Abstract
BACKGROUND
Asciminib is an allosteric inhibitor that binds a myristoyl site of the BCR-ABL1 protein, locking BCR-ABL1 into an inactive conformation through a mechanism distinct from those for all other ABL kinase inhibitors. Asciminib targets both native and mutated BCR-ABL1, including the gatekeeper T315I mutant. The safety and antileukemic activity of asciminib in patients with Philadelphia chromosome–positive leukemia are unknown.
METHODS
In this phase 1, dose-escalation study, we enrolled 141 patients with chronic-phase and 9 with accelerated-phase chronic myeloid leukemia (CML) who had resistance to or unacceptable side effects from at least two previous ATP-competitive tyrosine kinase inhibitors (TKIs). The primary objective was to determine the maximum tolerated dose or the recommended dose (or both) of asciminib. Asciminib was administered once or twice daily (at doses of 10 to 200 mg). The median follow-up was 14 months.
RESULTS
Patients were heavily pretreated; 70% (105 of 150 patients) had received at least three TKIs. The maximum tolerated dose of asciminib was not reached. Among patients with chronic-phase CML, 34 (92%) with a hematologic relapse had a complete hematologic response; 31 (54%) without a complete cytogenetic response at baseline had a complete cytogenetic response. A major molecular response was achieved or maintained by 12 months in 48% of patients who could be evaluated, including 8 of 14 (57%) deemed to have resistance to or unacceptable side effects from ponatinib. A major molecular response was achieved or maintained by 12 months in 5 patients (28%) with a T315I mutation at baseline. Clinical responses were durable; a major molecular response was maintained in 40 of 44 patients. Dose-limiting toxic effects included asymptomatic elevations in the lipase level and clinical pancreatitis. Common adverse events included fatigue, headache, arthralgia, hypertension, and thrombocytopenia.
CONCLUSIONS
Asciminib was active in heavily pretreated patients with CML who had resistance to or unacceptable side effects from TKIs, including patients in whom ponatinib had failed and those with a T315I mutation. (Funded by Novartis Pharmaceuticals; ClinicalTrials.gov number, NCT02081378.)
FAILURE OF TYROSINE KINASE INHIBITOR (TKI) therapy in patients with Philadelphia chromosome (Ph)–positive chronic myeloid leukemia (CML) may result from resistance to or unacceptable side effects from the drug or both. Currently approved TKIs mainly target the ATP-binding site of BCR-ABL1, and approximately half of clinical resistance is associated with the acquisition of mutations in this region of the kinase, resulting in conformational changes that render TKIs inactive.1–6 The “gatekeeper” T315I mutation, reported in approximately 20% of patients with mutations, is of particular concern because it is associated with resistance to all clinically available TKIs except ponatinib.7–9 Unacceptable side effects from TKIs also occur in approximately 25% of patients, with increasing recognition that patients receiving second- and third-generation TKIs are at risk for vascular and pulmonary toxic effects.10-14
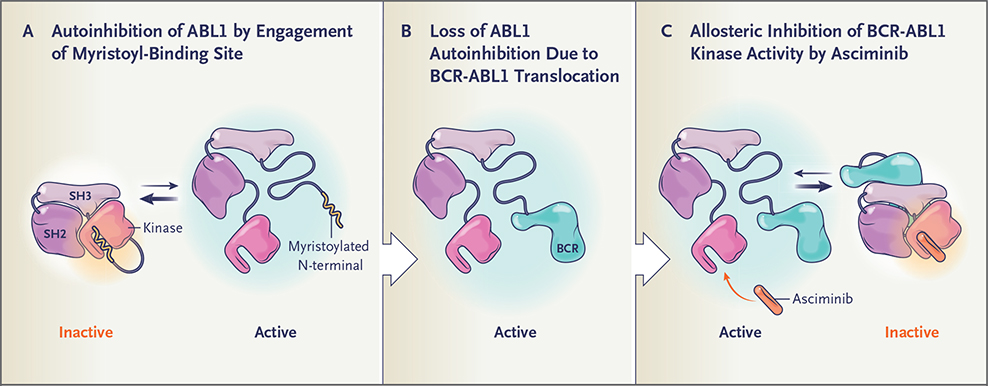
Asciminib (ABL001) is a potent, specific, orally bioavailable BCR-ABL1 inhibitor that is distinct from approved ABL1 kinase inhibitors in that it does not bind to the ATP-binding site of the kinase. In contrast, asciminib acts as an allosteric inhibitor and engages a vacant pocket at a site of the kinase domain normally occupied by the myristoylated N-terminal of ABL1 — a motif that serves as an allosteric negative regulatory element lost on fusion of ABL1 to BCR (Fig. 1). By binding the myristoyl site, asciminib mimics myristate and restores inhibition of kinase activity. Owing to the distinct conformation of the myristoyl pocket, asciminib has high selectivity for only ABL1 and, hypothetically, ABL2 kinases, with low-nanomolar–range activity against unmutated BCR-ABL1 and all clinically observed ATP-site mutants, including T315I.15,16 We hypothesized that asciminib may produce clinically significant responses in patients with CML in whom multiple approved TKIs have failed.
Figure 1. Binding of the Myristoyl Site of the BCR-ABL1 Protein by Asciminib.
Autoinhibition of the ABL1 kinase occurs through engagement of the myristoyl-binding site by the myristoylated N-terminal — a negative regulatory motif that locks the ABL1 kinase in the inactive state (Panel A). On fusion of ABL1 to BCR, the myristoylated N-terminal is lost and the ABL1 kinase is activated (Panel B). By allosterically binding the myristoyl site, asciminib mimics myristate and restores inhibition of BCR-ABL1 kinase activity (Panel C).
We conducted a phase 1 study to determine the safety, maximum tolerated dose or recommended dose, pharmacokinetics, and antileukemic activity of asciminib in patients with Ph-positive leukemia after failure of multiple approved TKIs. Here we report on patients with CML in the chronic or accelerated phase.
METHODS
STUDY OVERSIGHT
The study was designed collaboratively by the sponsor (Novartis Pharmaceuticals) and study investigators. The sponsor collected the data and analyzed them in conjunction with the authors. The first two authors wrote the first draft of the manuscript. Editorial support was provided by ArticulateScience and funded by the sponsor. All authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol (available with the full text of this article at NEJM.org).
PATIENTS
Patients were eligible if they were 18 years of age or older, had Ph-positive chronic-phase or accelerated-phase CML, and had hematologic, cytogenetic, or molecular disease that was relapsed or refractory to at least two different TKIs before study entry or had unacceptable side effects from the TKIs, as determined by investigators according to standard criteria.17 Patients with a BCR-ABL1 T315I mutation were eligible after they had received at least one TKI if no other effective therapy was available. Additional cohorts of patients were subsequently enrolled through a protocol amendment (Fig. S1 in the Supplementary Appendix, available at NEJM.org).
STUDY DESIGN
The primary objective was to determine the maximum tolerated dose or the recommended dose (or both) of asciminib administered twice daily in patients with chronic-phase or accelerated-phase CML. Secondary objectives included assessments of safety, pharmacokinetics, and efficacy. The study included a dose-escalation phase and an expansion phase for patients treated at either the maximum tolerated dose or the recommended dose.
PHARMACOKINETICS
Data on pharmacokinetics are presented for 110 patients from cycle 1, day 1 onward (Table S1). A two-compartment pharmacokinetic–pharmacodynamic model with linear elimination and distribution was used to inform the recommended dose selection (see the Supplementary Appendix).
ANTILEUKEMIC ACTIVITY
Complete blood counts were performed regularly to assess hematologic response. Bone marrow aspirations for morphologic and cytogenetic analyses were performed before therapy and during the study in all patients with BCR-ABL1 transcript levels of more than 1% on the International Scale (IS) or loss of a complete hematologic response, every 3 months in patients without a complete cytogenetic response, and as clinically indicated. Real-time quantitative reverse-transcriptase–polymerase-chain-reaction assays for molecular response were performed every 3 months and as clinically indicated.
Responses were defined according to standard criteria.18–22 Molecular responses were assessed with the ratio of BCR-ABL1 to ABL1 measured on the International Scale (BCR-ABL1IS).22 Molecular response was calculated for patients with typical b2a2 or b3a2 BCR-ABL1 transcripts only. Changes in molecular-response category from baseline were assessed with the use of intervals of 1-log changes in BCR-ABL1 transcript levels by 6 or 12 months (see the Supplementary Appendix). BCR-ABL1 myristoyl-pocket mutations were assessed by means of bidirectional Sanger and next-generation sequencing in patients who could be evaluated and who had molecular disease progression or loss of a complete cytogenetic response at any time and in patients who had received asciminib for at least 12 months at the time of analysis (Fig. S2). Molecular assessments were performed centrally (MolecularMD, Portland, Oregon).
STATISTICAL ANALYSIS
A Bayesian logistic-regression model23,24 was used to estimate the posterior distribution of probabilities of dose-limiting toxic effects at various doses after each patient cohort in dose escalation and to determine the maximum tolerated dose (Fig. S3).
RESULTS
PATIENTS
Treatment status and patient characteristics are shown in Table 1 and Table S2. From May 2014 through September 2017, a total of 141 patients with chronic-phase CML and 9 with accelerated-phase CML were treated with asciminib monotherapy, with a median follow-up of 59 weeks (range, 0.1 to 167) at the time of analysis. Of all 150 patients enrolled, 105 (70%) had received at least three previous TKIs. At study entry, 46 patients (31%) had at least one BCR-ABL1 kinase domain mutation, the most frequent being T315I (in 33 patients [22%]). As of September 1, 2017, a total of 110 patients (73%) were continuing study treatment. Of patients who discontinued treatment, 1 died (after a blast crisis developed).
Table 1.
Treatment Status and Demographic and Clinical Characteristics of the Patients at Baseline, According to Asciminib Dosing Schedule.*
| Variable | Chronic-Phase CML | Accelerated-Phase CML | |||||
|---|---|---|---|---|---|---|---|
| No T315I Mutation | T315I Mutation | No T315I Mutation | T315I Mutation | ||||
| 2×/Day (N = 68) | 1×/Day (N = 45) | Combined 1×/Day and 2×/Day (N = 113) | 200 mg 2×/Day (N = 9) | Combined 1×/Day and 2×/Day (N = 28) | Combined 1×/Day and 2×/Day (N = 4) | Combined 1×/Day and 2×/Day (N = 5) | |
| number (percent) | |||||||
| Continued to receive asciminib at time of analysis | 48 (71) | 40 (89) | 88 (78) | 9 (100) | 19 (68) | 2 (50) | 1 (20) |
| Discontinued asciminib | 20 (29) | 5 (11) | 25 (22) | 0 | 9 (32) | 2 (50) | 4 (80) |
| Adverse event | 6 (9) | 1 (2) | 7 (6) | 0 | 3 (11) | 0 | 0 |
| Death | 1 (1) | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Physician decision | 4 (6) | 2 (4) | 6 (5) | 0 | 1 (4) | 0 | 1 (20) |
| Progressive disease | 7 (10) | 2 (4) | 9 (8) | 0 | 3 (11) | 2 (50) | 3 (60) |
| Patient or guardian decision | 2 (3) | 0 | 2 (2) | 0 | 2 (7) | 0 | 0 |
| ECOG performance-status score† | |||||||
| 0 | 52 (76) | 30 (67) | 82 (73) | 5 (56) | 21 (75) | 3 (75) | 4 (80) |
| 1 | 15 (22) | 15 (33) | 30 (27) | 4 (44) | 6 (21) | 1 (25) | 1 (20) |
| 2 | 1 (1) | 0 | 1 (1) | 0 | 1 (4) | 0 | 0 |
| No. of previous TKIs | |||||||
| 1 | 2 (3) | 0 | 2 (2) | 0 | 4 (14) | 0 | 0 |
| 2 | 20 (29) | 10 (22) | 30 (27) | 2 (22) | 8 (29) | 1 (25) | 0 |
| ≥3 | 46 (68) | 35 (78) | 81 (72) | 7 (78) | 16 (57) | 3 (75) | 5 (100) |
| Previous TKI | |||||||
| Imatinib | 46 (68) | 37 (82) | 83 (73) | 7 (78) | 21 (75) | 4 (100) | 4 (80) |
| Nilotinib | 49 (72) | 37 (82) | 86 (76) | 7 (78) | 15 (54) | 3 (75) | 5 (100) |
| Dasatinib | 57 (84) | 41 (91) | 98 (87) | 8 (89) | 19 (68) | 4 (100) | 5 (100) |
| Bosutinib | 23 (34) | 20 (44) | 43 (38) | 1 (11) | 5 (18) | 1 (25) | 1 (20) |
| Radotinib | 5 (7) | 1 (2) | 6 (5) | 2 (22) | 4 (14) | 0 | 0 |
| Ponatinib | 20 (29) | 14 (31) | 34 (30) | 7 (78) | 15 (54) | 1 (25) | 2 (40) |
| BCR-ABL1 transcript | |||||||
| Typical | 63 (93) | 42 (93) | 105 (93) | 9 (100) | 27 (96) | 4 (100) | 5 (100) |
| Atypical | 3 (4) | 3 (7) | 6 (5) | 0 | 1 (4) | 0 | 0 |
| Unknown | 2 (3) | 0 | 2 (2) | 0 | 0 | 0 | 0 |
Percentages are based on the number of patients who received at least one dose of asciminib. Percentages may not total 100 because of rounding. CML denotes chronic myeloid leukemia, and TKI tyrosine kinase inhibitor.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores reflecting greater disability.
SAFETY PROFILE
In patients with CML treated on a twice-daily schedule, seven dose levels were investigated: 10 mg (1 patient), 20 mg (14 patients), 40 mg (35 patients), 80 mg (12 patients), 150 mg (13 patients), 160 mg (7 patients), and 200 mg (16 patients). Five dose-limiting toxic effects were reported: grade 3 elevations in the lipase level without clinical pancreatitis in 2 patients receiving 40 mg, grade 2 myalgia and arthralgia in 1 patient receiving 80 mg, grade 3 acute coronary syndrome in 1 patient receiving 150 mg, and grade 3 bronchospasm in 1 patient receiving 200 mg. In patients treated on a once-daily schedule, three dose levels were investigated: 80 mg (18 patients), 120 mg (22 patients), and 200 mg (12 patients). Three dose-limiting toxic effects occurred in patients receiving 200 mg: a grade 3 elevation in the lipase level associated with clinical pancreatitis, a grade 3 asymptomatic elevation in the lipase level, and grade 3 abdominal pain of undetermined cause.
Among all 150 patients who could be evaluated for safety, the most common nonhematologic adverse events that emerged during treatment regardless of dose schedule were asymptomatic elevations in the lipase or amylase level, rash, and constitutional symptoms (e.g., fatigue, nausea, headache, and arthralgia), of which 92% were grade 1 or 2. Hypertension, reported in 19% of patients, was the most commonly reported cardiovascular adverse event (Table 2).
Table 2.
Most Frequent Adverse Events That Emerged during Asciminib Monotherapy in Patients with Chronic-Phase or Accelerated-Phase CML.
| Event | All Grades (N = 150) | Grade 3 or 4 (N = 150) |
|---|---|---|
| number (percent) | ||
| Total* | 150 (100) | 90 (60.0) |
| Fatigue | 44 (29.3) | 2 (1.3) |
| Headache | 42 (28.0) | 1 (0.7) |
| Lipase increased | 40 (26.7) | 15 (10.0) |
| Arthralgia | 36 (24.0) | 2 (1.3) |
| Nausea | 36 (24.0) | 1 (0.7) |
| Diarrhea | 35 (23.3) | 0 |
| Rash | 35 (23.3) | 0 |
| Thrombocytopenia | 33 (22.0) | 14 (9.3) |
| Vomiting | 31 (20.7) | 4 (2.7) |
| Hypertension | 29 (19.3) | 14 (9.3) |
| Upper respiratory tract infection | 27 (18.0) | 0 |
| Abdominal pain | 25 (16.7) | 0 |
| Pain in arm or leg | 24 (16.0) | 0 |
| Pruritus | 24 (16.0) | 1 (0.7) |
| Back pain | 23 (15.3) | 2 (1.3) |
| Constipation | 21 (14.0) | 0 |
| Pyrexia | 21 (14.0) | 0 |
| Dizziness | 20 (13.3) | 1 (0.7) |
| Amylase increased | 19 (12.7) | 4 (2.7) |
| Cough | 19 (12.7) | 0 |
| Dyspnea | 19 (12.7) | 2 (1.3) |
| Myalgia | 19 (12.7) | 1 (0.7) |
| Anemia | 17 (11.3) | 11 (7.3) |
| Hypertriglyceridemia | 17 (11.3) | 4 (2.7) |
| Nasopharyngitis | 17 (11.3) | 0 |
| Alanine aminotransferase increased | 16 (10.7) | 4 (2.7) |
| Neutropenia | 16 (10.7) | 11 (7.3) |
| Abdominal pain, upper | 15 (10.0) | 0 |
| Aspartate aminotransferase increased | 15 (10.0) | 3 (2.0) |
| Bone pain | 15 (10.0) | 1 (0.7) |
| Insomnia | 15 (10.0) | 1 (0.7) |
| Edema, peripheral | 15 (10.0) | 0 |
| Hyperhidrosis | 14 (9.3) | 0 |
| Hypophosphatemia | 14 (9.3) | 2 (1.3) |
| Hyperglycemia | 13 (8.7) | 3 (2.0) |
| Noncardiac chest pain | 13 (8.7) | 1 (0.7) |
| Decreased appetite | 12 (8.0) | 1 (0.7) |
| Depression | 12 (8.0) | 0 |
| Dry eye | 12 (8.0) | 0 |
| γ-Glutamyltransferase increased | 12 (8.0) | 3 (2.0) |
| Hyperuricemia | 12 (8.0) | 2 (1.3) |
| Musculoskeletal pain | 12 (8.0) | 0 |
| Vision blurred | 12 (8.0) | 0 |
| Anxiety | 11 (7.3) | 2 (1.3) |
| Dry skin | 11 (7.3) | 0 |
| Flank pain | 11 (7.3) | 0 |
| Muscle spasms | 11 (7.3) | 0 |
| Oropharyngeal pain | 11 (7.3) | 0 |
| Weight increased | 11 (7.3) | 0 |
| Fall | 10 (6.7) | 1 (0.7) |
| Dyspepsia | 9 (6.0) | 0 |
| Hypokalemia | 9 (6.0) | 1 (0.7) |
| Influenza | 9 (6.0) | 1 (0.7) |
| Memory impairment | 9 (6.0) | 0 |
| Pleural effusion | 9 (6.0) | 4 (2.7) |
| Abdominal discomfort | 8 (5.3) | 0 |
| Blood creatinine increased | 8 (5.3) | 0 |
| Urinary tract infection | 8 (5.3) | 1 (0.7) |
Data are for all patients who received at least one dose of asciminib.
Clinical pancreatitis, marked by abdominal pain and elevation in the lipase level and confirmed by abdominal imaging, occurred in 5 patients (3 patients receiving 80 mg twice daily, 1 receiving 150 mg twice daily, and 1 receiving 200 mg once daily); three cases were reported as serious adverse events (Table S3). Four of 5 patients had a single episode of pancreatitis, and 1 patient had two episodes of pancreatitis while receiving a reduced dose of asciminib. All cases resolved within 5 to 10 days after discontinuation of asciminib, and the sole patient rechallenged with asciminib was able to continue treatment at a lower dose. Three of 5 patients had had pancreatitis when using a previous TKI. Asymptomatic biochemical elevations in the lipase or amylase level occurred in 35 additional patients across all doses except 10 mg twice daily. These events were self-limited and did not progress to clinical pancreatitis. A total of 10 patients required temporary dose interruptions, and 1 patient discontinued treatment. Hematologic toxic effects that emerged during treatment were common but were typically of grade 1 or 2 (Table 2).
PHARMACOKINETICS
For both twice-daily and once-daily schedules, the relationships between asciminib dose and both peak blood concentration and area under the curve for each dose level on days 1 and 29 were approximately dose proportional (Fig. S4A and S4B). The preliminary half-life was approximately 8 hours, which suggests a steady state by day 3. At a dose of 40 mg twice daily or 80 mg once daily, trough blood concentrations surpassed the preclinical 90% inhibitory concentration for phosphorylated signal transducer and activator of transcription 5 (pSTAT5) inhibition in a KCL-22 xenograft animal model (121 ng per milliliter). Pharmacokinetic–pharmacodynamic model–based predictions indicated that a dose of 40 mg twice daily would maintain 100% of patients without a T315I mutation above the preclinical 90% inhibitory concentration for pSTAT5 inhibition.
ANTILEUKEMIC ACTIVITY
Patients with Chronic-Phase CML without a T315I Mutation
Among 113 patients with chronic-phase CML without a T315I mutation receiving asciminib either once or twice daily, 34 of 37 patients (92%) without a complete hematologic response at baseline had a complete hematologic response (Table 3). Of 57 patients without a complete cytogenetic response at baseline, 31 (54%) had a complete cytogenetic response (Table 3) in a median time of 24 weeks (range, 4 to 126).
Table 3.
Hematologic, Cytogenetic, and Molecular Responses with Asciminib (Combined Once-Daily and Twice-Daily Schedules).*
| Variable | Chronic-Phase CML | Accelerated-Phase CML | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No T315I Mutation | T315I Mutation | No T315I Mutation | T315I Mutation | |||||||||
| Overall (N = 113)† | Response Achieved | Response Maintained | Overall (N = 28)† | Response Achieved | Response Maintained | Overall (N = 4)† | Response Achieved | Response Maintained | Overall (N = 5)† | Response Achieved | Response Maintained | |
| Median follow-up (range) — wk | 72 (0.1–167) | 37 (0.7–167) | 46 (15–72) | 16 (6–120) | ||||||||
| Patients remaining in the study — no. (%) | 88 (78) | 19 (68) | 2 (50) | 1 (20) | ||||||||
| Complete hematologic response — no./total no. (%)‡ | 34/37 (92) | 14/16 (88) | 3/3 (100) | 4/5 (80) | ||||||||
| Major cytogenetic response — no./total no. (%)‡§ | 85/110 (77) | 24/40 (60) | 61/70 (87) | 15/25 (60) | 11/20 (55) | 4/5 (80) | 0/4 | 0/2 | 0/2 | 1/5 (20) | 1/4 (25) | 0/1 |
| Complete cytogenetic response — no./total no. (%)‡§ | 77/110 (70) | 31/57 (54) | 46/53 (87) | 11/25 (44) | 9/22 (41) | 2/3 (67) | 0/4 | 0/2 | 0/2 | 1/5 (20) | 1/4 (25) | 0/1 |
| Major molecular response — no./total no. (%)‡¶ | ||||||||||||
| In all patients | ||||||||||||
| By 6 mo | 37/99 (37) | 19/80 (24) | 18/19 (95) | 5/20 (25) | 4/19 (21) | 1/1 (100) | 0/4 | 0/3 | 0/1 | 1/5 (20) | 1/5 (20) | 0 |
| By 12 mo | 44/91 (48) | 26/72 (36) | 18/19 (95) | 5/18 (28) | 4/17 (24) | 1/1 (100) | 0/4 | 0/3 | 0/1 | 1/5 (20) | 1/5 (20) | 0 |
| In patients with ≤2 previous TKIs∥ | ||||||||||||
| By 6 mo | 13/25 (52) | 5/15 (33) | 8/10 (80) | 4/10 (40) | 3/9 (33) | 1/1 (100) | 0/1 | 0/1 | 0 | |||
| By 12 mo | 15/25 (60) | 7/15 (47) | 8/10 (80) | 4/9 (44) | 3/8 (38) | 1/1 (100) | 0/1 | 0/1 | 0 | |||
| In patients with >2 previous TKIs** | ||||||||||||
| By 6 mo | 24/74 (32) | 14/64 (22) | 10/10 (100) | 1/10 (10) | 1/10 (10) | 0 | 0/3 | 0/2 | 0/1 | 1/5 (20) | 1/5 (20) | 0 |
| By 12 mo | 29/66 (44) | 19/56 (34) | 10/10 (100) | 1/9 (11) | 1/9 (11) | 0 | 0/3 | 0/2 | 0/1 | 1/5 (20) | 1/5 (20) | 0 |
| In patients with resistance to or unacceptable side effects from ponatinib†† | ||||||||||||
| By 6 mo | 7/17 (41) | 3/13 (23) | 4/4 (100) | 1/7 (14) | 1/7 (14) | 0/0 | 0/2 | 0/2 | ||||
| By 12 mo | 8/14 (57) | 4/10 (40) | 4/4 (100) | 1/6 (17) | 1/6 (17) | 0/0 | 0/2 | 0/2 | ||||
For definitions of hematologic, cytogenetic, and molecular responses, see the Methods section in the Supplementary Appendix.
Shown is the number of patients who received at least one dose of asciminib.
The total number is the number of patients who could be evaluated.
Data on cytogenetic responses are based on patients who presented with Philadelphia chromosome–positive CML at baseline. Calculation of the number of patients in whom a major cytogenetic response or complete cytogenetic response was achieved is based on patients not in the respective response category at baseline.
Molecular-response assessment is reported only for patients with the b2a2 or b3a2 transcripts; 7 patients had atypical BCR-ABL1 transcripts and were not included in the response assessment.
The numbers of patients who received at least one dose of asciminib were as follows: 34 with chronic-phase CML without a T315I mutation, 12 with chronic-phase CML with a T315I mutation, and 1 with accelerated-phase CML without a T315I mutation.
The numbers of patients who received at least one dose of asciminib were as follows: 79 with chronic-phase CML without a T315I mutation, 16 with chronic-phase CML with a T315I mutation, 3 with accelerated-phase CML without a T315I mutation, and 5 with accelerated-phase CML with a T315I mutation.
The numbers of patients who received at least one dose of asciminib were as follows: 18 with chronic-phase CML without a T315I mutation, 11 with chronic-phase CML with a T315I mutation, and 2 with accelerated-phase CML with a T315I mutation.
A major molecular response (BCR-ABL1IS ≤0.1%) was achieved or maintained by 6 months in 37 of 99 patients (37%) who could be evaluated and by 12 months in 44 of 91 patients (48%) who could be evaluated (Table 3 and Table S4). Among the latter group of 91 patients, 30 of 40 patients (75%) with a baseline BCR-ABL1IS of 1% or less had a major molecular response by 12 months, whereas 14 of 51 patients (27%) with a BCR-ABL1IS of more than 1% had a major molecular response by 12 months. Of 85 patients without a deep molecular response (i.e., BCR-ABL1IS ≤0.01%) at baseline, 17 (20%) had a deep molecular response by 12 months. By 12 months, 57 of 91 patients (63%) had an improvement in their molecular-response category (Table 4). In addition, a major molecular response was achieved or maintained by 12 months in 8 of 14 patients (57%) with chronic-phase CML who were deemed to have resistance to or unacceptable side effects from ponatinib.
Table 4.
Categorical Response Shift from Baseline in Patients with Chronic-Phase CML Treated with Asciminib.*
| Variable | No T315I Mutation | T315I Mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline BCR-ABL1IS† | Baseline BCR-ABL1IS† | ||||||||
| ≤0.01% (N = 6) | >0.01 to 0.1% (N = 13) | >0.1 to 1% (N = 22) | >1 to 10% (N = 21) | >10% (N = 42) | >0.01 to 0.1% (N = 1) | >0.1 to 1% (N = 2) | >1 to 10% (N = 5) | >10% (N = 19) | |
| Post-treatment BCR-ABL1IS by 6 mo | |||||||||
| Patients who could be evaluated‡ | 6 | 13 | 23 | 18 | 39 | 1 | 1 | 5 | 13 |
| Distribution — no. of patients (%)§ | |||||||||
| ≤0.01% | 6 (100) | 4 (31) | 5 (22) | 4 (22) | 1 (3) | 1 (100) | 0 | 0 | 0 |
| >0.01 to 0.1% | 0 | 8 (62) | 6 (26) | 1 (6) | 2 (5) | 0 | 1 (100) | 2 (40) | 1 (8) |
| >0.1 to 1% | 0 | 1 (8) | 12 (52) | 12 (67) | 7 (18) | 0 | 0 | 2 (40) | 1 (8) |
| >1 to 10% | 0 | 0 | 0 | 1 (6) | 12 (31) | 0 | 0 | 1 (20) | 2 (15) |
| >10% | 0 | 0 | 0 | 0 | 17 (44) | 0 | 0 | 0 | 9 (69) |
| Post-treatment BCR-ABL1IS by 12 mo | |||||||||
| Patients who could be evaluated‡ | 6 | 13 | 21 | 17 | 34 | 1 | 1 | 5 | 11 |
| Distribution — no. of patients (%)§ | |||||||||
| ≤0.01% | 6 (100) | 5 (38) | 6 (29) | 5 (29) | 1 (3) | 1 (100) | 0 | 2 (40) | 0 |
| >0.01 to 0.1% | 0 | 7 (54) | 6 (29) | 3 (18) | 5 (15) | 0 | 1 (100) | 0 | 1 (9) |
| >0.1 to 1% | 0 | 1 (8) | 9 (43) | 8 (47) | 6 (18) | 0 | 0 | 2 (40) | 1 (9) |
| >1 to 10% | 0 | 0 | 0 | 1 (6) | 12 (35) | 0 | 0 | 1 (20) | 1 (9) |
| >10% | 0 | 0 | 0 | 0 | 10 (29) | 0 | 0 | 0 | 8 (73) |
Percentages may not total 100 because of rounding. BCR-ABL1IS denotes the ratio of BCR-ABL1 to ABL1 measured on the International Scale.
The number of patients is the number who received at least one dose of asciminib in each category of BCR-ABL1 transcript level at baseline. A total of 10 patients with chronic-phase CML (9 without a T315I mutation and 1 with a T315I mutation) had a missing BCR-ABL1IS value at baseline.
Shown is the number of patients who could be evaluated in each category of baseline BCR-ABL1 transcript level who had undergone assessment of molecular response at 6 (or 12) months after treatment or who had a major molecular response within 6 (or 12) months. For a detailed definition of molecular response, see the Methods section in the Supplementary Appendix. Response assessment is reported only for patients with the b2a2 or b3a2 transcripts.
Percentages were calculated on the basis of the number of patients who could be evaluated.
Among 44 patients in whom a major molecular response was either achieved or maintained, all but 4 continued to receive treatment at the time of analysis; the median time in which a major molecular response was achieved was 20 weeks (range, 2 to 120), and the median duration of response was more than 61 weeks (range, 4 to 154). In these 4 patients, the major molecular response was lost between 28 and 100 weeks of treatment; 2 of them remained in the study with a complete cytogenetic response. A total of 21 of 64 patients (33%) without detectable mutations and 5 of 8 (62%) with mutations other than T315I had a major molecular response by 12 months. Hematologic, cytogenetic, and molecular responses were noted across all doses of asciminib administered on once-daily or twice-daily schedules.
Patients with Chronic-Phase CML with a T315I Mutation
Among 28 patients with chronic-phase CML harboring a T315I mutation, 14 of 16 (88%) without a complete hematologic response at baseline had a complete hematologic response (Table 3). Of 22 patients without a complete cytogenetic response at baseline, 9 (41%) had a complete cytogenetic response (Table 3) in a median time of 8 weeks (range, 4 to 33).
A major molecular response was achieved in 4 of 17 patients (24%) and maintained in 1 of 1 patient (100%) by 12 months, with 9 of 18 (50%) showing improvement in their molecular-response category by 12 months (Table 4). Of 5 patients with a T315I mutation who were deemed to have resistance to ponatinib, 1 (20%) had a major molecular response by 12 months. Three of 4 patients (75%) with chronic-phase CML with a T315I mutation who had a major molecular response received a dose of more than 150 mg twice daily (Table S5). All 5 patients with a T315I mutation in whom a major molecular response was either achieved or maintained continued to receive treatment and were having a response at the time of analysis; the median time in which a major molecular response was achieved was 14 weeks (range, 4 to 20), and the median duration of response was more than 25 weeks (range, 12 to 96).
Patients with Accelerated-Phase CML
Among nine patients with accelerated-phase CML, seven of eight (88%) with hematologic disease at baseline had a complete hematologic response, and one of nine (11%) had a major molecular response, with responses maintained during therapy for a median of more than 11 weeks (Table 3).
Development of Myristoyl-Pocket Mutations
New myristoyl-pocket mutations were detected in 2 of 20 patients who had disease progression during asciminib treatment and in 2 of 66 patients without evidence of disease progression who had received asciminib for at least 12 months at the time of analysis. Four patients whose disease progressed before 12 months were not screened for mutations owing to sample unavailability. One patient with chronic-phase CML with a BCR-ABL1IS of 8.1% and a baseline E255K mutation received asciminib at a dose of 40 mg twice daily. The patient had a major molecular response by 6 months but eventually discontinued the study with progressive disease associated with a new myristoyl-pocket G463S mutation at week 50 of treatment (Fig. S5). Details of the other 3 patients are presented in the Supplementary Appendix.
DISCUSSION
Asciminib had substantial and durable clinical activity in a heavily pretreated population of patients with chronic-phase or accelerated-phase CML in whom treatment with currently available ATP-competitive TKIs had failed. Major side effects were asymptomatic elevations in the lipase or amylase level, rash, and constitutional symptoms (e.g., fatigue, nausea, headache, and arthralgia), most of which were of grade 1 or 2 and appeared to be equivalent in patients receiving asciminib twice daily and in those receiving the drug once daily. A maximum tolerated dose was not reached. Clinical pancreatitis was noted in 3% of patients overall, only at asciminib doses of more than 40 mg twice daily, and was manageable with dose modifications. Myelosuppression was uncommon and mostly of grade 1 and 2. Among patients with chronic-phase CML without a T315I mutation, the incidences of complete cytogenetic response and major molecular response at 12 months were 70% and 48%, respectively. Among patients who entered the study with a BCR-ABL1IS of 0.1% or less at baseline, a deep molecular response was achieved or maintained in 60% during the study.
Although TKI therapy has transformed the natural history of CML,25 many patients have TKI failure — frequently due to the emergence of resistance mutations in the BCR-ABL1 kinase domain.8,9,26–29 In our study, asciminib showed activity in patients with or without BCR-ABL1 kinase domain mutations.
Complete cytogenetic and major molecular responses were achieved in patients with chronic-phase CML with a T315I mutation, with the majority of those who had a response receiving asciminib doses of more than 150 mg twice daily, which was higher than the doses required to achieve responses in patients without a T315I mutation. This finding mirrors preclinical in vitro observations, in which the concentrations of asciminib that were required to achieve half the maximum inhibitory concentration were 5 to 10 times higher in cell lines expressing T315I-mutated BCR-ABL1 than in cell lines expressing non–T315I-mutated BCR-ABL1.15 These clinical responses are important because, currently, only ponatinib yields meaningful clinical benefit for patients with a T315I mutation; however, the vascular events that are associated with ponatinib often limit its use. Furthermore, a major molecular response was achieved in some patients with CML who were deemed to have resistance to or unacceptable side effects from ponatinib, which indicates a benefit in patients with limited effective therapeutic options other than stem-cell transplantation.
Asciminib was developed to bind to the myristoyl pocket — a previously unexploited feature of ABL1 and ABL2 kinases that is key to physiological autoinhibition of the native kinase. Given the extensive homology between the ATP-binding sites of many human kinases, targeting the myristoyl pocket is predicted to achieve superior selectivity and, hence, reduced toxicity. Asciminib exhibits both in vitro potency similar to that of second-generation TKIs and a resistance profile distinct from that of catalytic-site inhibitors.15,16 Although recent in vitro work suggested the potential for a high rate of emergent mutations with inhibition of the myristoyl pocket of BCR-ABL1,30 our early clinical experience does not support this prediction. To date, myristoyl-pocket mutations have been detected in only 4 of 86 patients receiving asciminib, and clinical responses were maintained in the majority of patients who had a response.
In our study, asciminib monotherapy showed durable clinical activity in most patients with chronic-phase CML. Low-grade, reversible toxic effects occurred in a minority of patients.
Supplementary Material
Acknowledgments
Supported by Novartis Pharmaceuticals.
Footnotes
Contributor Information
T.P. Hughes, South Australian Health and Medical Research Institute and the University of Adelaide, Adelaide, SA, Australia
M.J. Mauro, Memorial Sloan Kettering Cancer Center, New York
J.E. Cortes, University of Texas M.D. Anderson Cancer Center, Houston
H. Minami, Kobe University Graduate School of Medicine, Kobe, Japan
D. Rea, Hôpital Saint-Louis, Paris, France
D.J. DeAngelo, Dana–Farber Cancer Institute, Boston
M. Breccia, Sapienza University, Rome
Y.-T. Goh, Singapore General Hospital, Singapore
M. Talpaz, University of Michigan Comprehensive Cancer Center, Ann Arbor
A. Hochhaus, Universitätsklinikum Jena, Jena, Germany
P. le Coutre, Charité Hospital, Berlin, Germany
O. Ottmann, University of Cardiff, Cardiff, United Kingdom
M.C. Heinrich, Veterans Affairs Portland Health Care System, Portland Oregon Health and Science University Knight Cancer Institute, Portland.
J.L. Steegmann, Hospital de la Princesa and Instituto de Investigación Sanitaria Princesa, Madrid
M.W.N. Deininger, Huntsman Cancer Institute, University of Utah, Salt Lake City
J.J.W.M. Janssen, Amsterdam University Medical Centers, VU University Medical Center, Amsterdam
F.-X. Mahon, University of Bordeaux, Bordeaux, France
Y. Minami, National Cancer Center Hospital East, Chiba, Japan
D. Yeung, South Australian Health and Medical Research Institute and the University of Adelaide, Adelaide, SA, Australia
D.M. Ross, South Australian Health and Medical Research Institute and the University of Adelaide, Adelaide, SA, Australia
M.S. Tallman, Memorial Sloan Kettering Cancer Center, New York
J.H. Park, Memorial Sloan Kettering Cancer Center, New York
B.J. Druker, Oregon Health and Science University Knight Cancer Institute, Portland
D. Hynds, Novartis Pharma, Basel, Switzerland
Y. Duan, Novartis Pharma, Basel, Switzerland
C. Meille, Novartis Pharma, Basel, Switzerland
F. Hourcade-Potelleret, Novartis Pharma, Basel, Switzerland
K.G. Vanasse, Novartis Pharma, Basel, Switzerland
F. Lang, Department for Hematology–Oncology, Goethe University Hospital, Frankfurt am Main, Germany
D.-W. Kim, Seoul St. Mary’s Hematology Hospital, Catholic University of Korea, Seoul, South Korea
REFERENCES
- 1.Hughes TP, Saglio G, Quintás-Cardama A, et al. BCR-ABL1 mutation development during first-line treatment with dasatinib or imatinib for chronic myeloid leukemia in chronic phase. Leukemia 2015; 29: 1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002; 2: 117–25. [DOI] [PubMed] [Google Scholar]
- 3.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003; 102:276–83. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 2012; 119: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007; 110:4005–11. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011; 12: 841–51. [DOI] [PubMed] [Google Scholar]
- 7.Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A 2005;102: 11011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood 2007; 110: 2242–9. [DOI] [PubMed] [Google Scholar]
- 9.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutationbased resistance. Cancer Cell 2009; 16: 401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valent P, Hadzijusufovic E, Scherntha-ner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015; 125: 901–6. [DOI] [PubMed] [Google Scholar]
- 11.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015; 33:4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saussele S, Krauss MP, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 2015; 126: 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep 2016; 11: 71–9. [DOI] [PubMed] [Google Scholar]
- 14.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016; 30:1648–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wylie AA, Schoepfer J, Jahnke W, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 2017; 543:733–7. [DOI] [PubMed] [Google Scholar]
- 16.Schoepfer J, Jahnke W, Berellini G, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem 2018; 61:8120–35. [DOI] [PubMed] [Google Scholar]
- 17.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome–positive leukemias. N Engl J Med 2006; 354: 2531–41. [DOI] [PubMed] [Google Scholar]
- 19.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008;112: 3330–8. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002;346: 645–52. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–9. [DOI] [PubMed] [Google Scholar]
- 22.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 1998; 17: 1103–20. [DOI] [PubMed] [Google Scholar]
- 24.Neuenschwander B, Branson M, Gspon-er T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 2008;27: 2420–39. [DOI] [PubMed] [Google Scholar]
- 25.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood 2012; 119: 1981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome–positive leukemias. N Engl J Med 2012; 367: 2075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintás-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control 2009; 16:122–31. [DOI] [PubMed] [Google Scholar]
- 28.Apsel Winger B, Shah NP. PPARγ: welcoming the new kid on the CML stem cell block. Cancer Cell 2015; 28: 409–11. [DOI] [PubMed] [Google Scholar]
- 29.Khorashad JS, Anand M, Marin D, et al. The presence of a BCR-ABL mutant allele in CML does not always explain clinical resistance to imatinib. Leukemia 2006; 20: 658–63. [DOI] [PubMed] [Google Scholar]
- 30.Lee BJ, Shah NP. Identification and characterization of activating ABL1 1b kinase mutations: impact on sensitivity to ATP-competitive and allosteric ABL1 inhibitors. Leukemia 2017; 31: 1096–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.