c.202T–deficient homo/hemizygotes, 890 (13.0%), whereas the comparable figures for the 10 179 controls were 7422 (72.9%), 1612 (15.8%), and 1145 (11.3%), respectively. The odds of admission during the neonatal period overall were lower among G6PD c.202T heterozygous females compared with wild-type homo/hemizygotes, whereas the odds of admission with jaundice were significantly raised (Table 4). The odds of jaundice were further raised in G6PD c.202–deficient homo/hemizygotes (Table 4). During the postneonatal period, the odds of admission to hospital for any reason, or with a positive malaria slide, or with very severe pneumonia were all lower among G6PD c.202T heterozygous females than in wild-type homo/hemizygotes, whereas the odds of admission with all-cause bacteremia were raised (Table 4). The association with bacteremia varied by organism. Although the odds for all-cause bacteremia among female heterozygotes were 1.45 (95% confidence interval [CI] 1.03-2.03), this appeared to be driven by a particular risk of Gram-positive bacteremia, the adjusted odds ratios for Gram-positive bacteremia being 1.34 (0.84-2.13; P = .22) and 1.58 (1.03-2.42; 0.038) among G6PD c.202T–deficient heterozygotes and homo/hemizygotes, respectively. Although the odds for the largest group of gram-positive organisms, S pneumoniae, were also raised (1.45; 0.78-2.69 and 1.53; 0.90-2.62, respectively), they did not reach statistical significance (P = .24 and P = .12, respectively). We found no significant evidence for a protective effect of G6PD c.202T–deficient homo/hemizygosity against any syndrome individually, but it was positively associated with admission to hospital with jaundice, severe pneumonia, and meningitis/encephalitis and with an increased odds of blood transfusion or death.
Table 4.
Odds ratios for specific clinically and laboratory-based syndromes
| Diagnosis | G6PD c.202T heterozygous girls | G6PD c.202T homozygous girls and hemizygous boys |
||
|---|---|---|---|---|
| OR (95% CI)* | P | OR (95% CI)* | P | |
| Clinical syndromes* | ||||
| All-cause hospital admissions | 0.81 (0.73-0.90) | <.0001 | 1.10 (0.98-1.19) | .13 |
| Neonatal | ||||
| All-cause neonatal admissions | 0.65 (0.47-0.90) | .009 | 1.03 (0.77-1.38) | .84 |
| Neonatal bacteremia | 1.64 (0.70-3.86) | .25 | 1.26 (0.59-2.89) | .59 |
| Neonatal jaundice | 1.70 (1.12-2.58) | .013 | 3.06 (2.30-4.08) | <.0001 |
| Postneonatal | ||||
| All-cause postneonatal admissions | 0.81 (0.73-0.90) | <.0001 | 1.02 (0.92-1.14) | .65 |
| Bacteremia | 1.45 (1.03-2.03) | .033 | 1.12 (0.81-1.56) | .49 |
| Severe malaria | 0.88 (0.64-1.21) | .43 | 0.86 (0.60-1.24) | .42 |
| Malaria | 0.88 (0.74-1.03) | .12 | 0.97 (0.83-1.14) | .75 |
| Severe anemia | 1.06 (0.79-1.43) | .72 | 1.13 (0.85-1.51) | .40 |
| Meningitis/encephalitis | 0.88 (0.74-1.05) | .15 | 1.22 (1.05-1.44) | .012 |
| Severe malnutrition | 1.17 (0.94-1.47) | .17 | 1.01 (0.78-1.30) | .94 |
| Very severe pneumonia | 0.83 (0.74-0.94) | .004 | 1.00 (0.89-1.12) | .96 |
| Severe pneumonia | 0.70 (0.40-1.22) | .21 | 1.77 (1.15-2.70) | .009 |
| Gastroenteritis | 0.91 (0.76-1.10) | .33 | 0.98 (0.83-1.16) | .83 |
| Jaundice | 0.87 (0.39-1.97) | .75 | 2.47 (1.41-4.33) | .002 |
| Other | 0.81 (0.61-1.07) | .13 | 0.81 (0.63-1.04) | .093 |
| Nonmutually exclusive syndromes | ||||
| Severe anemia | 1.03 (0.75-1.40) | .87 | 1.09 (0.80-1.48) | .59 |
| Malaria blood film positive | 0.79 (0.68-0.93) | .004 | 0.99 (0.85-1.15) | .87 |
| Transfused | 0.91 (0.68-1.23) | .55 | 1.66 (1.29-2.15) | <.0001 |
| Died | 0.74 (0.53-1.05) | .93 | 1.37 (1.03-1.82) | .03 |
OR, odds ratio.
Some children displayed >1 clinical feature and therefore appear more than once. ORs derived through comparison of allele frequencies in cases vs community controls by logistic regression with adjustment for division of residence, sex, and HbS genotype. We found no evidence for an interaction between HbS and G6PD c202 genotype with regard to malaria risk.
Discussion
We have investigated the effect of G6PD c.202T genotype on the risk of a range of common childhood diseases in a well-characterized population on the coast of Kenya. Based on data from our case-control analyses, the risks of both all-cause and malaria-positive hospital admission were significantly lower (19% and 21%, respectively) in G6PD c.202T heterozygous girls than in G6PD c.202T homo/hemizygous wild-type children, whereas no impact was seen of G6PD c.202T–deficient homo/hemizygosity. Conversely, the risk and severity of neonatal jaundice were significantly higher in both G6PD c.202T heterozygous girls and G6PD c.202T–deficient homo/hemizygotes, whereas the risk of gram-positive bacteremia was raised in female heterozygotes. We saw no evidence for an increased risk of acute intravascular hemolysis or severe anemia among children in either of the G6PD-deficiency groups.
G6PD deficiency is extremely common, affecting >2.5 billion people worldwide.3 Allele frequencies of up to 32.5% are found in some parts of sub-Saharan Africa, where G6PD c.202T is the most important cause.3,17 Although G6PD c.202T is thought to be asymptomatic in the majority of those affected,2 this conclusion is based on limited epidemiological evidence, most previous studies having been conducted in the context of specific questions, most notably relating to favism, the risk of hemolysis following the ingestion of specific drugs,4,18,19 or the influence of G6PD deficiency on malaria.20,21 To the best of our knowledge, the impact of G6PD c.202T on a wide range of child health outcomes in Africa has not been investigated previously through a single epidemiological study.
First, we found no evidence to suggest that severe or acute intravascular hemolysis was a significant problem in children in either of the G6PD c.202T deficiency groups under the epidemiological circumstances that prevailed during the study period. We saw no difference in admission Hb concentrations or in the prevalence of severe anemia by G6PD genotype, and although postneonatal jaundice, a potential consequence of hemolysis, was significantly more common, it was only seen in a handful of individuals. Acute hemolysis based on clinical history was also uncommon and in most cases was attributable to malaria, although the fact that few episodes were identified suggests that this method was not particularly sensitive. Although G6PD c.202T deficiency can result in severe hemolysis under specific circumstances, most notably on exposure to high doses of strongly oxidant drugs, such as primaquine22-24 and dapsone,4 previous reports that have not described specific trigger factors have been extremely limited.25-27 Unfortunately, the absence of detailed data on preadmission drug exposure does not allow us to address this issue directly in our current study; however, the absence of a signal supports the conclusion that acute intravascular hemolysis among children with G6PD c.202T deficiency is rare under normal circumstances.
Our study did show, however, that in our study population G6PD c.202 deficiency was strongly associated with neonatal jaundice, being significantly more common in both heterozygous girls and homo/hemizygous girls and boys than in wild-type homozygous children. More than 40% of all the babies admitted with detectable neonatal jaundice were either heterozygous or homo/hemizygous for G6PD c.202. Moreover, where jaundice was present, it was significantly more severe, median bilirubin levels being over 3 times higher in homo/hemizygotes than in wild-type homozygotes. Although all cases in our current study were safely managed in our well-resourced facility, our findings suggest that both historically and in less well-resourced facilities or in the community today, G6PD c.202 deficiency could be a significant cause of long-term neurological damage due to neonatal hyperbilirubinemia.28 Our data support the conclusion made by others29 that G6PD c.202 deficiency does not cause neonatal jaundice through a mechanism that predominantly involves hemolysis. Although mean Hb concentrations were significantly lower in homo/hemizygous-deficient than in wild-type homo/hemizygous wild-type neonates, the difference was small (138 g/L vs 146 g/L) and the prevalence of neither anemia nor severe anemia was significantly increased. Previous studies have suggested that impairment of bilirubin conjugation and clearance by the liver is etiologically more important than hemolysis.29 Unfortunately, differential bilirubin concentrations, that might have allowed us to interrogate this question in further detail, were not measured in our current study.
Our observation that G6PD-deficient children are at increased risk of bacteremia, and of gram-positive bacteremia in particular, aligns with observations from a previous study in which we investigated the risk of S pneumoniae bacteremia in greater detail.30 In our earlier paper, which included a larger number of bacteremic cases recruited over a longer period of time, we found that G6PD c.202 was associated with a raised risk of S pneumoniae bacteremia via a mechanism that was dependent on malaria, a finding that was consistent with either an additive or a recessive model. Although we noted that G6PD c.202 heterozygous females were significantly more likely to be admitted with severe malnutrition, this appeared to be explained by the significantly higher risk of severe malnutrition in girls overall. It is unclear why this should be the case given that a sex bias toward girls has not been commonly described in previous studies of malnutrition. Whatever the cause, the increased risk of malnutrition did not appear to explain the association between heterozygosity and the risk of invasive bacterial infections given that we found no significant relationship between sex and bacterial infections.
The results of our current study also support those from a previous study with regard to the protective role of G6PD deficiency against malaria.21 In our current study, which was conducted in the same population as our previous study but within a different group of children, we found that G6PD c.202 heterozygous girls were significantly less likely to be admitted with a positive blood film for malaria, whereas G6PD c.202–deficient homo/hemizygotes were more likely to receive a blood transfusion during the course of their admission. These observations support the conclusion that the protective effect of G6PD c.202 heterozygosity against malaria is balanced by an increased risk of severe anemia in G6PD c.202–deficient homo/hemizygotes.21 Although this conclusion supports that of some previous studies,31-34 others have suggested that there is either no effect of G6PD deficiency on malaria risk35,36 or that protection is afforded to males and females in various combinations.15,20,37 A key strength of ours over some of these previous studies was its size, and therefore, the power of our study to detect significant differences. Moreover, we used G6PD genotyping rather than phenotyping, which is difficult to interpret in heterozygous females because of random X-chromosome inactivation.38 Finally, our study was conducted in an area where 85% of the variability in G6PD activity is explained by the G6PD c.202T mutation, which alone is responsible for virtually all of the G6PD deficiency39: allelic heterogeneity means that other G6PD variants can have widely differing effects on G6PD activity,38 making it more challenging to interpret studies that are conducted in areas with multiple G6PD variants. However, our study did have some limitations, which include the fact that it was somewhat opportunistic, capitalizing on the existence of data and samples collected for other purposes. As a result, participants were recruited over a 2-year period during which the transmission of malaria and other diseases varied.40 Second, although our control participants were recruited from the same area as cases, they were younger and were recruited several years later than the cases, meaning that we could not correct for age. However, we believe that this would be more likely to result in negative rather than positive confounding because any positive selection for G6PD-deficiency alleles by malaria would result in a rising prevalence by age and over time, which would have the effect of enhancing rather than reducing the differences that we have observed.
Although there was some degree of overlap between the cases included in the current study and cases recruited to two of our previously published studies, both of the earlier studies were conducted with the aim of investigating the relationship between G6PD deficiency and specific conditions and the degree of that overlap was small. Our previous paper on severe malaria21 included 2220 cases admitted between 1995 and 2008, whereas that on pneumococcal bacteremia involved 429 cases admitted between 1998 and 2010.30 In the current study, we included all children (cases = 6829) who were recruited, for any reason, during the 2-year period between January 2003 and December 2004, a sample that included 483 children (7% of cases in the current study) who presented with either severe malaria or pneumococcal bacteremia and therefore were also included as cases in the previous studies. Although the restricted recruitment period in our current study means that it is less well powered to investigate associations between G6PD deficiency and those 2 specific diagnoses, that was not our aim. Our current study was broadly hypothesis free and was aimed at identifying within which of many clinical phenotypic groups the G6PD-deficient children were to be found. A second issue of potential concern is the fact that, although collected from the same geographic area, for practical and pragmatic reasons the controls for our study were recruited during a different time period from cases and, unlike cases, included only children <1 year old. This would be a major issue if the population structure with regard to G6PD-deficiency alleles changed significantly within a single generation; however, current levels of mortality within the study population (recently estimated at 2.4 [2.0-2.8]/1000 person-years in the postneonatal period among children without sickle cell disease6) coupled with the degree to which G6PD deficiency is likely to affect mortality, make it highly unlikely that this design would result in significant bias.
In summary, in a large observational and case-control study conducted in Kenya, we have shown that although G6PD c.202 deficiency is not associated with a major risk of severe intravascular hemolysis, it is responsible for almost half of all cases of neonatal jaundice. We conclude that the G6PD c.202 allele is under balancing selection in Africa, positive selection by malaria for heterozygous females being counterbalanced by an increased risk of neonatal jaundice and gram-positive bacteremia in both deficiency groups and by an increased risk of anemia among homo/hemizygotes.
Acknowledgments
The authors thank Johnstone Makale, Emily Nyatichi, and Metrine Tendwa for laboratory support, Kevin Marsh for his support in developing the surveillance platform, all members of staff at KCH, and the KEMRI–Wellcome Trust Research Programme, Kilifi, who helped with data and sample collection, and the study participants and their parents for participating in this study.
This work was funded through the Developing Excellence in Leadership, Training and Science in Africa Initiative (DEL-15-003), an independent funding scheme of the African Academy of Sciences’Alliance for Accelerating Excellence in Science in Africa and that is supported by the New Partnership for Africa’s Development Planning and Coordinating Agency with funding from the Wellcome Trust (203077/Z/16/Z) and the UK government (S.U.). This work is also funded through Senior Research Fellowships from the Wellcome Trust (091758, 202800, 098532, 214320) (T.N.W. and J.A.G.S.), who also provided core support to the KEMRI–Wellcome Trust Research Programme in Kilifi (203077).
This paper is published with permission from the Director of the Kenya Medical Research Institute.
Footnotes
Requests for access to appropriately anonymized data from this study can be made by application to the data access committee at the KEMRI–Wellcome Trust Research Programme by e-mail to mmunene@kemri-wellcome.org. The authors declare that all other data supporting the findings of this study are available within the article, and its supplemental data files are available from the authors upon request.
Authorship
Contribution: T.N.W., S.U., J.A.G.S., and K.M. designed the study and conducted the literature review; A.W.M. and K.O.A. assisted with sample preparation and analysis; M.S., N.M., N.P., and B.T. assisted with the collection and interpretation of clinical data; T.N.W., C.M.N., and G.N. analyzed data; all authors helped to interpret the data; T.N.W. and S.U. wrote the first draft of the report; and all authors contributed to editing the final version.
Conflict--interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas N. Williams, KEMRI–Wellcome Trust Research Programme, Centre for Geographic Medicine Research-Coast, PO Box 230, Kilifi 80108, Kenya; e-mail: tom.williams@imperial.ac.uk; and Sophie Uyoga, KEMRI–Wellcome Trust Research Programme, Centre for Geographic Medicine Research-Coast, PO Box 230, Kilifi 80108, Kenya; e-mail: suyoga@kemri-wellcome.org.
References
- 1.WHO Working Group Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ. 1989;67(6):601-611. [PMC free article] [PubMed] [Google Scholar]
- 2.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64-74. [DOI] [PubMed] [Google Scholar]
- 3.Howes RE, Piel FB, Patil AP, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9(11):e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pamba A, Richardson ND, Carter N, et al. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120(20):4123-4133. [DOI] [PubMed] [Google Scholar]
- 5.Scott JA, Bauni E, Moisi JC, et al. Profile: the Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol. 2012;41(3):650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyoga S, Macharia AW, Mochamah G, et al. The epidemiology of sickle cell disease in children recruited in infancy in Kilifi, Kenya: a prospective cohort study. Lancet Glob Health. 2019;7(10):e1458-e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352(1):39-47. [DOI] [PubMed] [Google Scholar]
- 8.Pedro R, Akech S, Fegan G, Maitland K. Changing trends in blood transfusion in children and neonates admitted in Kilifi District Hospital, Kenya [published correction appears in Malar J. 2010;9:364]. Malar J. 2010;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. 2nd ed Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 10.The World Health Organization Severe malaria. Trop Med Int Health. 2014;19(suppl 1):7-131. [DOI] [PubMed] [Google Scholar]
- 11.Scott J Anthony G, Wonodi Chizoba, Moïsi Jennifer C, et al. ; Pneumonia Methods Working Group . The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clin Infect Dis. 2012;54(suppl 2):S109-S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Guideline Updates on the management of severe acute malnutrition in infants and children. WHO Guideline; 2013. [PubMed] [Google Scholar]
- 13.Macharia AW, Mochamah G, Uyoga S, et al. The clinical epidemiology of sickle cell anemia in Africa. Am J Hematol. 2018;93(3):363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams TN, Wambua S, Uyoga S, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106(1):368-371. [DOI] [PubMed] [Google Scholar]
- 15.Suchdev PS, Ruth LJ, Earley M, Macharia A, Williams TN. The burden and consequences of inherited blood disorders among young children in western Kenya. Matern Child Nutr. 2014;10(1):135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyoga S, Macharia AW, Ndila CM, et al. The indirect health effects of malaria estimated from health advantages of the sickle cell trait. Nat Commun. 2019;10(1):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howes RE, Dewi M, Piel FB, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J. 2013;12(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastiaens GJH, Tiono AB, Okebe J, et al. Safety of single low-dose primaquine in glucose-6-phosphate dehydrogenase deficient falciparum-infected African males: two open-label, randomized, safety trials. PLoS One. 2018;13(1):e0190272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman J, Allen E, Workman L, et al. Safety and tolerability of single low-dose primaquine in a low-intensity transmission area in South Africa: an open-label, randomized controlled trial. Malar J. 2019;18(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376(6537):246-249. [DOI] [PubMed] [Google Scholar]
- 21.Uyoga S, Ndila CM, Macharia AW, et al. ; MalariaGEN Consortium . Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case-control and a cohort study. Lancet Haematol. 2015;2(10):e437-e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dern RJ, Beutler E, Alving AS. The hemolytic effect of primaquine. II. The natural course of the hemolytic anemia and the mechanism of its self-limited character. J Lab Clin Med. 1954;44(2):171-176. [PubMed] [Google Scholar]
- 23.Alving AS, Johnson CF, Tarlov AR, Brewer GJ, Kellermeyer RW, Carson PE. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Piasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull World Health Organ. 1960;22:621-631. [PMC free article] [PubMed] [Google Scholar]
- 24.Coatney GR, Alving AS, Jones R Jr., et al. Korean vivax malaria. V. Cure of the infection by primaquine administered during long-term latency. Am J Trop Med Hyg. 1953;2(6):985-988. [PubMed] [Google Scholar]
- 25.Galiano S, Gaetani GF, Barabino A, et al. Favism in the African type of glucose-6-phosphate dehydrogenase deficiency (A-). BMJ. 1990;300(6719):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odièvre MH, Danékova N, Mesples B, et al. Unsuspected glucose-6-phosphate dehydrogenase deficiency presenting as symptomatic methemoglobinemia with severe hemolysis after fava bean ingestion in a 6-year-old boy. Int J Hematol. 2011;93(5):664-666. [DOI] [PubMed] [Google Scholar]
- 27.Calabrò V, Cascone A, Malaspina P, Battistuzzi G. Glucose-6-phosphate dehydrogenase (G6PD) deficiency in southern Italy: a case of G6PD A(-) associated with favism. Haematologica. 1989;74(1):71-73. [PubMed] [Google Scholar]
- 28.Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. 2002;140(4):396-403. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan M, Rubaltelli FF, Hammerman C, et al. Conjugated bilirubin in neonates with glucose-6-phosphate dehydrogenase deficiency. J Pediatr. 1996;128(5 Pt 1):695-697. [DOI] [PubMed] [Google Scholar]
- 30.Gilchrist JJ, Uyoga S, Pirinen M, et al. ; Kenyan Bacteraemia Study Group . Risk of pneumococcal bacteremia in Kenyan children with glucose-6-phosphate dehydrogenase deficiency. BMC Med. 2020;18(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luzzatto L, Usanga FA, Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science. 1969;164(3881):839-842. [DOI] [PubMed] [Google Scholar]
- 32.Mockenhaupt FP, Mandelkow J, Till H, Ehrhardt S, Eggelte TA, Bienzle U. Reduced prevalence of Plasmodium falciparum infection and of concomitant anaemia in pregnant women with heterozygous G6PD deficiency. Trop Med Int Health. 2003;8(2):118-124. [DOI] [PubMed] [Google Scholar]
- 33.Bienzle U, Ayeni O, Lucas AO, Luzzatto L. Glucose-6-phosphate dehydrogenase and malaria. Greater resistance of females heterozygous for enzyme deficiency and of males with non-deficient variant. Lancet. 1972;1(7742):107-110. [DOI] [PubMed] [Google Scholar]
- 34.Clarke GM, Rockett K, Kivinen K, et al. ; MalariaGEN Consortium . Characterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemia. eLife. 2017;6:e15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell RD, Brewer GJ, DeGowin RL, Carson PE. Effects of glucose-6-phosphate dehydrogenase deficiency upon the host and upon host-drug-malaria parasite interactions. Mil Med. 1966;131(9):suppl:1039-1056. [PubMed] [Google Scholar]
- 36.Martin SK, Miller LH, Alling D, et al. Severe malaria and glucose-6-phosphate-dehydrogenase deficiency: a reappraisal of the malaria/G-6-P.D. hypothesis. Lancet. 1979;1(8115):524-526. [DOI] [PubMed] [Google Scholar]
- 37.Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 2007;4(3):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30(2):373-393. [DOI] [PubMed] [Google Scholar]
- 39.Shah SS, Macharia A, Makale J, et al. Genetic determinants of glucose-6-phosphate dehydrogenase activity in Kenya. BMC Med Genet. 2014;15(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott JA, Berkley JA, Mwangi I, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378(9799):1316-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]