Key Points
The ASH Research Collaborative COVID-19 Registry for Hematology collects data on patients with hematologic diseases and COVID-19.
Among the first 250 patients, mortality was 28%, and in several patients, a decision was made to forgo intensive care unit admission.
Abstract
Coronavirus disease 2019 (COVID-19) is an illness resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged in late 2019. Patients with cancer, and especially those with hematologic malignancies, may be at especially high risk of adverse outcomes, including mortality resulting from COVID-19 infection. The ASH Research Collaborative COVID-19 Registry for Hematology was developed to study features and outcomes of COVID-19 infection in patients with underlying blood disorders, such as hematologic malignancies. At the time of this report, data from 250 patients with blood cancers from 74 sites around the world had been entered into the registry. The most commonly represented malignancies were acute leukemia (33%), non-Hodgkin lymphoma (27%), and myeloma or amyloidosis (16%). Patients presented with a myriad of symptoms, most frequently fever (73%), cough (67%), dyspnea (50%), and fatigue (40%). Use of COVID-19–directed therapies, such as hydroxychloroquine (n = 76) or azithromycin (n = 59), was common. Overall mortality was 28%. Patients with a physician-estimated prognosis from the underlying hematologic malignancy of <12 months at the time of COVID-19 diagnosis and those with relapsed/refractory disease experienced a higher proportion of moderate/severe COVID-19 disease and death. In some instances, death occurred after a decision was made to forgo intensive care unit admission in favor of a palliative approach. Taken together, these data support the emerging consensus that patients with hematologic malignancies experience significant morbidity and mortality resulting from COVID-19 infection. Batch submissions from sites with high incidence of COVID-19 infection are planned to support future analyses.
Visual Abstract
Introduction
Coronavirus disease 2019 (COVID-19), an illness resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in late 2019 and was declared a global pandemic on 11 March 2020.1 Individuals infected with COVID-19 experience a range of symptoms, including fever, cough, dyspnea, anosmia, and gastrointestinal symptoms, among others. Although some individuals are asymptomatic, others become critically ill, requiring intensive care, and many have died as a result of the disease. As of 1 July 2020, the COVID-19 pandemic had resulted in >10 million identified cases and 508 000 confirmed deaths worldwide.2
Early in the pandemic, reports emerged that patients with active or resolved cancers may be at particularly high risk of adverse outcomes from COVID-19.3 This finding was especially concerning to patients with hematologic malignancies and those providing care for them and potentially affected shared management decision making. Many patients with blood cancers have an underlying immune dysfunction and are uniquely vulnerable to viral and other infections.4 Additionally, blood cancer treatments, including cytotoxic agents, immunomodulators, hematopoietic stem cell transplantation, and chimeric antigen receptor T-cell therapy, are profoundly immunosuppressive. Many patients with hematologic malignancies have risk factors of particular concern in the context of COVID-19 infection, including advanced age, underlying or treatment-induced comorbid illnesses like hypertension and diabetes, and chronic lymphopenia. Complications of COVID-19 include hypercoagulability and thrombosis,5 a finding with major implications for patients with cancer who already experience elevated risk of venous thromboembolic events.6
For patients with hematologic malignancies, overall risk of morbidity and mortality resulting from COVID-19 infection, as well as how this risk varies as a function of age, disease status, type of malignancy, and cancer therapy, has not yet been well defined. There is a need for this information among patients, clinicians, and those involved in decisions surrounding resource allocation (intensive care unit [ICU] beds, ventilators) in geographic areas hard hit by the pandemic.
The ASH Research Collaborative was established by the American Society of Hematology in 2018. The ASH Research Collaborative COVID-19 Registry for Hematology was developed to address the paucity of data on management and outcomes in patients with hematologic malignancies and COVID-19.7 The registry is an online, open, observational registry collecting patient-level data on individuals with hematologic malignancies and COVID-19 infection. This first report of the ASH Research Collaborative COVID-19 Registry for Hematology describes data from the first 250 patients with hematologic malignancies entered into the registry.
Methods
Study design and participants
The ASH Research Collaborative COVID-19 Registry for Hematology opened for data collection on 1 April 2020, and data in this report were collected through 8 July 2020. The registry is a global effort and was developed by the ASH Research Collaborative; it is housed on a secure data platform hosted by Prometheus Research, an IQVIA company. The ASH Research Collaborative COVID-19 Registry collects data from patients of all ages with a current or history of hematologic disease and either a laboratory-confirmed or presumptive diagnosis of SARS-CoV-2 infection. The current report is limited to malignant hematologic diseases only. Contributors were individual providers or designees submitting data on behalf of providers. Data were submitted through individual case entry or batch data submission. Individual data submissions were entered on a voluntary basis using the online ASH Research Collaborative COVID-19 Registry data entry platform. There were no geographic restrictions on originating site of data contribution. Case data were entered retrospectively (post–COVID-19 infection) or concurrently with COVID-19 infection. Because data submission could be individual provider dependent and voluntary, cases were not necessarily consecutive cases from a contributing site and do not necessarily represent the entirety of cases seen at a contributing site. The denominator of cases at each site is not known.
The registry and this analysis were reviewed and approved by the Western Institutional Review Board (IRB), a central IRB, and received an exemption determination under 45 CFR §46.104(d)(4) and waiver of Health Insurance Portability and Accountability Act authorization. Data contributors could seek exemption determinations from local IRBs that did not recognize the central IRB exemption determination. Procedures were followed to comply with General Data Protection Regulation requirements. Study procedures were conducted according to the principles of the Declaration of Helsinki.
Procedures
The ASH Research Collaborative COVID-19 Registry collects deidentified data across several broad categories relevant to patients with COVID-19 and hematologic diseases. Patient information at the time of this report included country of residence, age at time of COVID-19 diagnosis, sex, race/ethnicity, smoking status, vaping status, and 17 specific comorbidities. COVID-19 information included method of diagnosis, COVID-19 severity (defined as mild, moderate, or severe), COVID-19 symptoms, treatments specifically given for COVID-19, duration of symptoms, neutrophil and lymphocyte counts at diagnosis, whether a decision was made to forgo ICU admission in favor of a palliative approach, and whether death occurred. Severity was defined as mild (outpatient-level severity), moderate (hospitalization-level severity), or severe (ICU-level severity). Hematologic malignancy information included data about whether the patient had ≥1 of the following malignancies: acute myeloid leukemia, acute promyelocytic leukemia, acute lymphoblastic leukemia, myelodysplastic syndromes, myelofibrosis, other myeloproliferative neoplasm, Hodgkin lymphoma, aggressive non-Hodgkin lymphoma, chronic lymphocytic leukemia, chronic myeloid leukemia, indolent non-Hodgkin lymphoma, mantle cell lymphoma, multiple myeloma, amyloid light-chain amyloidosis, or POEMS syndrome. Additional data captured included prior treatment received, recency of cancer treatments, intent of cancer therapy, hematologic disease status at time of COVID-19 infection, estimated cancer prognosis before infection, and any changes to the hematologic management plan as a result of the COVID-19 pandemic or COVID-19 diagnosis.
A full version of the data collection form at the time of article submission is available in the data supplement, and a current version can be accessed from the ASH Research Collaborative COVID-19 Registry Web site.7
Statistical analysis
Analyses are descriptive in nature and include point estimates and exact binomial confidence intervals (CIs). Data have been binned to preserve deidentification of cases. Summaries are based only on those entries for which the data items of interest were reported. Fisher’s exact tests were performed to assess differences in outcome by severity of infection. Nominal 2-sided P < .05 was considered statistically significant.
Results
Baseline characteristics
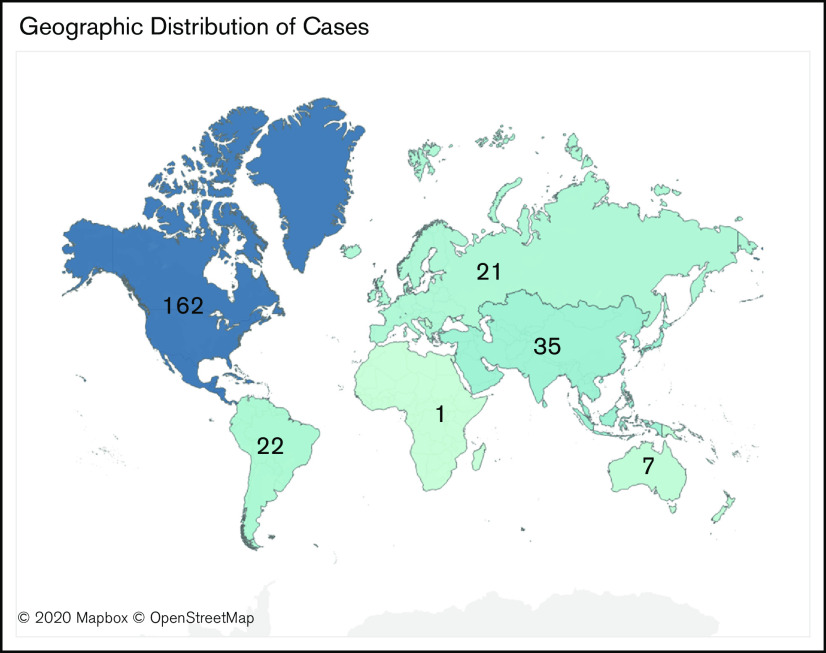
Data from the first 250 patient entries are included in this initial report. The diagnosis of COVID-19 was confirmed with a polymerase chain reaction–based test in 98% of reported cases and was otherwise presumptive. Location data were available for 248 cases (Figure 1). Most patient entries (n = 162; 65%) were from North America, whereas smaller but significant contributions were from Asia (n = 35; 14%), South America (n = 22; 9%), and Europe (n = 21; 8%). Seven patients were from Oceania, and 1 was from Africa. A total of 74 sites submitted data, and the most frequently submitting site contributed 29 cases to the total.
Figure 1.
Geographic distribution of cases reported to the ASH Research Collaborative COVID-19 Registry for Hematology (n = 248).
Baseline patient characteristics are shown in Table 1. At COVID-19 diagnosis, 78% of patients were age >40 years, and 36% were age >70 years. Slightly more than half (57%) were male, and among those with reported race, 42% were White, 14% were Black/African American, and 12% were Asian (Hispanic ethnicity was not collected for the first 193 patients). A total of 138 patients (55%) had ≥1 reported comorbidities; hypertension (54%) and diabetes (33%) were reported most commonly. Approximately 25% of patients were current or former smokers. The most commonly represented malignancies were acute leukemia (33%), non-Hodgkin lymphoma (27%), and myeloma or amyloidosis (16%). Physician-estimated prognosis for survival before COVID-19 infection was >12 months for 62% of patients.
Table 1.
Baseline characteristics of all patients with COVID-19 and hematologic malignancies
| n (%) | |
|---|---|
| Age, y | |
| <19 | 20 (8) |
| 19-39 | 31 (12) |
| 40-69 | 105 (42) |
| >70 | 89 (36) |
| No response | 5 (2) |
| Sex | |
| Male | 143 (57) |
| Female | 102 (41) |
| No response | 5 (2) |
| Race/ethnicity | |
| Asian | 29 (12) |
| Black/African American | 35 (14) |
| White | 105(42) |
| Other/unknown/no response | 81 (32) |
| Comorbidity* | |
| Diabetes | 46 (33) |
| Hypertension | 75 (54) |
| Heart disease | 35 (25) |
| Lung disease | 25 (18) |
| Kidney disease | 29 (21) |
| Other | 43 (33) |
| Smoking status | |
| Current | 10 (4) |
| Former | 54 (22) |
| Never | 109 (44) |
| Unknown/no response | 77 (31) |
| Hematologic malignancy | |
| Acute leukemia | 82 (33) |
| Chronic lymphocytic leukemia | 29 (12) |
| Hodgkin lymphoma | 11 (4) |
| Non-Hodgkin lymphoma | 68 (27) |
| Myeloproliferative neoplasm | 24 (10) |
| Myeloma or amyloidosis | 40 (16) |
| Estimated pre–COVID-19 prognosis, mo | |
| <3 | 10 (4) |
| 3-6 | 22 (9) |
| 6-12 | 15 (6) |
| >12 | 154 (62) |
| No response | 49 (20) |
Comorbidities: heart disease included congestive heart failure, coronary artery disease, and other chronic heart diseases; lung disease included chronic obstructive pulmonary disease/emphysema and other chronic lung diseases; kidney disease included chronic renal insufficiency.
Presenting symptoms and treatment of COVID-19
As shown in Table 2, we investigated presenting features of patients with COVID-19 infection. The most common presenting symptoms were fever (73%), cough (67%), dyspnea (50%), and fatigue (40%). Forty-six patients (18%) had symptoms that persisted >3 weeks from time of first onset to resolution or death.
Table 2.
Symptoms of COVID-19
| All Patients (N = 250), n (%) | Moderate or severe COVID-19 (n = 168), n (%) | |
|---|---|---|
| Symptom | ||
| Fever | 171 (73) | 131 (80) |
| Cough | 155 (67) | 107 (65) |
| Shortness of breath | 117 (50) | 95 (58) |
| Fatigue | 93 (40) | 76 (46) |
| Myalgias | 51 (22) | 38 (23) |
| Headache | 39 (17) | 23 (14) |
| Diarrhea | 32 (14) | 25 (15) |
| Nausea/vomiting | 21 (9) | 14 (9) |
| Rhinorrhea | 21 (9) | 8 (5) |
| Anosmia | 13 (6) | 6 (4) |
| Confusion | 14 (6) | 13 (8) |
| Diaphoresis | 13 (6) | 10 (6) |
| Abdominal pain | 12 (5) | 10 (6) |
| Weight loss | 5 (2) | 2 (1) |
| No symptoms | 7 (3) | 2 (1) |
| Unknown/no response | 17 (7) | 4 (2) |
| Time to symptom resolution, d | ||
| ≤2 | 24 (10) | 18 (11) |
| 3-5 | 39 (16) | 24 (14) |
| 6-10 | 34 (14) | 23 (14) |
| 11-20 | 56 (22) | 44 (26) |
| 21-30 | 30 (12) | 24 (14) |
| >30 | 16 (6) | 11 (7) |
| No symptoms | 14 (6) | 3 (2) |
| Unknown/no response | 37 (15) | 21 (13) |
Patients were included only if symptoms or no symptoms were reported.
COVID-19–specific therapies for patients with at least moderate-severity disease are shown in Table 3. The most common COVID-19–specific therapies in this data set were hydroxychloroquine and azithromycin, used in 76 and 59 patients, respectively.
Table 3.
COVID-19–specific therapies received
| n (%) | |
|---|---|
| Hydroxychloroquine | 76 (64) |
| Azithromycin | 59 (50) |
| Tocilizumab | 15 (13) |
| Lopinavir and ritonavir | 12 (10) |
| Convalescent plasma | 11 (9) |
| Favipravir | 11 (9) |
| Remdesivir | 5 (4) |
| IVIG | 4 (3) |
| Chloroquine | 3 (3) |
| Other/unknown | 39 (33) |
Associations of variables of interest with COVID-19 outcomes are illustrated in Table 4. Older age was associated with moderate or severe disease (P < .001), a decision to forgo ICU care in favor of a palliative approach (P < .001), and mortality (P = .01). Male sex was also associated with more frequent preference for a palliative approach (P = .03) and mortality (P = .008).
Table 4.
Associations of selected variables with moderate or severe COVID-19, decision to forgo ICU care, and mortality
| COVID-19 severity of moderate or severe* (n = 245), n/N (%) | ICU forgone in favor of palliative care* (n = 244), n/N (%) | Mortality (n = 245), n/N (%) | |
|---|---|---|---|
| Age, y | |||
| <19 | 4/19 (21) | 1/18 (6) | 2/18 (11) |
| 19-39 | 16/31 (52) | 0/30 (0) | 4/30 (13) |
| 40-70 | 77/104 (74) | 13/104 (13) | 28/105 (27) |
| >70 | 66/86 (77) | 25/87 (29) | 34/87 (39) |
| No response | 5/5 (100) | 1/5 (20) | 2/5 (40) |
| Sex | |||
| Male | 98/140 (70) | 30/141 (21) | 49/141 (35) |
| Female | 65/100 (65) | 10/98 (10) | 19/99 (19) |
| No response | 5/5 (100) | 0/5 (0) | 2/5 (40) |
| Race | |||
| Asian | 22/28 (79) | 10/29 (34) | 12/29 (41) |
| Black/African American | 22/34 (65) | 1/35 (3) | 6/35 (17) |
| White | 66/104 (63) | 20/102 (20) | 29/102 (28) |
| Hispanic/Latino/other | 39/57 (68) | 18/57 (32) | 18/58 (31) |
| Unknown/no response | 19/22 (86) | 3/22 (11) | 5/21 (24) |
| Comorbidity | |||
| Diabetes | 36/45 (80) | 10/46 (22) | 15/46 (33) |
| Hypertension | 51/72 (71) | 9/75 (12) | 22/75 (29) |
| Heart disease | 25/34 (74) | 10/35 (29) | 19/35 (54) |
| Lung disease | 15/23 (65) | 5/24 (21) | 9/24 (38) |
| Kidney disease | 23/27 (85) | 8/28 (29) | 13/28 (46) |
| Other | 36/46 (78) | 9/46 (20) | 16/46 (35) |
| ≥1 comorbidity | 96/134 (72) | 28/137 (20) | 45/137 (33) |
| No response | 72/111 (65) | 12/107 (11) | 25/108 (23) |
| Smoking status | |||
| Current | 9/10 (90) | 1/10 (10) | 5/10 (50) |
| Former | 34/52 (65) | 5/54(9) | 13/54 (24) |
| Never | 64/109 (59) | 15/106 (14) | 21/107 (20) |
| Unknown | 16/18 (89) | 5/18 (28) | 6/18 (33) |
| No response | 45/56 (80) | 14/56 (25) | 25/56 (45) |
| Hematologic malignancy | |||
| Acute leukemia | 50/80 (63) | 16/79 (20) | 26/79 (33) |
| CLL | 21/29 (72) | 3/29 (10) | 8/29 (28) |
| HL | 5/11 (45) | 0/11 (0) | 4/11 (36) |
| NHL | 47/68 (69) | 10/67 (15) | 16/67 (24) |
| MPN | 19/24 (79) | 4/23 (17) | 6/24 (25) |
| MM or AL amyloid | 30/37 (81) | 7/39 (18) | 11/39 (28) |
| All respondents | 168/245 (69) | 40/244 (16) | 70/245 (29) |
| Treatment received in year before COVID-19 diagnosis among treated patients | |||
| Cytotoxic chemotherapy | 60/82 (73) | 11/83 (13) | 30/83 (36) |
| Immunotherapy | 31/35 (89) | 10/35 (29) | 15/35 (43) |
| Targeted therapy | 40/52 (77) | 6/52 (12) | 16/53 (30) |
| Other | 73/96 (75) | 20/95 (21) | 31/95 (33) |
| Unknown | 1/1 (100) | 0/1 (0) | 0/1 (0) |
| All respondents | 130/179 (73) | 31/177 (18) | 59/178 (33) |
| Pre–COVID-19 prognosis, mo | |||
| ≤12 | 38/46 (83) | 21/45 (47) | 28/45 (62) |
| >12 | 102/153 (67) | 17/151 (11) | 34/152 (22) |
| No response | 28/46 (61) | 2/48 (4) | 8/48 (17) |
| Malignancy status | |||
| Initial treatment | 42/58 (72) | 12/59 (20) | 19/59 (32) |
| Remission | 50/87 (57) | 7/87 (8) | 16/87 (18) |
| Relapsed/refractory | 28/34 (82) | 10/34 (29) | 16/34 (47) |
| Stable, not in remission | 37/54 (69) | 9/52 (17) | 16/53 (30) |
| No response | 11/12 (92) | 2/12 (17) | 3/12 (25) |
| Mortality | |||
| Recovered | 94/166 (57) | 5/167 (3) | |
| Death | 68/70 (97) | 35/70 (50) | |
| Unknown | 5/6 (83) | 5/7 (71) | |
| No response | 1/3 (33) | 0/0 | |
| ICU forgone in favor of palliative care | |||
| Yes | 37/40 (93) | 35/40 (88) | |
| No | 123/192 (64) | 33/194 (17) | |
| Unknown | 7/9 (78) | 2/10 (20) | |
| No response | 1/4 (25) | 0/1 (0) |
AL, amyloid light chain; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; MM, multiple myeloma; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma.
Cases were included for an outcome only if information about that outcome was reported.
Outcomes of COVID-19 infection
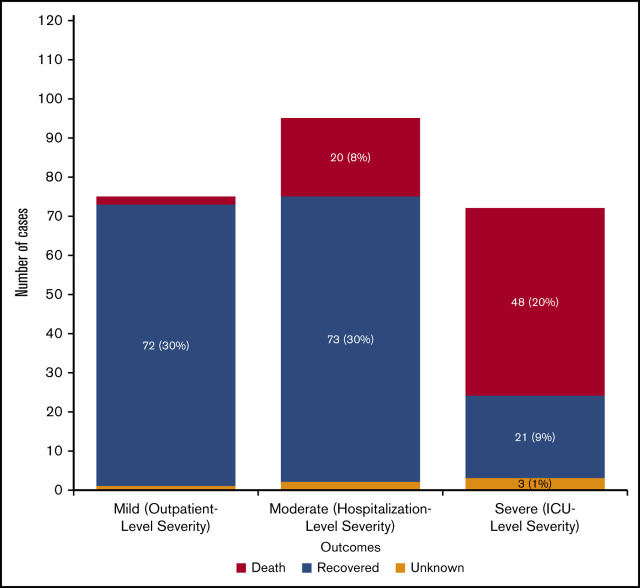
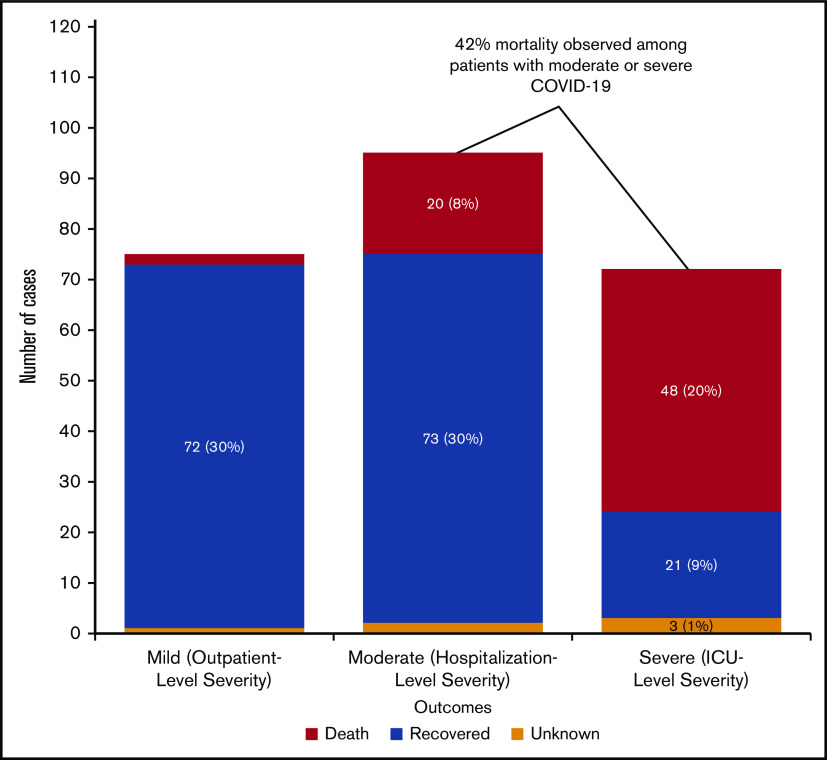
Overall outcomes for patients are shown in Figure 2. Seventy-seven patients (31%) had mild-severity, 96 (38%) had moderate-severity, and 72 (29%) had severe COVID-19 infection. Two patients (3%) with mild-severity infection died, 20 (22%) with moderate-severity infection died, and 48 (70%) with severe infection died. Overall, mortality for the entire cohort was 28% (95% exact binomial CI, 23% to 34%); among adults (age >18 years), mortality was 30% (95% exact binomial CI, 24% to 36%). Among those patients with moderate or severe COVID-19, mortality was 42% (95% exact binomial CI, 34% to 50%). Clinicians provided information on whether there was a decision to forgo ICU admission in favor of a palliative approach. Of those patients with at least moderately severe COVID-19 for whom ICU admission was forgone in favor of palliative care (n = 37), 33 died (89%). Of those with at least moderate-severity infection who did not forgo ICU admission (n = 131), 35 died (27%; P < .001 for comparison of mortality in those who did or did not forgo ICU admission).
Figure 2.
COVID-19 severity and mortality among patients with hematologic malignancies (n = 242). Patients for whom severity was not known were excluded.
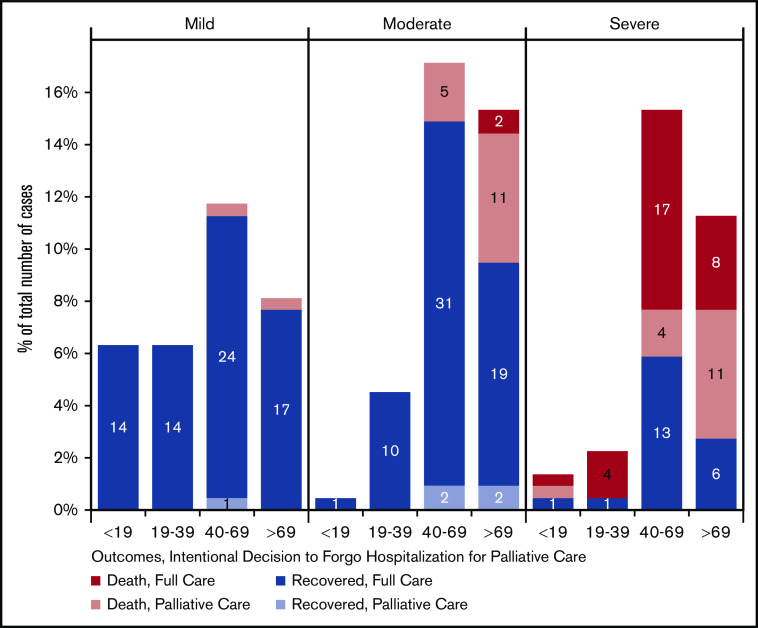
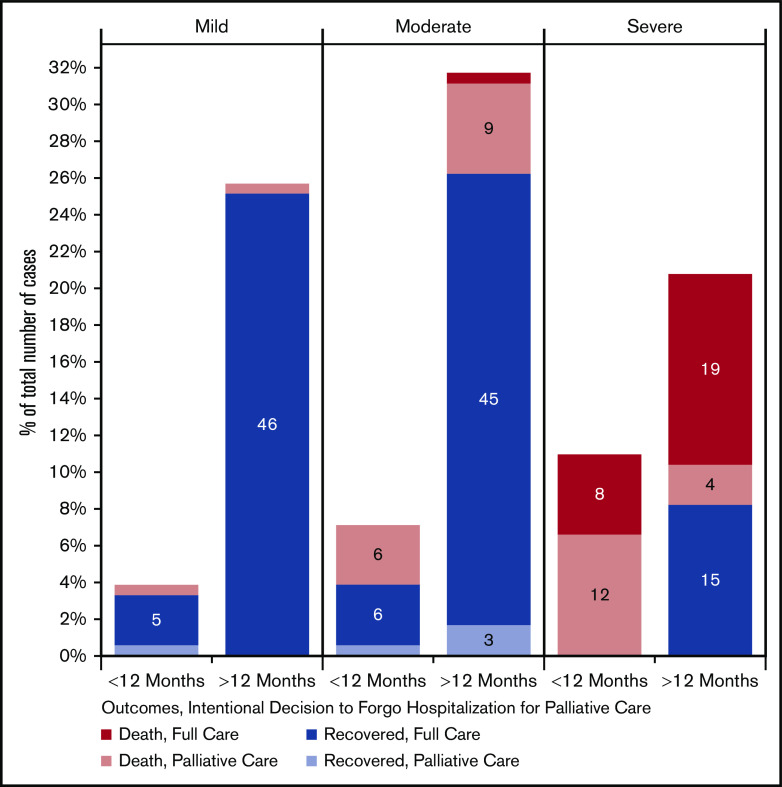
We also looked at COVID-19 severity, outcomes, and decision to forgo ICU care as a function of age, as shown in Figure 3. We found that in patients with moderate-severity infection, most deaths occurred when ICU-level care was forgone in favor of a palliative approach. Among those with severe disease, most deaths occurred in those age >40 years and represented a mixture of deaths occurring despite maximal supportive management or in the context of a decision to pursue a palliative approach. Figure 4 shows similar data in relation to physician-estimated prognosis. In those patients with mild COVID-19 infection and estimated prognosis of >12 months, there were no deaths. In contrast, in patients with severe infection and estimated pre–COVID-19 prognosis of <12 months, there were no survivors. In the 21 cases of severe infection in patients with favorable prognosis, 10 (48%) survived. Pre–COVID-19 prognosis of <12 months was associated with moderate or severe disease, increased rate of forgoing ICU admission in favor of palliative treatment, and increased mortality (P < .001 for each).
Figure 3.
COVID-19 severity, mortality, and decision to forgo ICU among patients with hematologic malignancies by patient age (n = 222). Patients for whom severity outcomes were not known were excluded.
Figure 4.
COVID-19 severity, mortality, and decision to forgo ICU among patients with hematologic malignancies by estimated pre–COVID-19 prognosis (n = 183). Patients were included only if estimated prognosis was provided.
Discussion
In this report, we describe the first 250 cases of patients with hematologic malignancy and COVID-19 that were entered into the ASH Research Collaborative COVID-19 Registry. We believe that several findings are important to highlight.
First, consistent with reports from other data sets of patients with COVID-19 and cancer, our data set suggests that patients with hematologic malignancies have higher mortality resulting from COVID-19 than reported in the general population. We report an overall mortality of 28%, increasing to 42% for those with COVID-19 severity sufficient to necessitate hospital-level care. A recently published study from Italy that included 536 patients with hematologic malignancies and COVID-19 infection reported mortality in this cohort of 37%, with higher risk among those with older age, progressive disease, or severe infection. The standardized mortality ratio for those with hematologic malignancies and COVID-19 was 2.04 (95% CI, 1.77-2.34) in comparison with the general Italian population with COVID-19.8 In the first published report from the COVID-19 and Cancer Consortium, mortality among 928 analyzed adult patients with any malignancy was 13%, with 23% mortality for any admission to the hospital and 38% mortality for admission to the ICU.9 Among 800 patients with cancer included in the UK Coronavirus Cancer Monitoring Project, reported mortality in the overall cohort was 28%.10 A multicenter study in China of 205 patients with cancer reported mortality of 20%, with 41% mortality in the 22 patients with hematologic malignancies who were included.11 The TERAVOLT registry of patients with thoracic malignancies and COVID-19 infection reported 33% mortality among 200 included patients and 34% mortality for those admitted to the hospital.12 Hematologic malignancy data have been less frequently reported, although subset data from larger cohorts and disease-specific data are now emerging. For example, the UK Myeloma Forum reported 75 patients with COVID-19 and multiple myeloma, with mortality of 55%.13
The ASH Research Collaborative COVID-19 Registry for Hematology provides a unique and ongoing global resource for data collection regarding COVID-19 infection in patients with hematologic malignancies. The registry contains granular data about hematologic malignancy subtypes, stages, outcomes, symptoms, and other variables of interest that will facilitate more detailed analyses as the data set matures. In the current analysis, we found that although mortality seemed increased for patients with hematologic malignancies and COVID-19, the risks were not evenly distributed across the population of patients with blood cancers. As expected, morbidity and mortality were common in patients who were older, had relapsed/refractory disease, lived with comorbidities, and had an estimated pre–COVID-19 prognosis of <12 months. Nonetheless, outcomes were sometimes unpredictable. For example, there were some patients who were older or had limited prognosis yet experienced mild- or moderate-severity disease and recovered. Also, although most patients age <40 years with a pre–COVID-19 prognosis of >12 months and receiving treatment at the time of diagnosis lived, there were still some in this group who died as result of COVID-19 (13%). These findings suggest that COVID-19 may result in substantial years of life lost in patients with hematologic malignancies.
The heterogeneity of COVID-19 outcomes in patients with hematologic malignancies takes on particular importance when decisions are made about resource allocation and provision of intensive supportive care. In our cohort, 40 patients (16%) forwent intensive care in favor of a palliative approach, and most of these patients died. The decision to forgo ICU care represented a significant proportion of deaths in patients who were older or had limited prognosis. In patients with physician-estimated prognosis of <12 months who had severe infection, all 11 died, and forgoing ICU care represented 8 of these deaths. Conversely, approximately half of patients (10 of 21) with severe disease and estimated prognosis of >12 months who received maximal supportive care survived. Decisions about forgoing ICU care in patients with hematologic malignancies are notoriously complex, even outside of the COVID-19 pandemic. Several teams have tried to understand predictors of survival in patients with cancer receiving intensive care, which can be challenging.14-17 In the context of the COVID-19 pandemic, many geographic areas have experienced scarcity of intensive care beds and ventilators, leading to discussions about triage algorithms to determine which populations should be prioritized for these resources.18,19 Decisions about ICU care are also informed by patient preferences. Many patients may have had advance directives in place or opted not to pursue maximal supportive care once diagnosed with severe COVID-19 infection. We have no data in our registry to inform the context within which ICU decisions were made, but given the prevalence of this practice pattern, we believe that this is an important area for future research.
Several additional findings from our cohort are also noteworthy. Patients with hematologic malignancies and COVID-19 infection presented with a myriad of symptoms, suggesting that a high index of suspicion for COVID-19 should be entertained during periods of high disease prevalence in the overall population. We also found that patients with COVID-19 infection received a wide variety of COVID-19–directed therapies. The most common of these were hydroxychloroquine and azithromycin, in addition to a number of other treatment approaches. Although we do not know which of these treatments were offered on a clinical trial, it is reasonable to assume that this percentage was low, given historically low rates of enrollment of cancer patients onto clinical trials before the COVID-19 pandemic.20,21 Off-label use of COVID-19–directed therapies in patients with hematologic malignancies could be potentially problematic because of unexpected toxicities of these agents,22 potential drug-drug interactions in patients with cancer,23,24 and emerging data suggesting ineffectiveness or potential harm from these treatment approaches in COVID-19 and cancer.7,25
We acknowledge methodologic limitations to our study. Most importantly, this is a voluntary registry of nonconsecutively reported cases. Therefore, the true denominator of cases from a given site was not known, and the potential for recall bias or other forms of respondent bias existed. Many of our sites were tertiary academic centers, and our data may have also been subject to referral bias, thus not reflective of the overall population of patients with hematologic malignancies. The potential for duplicate data entry existed, although the location of respondents was known, and steps were taken to minimize this possibility. For all of these reasons, caution should be used in interpreting our results. Nonetheless, our findings are similar to reports in other cancer populations.
We believe that our findings point toward new directions to confirm and expand upon the results reported here. First, we envision ways to address the denominator concern. Batch data submissions from participating sites, in which a site representative collates and enters cases from across the clinical activity of that location, are currently under way. These offer the opportunity to come closer to the true case volume of hematologic malignancies and COVID-19 at a location, rather than relying solely on individual provider data submission. Beyond this, there may be opportunities to explore direct electronic health record integrations with sites, consistent with the overall vision of the ASH Research Collaborative Data Hub. This approach would allow for informatics-based approaches to facilitate case identification through flags in structured electronic health record data. In the long run, population-based seroprevalence studies may provide the most complete information about COVID-19 incidence and outcomes among patients with hematologic malignancies. These studies are under way in the general population, but not yet at scale.
One of the strengths of the ASH Research Collaborative COVID-19 Registry is that it represents an ongoing resource that is updated in real time as the pandemic continues to evolve and is immediately made available as a data source for treating clinicians. Using current ASH Research Collaborative COVID-19 Registry infrastructure, we anticipate additional analyses as data collection continues. As each specific disease subpopulation accumulates case entries, we will be able to explore whether particular hematologic malignancies or treatments received confer elevated risk for adverse COVID-19 outcomes, which may help to guide decisions about adjustment or deferral of certain therapies during times of high COVID-19 prevalence or in patients with recent COVID-19 infection. We can provide insight into the safety and efficacy of COVID-19–directed therapies in patients with hematologic malignancies who experience moderate or severe infection, and this will be particularly important as treatment of patients with COVID-19 continues to evolve. Insights from the ASH Research Collaborative COVID-19 Registry can also inform consensus practice recommendations for the care of patients with hematologic conditions. For example, an international expert panel recently published management considerations for adult patients with acute leukemias and myeloid neoplasms in the COVID-19 era.26 Future efforts like these will be able to incorporate empiric data from the ASH Research Collaborative COVID-19 Registry to enhance and refine recommendations.
As the registry expands to include nonmalignant underlying conditions and complications of infection, we can evaluate the prevalence of thrombosis and the potential role of thromboprophylaxis in patients with COVID-19 infection, with or without underlying hematologic malignancies. We can also consider follow-up studies in registry patients to determine functional and disease-specific outcomes. Lastly, the publicly available data visualizations on the ASH Research Collaborative COVID-19 Registry Web site are easily accessed, are updated in real time, and may be a useful way for providers to help understand and explain COVID-19 risks and outcomes to patients.
On the basis of our findings, we believe that there are already a few key recommendations that we can make. Our data demonstrate a high risk for severe COVID-19 infection and mortality in patients with hematologic malignancies. Therefore, our findings illustrate the importance of protecting this vulnerable population from COVID-19 exposure. Many sites have adopted approaches ranging from enhanced use of telemedicine to on-site physical distancing, and these practices should continue as long as COVID-19 prevalence remains high. We see no reason based on our data to withhold intensive life-supporting interventions from patients with underlying hematologic malignancies and a favorable prognosis, if aggressive supportive care is consistent with patient preferences. Finally, when vaccines become available, we believe that it will be important to prioritize access for patients with hematologic malignancies and study efficacy in this population.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Jeremy Warner, Michael Kappelman, and Julie Panepinto for their advice during the early stages of formation of the ASH Research Collaborative COVID-19 Registry.
Footnotes
Presented in abstract form at the 62nd annual meeting of the American Society of Hematology, 5-8 December 2020.
Information from the ASH Research Collaborative COVID-19 Registry is publicly displayed on the ASH Research Collaborative Web site, and inquiries about detailed raw data can be sent to the corresponding author.
Authorship
Contribution: W.A.W. and L.K.H. conceived of and codesigned the database; W.A.W., D.S.N., L.K.H., and J.C.T. contributed to data analysis; W.A.W. and L.K.H. wrote the manuscript; and all authors critically reviewed and revised the manuscript and provided final approval.
Conflict--interst disclosure: D.S.N. received research support from Pharmacyclics; and holds stock in Madrigal. W.A.W. received research support from Pfizer; provided consulting for Teladoc; was an advisor for Koneksa Health and Elektra Labs; and received honoraria from the ASH Research Collaborative. L.K.H. received research support from Gilead. N.A.P. provided consulting for AstraZeneca, BMS, Merck, Genentech, Amgen, Eli Lilly, and G1 therapeutics. M.S.T. received research support from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; served on advisory boards for AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, Jazz Pharma, Roche, Biosight, and Novartis; and received royalties from UpToDate. C.M.N. provided consulting for Celgene and Novartis. A.D.G. served on advisory boards for AbbVie, Aptose, Celgene, Daiichi Sankyo, and Genentech; received research support from AbbVie, ADC Therapeutics, Aprea, Aptose, AROG, Celularity, Daiici Sankyo, and Pfizer; and received honoraria from Dava Oncology. L.H.S. received consulting or honoraria from Roche/Genentech, AbbVie, Amgen, Apobiologix, AstraZeneca, Acerta, Celgene, Gilead, Janssen, Kite Karyopharm, Lundbeck, Merck, Morphosys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Verastem. The remaining authors declare no competing financial interests.
Correspondence: William A. Wood, Division of Hematology, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Campus Box 7305, 3rd Floor, Physicians Office Building, 170 Manning Dr, Chapel Hill, NC 27599-7305; e-mail: wawood@med.unc.edu.
References
- 1.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. Accessed 23 September 2020.
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4):e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana L, Strasfeld L. Respiratory virus infections of the stem cell transplant recipient and the hematologic malignancy patient. Infect Dis Clin North Am. 2019;33(2):523-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AYY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23suppl 1):I17-I21. [DOI] [PubMed] [Google Scholar]
- 7.ASH Research Collaborative ASH Research Collaborative COVID-19 Registry for Hematology. https://www.ashresearchcollaborative.org/s/covid-19-registry. Accessed 23 September 2020.
- 8.Passamonti F, Cattaneo C, Arcaini L, et al. ; ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study [published online ahead of print 13 August 2020]. Lancet Haematol. doi:10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer NM, Choueiri TK, Shah DP, et al. ; COVID-19 and Cancer Consortium . Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study [published correction appears in Lancet. 2020;396(10253):758]. Lancet. 2020;395(10241):1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, Cazier JB, Angelis V, et al. ; UK Coronavirus Monitoring Project Team . COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang K, Sheng Y, Huang C, et al. . Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garassino MC, Whisenant JG, Huang L-C, et al. ; TERAVOLT investigators . COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook G, John Ashcroft A, Pratt G, et al. ; United Kingdom Myeloma Forum . Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190(2):e83-e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit Care Med. 2003;31(1):104-112. [DOI] [PubMed] [Google Scholar]
- 15.Soares M, Fontes F, Dantas J, et al. . Performance of six severity-of-illness scores in cancer patients requiring admission to the intensive care unit: a prospective observational study. Crit Care. 2004;8(4):R194-R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owczuk R, Wujtewicz MA, Sawicka W, Wadrzyk A, Wujtewicz M. Patients with haematological malignancies requiring invasive mechanical ventilation: differences between survivors and non-survivors in intensive care unit. Support Care Cancer. 2005;13(5):332-338. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Cheng Q, Yang Q, et al. . Prognosis-related factors in intensive care unit (ICU) patients with hematological malignancies: A retrospective cohort analysis in a Chinese population. Hematology. 2015;20(9):494-503. [DOI] [PubMed] [Google Scholar]
- 18.Phua J, Weng L, Ling L, et al. ; Asian Critical Care Clinical Trials Group . Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations [published correction appears in Lancet Respir Med. 2020;8(5):e42]. Lancet Respir Med. 2020;8(5):506-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergano M, Bertolini G, Giannini A, et al. . Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Crit Care. 2020;24(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steensma DP, Brunner AM, DeZern AE, et al. . Low clinical trial accrual of patients with myelodysplastic syndromes: causes and potential solutions. Cancer. 2018;124(24):4601-4609. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine, Board on Health Care Services, Committee on Cancer Clinical Trials and the NCI Cooperative Group Program In: Nass SJ, Moses HL, Mendelsohn J, eds. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leeuwen RWF, Jansman FGA, van den Bemt PMLA, et al. . Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015;26(5):992-997. [DOI] [PubMed] [Google Scholar]
- 24.Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56(3):286-290. [DOI] [PubMed] [Google Scholar]
- 25.Rivera DR, Peters S, Panagiotou OA, et al. ; COVID-19 and Cancer Consortium. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: A COVID-19 and Cancer Consortium (CCC19) cohort study [published online ahead of print 22 July 2020]. Cancer Discov. doi:10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeidan AM, Boddu PC, Patnaik MM, et al. . Special considerations in the management of adult patients with acute leukaemias and myeloid neoplasms in the COVID-19 era: recommendations from a panel of international experts. Lancet Haematol. 2020;7(8):e601-e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.