Abstract
Background:
We examined how the relationship between education and latelife cognitive impairment (defined as a Mini Mental State Examination score below 24) is influenced by age, sex, ethnicity, and Apolipoprotein E epsilon 4 (APOE*4).
Methods:
Participants were 30,785 dementia-free individuals aged 55–103 years, from 18 longitudinal cohort studies, with an average follow-up ranging between 2 and 10 years. Pooled hazard ratios were obtained from multilevel parametric survival analyses predicting cognitive impairment (CI) from education and its interactions with baseline age, sex, APOE*4 and ethnicity. In separate models, education was treated as continuous (years) and categorical, with participants assigned to one of four education completion levels: Incomplete Elementary; Elementary; Middle; and High School.
Results:
Compared to Elementary, Middle (HR = 0.645, P = 0.004) and High School (HR = 0.472, P < 0.001) education were related to reduced CI risk. The decreased risk of CI associated with Middle education weakened with older baseline age (HR = 1.029, P = 0.056) and was stronger in women than men (HR = 1.309, P = 0.001). The association between High School and lowered CI risk, however, was not moderated by sex or baseline age, but was stronger in Asians than Whites (HR = 1.047, P = 0.044), and significant among Asian (HR = 0.34, P < 0.001) and Black (HR = 0.382, P = 0.016), but not White, APOE*4 carriers.
Conclusion:
High School completion may reduce risk of CI associated with advancing age and APOE*4. The observed ethnoregional differences in this effect are potentially due to variations in social, economic, and political outcomes associated with educational attainment, in combination with neurobiological and genetic differences, and warrant further study.
Keywords: Cognitive decline, Education, Ageing, Sex, Age, Ethnicity
1. Introduction
The attainment of higher levels of education is related to decreased dementia incidence (Caamano-Isorna, Corral, Montes-Martinez, & Takkouche, 2006; Meng & D’Arcy, 2012; Sharp & Gatz, 2011; Valenzuela & Sachdev, 2006; Xu et al., 2016), and attenuated cognitive decline (Albert et al., 1995; Alley, Suthers, & Crimmins, 2007; Anstey & Christensen, 2000a, 2000b; Anstey, Hofer, & Luszcz, 2003; Bosma, van Boxtel, Ponds, Houx, & Jolles, 2003; Christensen et al., 1997; Colsher & Wallace, 1991; Hall et al., 2007; Leibovici, Ritchie, Ledésert, & Touchon, 1996; Zahodne, Stern, & Manly, 2015). It has been theorised that this is in part due to education building cognitive reserve (CR) or brain reserve capacity. Cognitive reserve may be characterised as more efficient brain networks and greater neural capacity, making the brain less prone to disruption. It may also reflect better compensatory neural processes in the face of neural damage, including the use of alternative brain structures and neural pathways which are normally unused by those with intact brains (Meng & D’Arcy, 2012; Stern, Albert, Tang, & Tsai, 1999). The cognitive reserve hypothesis is supported by studies finding higher levels of brain pathology and degraded functioning among AD sufferers with high versus low educational attainment, despite having comparable levels of cognitive impairment (Fratiglioni & Wang, 2007). Because of this cognitive reserve, individuals are better able to tolerate age-related neuropathology without the expression of marked cognitive impairment(Meng & D’Arcy, 2012; Stern et al., 1999). As the degree of neuropathology advances over time, however, it may be so severe that the brain can no longer compensate for the underlying physical damage, thus leading to accelerated cognitive decline (Stern et al., 1999). This would imply that higher education may not be related to attenuated decline at very old ages (Van Dijk, Van Gerven, Van Boxtel, Van Der Elst, & Jolles, 2008). This hypothesis has been supported by some(Butler, Ashford, & Snowdon, 1996; Schmand et al., 1997), but not all studies (Farmer, Kittner, Rae, Bartko, & Regier, 1995; Van Dijk et al., 2008), highlighting the need for clarification.
Recent studies also indicate that the relationship between education and attenuated cognitive decline is curvilinear, meaning that after a certain level of educational attainment, additional education does not contribute to further significant reductions in cognitive decline(Wilson et al., 2009). Two U.S. based studies have suggested that this may occur after completion of 8–9 years (Lyketsos, Chen, & Anthony, 1999; Zahodne et al., 2015) of formal education (i.e., middle school). Whether this inference can be generalised to different cohorts and settings remains to be determined.
Compared to men, women with lower education are more likely to develop dementia (Launer et al., 1999; Ott et al., 1999; Russ et al., 2013; Sharp & Gatz, 2011); however, it is currently unclear if they also decline faster. Ethnoregional differences in the relationship between education and cognitive decline are also unclear, particularly differences to Asian groups, as prior studies have mainly compared White and Black Americans (Fitzpatrick et al., 2004; Lopez et al., 2017; Sachs-Ericsson & Blazer, 2005). Finally, there are mixed results surrounding whether education can offset faster cognitive decline associated with carriage of the Apolipoprotein E epsilon 4 (APOE*4) allele (Duara et al., 1996; Seeman et al., 2005; Shadlen et al., 2005; Vermeiren et al., 2013; Winnock et al., 2002). Studies incorporating larger, and more diverse study samples may help to clarify these important issues.
Therefore, we aimed to pool harmonised data from 18 independent research studies participating in COSMIC (Cohort Studies of Memory in an International Consortium) to clarify the nature of the relationship between education and cognitive decline in an ethnically diverse group of older adults, and to determine how this relationship is moderated by age, sex, ethnicity, and APOE*4 carriage.
2. Methods
2.1. Study selection
We collected datasets for this meta-analysis from independent research studies participating in COSMIC; a consortium which combines data from longitudinal, population-based cohort studies of older adults to identify factors that moderate the risk of cognitive decline(Sachdev et al., 2013). Studies were included if they collected the following Individual Participant Data (IPD) at baseline: age, sex, education (in years), data for four dementia risk factors to be treated as covariates (i.e., hypertension, diabetes, history of cardiovascular disease, history of stroke), score for a test of general mental status or cognition (typically the Mini Mental State Examination [MMSE]), self-reported ethnicity, and dementia status (details of participating studies are provided in eTable 1). For studies lacking IPD for ethnicity, participants’ ethnicity was assigned as the majority ethnicity of the study sample (as informed by each study’s lead investigators). Criteria used to diagnose dementia as well as risk factor data available in each study, and how this was harmonised across studies is provided in eTable 2. Participants that did not have the requisite data specified above, or who had dementia at baseline were excluded from all analyses. This project was approved by the University of New South Wales Human Research Ethics Committee (HC 12446 and HC 17292). All cohorts contributing data to this meta-analysis had prior ethics approval (see eTable 3 in the Supplement).
2.2. Outcome measure
The MMSE (Folstein, Folstein, & McHugh, 1975) was the primary outcome measure in this study, which was administered in all but three studies. In these three studies an alternative test of general mental status was administered, and scores converted to MMSE scores using published algorithms or co-calibration tables (see eTable 2). MMSE scores were then converted to a binary indicator, where scores ≤ 23 indicated the presence of cognitive impairment (Tombaugh & McIntyre, 1992a, 1992b). This cut-off has shown good sensitivity and specificity for the classification of dementia (Kochhann, Varela, Lisboa, & Chaves, 2010).
2.3. Synthesis methods
A one-step IPD meta-analysis was conducted using multilevel parametric survival analysis to examine the relationship between education and risk of cognitive impairment (CI). The multilevel model included two levels (i.e., participants nested in studies). Multivariate survival analysis with ordered failure events was used to account for participants who were cognitively impaired at multiple study waves (Andersen & Gill, 1982; Twisk, 2013). For all participants, event time was the time (in years) at which cognitive impairment, or when censoring occurred. A start time variable coded the time when participants began being at risk of cognitive impairment. For censored observations, start time was 0 (i.e., time of entry into the study). For participants who experienced CI, on their subsequent dataset row, start time was the time point when they were last cognitively impaired (i.e., their most recent “failure”).
Models were fit using the mestreg package in Stata 15 (StataCorp., 2013). Model terms included education in years (centred at the mean of 8.9 years), education2, baseline age (centred at the median age of 70 years), and the interaction between both education and education2 with baseline age (i.e., education x age; education2 x age). Covariates were sex, hypertension, diabetes, history of cardiovascular disease, history of stroke and MMSE score at baseline. To examine whether associations with education differed between sexes, two additional interactions were included: education x sex, and education2 x sex. Robust standard errors were calculated which adjusted for multiple CI events in individuals. Based on tests of model fit using likelihood ratio tests, random effects for the intercept and slope (i.e., for education), and their covariance, were included in the analysis, and a Weibull distribution was selected.
The above analyses were repeated treating education as categorical. We categorised participants into the following levels of educational attainment: (i) Incomplete Elementary; (ii) Elementary; (iii) Middle Level/Incomplete High School; and (iv) High School. The Elementary category was treated as the reference group. If a study collected categorical educational data, participants were assigned to one of the above-mentioned categories using this data. In studies, with minimal or no available categorical data, we used the continuous years of education data to assign participants to an educational achievement level based on cut-offs specific to the country where the study was conducted (see eTable 4), which were obtained from the Scholaro website (https://www.scholaro.com).
To explore ethnic differences, we included a categorical variable in the analysis that coded ethnicity (0 = White, 1 = East/Southeast Asian, 2 = Black), and all interactions (i.e., 2-way, and 3-way) with education category and baseline age. Whites were treated as the reference. To examine whether APOE*4 carriage moderated the association between education and cognitive impairment, we repeated the analyses above, and included a binary indicator for APOE*4 (1 = carrier, 0 = non-carrier) and all interactions with education category and baseline age. To test for ethnic differences in the interaction between APOE*4 and education, we included all interactions between ethnicity, APOE*4, and education category.
To minimise bias associated with non-random attrition, we incorporated inverse probability weighting (IPW) into all analyses (Buchanan, Hudgens, Cole, Lau, & Adimora, 2014). To calculate IPWs, logistic regression was used to regress a binary indicator of missingness (1 = not missing; 0 = missing) for each outcome at each wave on participants’ sex, age at last measurement, years of education (and all interactions between age, sex, and education), presence of hypertension, diabetes, heart disease, and stroke, and their MMSE score, age, and time in study from their most recently completed data collection point. Predicted probabilities from each model were converted to stabilised IPWs and used as a scale weight in the analysis (Singer & Willet, 2003; Tabachnick, 2007; Thoemmes & Ong, 2015).
3. Results
3.1. Participant characteristics
Baseline demographic characteristics are shown Table 1. The average follow-up duration ranged between 2 and 10 years, and the mean age at baseline ranged between 63 and 81 years. The proportion of participants lost to follow-up ranged between 1% (Tajiri) and 99.9 % (EAS). The rate of attrition was positively associated with the length of follow up (R = 0.9, P < 0.001). In all but two studies (PATH, MoVIES), females outnumbered males. Mean years of education ranged between 2.8 and 13.9 years across studies, with an overall mean of 9 years. For 10 studies, the majority educational attainment level was at least Middle school, whereas for the remaining 8 studies, most participants had either a complete or incomplete Elementary education. The proportion of participants who were APOE*4 carriers ranged between 13.3 and 25.2 %. IPD for ethnicity was available in six studies (Bambui, EAS, MoVIES, PATH, SydneyMAS, CHAS). In ten studies, the majority of the sample (i.e., > 60 %) was White. Six studies included Asian participants. In three of these studies, the entire sample comprised of Asians. Five studies had Black participants, with the proportion ranging between 0.2 to 27.1% of the study sample. The proportion of participants that experienced cognitive impairment ranged from 2% (PATH) to 62 % (Bambui).
Table 1.
Descriptive Statistics of Each Included Study at Baseline.
| Study | Sex | Education | |
Covariates |
Ethnicity |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Lost to follow up |
Impaired | Female | Age, y | Follow up, y |
Education, y | Incomplete Elementary |
Elementary | Middle Level |
High School |
CVD | DIAB | HT | Stroke |
APOE*4 carrier |
White Asian | Black Other | Missing | |||
| Analyseda | (%) | N (%) | % | M (SD) | M (SD) | M (SD) | %b | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| BAMBUI c | 1329 | 72.4 | 827 (62.2) | 62.4 | 68.6 (6.9) | 10.1 (4.7) | 2.9 (3) | 88.1 | 5.3 | 4.2 | 2 | 15.5 | 14.8 | 68.2 | 3.5 | 25.2 | 60.7 | 2.3 | 36.9 | ||
| CAS | 1464 | 0 | 222 (15.2) | 66.0 | 73.7 (6.4) | 4.6 (0.9) | 9.5 (4.7) | 21.8 | 32.2 | - | 46 | 29.5 | 31.8 | 75.7 | 6.0 | 16.7 | 67.9 | 16.3 | 10.4 | 5.4 | |
| CFAS | 8253 | 63.2 | 1951 (23.6) | 58.6 | 74.3 (6.4) | 4.9 (3.8) | 10.1 (2.3) | 0.7 | 5.4 | 65.5 | 28.5 | 17.3 | 5.5 | 32.5 | 5.9 | 24.0 | 64.7 | 0.2 | 0.1 | 35.0 | |
| EAS | 1220 | 99.8 | 220 (18) | 61.6 | 78.1 (5.4) | 4.4 (3.3) | 13.6 (3.5) | 0.4 | 3.5 | 16.6 | 79.5 | 33.7 | 16.2 | 63.9 | 9.0 | 22.2 | 0.4 | 68.5 | 27.1 | 3.9 | |
| ESPRIT d | 1916 | 36.3 | 220 (11.5) | 59.3 | 72.9 (5.4) | 6 (2.3) | 10.4 (3.7) | 23.4 | 18.2 | 10.7 | 47.7 | 19.5 | 8.8 | 71.4 | 3.2 | 19.3 | 100.0 | ||||
| HELIAD d | 419 | 0 | 69 (16.5) | 59.2 | 71.9 (5.8) | 2.8 (0.6) | 6.3 (3.2) | 23.4 | 60.9 | 3.1 | 12.6 | 21.0 | 15.8 | 64.9 | 6.9 | 17.2 | 100.0 | ||||
| HK-MAPS d | 561 | 24.4 | 169 (30.1) | 54.4 | 72 (7) | 4.6 (1.5) | 5 (4.7) | 55.4 | 19.6 | 10 | 15 | 16.4 | 18.2 | 49.9 | 7.3 | 13.3 | 100.0 | ||||
| Invece.Ab d | 977 | 0 | 32 (3.3) | 52.6 | 72.1 (1.3) | 2.2 (0.2) | 7.1 (3.3) | 7.2 | 51.1 | 31.7 | 10 | 27.3 | 18.0 | 60.6 | 7.6 | 18.4 | 100.0 | ||||
| KLOSCAD d | 4331 | 0 | 787 (18.2) | 56.3 | 69.3 (6.2) | 2 (0.3) | 8.7 (5.3) | 21.4 | 25.9 | 13.3 | 39.4 | 13.2 | 26.9 | 61.2 | 9.4 | 25.4 | 100.0 | ||||
| LEILAd | 766 | 93.6 | 208 (27.2) | 73.4 | 81.1 (4.6) | 5.4 (3.3) | 12 (1.8) | 21.5 | 78.3 | 8.1 | 22.8 | 81.7 | 6.5 | 16.0 | 100.0 | ||||||
| MoVIES | 368 | 83.2 | 201 (54.6) | 49.2 | 74.6 (6.1) | 7.9 (4.1) | 10.6 (2.7) | 1.4 | 33.7 | 16 | 48.9 | 41.3 | 14.1 | 70.4 | 9.2 | 25.1 | 96.7 | 3.3 | |||
| PATH | 2212 | 13.5 | 44 (2) | 48.4 | 62.5 (1.5) | 7.5 (1.4) | 13.9 (2.7) | 0.8 | 10.1 | 35.4 | 53.8 | 14.8 | 7.1 | 65.7 | 3.9 | 27.1 | 96.1 | 2.4 | 0.0 | 1.5 | |
| SALSA d | 1438 | 47.1 | 359 (25) | 58.3 | 70.1 (6.6) | 6.3 (2.4) | 7.6 (5.4) | 33.4 | 17 | 17.5 | 32.1 | 22.2 | 31.4 | 67.5 | 8.2 | 14.2 | 100.0 | ||||
| SGS d | 842 | 0 | 47 (5.6) | 57.2 | 72.8 (5.6) | 2 (0) | 11.4 (2.6) | 0.5 | 8.1 | 36.5 | 55 | 12.4 | 12.7 | 37.2 | 3.3 | b | 100.0 | ||||
| SLASI d | 432 | 44.2 | 41 (9.5) | 62.5 | 64.7 (6.7) | 2.9 (1.2) | 7 (4.4) | 32.1 | 21 | 9.6 | 37.3 | 10.0 | 12.8 | 60.7 | 3.0 | 16.6 | 100.0 | ||||
| SydneyMAS | 891 | 25.5 | 76 (8.5) | 54.3 | 78.5 (4.7) | 5.2 (1.4) | 11.7 (3.5) | 2.1 | 42.6 | 20.1 | 35.1 | 28.5 | 15.3 | 82.9 | 3.9 | 22.7 | 98.0 | 1.0 | 1.0 | ||
| ZARADEMP | 3161 | 25.3 | 521 (16.5) | 55.5 | 71.9 (8.7) | 4.1 (1.2) | 7.6 (3.9) | 41.5 | 40.2 | 3.7 | 14.6 | 6.7 | 12.3 | 67.8 | 4.8 | b | 100.0 | ||||
| Tajiri c | 98 | 0 | 18 (18.4) | 57.1 | 71.1 (3.9) | 5 (0) | 8.1 (1.8) | 5.1 | 76.5 | 13.3 | 5.1 | 2.0 | 10.2 | 71.4 | 0 | b | 100.0 | ||||
Abbreviations: APOE*4, Apolipoprotein E ε4; Bambui, Bambui Cohort Study of Aging; CHAS, Cuban Health and Alzheimer Study; CVD, cardiovascular disease history; DIAB, diabetes, EAS, Einstein Aging Study; ESPRIT, Etude Sante Psychologique et Traitement; HELIAD, Hellenic Longitudinal Investigation of Aging and Diet; HK-MAPS, Hong Kong Memory and Ageing Prospective Study; HT, hypertension; Invece.Ab, Invecchiamento Cerebrale in Abbiategrasso; KLOSCAD, Korean Longitudinal Study on Cognitive Aging and Dementia; LEILA75+, Leipzig Longitudinal Study of the Aged; MoVIES, Monongahela Valley Independent Elders Survey; PATH, Personality and Total Health Through Life Project; SALSA, Sacramento Area Latino Study on Aging; SGS, Sasaguri Genkimon Study; SLASI, Singapore Longitudinal Ageing Studies; SydneyMAS, Sydney Memory and Ageing Study; ZARADEMP, Zaragoza Dementia Depression Project.
Refers to the number of participants used in survival analysis. This includes participants with data for age at baseline, sex, education, all covariates, and have valid time-to-event information. Participants that dropped out at the initial wave are excluded from survival analyses because no time to event information is available.
Values in percentages are in relation to analysed sample (i.e., those with time-to-event information). Percentages may sum to less or more than 100 due to rounding error. Current smoking and high cholesterol were not used as covariates in the analysis as data for these variables was not available in all studies (Bambui did not have data on individuals who were non-smokers, and the KLOSCAD, LEILA, and MoVIES studies lacked data on high cholesterol).
Data relating to ethnicity in the Bambui study derived from a variable coding the skin colour of participants. For the purposes of ethnoregional analyses, however, the ethnicity of all participants in Bambui was regarded as Brazilian, as advised by the chief investigators of this study, and consequently all participants in this study were excluded from comparisons between Whites and Asians.
IPD for ethnicity was not available in these studies. Participants were assigned to the majority ethnic group of the study sample based on the recommendations of each study’s lead investigator(s).
3.2. Association between education and cognitive impairment
Results are displayed in Table 2. Overall, more years of education was related to decreased CI risk (HR = 0.881, P < 0.001). The quadratic term for education (education2), was small, but significant (HR = 1.003, P = 0.031), implying that the association between education and decreased CI risk was less pronounced at higher levels of education. Both the linear (HR = 1.006, P < 0.001) and quadratic terms (HR = 0.999, P = 0.002) for education significantly weakened with older baseline age but were not moderated by sex.
Table 2.
Results of Parametric Survival Analysis Examining Association Between Education and Risk of Cognitive Impairment and Moderation by Age, Sex, and APOE*4.
| HR (95 % confidence interval) | P | |
|---|---|---|
| Continuous | ||
| Education | 0.881, (0.847–0.916) | 0.000 |
| Education x Age | 1.006, (1.003–1.01) | 0.000 |
| Education effect at 80 y | 0.94, (0.913–0.968) | 0.000 |
| Education effect at 60 y | 0.826, (0.773–0.883) | 0.000 |
| Education2 | 1.003, (1–1.006) | 0.031 |
| Education2 x Age | 0.999, (0.999–1) | 0.002 |
| Education2 effect at 80 y | 0.997, (0.991–1.004) | 0.409 |
| Education2 effect at 60 y | 1.009, (1.007–1.012) | 0.000 |
| Education x Sex | 1.026, (0.997–1.055) | 0.074 |
| Education2 x Sex | 1.001, (0.995–1.008) | 0.743 |
| Categorical | ||
| Incomplete Elementary | 1.645 (1.324–2.045) | 0.000 |
| Incomplete Elementary x Age | 0.955 (0.933–0.978) | 0.000 |
| Old | 1.041 (0.771–1.406) | 0.794 |
| Young | 2.601 (1.854–3.648) | 0.000 |
| Incomplete Elementary x Sex | 1.002 (0.754–1.33) | 0.991 |
| Female | 1.625 (1.328–1.988) | 0.000 |
| Male | 1.627 (1.163–2.277) | 0.005 |
| Middle Level | 0.645 (0.479–0.87) | 0.004 |
| Middle Level x Age | 1.038 (1.009–1.067) | 0.009 |
| Old | 0.937 (0.765–1.147) | 0.527 |
| Young | 0.444 (0.259–0.763) | 0.003 |
| Middle Level x Sex | 1.309 (1.109–1.545) | 0.001 |
| Female | 0.58 (0.438–0.769) | 0.000 |
| Male | 0.76 (0.533–1.083) | 0.128 |
| High School | 0.472 (0.312–0.715) | 0.000 |
| High School x Age | 1.029 (0.999–1.061) | 0.056 |
| Old | 0.631 (0.489–0.815) | 0.000 |
| Young | 0.353 (0.18–0.694) | 0.003 |
| High School x Sex | 1.215 (0.968–1.525) | 0.093 |
| Female | 0.437 (0.296–0.643) | 0.000 |
| Male | 0.53 (0.326–0.862) | 0.011 |
| High School versus Middle Level | 0.732 (0.599–0.894) | 0.002 |
| College versus High School (some College) | 0.762 (0.487–1.192) | 0.234 |
| College v Middle | 0.595 (0.345–1.026) | 0.062 |
| College v Elementary | 0.381 (0.184–0.788) | 0.009 |
| Education and APOE*4 | ||
| Incomplete Elementary | ||
| APOE*4 x Incomplete Elementary | 0.906 (0.66–1.244) | 0.543 |
| Incomplete Elementary (NC) | 1.526 (1.226–1.901) | 0.000 |
| Incomplete Elementary (C) | 1.383 (0.953–2.008) | 0.088 |
| APOE*4 x Incomplete Elementary x Age | 0.998 (0.956–1.04) | 0.910 |
| APOE*4 x Incomplete Elementary x Sex | 0.928 (0.602–1.432) | 0.737 |
| Middle | ||
| APOE*4 x Middle | 1.505 (0.946–2.394) | 0.084 |
| Middle (NC) | 0.496 (0.316–0.78) | 0.002 |
| Middle (C) | 0.747 (0.454–1.229) | 0.251 |
| APOE*4 x Middle x Age | 0.977 (0.929–1.027) | 0.355 |
| APOE*4 x Middle x Sex | 0.961 (0.577–1.6) | 0.879 |
| High School | ||
| APOE*4 x High School | 1.002 (0.62–1.619) | 0.994 |
| High School (NC) | 0.519 (0.316–0.851) | 0.009 |
| High School (C) | 0.52 (0.324–0.834) | 0.007 |
| APOE*4 x High School x Age | 1.014 (0.973–1.058) | 0.505 |
| APOE*4 x High School x Sex | 1.458 (0.868–2.45) | 0.154 |
Abbreviations: APOE*4, Apolipoprotein E ε4; C, APOE*4 carrier; NC, APOE*4 non-carrier.
We further explored this nonlinearity by examining education categorically. With Elementary education as the reference, the risk of CI was significantly higher for incomplete Elementary (HR = 1.645, P < 0.001), and lower for both Middle (HR = 0.645, P = 0.004) and High School education (HR = 0.472, P < 0.001; See Fig. 1A). Additional comparisons showed that CI risk was lower for High School versus Middle education (HR = 0.73, P = 0.002). Furthermore, we separated the High School category into those that did and did not complete College Education and found no difference in CI risk between these groups (HR = 0.762, P = 0.234).
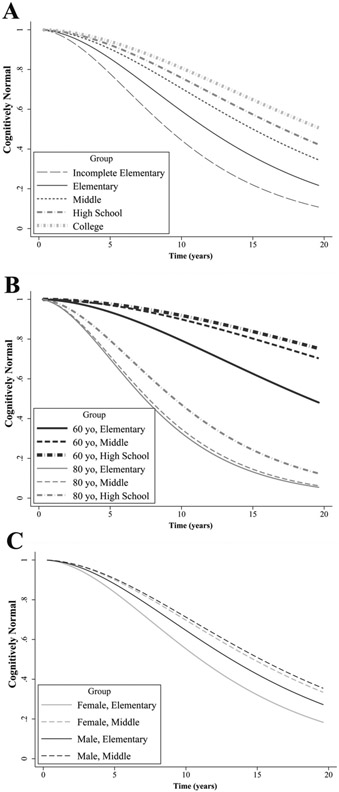
Fig. 1.
Relationship between education and risk of cognitive impairment (i.e., MMSE ≤ 23).A) Proportion of participants cognitively unimpaired over time for participants in each of the educational attainment categories. B) Proportion of cognitively unimpaired participants aged 60 and 80 years at baseline with either an Incomplete Elementary, Elementary, or High School education. C) Proportion of cognitively unimpaired men and women at baseline with either an Elementary or Middle education.
Differences in CI risk between both Incomplete Elementary (HR = 0.955, P < 0.001; Fig. 1B) and Middle education (HR = 1.038, P < 0.001; Fig. 1C) compared to Elementary education significantly weakened with older baseline age, but not so for High School education (HR = 1.029, P = 0.056). Simple effect comparisons showed that among 60 but not 80-year olds, the risk of CI was higher for incomplete Elementary (HR = 2.601, P < 0.001), and lower for Middle versus Elementary education (HR = 0.444, P = 0.003). High School education, however, was related to decreased CI risk in both 60 (HR = 0.353, P < 0.003) and 80-year olds (HR = 0.631, P < 0.001).
Examining sex differences, the reduction in CI risk associated with Middle versus Elementary education was significant for women (HR = 0.58, P < 0.001) but not men (HR = 0.76, P = 0.128), and this sex difference was significant (HR = 1.309, P = 0.001) as shown in Fig. 1C. There were no sex differences in CI risk for either the Incomplete Elementary or High School categories (versus Elementary education).
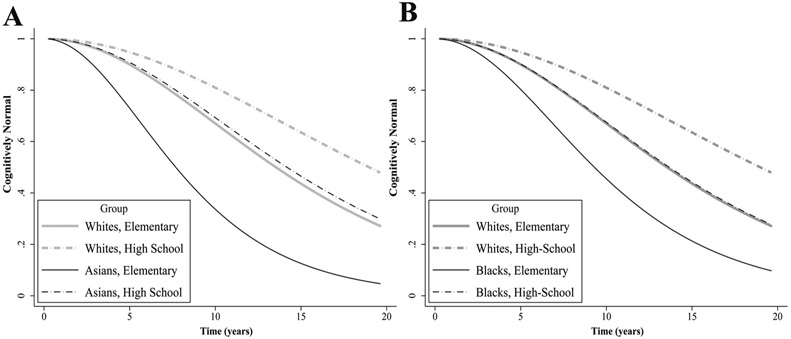
As shown in Table 3, in both Asians and Whites, incomplete Elementary education was related to a significant increase in CI risk, whereas Middle and High School education were both related to a significant decrease in CI risk, relative to Elementary education. The reduction in CI risk associated with High School education, however, was significantly larger in Asians than Whites (HR = 0.575, P = 0.002), as can be seen in Fig. 2A. In addition, the reduction in CI risk associated with High School completion weakened with older baseline age to a stronger degree in Asians than Whites (HR = 1.047, P = 0.044).
Table 3.
Ethnicity Differences in the Association Between Education and Risk of Cognitive Impairment, and oderation by Age and APOE*4.
| Asians | Blacks | Whites | ||||
|---|---|---|---|---|---|---|
| HR (95 % confidence interval) | p | HR (95 % confidence interval) | p | HR (95 % confidence interval) | p | |
| Education | ||||||
| Incomplete Elementary | ||||||
| Incomplete Elementary x Ethnicity | 0.87 (0.676–1.119) | 0.278 | 0.866 (0.431–1.743) | 0.688 | 1a | |
| Within Ethnic Group: Incomplete Elementary | 1.631 (1.535–1.734) | 0.000 | 1.625 (0.768–3.437) | 0.204 | 1.875 (1.454–2.418) | 0.000 |
| Incomplete Elementary x Age | 0.977 (0.942–1.013) | 0.208 | 0.993 (0.908–1.087) | 0.879 | 1a | |
| Within Ethnic Group: Incomplete Elementary x Age | 0.943 (0.911–0.977) | 0.001 | 0.959 (0.88–1.045) | 0.341 | 0.966 (0.954–0.978) | 0.000 |
| Middle | ||||||
| Middle Level x Ethnicity | 0.984 (0.675–1.434) | 0.934 | 1.664 (0.759–3.649) | 0.204 | 1a | |
| Within Ethnic Group: Middle | 0.595 (0.405–0.875) | 0.008 | 1.006 (0.443–2.287) | 0.988 | 0.605 (0.507–0.721) | 0.000 |
| Middle Level x Age x Ethnicity | 0.972 (0.944–1.001) | 0.058 | 1.014 (0.887–1.16) | 0.837 | 1a | |
| Within Ethnic Group: Middle x Age | 1.015 (0.996–1.035) | 0.121 | 1.059 (0.927–1.211) | 0.399 | 1.044 (1.018–1.072) | 0.001 |
| High School | ||||||
| High School x Ethnicity | 0.575 (0.406–0.815) | 0.002 | 0.909 | 0.685 | 1a | |
| Within Ethnic Group: High School | 0.29 (0.238–0.354) | 0.000 | 0.459 (0.287–0.734) | 0.001 | 0.505 (0.363–0.704) | 0.000 |
| High School x Age x Ethnicity | 1.047 (1.001–1.094) | 0.044 | 1.002 (0.925–1.085) | 0.968 | 1a | |
| Within Ethnic Group: High School x Age | 1.078 (1.038–1.12) | 0.000 | 1.032 (0.959–1.11) | 0.404 | 1.03 (1.008–1.052) | 0.007 |
| Education and APOE*4 | ||||||
| Incomplete Elementary | ||||||
| APOE x Incomplete Elementary x Ethnicity | 0.555 (0.34–0.905) | 0.018 | b | 1a | ||
| Within ethnicity: APOE*4 x Incomplete Elementary | 0.725 (0.496–1.06) | 0.097 | b | 1.306 (0.854–1.997) | 0.217 | |
| Incomplete Elementary (NC) | 1.572 (1.161–2.129) | 0.003 | b | 1.834 (1.269–2.65) | 0.001 | |
| Incomplete Elementary (C) | 1.139 (0.669–1.939) | 0.631 | b | 2.395 (1.325–4.329) | 0.004 | |
| Middle Level | ||||||
| APOE x Middle x Ethnicity | 0.304 (0.182–0.507) | 0.000 | 1.039 (0.683–1.58) | 0.859 | 1a | - |
| Within Ethnicity: APOE*4 x Middle Level | 0.69 (0.505–0.943) | 0.020 | 2.366 (1.435–3.901) | 0.001 | 2.271 (1.457–3.538) | 0.000 |
| Middle (NC) | 0.493 (0.403–0.602) | 0.000 | 0.634 (0.295–1.362) | 0.243 | 0.382 (0.275–0.529) | 0.000 |
| Middle (C) | 0.34 (0.257–0.449) | 0.000 | 1.5 (0.957–2.352) | 0.077 | 0.867 (0.545–1.379) | 0.547 |
| High School | ||||||
| APOE*4 x High School x Ethnicity | 0.636 (0.362–1.118) | 0.116 | 0.731 (0.316–1.692) | 0.465 | 1a | |
| Within Ethnicity: APOE x High School | 0.896 (0.676–1.187) | 0.444 | 0.83 (0.322–2.138) | 0.700 | 1.408 (0.804–2.466) | 0.231 |
| High School (NC) | 0.27 (0.182–0.4) | 0.000 | 0.461 (0.16–1.324) | 0.150 | 0.448 (0.268–0.751) | 0.002 |
| High School (C) | 0.242 (0.204–0.287) | 0.000 | 0.382 (0.175–0.834) | 0.016 | 0.632 (0.349–1.143) | 0.129 |
Abbreviations: APOE*4, Apolipoprotein E ε4; C, APOE*4 carrier; NC, APOE*4 non-carrier.
Reference Group.
Because of the small numbers of Black APOE*4 carriers in the low education groups, the Elementary and Incomplete Elementary groups were collapsed and treated as the reference education group.
Fig. 2.
Ethnoregional differences in risk of cognitive impairment between education groups. A) Proportion of cognitively unimpaired White and Asian participants with an Elementary or High School education. B) Proportion of cognitively unimpaired White and Black participants with an Elementary or High School education.
Although differences between Whites and Blacks were not significant for each of the comparisons, Table 3 shows that neither Middle (versus Elementary), nor Elementary (versus incomplete Elementary) education were related to significant reductions in CI risk among Blacks. High School versus Elementary education, however, was related to significant reduction CI risk among Blacks (HR = 0.459, P = 0.001), as shown in Fig. 2B.
As shown in Table 2, APOE*4 carriage did not moderate differences in CI risk between education levels (treating Elementary education as the reference), and these associations were not moderated by baseline age or sex. Results in Table 2 nonetheless show that Elementary (versus incomplete Elementary) and Middle (versus Elementary) education were related to significant reductions in CI risk in non-carriers only. High School completion, however, was associated with a lowered risk of CI compared to Elementary education in both APOE*4 carriers (HR = 0.52, P = 0.007) and non-carriers (HR = 0.519, P = 0.009).
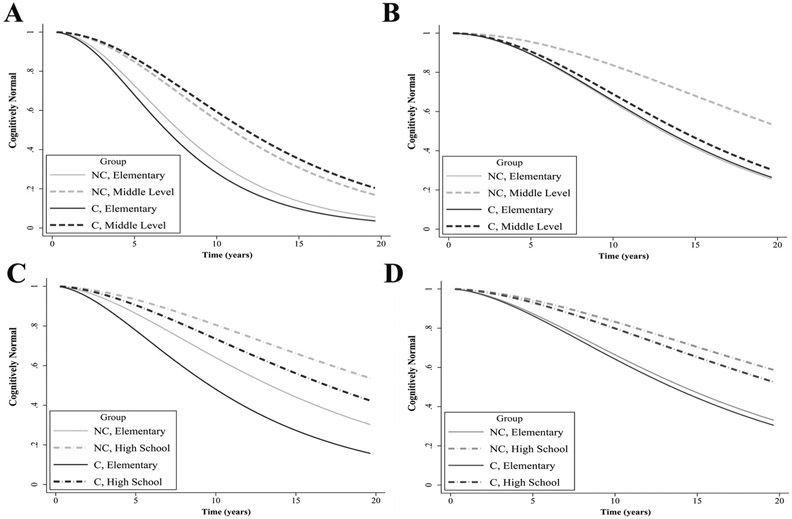
As shown in Table 3, APOE*4 carriage moderated the associations between Incomplete Elementary (HR = 0.555, P = 0.018) and Middle education (HR = 0.304, P < 0.001) with CI risk differently in Whites and Asians. In Whites, Incomplete versus Complete Elementary education was associated with increased CI risk in both APOE*4 carriers (HR = 2.395, P = 0.004) and non-carriers (HR = 1.834, P = 0.001). In Asians, however, this difference was significant for non-carriers only (HR = 1.572, P = 0.003). As shown in Fig. 3A, however, Middle level (versus Elementary) education was significantly related to decreased CI risk in both Asian APOE*4 carriers (HR = 0.34, P < 0.001) and non-carriers (HR = 0.493, P < 0.001); whereas in Whites, as shown in Fig. 3B, this reduction in CI risk only emerged for non-carriers (HR = 0.382, P < 0.001). Similar results emerged for the comparison between High School and Elementary education, although the difference between Whites and Asians was not significant.
Fig. 3.
Interaction between educational level and carriage of APOE*4 on the risk of cognitive impairment in ethnoregional groups. A) Proportion of cognitively unimpaired Asian APOE*4 carriers and non-carriers with an Elementary or Middle education. B) Proportion of cognitively unimpaired White APOE*4 carriers and non-carriers with an Elementary or Middle education. C) Proportion of cognitively unimpaired Black APOE*4 carriers and non-carriers with an Elementary or High School education. D) Proportion of cognitively unimpaired White APOE*4 carriers and non-carriers with an Elementary or High School education. Abbreviations: C, APOE*4 carriers; NC, APOE*4 non-carriers.
The interaction between APOE*4 and education on CI risk did not significantly differ between Whites and Blacks, at any educational level. Nonetheless, results in Table 3 indicate that Elementary and Middle education were unrelated to decreased CI risk in both Black APOE*4 carriers and non-carriers. High School education was related to decreased CI risk in Black APOE*4 carriers (HR = 0.382, P = 0.016), but not non-carriers, as can be seen in Fig. 3C. In Whites (Fig. 3D), the opposite was found, with High School education being associated with a significant decrease in CI risk in non-carriers (HR = 0.448, P = 0.002), but not in APOE*4 carriers.
4. Discussion
In this IPD meta-analysis, years of education was associated with a significant reduction in the risk of cognitive impairment (CI). This association, however, was non-linear, indicating that with even more years of education, the reduction in CI risk was less pronounced. Categorical analyses clarified these findings, indicating that increasing levels of educational attainment (i.e., Elementary versus Incomplete Elementary, Middle versus Elementary, and High School versus Middle) were each significantly related to decreases in CI risk. Additional analyses revealed, however, that completion of College did not decrease CI risk relative to the completion of High School. Expanding upon previous systematic reviews (Caamano-Isorna et al., 2006; Meng & D’Arcy, 2012; Sharp & Gatz, 2011; Valenzuela & Sachdev, 2006; Xu et al., 2016), our results imply that the association between education and reduction in cognitive impairment risk is not monotonically linear, in line with recent studies conducted in individual cohorts (Lyketsos et al., 1999; Wilson et al., 2009; Zahodne et al., 2015).
Our results align with those of Zahodne et al. (2015), who found that years of education both before (i.e., early education) and after middle school (i.e., late education) was related to attenuated cognitive decline. Importantly, they found that the association between education and cognitive decline after middle school was mediated entirely by income. They proposed that early education promotes the development of critical skills, as well as cognitive and neural development in a sensitive period of childhood, which directly contribute to latelife protection against cognitive decline. In contrast, late education influences cognitive reserve indirectly by shaping employment opportunities and income, which contribute to latelife protection against cognitive decline by increasing access to quality health care and leisure opportunities, and reducing exposure to life stressors (Zahodne et al., 2015). For the historical periods relevant to our cohorts, differences in occupational and economic outcomes between High School and college graduates were relatively small (Baum, 2014; Taylor et al., 2014). This may therefore explain why the risk of cognitive impairment was comparable between those with a High School versus College education in our study.
The reduced risk of cognitive impairment associated with Elementary (vs. Incomplete Elementary education) and Middle (vs. Elementary) education weakened with older baseline age. Namely, both associations were significant in those aged 60, but not among those aged 80 years at baseline. Schmand et al. (1997) found that high versus low education was associated with significant reduction in the magnitude of cognitive decline among participants in the youngest (i.e., 65–70 years), but not in the oldest (i.e., 80+ years) age group. Similarly, Butler et al. found that holding a bachelor’s degree was associated with a larger reduction in the amount of MMSE decline in nuns aged 75–84, compared to those aged 85+ at baseline(Butler et al., 1996). Interestingly, however, we found that High School (versus Elementary) education was associated with attenuated risk of cognitive impairment in both 60 and 80-year-old participants, which is broadly in line with the cognitive reserve hypothesis (Meng & D’Arcy, 2012; Stern, Alexander, Prohovnik, & Mayeux, 1992, 1999). Namely, in 80-year-olds, the level of neurodegeneration may have been so severe that lower levels of educational attainment (i.e., Elementary, and Middle) were insufficient to compensate for age-related cognitive deficits. However, having a High School education may have provided 80-year-olds with sufficient reserve to compensate for their higher degree of neurodegeneration, thus contributing to significant reductions in cognitive impairment risk relative to those with only Elementary education.
Some studies have found no moderating effect of baseline age on the relationship between education and cognitive decline. These studies, however, have used either very low cut-offs to define the younger and older-aged groups (i.e., 65 years) (Farmer et al., 1995), examined education solely as a continuous variable (Farmer et al., 1995; Van Dijk et al., 2008), or limited their analyses to linear associations(Van Dijk et al., 2008). On the other hand, not examining age as a moderator may explain why non-significant associations between education and cognitive decline were found in previous studies (Christensen et al., 2001; Seeman et al., 2005; Van Dijk et al., 2008; Winnock et al., 2002).
Overall, we found that the protective association between Middle education and cognitive impairment was significant in women but not men. Two previous studies examining sex differences found that low education was related to increased dementia risk in women but not men (Launer et al., 1999; Ott et al., 1999). Relatedly, an IPD meta-analysis found that leaving full-time education before versus after the age of 15 was associated with an increased risk of dementia death in women and not men (Russ et al., 2013). These sex differences have been attributed to larger socioeconomic discrepancies between those with low versus high education among women than men (Ott et al., 1999). Namely, women with low education are more likely to have worse occupational attainment, lower income, poorer health, fewer leisure opportunities, and consequently poorer cognitive outcomes than low educated men (Ott et al., 1999; Sharp & Gatz, 2011). This is evident in Fig. 1D, which indicated that the sex difference was primarily attributable to larger cognitive impairment among the Elementary (low) educated women than men.
Regarding ethnoregional differences, there was a larger difference in CI risk between the Elementary and High School education categories in Asians than Whites. Historically, in some Asian countries (e.g. Japan), those that received a High School education likely had better socioeconomic status than those who did not (Sorensen, 1994). In addition, educational systems in Hong Kong and South Korea were in disarray around the time of the Second World War (WWII), and illiteracy rates were high (Sorensen, 1994) implying that the quality of early education was poor in Asian countries during this period. Collectively, this suggests that the discrepancy between Elementary and High School education reflects wider gaps in educational quality and socioeconomic outcomes among Asians than Whites, hence leading to larger differences in cognitive performance between those with an Elementary versus High School education in Asians compared to Whites.
In contrast to Whites, in Blacks there was no significant reduction in CI risk associated with an Elementary (versus Incomplete Elementary) or Middle (versus Elementary) education, implying that early education was not protective in this group. This is possibly reflective of historical gaps in access to quality education between Blacks and Whites (Boozer, Krueger, & Wolkon, 1992; Carvalho et al., 2015), such that critical skills (e.g., reading, writing) were not adequately instilled among Blacks. Indeed, studies have found that differences in cognitive outcomes between Whites and Blacks are reduced after controlling for illiteracy (Carvalho et al., 2015; Sachs-Ericsson & Blazer, 2005). Interestingly, however, High School completion was associated with a significant reduction in CI risk among Blacks, and the size of this effect comparable to Whites. This aligns with Shadlen et al. (2006), who found that dementia incidence was significantly lower for Black Americans who had more (versus less) than 10 years of education. Fitzpatrick et al. (2004), however, found no significant differences in dementia incidence for Blacks with and without a High School education, although they didn’t control for vascular risk factors or baseline cognition. Our results suggest that only late education reduced CI risk among Blacks. Hall, Gao, Unverzagt, and Hendrie (2000)) argued that, historically, for a Black American to attain an education beyond Middle school, they likely would have overcome economic deprivation, poverty, rural life, and acquired psychological resilience, collectively building their cognitive reserve, and thereby reducing their risk of cognitive impairment. We emphasise, however, that without access to relevant data, these proposed mechanisms are speculative at best.
Finally, we found no significant differences in the relationship between educational attainment and CI between APOE*4 carriers and non-carriers. Results showed, however, that only High School attainment was related to reduced CI risk in APOE*4 carriers, implying that a higher degree of cognitive reserve is needed to counteract faster cognitive decline associated with APOE*4. Similarly, Shadlen et al. (2005) found that there were greater reductions in the magnitude of cognitive decline in APOE*4 homozygotes with increasing years of education. Our ethnoregional analyses, however, indicated that High School completion was related to a reduced risk of cognitive impairment (relative to Elementary school) in Asian and Black, but not White, APOE*4 carriers. Furthermore, Middle education was related to decreased CI risk in Asian APOE*4 carriers. As discussed above, differences between lower and higher levels of education, in particular completing versus not completing High School, may reflect wider gaps in socioeconomic status (Hall et al., 2000; Sharp & Gatz, 2011), literacy (Sachs-Ericsson & Blazer, 2005), occupational complexity (Andel, Vigen, Mack, Clark, & Gatz, 2006), income (Zahodne et al., 2015), and health outcomes (Williams, Priest, & Anderson, 2016) in Asians and Blacks than in Whites. Each of these factors is associated with reduced dementia incidence (Andel et al., 2006; Russ et al., 2013; Sharp & Gatz, 2011). This implies that higher educational attainment is correlated with larger reductions in multiple dementia risk factors in Blacks and Asians compared to Whites. Consequently, this may explain why High School attainment was associated with significant attenuation of CI risk in Asian and Black, but not White APOE*4 carriers. Interestingly, Elementary versus Incomplete Elementary education was related to reduced CI risk in White APOE*4 carriers only. We speculate that poor-quality Elementary Education available to Asians and Blacks in previous decades may not have built a sufficient level of cognitive reserve to overcome APOE*4-mediated cognitive impairments in these ethnic groups.
Because of limited access to relevant data, it is not possible for us to disentangle whether the reductions in cognitive impairment are primarily due to cognitive reserve or other variables that education is a proxy for (e.g., income, socioeconomic status, access to healthcare). This also precludes our ability to investigate more complex research questions, including the factors that mediate reductions in CI risk associated with education. We were also not able to control for literacy levels, which may (in part) account for the observed ethnic, gender, and age (and/or cohort) differences in the relationship between education and cognitive impairment. Our study is, however, strengthened by our large and diverse sample; our ability to control for several possible confounders/vascular risk factors; use of inverse probability weighting to reduce bias in parameter estimates due to non-random attrition; and the availability of educational IPD, enabling us to classify participants into finer-grained, educational categories. The cut-offs used to classify participants into these levels of achievement, furthermore, were tailored to the specific educational system of that country, thus enhancing the generalisability of our findings.
In this IPD meta-analysis of 18 population-based studies, we found that compared to Elementary education, attainment of a Middle or High School education was related to significant reduction in the risk of cognitive impairment. There was, however, no difference in CI risk between those with a complete High School or Tertiary education. The decreased CI risk associated with Middle education weakened with older baseline age and was stronger in women than men. The reduced risk of CI associated with High School completion, however, was unrelated to sex or baseline age, but emerged stronger in Asians than Whites. Finally, High School completion was related to reduced CI risk in APOE*4 carriers, specifically among those of Asian and Black ethnicity. High School completion may potentially reduce the risk of CI associated with advancing age and carriage of APOE*4 among non-White ethnic groups. The emergent ethnic differences may be attributable to historically wider gaps in socioeconomic status, employment opportunities, income, and health between low and high educated individuals in Asian and Black, versus White populations. Limited access to this data precludes us from making definitive conclusions about ethnic differences in how education influences the trajectory of cognitive decline in late adulthood. This limitation points to the challenges of studying ethnic differences in cognitive ageing, particularly in relation to examining differential impacts of risk and protective factors between and within ethnic groups (Brewster et al., 2019). A complete and unbiased analysis of risk factors take into account ethnoregional differences in genetics (e.g., ethnicity-specific genetic risk factors for Alzheimer’s Disease), biology, early life deprivation, neighbourhood characteristics (e.g., economically disadvantaged, ethnically homogenous), social and political history (e.g., experiences of discrimination and policies promoting inequality), health outcomes (e.g., vulnerability to cerebrovascular risk factors), income and employment across the lifespan, attitudes to the care of older adults with dementia (e.g., nursing home versus family care) and a wide range of other factors (Glymour & Manly, 2008). Historically, however, studies of normative ageing have been limited to non-Latino white participants, and therefore access to this rich data has been limited. Furthermore, cognitive tests used to inform dementia diagnoses are sensitive to educational attainment adding further complexity to unbiased assessment of cognitive decline across ethnic groups (Glymour & Manly, 2008).
Besides data and measurement limitations, the consideration of complex social, political, and biological factors and how they interact with risk and protective factors (e.g., education) of cognitive ageing calls for the greater use of complex systems models, where interrelations and interactions among multiple levels of relevant variables can be modelled (Brewster et al., 2019). Here the impact of risk factors on cognitive outcomes can be examined in the context of a complex interplay of biological, clinical, political, and social factors. Such models have been used to examine determinants of racial and ethnic disparities in obesity and HIV transmission, and their application to cognitive ageing provides a promising avenue for future research.
Supplementary Material
Acknowledgments
Funding Sources
Funding for COSMIC comes from a National Health and Medical Research Council of Australia Program Grant (ID 1093083), the National Institute On Aging of the National Institutes of Health under Award Number RF1AG057531, and philanthropic contributions to The Dementia Momentum Fund (UNSW Project ID PS38235). Funding for each of the contributing studies is as follows: Bambui: The Brazilian Ministry of Health (Department of Science and Technology), the Brazilian Ministry of Science and Technology (National Fund for Scientific and Technological Development, Funding of Studies, Brazilian National Research Council) and the Minas Gerais State Research Foundation; CFAS: Major awards from the Medical Research Council and the Department of Health, UK; CHAS: The Wellcome Trust Foundation (GR066133 and GR08002) and the Cuban Ministry of Public Health; EAS: Supported in part by National Institutes of Health grants NIA 2 P01 AG03949, the Leonard and Sylvia Marx Foundation, and the Czap Foundation; ESPRIT: Novartis; HELIAD: IIRG-09133014 from the Alzheimer’s Association; 189 10276/8/9/2011 from the ESPAEU program Excellence Grant (ARISTEIA), which is co-funded by the European Social Fund and Greek National resources, and ΔΥ2β/οικ.51657/14.4.2009 from the Ministry for Health and Social Solidarity (Greece); HK-MAPS: The Mei Family Trust; Invece.Ab: Financed with own funds and supported in part by “Federazione Alzheimer Italia”, Milan, Italy; KLOSCAD: The Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea [Grant No. HI09C1379 (A092077)]; LEILA75+: The Interdisciplinary Centre for Clinical Research at the University of Leipzig (Interdisziplinäres Zentrum für Klinische Forschung/IZKF; grant 01KS9504); MoVIES: Grant # R01AG07562 from the National Institute on Aging, National Institutes of Health, United States Department of Health and Human Services; PATH: National Health and Medical Research Council of Australia grants 973302, 179805, 157125 and 1002160; SALSA: NIH grants AG12975, T32 AG049663, ES023451; Carolina Population Center (CPC) Funding: CPC Center grant (the P2C Center grant from NIH): P2C HD050924. CPC NICHD-NRSA Population Research Training (the T32 Training grant from NIH): T32 HD007168, Biosocial Training Grant: T32 HD091058; SGS: JSPS KAKENHI Grant Number JP17K09146; SLASI: Agency for Science Technology and Research (A*STAR) Biomedical Research Council (BMRC) [Grants: 03/1/21/17/214 and 08/1/21/19/567] and the National Medical Research Council [Grant: NMRC/1108/2007]; Sydney MAS: National Health & Medical Research Council of Australia Program Grant (ID 350833); ZARADEMP: Supported by grants from the Fondo de Investigacion Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128), and the Fondo Europeo de Desarrollo Regional (FEDER) of the European Union and Gobierno de Aragón, Group #19.
Declaration of Competing Interest
Richard B. Lipton Is the Edwin S. Lowe Professor of Neurology at the Albert Einstein College of Medicine in New York. He receives research support from the NIH: 2PO1 AG003949 (mPI), 5U10 NS077308 (PI), RO1 NS082432 (Investigator), 1RF1 AG057531 (Site PI), RF1 AG054548 (Investigator), 1RO1 AG048642 (Investigator), R56 AG057548 (Investigator), K23 NS09610 (Mentor), K23AG049466 (Mentor), 1K01AG054700 (Mentor). He also receives support from the Migraine Research Foundation and the National Headache Foundation. He serves on the editorial board of Neurology, senior advisor to Headache, and associate editor to Cephalalgia. He has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from: American Academy of Neurology, Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, Vedanta. He receives royalties from Wolff’s Headache 7th and 8th Edition, Oxford Press University, 2009, Wiley and Informa. Henry Brodaty is on the Advisory Committee for Nutricia Australia; Clinincal Advisory Committee, Montefiore Home; Medical Advisory Committee, Cranbrook Care. Nikolaos Scarmeas reports personal fees from Merck Consumer Health and the NIH outside the submitted work. Mary Ganguli was on Biogen Inc.’s “Patient Journey Advisory Group” in 2016 and 2017. Allison E. Aiello is a consultant for Kinsa Inc. and has received an unrestricted gift from Gojo Inc. Henry Brodaty is on the Advisory Board of Nutricia Australia.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.archger.2020.104112.
References
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, et al. (1995). Predictors of cognitive change in older persons: Mac Arthur studies of successful aging. Psychology and Aging, 10, 578–589. [DOI] [PubMed] [Google Scholar]
- Alley D, Suthers K, & Crimmins E (2007). Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging, 29, 73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andel R, Vigen C, Mack WJ, Clark LJ, & Gatz M (2006). The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. Journal of the International Neuropsychological Society: JINS, 12, 147–152. [DOI] [PubMed] [Google Scholar]
- Andersen PK, & Gill RD (1982). Cox’s regression model for counting processes: A large sample study. Annals of Statistics, 10, 1100–1120. [Google Scholar]
- Anstey K, & Christensen H (2000a). Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology, 46, 163–177. [DOI] [PubMed] [Google Scholar]
- Anstey K, & Christensen H (2000b). Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology, 46, 163–177. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Hofer SM, & Luszcz MA (2003). A latent growth curve analysis of latelife sensory and cognitive function over 8 years: Evidence for specific and common factors underlying change. Psychology and Aging 18, 714–726. [DOI] [PubMed] [Google Scholar]
- Baum S (2014). Higher education earnings premium: Value, variation, and trends. Washington, D.C: The Urban Institute. [Google Scholar]
- Boozer MA, Krueger AB, & Wolkon S (1992). Race and School Quality Since Brown vs. Board of Education. National Bureau of Economic research working paper series No. 4109. [Google Scholar]
- Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJH, & Jolles J (2003). Education and age-related cognitive decline: The contribution of mental workload. Educational Gerontology, 29, 165–173. [Google Scholar]
- Brewster P, Barnes L, Haan M, Johnson JK, Manly JJ, Nápoles AM, et al. (2019). Progress and future challenges in aging and diversity research in the United States. Alzheimer’s & Dementia, 15, 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan AL, Hudgens MG, Cole SR, Lau B, & Adimora AA (2014). Worth the weight: Using inverse probability weighted Cox models in AIDS research. AIDS Research and Human Retroviruses, 30, 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SM, Ashford JW, & Snowdon DA (1996). Age, education, and changes in the mini-mental state exam scores of older women: Findings from the nun study. Journal of the American Geriatrics Society, 44, 675–681. [DOI] [PubMed] [Google Scholar]
- Caamano-Isorna F, Corral M, Montes-Martinez A, & Takkouche B (2006). Education and dementia: A meta-analytic study. Neuroepidemiology, 26, 226–232. [DOI] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, et al. (2015). Deconstructing racial differences: The effects of quality of education and cerebrovascular risk factors. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Hofer S, Mackinnon A, Korten A, Jorm A, & Henderson A (2001). Age is no kinder to the better educated: Absence of an association investigated using latent growth techniques in a community sample. Psychological Medicine, 31, 15–28. [DOI] [PubMed] [Google Scholar]
- Christensen H, Korten AE, Jorm AF, Henderson AS, Jacomb PA, Rodgers B, et al. (1997). Education and decline in cognitive performance: Compensatory but not protective. International Journal of Geriatric Psychiatry, 12, 323–330. [DOI] [PubMed] [Google Scholar]
- Colsher PL, & Wallace RB (1991). Longitudinal application of cognitive function measures in a defined population of community-dwelling elders. Annals of Epidemiology, 1, 215. [DOI] [PubMed] [Google Scholar]
- Duara R, Barker WW, Lopez-Alberola R, Loewenstein DA, Grau LB, Gilchrist D, et al. (1996). Alzheimer’s disease: Interaction of apolipoprotein E genotype, family history of dementia, gender, education, ethnicity, and age of onset. Neurology, 46, 1575–1579. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Kittner SJ, Rae DS, Bartko JJ, & Regier DA (1995). Education and change in cognitive function. The epidemiologic catchment area study. Annals of Epidemiology, 5, 1–7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JCS, et al. (2004). Incidence and prevalence of dementia in the cardiovascular health study. Journal of the American Geriatrics Society, 52, 195–204. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, & Wang HX (2007). Brain reserve hypothesis in dementia. Journal of Alzheimer’s Disease: JAD, 12, 11–22. [DOI] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. [DOI] [PubMed] [Google Scholar]
- Hall BC, Derby JC, Levalley BA, Katz BM, Verghese BJ, & Lipton BR (2007). Education delays accelerated decline on a memory test in persons who develop dementia. Neurology, 69, 1657–1664. [DOI] [PubMed] [Google Scholar]
- Hall KS, Gao S, Unverzagt FW, & Hendrie HC (2000). Low education and childhood rural residence: Risk for Alzheimer’s disease in African Americans. Neurology, 54, 95–99. [DOI] [PubMed] [Google Scholar]
- Kochhann R, Varela JS, Lisboa CSM, & Chaves MLF (2010). The mini mental state examination: Review of cutoff points adjusted for schooling in a large Southern Brazilian sample. Dementia & Neuropsychologia, 4, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer JL, Andersen EK, Dewey AM, Letenneur ML, Ott MA, Amaducci ML, et al. (1999). Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. Neurology, 52, 78–84. [DOI] [PubMed] [Google Scholar]
- Leibovici D, Ritchie K, Ledésert B, & Touchon J (1996). Does education level determine the course of cognitive decline? Age and Ageing, 25, 392. [DOI] [PubMed] [Google Scholar]
- Lopez ME, Turrero A, Delgado ML, Rodriguez-Rojo IC, Arrazola J, Barabash A, et al. (2017). APOE epsilon4 genotype and cognitive reserve effects on the cognitive functioning of healthy elders. Dementia and Geriatric Cognitive Disorders, 44, 328–342. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Chen LS, & Anthony JC (1999). Cognitive decline in adulthood: An 11.5-year follow-up of the Baltimore epidemiologic catchment area study. The American Journal of Psychiatry, 156, 58–65. [DOI] [PubMed] [Google Scholar]
- Meng X, & D’Arcy C (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7, Article e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Van Rossum CTM, Van Harskamp F, Van De Mheen H, Hofman A, & Breteler MMB (1999). Education and the incidence of dementia in a large population-based study: The Rotterdam study. Neurology, 52, 663–666. [DOI] [PubMed] [Google Scholar]
- Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, & Batty GD (2013). Socioeconomic status as a risk factor for dementia death: Individual participant meta-analysis of 86 508 men and women from the UK. The British Journal of Psychiatry: The Journal of Mental Science, 203, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Rockwood K, Xiao S, et al. (2013). COSMIC (Cohort Studies of Memory in an International Consortium): An international consortium to identify risk and protective factors and biomarkers of cognitive ageing and dementia in diverse ethnic and sociocultural groups. BMC Neurology, 13, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson N, & Blazer DG (2005). Racial differences in cognitive decline in a sample of community-dwelling older adults: The mediating role of education and literacy. The American Journal of Geriatric Psychiatry, 13, 968–975. [DOI] [PubMed] [Google Scholar]
- Schmand B, Smit J, Lindeboom J, Smits C, Hooijer C, Jonker C, et al. (1997). Low education is a genuine risk factor for accelerated memory decline and dementia. Journal of Clinical Epidemiology, 50, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, & Guralnik JM (2005). Education and APOE-e4 in longitudinal cognitive decline: MacArthur studies of successful aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60, P74–83. [DOI] [PubMed] [Google Scholar]
- Shadlen M-F, Larson EB, Wang L, Phelan EA, McCormick WC, Jolley L, et al. (2005). Education modifies the effect of apolipoprotein epsilon 4 on cognitive decline. Neurobiology of Aging, 26, 17–24. [DOI] [PubMed] [Google Scholar]
- Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, & Jackson S (2006). Education, cognitive test scores, and black-white differences in dementia risk. Journal of the American Geriatrics Society, 54, 898–905. [DOI] [PubMed] [Google Scholar]
- Sharp ES, & Gatz M (2011). Relationship between education and dementia: An updated systematic review. Alzheimer Disease and Associated Disorders, 25, 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JB, & Willet JB (2003). Applied longitudinal data analysis. New York: Oxford University Press. [Google Scholar]
- Sorensen CW (1994). Success and education in South Korea. Comparative Education Review, 38, 10–35. [Google Scholar]
- StataCorp (2013). Stata statistical software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- Stern Y, Albert S, Tang MX, & Tsai WY (1999). Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology, 53, 1942–1947. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, & Mayeux R (1992). Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of Neurology, 32, 371–375. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG (2007). In Fidell LS (Ed.). Using multivariate statistics(5th ed). Pearson/A&B, Boston [Mass.]: Pearson International ed. ed. Boston Mass. [Google Scholar]
- Taylor P, Parker K, Morin R, Fry R, Pattern E, & Brown A (2014). The rising cost of not going to college, numbers, facts, and trends shaping the world. Washington, D.C: Pew Research Centre. [Google Scholar]
- Thoemmes F, & Ong AD (2015). A primer on inverse probability of treatment weighting and marginal structural models. Emerging Adulthood, 4, 40–59. [Google Scholar]
- Tombaugh TN, & McIntyre NJ (1992a). The mini-mental state examination: A comprehensive review. Journal of the American Geriatrics Society, 40, 922–935. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, & McIntyre NJ (1992b). The mini-mental State examination: A comprehensive review. Journal of the American Geriatrics Society, 40, 922–935. [DOI] [PubMed] [Google Scholar]
- Twisk JR (2013). Applied longitudinal data analysis for epidemiology. Padstow, Cornwall: Cambridge University Press. [Google Scholar]
- Valenzuela MJ, & Sachdev P (2006). Brain reserve and dementia: A systematic review. Psychological Medicine, 36, 441–454. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Van Gerven PWM, Van Boxtel MPJ, Van Der Elst W, & Jolles J (2008). No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht aging study. Psychology and Aging, 23, 119–130. [DOI] [PubMed] [Google Scholar]
- Vermeiren AP, Bosma H, Visser PJ, Zeegers MP, Graff C, Ewers M, et al. (2013). The association between APOE epsilon4 and Alzheimer-type dementia among memory clinic patients is confined to those with a higher education. The DESCRIPA study. Journal of Alzheimer’s Disease: JAD, 35, 241–246. [DOI] [PubMed] [Google Scholar]
- Williams DR, Priest N, & Anderson NB (2016). Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychology, 35, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, & Evans DA (2009). Educational attainment and cognitive decline in old age. Neurology, 72, 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, & Dartigues JF (2002). Longitudinal analysis of the effect of apolipoprotein E epsilon4 and education on cognitive performance in elderly subjects: The PAQUID study. Journal of Neurology, Neurosurgery, and Psychiatry, 72, 794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Tan L, Wang H-F, Tan M-S, Tan L, Li J-Q, et al. (2016). Education and risk of dementia: Dose-response meta-analysis of prospective cohort studies. Molecular Neurobiology, 53, 3113–3123. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, & Manly JJ (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology, 29, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.