Abstract
Background
Using a multicenter, active surveillance network from 2 rotavirus seasons (2012 and 2013), we assessed the vaccine effectiveness of RV5 (RotaTeq) and RV1 (Rotarix) rotavirus vaccines in preventing rotavirus gastroenteritis hospitalizations and emergency department (ED) visits for numerous demographic and secular strata.
Methods
We enrolled children hospitalized or visiting the ED with acute gastroenteritis (AGE) for the 2012 and 2013 seasons at 7 medical institutions. Stool specimens were tested for rotavirus by enzyme immunoassay and genotyped, and rotavirus vaccination histories were compared for rotavirus-positive cases and rotavirus-negative AGE controls. We calculated the vaccine effectiveness (VE) for preventing rotavirus associated hospitalizations and ED visits for each vaccine, stratified by vaccine dose, season, clinical setting, age, predominant genotype, and ethnicity.
Results
RV5-specific VE analyses included 2961 subjects, 402 rotavirus cases (14%) and 2559 rotavirus-negative AGE controls. RV1-specific VE analyses included 904 subjects, 100 rotavirus cases (11%), and 804 rotavirus-negative AGE controls. Over the 2 rotavirus seasons, the VE for a complete 3-dose vaccination with RV5 was 80% (confidence interval [CI], 74%–84%), and VE for a complete 2-dose vaccination with RV1 was 80% (CI, 68%–88%).
Statistically significant VE was observed for each year of life for which sufficient data allowed analysis (7 years for RV5 and 3 years for RV1). Both vaccines provided statistically significant genotype-specific protection against predominant circulating rotavirus strains.
Conclusions
In this large, geographically and demographically diverse sample of US children, we observed that RV5 and RV1 rotavirus vaccines each provided a lasting and broadly heterologous protection against rotavirus gastroenteritis.
Keywords: rotavirus vaccine, RV1-Rotarix, RV5-RotaTeq, acute gastroenteritis, surveillance
Two rotavirus vaccines now routinely administered to US infants were found to be highly effective in preventing rotavirus gastroenteritis in prelicensure clinical trials [1–4]. RotaTeq ([RV5] –Merck and Company, Whitehouse Station, New Jersey) is a live, attenuated vaccine containing five reassortant rotaviruses derived from human and bovine parent strains that express human outer capsid proteins of common circulating strains (G1, G2, G3, G4, and P[8]). RV5 was licensed in the United States and recommended for universal vaccination of infants by the Advisory Committee on Immunization Practices (ACIP) in 2006 with a recommended schedule of three oral doses administered at ages 2, 4, and 6 months. Rotarix ([RV1] – GlaxoSmithKline Biologicals, Rixensart, Belgium) was licensed and recommended by ACIP in the United States in 2008. RV1 contains the live, attenuated monovalent G1 P[8] human rotavirus strain and is administered according to the ACIP recommended schedule of 2 doses given orally at age 2 and 4 months [5].
Previous rotavirus vaccine effectiveness (VE) studies have demonstrated that these vaccines perform well in preventing severe rotavirus gastroenteritis among US children [6–9]. Furthermore, substantial evidence has accumulated that the US rotavirus vaccination program has led to a dramatically decreased incidence of rotavirus gastroenteritis during the post-licensure era [10–14]. Therefore, it is increasingly challenging to provide post-licensure vaccine assessments holding robust statistical power to offer a precise understanding of rotavirus VE and genotype-specific effectiveness. In particular, because of its later implementation, limited information on RV1 effectiveness in US children is available.
Using a well-powered and geographically diverse active rotavirus surveillance network we assessed the VE of both RV5 and RV1 in preventing rotavirus acute gastroenteritis (AGE) hospitalization and emergency department (ED) visits among US children during 2 rotavirus seasons (2012 and 2013).
METHODS
Definition and Enrollment of Subjects
Active surveillance methods have been previously published for the New Vaccine Surveillance Network (NVSN), funded by the US Centers for Disease Control and Prevention (CDC) [8, 15, 16]. Seven surveillance sites participated, including Children’s Mercy Hospitals and Clinics (Kansas City, Missouri [“Kansas City”]), UCSF Benioff Children’s Hospital, Oakland (Oakland, California [“Oakland”]), Texas Children’s Hospital (Houston, Texas [“Houston”]), Seattle Children’s Hospital (Seattle, Washington [“Seattle”]), Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio [“Cincinnati”]), Vanderbilt University Medical Center (Nashville, Tennessee [“Nashville”]), and the University of Rochester Medical Center (Rochester, New York [“Rochester”]). Institutional review board approvals were obtained from CDC and from each study site.
Children less than 8 years of age were enrolled if they were hospitalized or visited the ED from 1 December 2011 through 30 November 2012 (hereafter “2012”) and 1 December 2012 through 30 November 2013 (hereafter “2013”) with diarrhea (≥3 episodes within 24 hours) and/or vomiting (≥1 episode within 24 hours) and with informed consent from a parent or guardian. Enrolled subjects were screened for pre-existing conditions and excluded from eligibility if they had such indications including a noninfectious cause, a history of immune deficiency, previous enrollment for the same AGE episode, or transfer from another hospital. Children enrolled in the ED but subsequently hospitalized for the illness were categorized as inpatients. Children who were eligible but unenrolled for any reason were compared with children who were eligible and who consented to enrollment, in order to assess any potential enrollment bias.
Specimen Collection and Case Determination
Whole stool specimens were obtained within 10 days of symptom onset, with >95% of specimens obtained within 7 days of onset. Rotavirus testing was performed using Premier Rotaclone enzyme immunoassay (EIA) (Meridian Bioscience, Inc., Cincinnati, Ohio) at each surveillance site. Rotavirus strains were genotypically characterized using reverse transcription polymerase chain reaction (RT-PCR) and nucleotide sequencing at CDC [17]. Specimens without rotavirus amplification by RT-PCR were retested by EIA at CDC to confirm positive results. Specimens failing to confirm by repeat EIA testing at CDC were considered rotavirus negative in our analytical dataset.
Cases were defined as children with AGE symptoms who were either hospitalized or seen in the ED, having a rotavirus test-positive stool specimen. Data from cases were compared with children with AGE whose specimens tested negative for rotavirus (“controls”).
Descriptive Analyses
Demographic and socioeconomic data for both cases and controls were compared by Wilcoxon Rank-Sum tests for continuous variables and χ2 tests for categorical variables.
We assessed the clinical severity of subjects’ illnesses by calculating a modified 20-point Vesikari Severity Scores (modified-VSS) [18]. This method has been validated to accurately estimate the severity of AGE illness in this US pediatric population during the rotavirus post-licensure period [19], using an assessment of dehydration that is concordant with the World Health Organization Integrated Management of Childhood Illness (IMCI) dehydration assessment at the time of enrollment [20]. For the subset of children receiving rotavirus vaccines and having full clinical data, we calculated modified-VSS categories (mild {score <=10}, moderate {score 11–15} and severe {score >=16}).
Vaccine Effectiveness Analyses
Vaccine effectiveness was calculated using the formula: VE = (1−odds ratio) × 100 to estimate the preventive effect of rotavirus vaccines upon rotavirus-associated hospitalizations and ED visits. Stratified VE estimates were calculated for each vaccine type by vaccine dose number, secular factors (clinical setting, season, predominant rotavirus genotype), and subject factors (age and ethnicity). We also calculated VE estimates for each modified-VSS category. The adjusted odds ratio and 95% confidence intervals (CIs) were calculated by logistic regression and were adjusted for month/year of birth, month/year of symptom onset, and surveillance site. Tests were 2-sided and P-values <.05 were considered significant.
Rotavirus immunization status was verified by contacting the subjects’ primary care providers and through regional immunization information systems. Vaccine doses were defined as valid if given ≥14 days before onset of symptoms for the cases and controls. Subjects were required to be born on, or after, 1 April 2006 for RV5 analyses and on, or after, 1 August 2008 for RV1 analyses to ensure vaccine eligibility following Food and Drug Administration (FDA) licensure. We restricted analyses to cases and controls who had reached the maximum ACIP-recommended age for completion of the vaccine series within the recommended age window (maximum age for the last dose being 8 months and 0 days) to control for residual confounding by age at the time of last dose for both vaccine types [5]. We allowed for replacement of controls from the pooled sample of children with AGE to both the RV1 and RV5 analytical datasets, so long as conforming to the eligibility criteria established in Figure 1. Our study focused upon the independent effectiveness of RV5 and RV1, and subjects having mixed doses of both RV5 and RV1 were excluded from analyses.
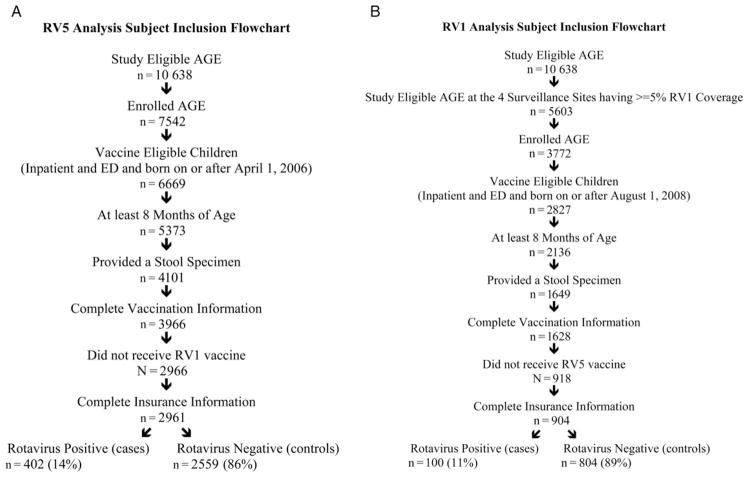
Figure 1.
RV5 and RV1 Analysis Subject Inclusion Flowchart. Abbreviations: AGE, acute gastroenteritis; ED, emergency department.
RESULTS
Characteristics of Cases and Controls
RV5-specific VE analyses included 402 rotavirus cases and 2559 controls. RV1-specific VE analyses included 100 rotavirus cases and 804 controls. These subjects included 147 rotavirus test-positive cases who received a full course of RV5 and 36 who had received a full course of RV1 (Figure 1).
In both RV5 and RV1 analyses, rotavirus cases were significantly older than controls (P < .001). For both vaccine analyses, cases were more often privately insured than controls, and a higher proportion of cases were enrolled in the 2013 season compared with the 2012 season. Fewer than 5% of enrolled subjects received RV1 vaccine in the Seattle, Houston, and Nashville sites, whereas all 7 sites had at least 5% RV5 vaccine coverage (P < .001). His-panic ethnicity, gender, and clinical setting were significantly different between cases and controls for the RV5 analyses but not for the RV1 analyses. Race was not statistically different between cases and controls for either VE analysis (Table 1).
Table 1.
Description of New Vaccine Surveillance Network Rotavirus Cases and Controls in RV5 and RV1 Analytical Datasets
| Variables | RV5 Analysis | P Value | RV1 Analysis | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Rotavirus Cases (n = 402) | Controls (n = 2559) | Rotavirus Cases (n = 100) | Controls (n = 804) | |||||||
|
|
|
|
|
|||||||
| n | Percent | n | Percent | n | Percent | n | Percent | |||
| Age (in months) | <.001 | <.001 | ||||||||
|
| ||||||||||
| Median | 35 | Range (8–82) | 26 | Range (8–89) | 24 | Range (8–56) | 18 | Range (8–62) | ||
|
| ||||||||||
| Gender | .041 | .157 | ||||||||
|
| ||||||||||
| Male | 203 | 50.5 | 1432 | 59.7 | 47 | 47.0 | 438 | 54.5 | ||
|
| ||||||||||
| Female | 199 | 49.5 | 1127 | 48.8 | 53 | 53.0 | 366 | 45.5 | ||
|
| ||||||||||
| Race | .407 | .419 | ||||||||
|
| ||||||||||
| White | 253 | 62.9 | 1528 | 59.7 | 39 | 39.0 | 269 | 33.5 | ||
|
| ||||||||||
| Black | 89 | 22.1 | 647 | 25.3 | 40 | 40.0 | 391 | 48.6 | ||
|
| ||||||||||
| Other | 60 | 14.9 | 384 | 15.0 | 21 | 21.0 | 144 | 17.9 | ||
|
| ||||||||||
| Ethnicity | .024 | .775 | ||||||||
|
| ||||||||||
| Hispanic | 152 | 37.8 | 1115 | 43.6 | 21 | 21.0 | 191 | 23.8 | ||
|
| ||||||||||
| Non-Hispanic | 248 | 61.7 | 1441 | 56.3 | 79 | 79.0 | 612 | 76.1 | ||
|
| ||||||||||
| Other/Unknown | 2 | 0.5 | 3 | 0.1 | 0 | 0.0 | 1 | 0.1 | ||
|
| ||||||||||
| Insurance | <.001 | .018 | ||||||||
|
| ||||||||||
| Private | 1268 | 31.8 | 555 | 21.6 | 19 | 19.0 | 88 | 10.9 | ||
|
| ||||||||||
| Public/None | 274 | 68.2 | 2006 | 78.4 | 81 | 81.0 | 716 | 89.1 | ||
|
| ||||||||||
| Clinical Setting | .038 | .303 | ||||||||
|
| ||||||||||
| Inpatient | 66 | 16.4 | 324 | 12.7 | 19 | 19.0 | 121 | 15.0 | ||
|
| ||||||||||
| ED | 336 | 83.6 | 2235 | 87.3 | 81 | 81.0 | 683 | 85.0 | ||
|
| ||||||||||
| Season | <.001 | <.001 | ||||||||
|
| ||||||||||
| 2012 | 77 | 19.1 | 1105 | 43.2 | 15 | 15.0 | 330 | 41.0 | ||
|
| ||||||||||
| 2013 | 325 | 80.9 | 1454 | 56.8 | 85 | 85.0 | 474 | 59.0 | ||
|
| ||||||||||
| NVSN Site | <.001 | <.001 | ||||||||
|
| ||||||||||
| Oakland | 58 | 14.4 | 258 | 10.1 | 20 | 20.0 | 54 | 6.7 | ||
|
| ||||||||||
| Seattle | 52 | 12.9 | 248 | 9.7 | ф | ф | ||||
|
| ||||||||||
| Kansas City | 46 | 11.4 | 382 | 14.9 | 46 | 46.0 | 425 | 52.9 | ||
|
| ||||||||||
| Houston | 125 | 31.1 | 785 | 30.7 | ф | ф | ||||
|
| ||||||||||
| Nashville | 79 | 19.7 | 543 | 21.2 | ф | ф | ||||
|
| ||||||||||
| Cincinnati | 21 | 5.2 | 257 | 10.0 | 23 | 23.0 | 270 | 33.6 | ||
|
| ||||||||||
| Rochester | 21 | 5.2 | 86 | 3.4 | 11 | 11.0 | 55 | 6.8 | ||
ф = Insufficient observations.
Abbreviations: ED, emergency department; NVSN, New Vaccine Surveillance Network.
Modified-Vesikari Severity Scores for Vaccinated Subjects
Clinical severity was assessed for a subset of 2091 children with AGE having complete clinical and laboratory data. Severity of illness was mild, moderate, and severe for 1123 (54%), 831 (40%), and 137 (6%) of the children, respectively. In this subset, the median severity score for cases was 13 (classified as moderate), significantly higher than for controls (median = 10, classified as mild) (P < .0001). Of the cases assessed for clinical severity, approximately 28%, 62%, and 10% were categorized as being mildly, moderately, and severely ill, compared with 56%, 38%, and 6%, respectively, of controls (P < .0001).
Rotavirus Vaccine Effectiveness by Vaccine Dose
In our aggregated data, receiving any vaccine dose of either RV5 or RV1 provided 78% (CI, 72%–82%) protection against severe rotavirus gastroenteritis requiring hospitalization or an ED visit.
A complete 3-dose vaccination with RV5 provided a VE of 80% (CI, 74%–84%), and a complete 2-dose vaccination with RV1 also provided VE of 80% (CI, 68%–88%). RV5 VE for a single dose was 68% (CI, 45%–82%) and 78% (CI, 66%–85%) for a second dose. The single dose VE for RV1 was 96% (CI, 67%–99%) (Table 2). In comparing VE of RV5 and RV1, we did not find any statistical difference in protection for full vaccination (P = 1.00) or for receiving any vaccine dose (P = .292).
Table 2.
Stratified Vaccine Effectiveness and 95% Confidence Intervals for RV5 and RV1, 2012–2013
| Stratum | RV5 | RV1 | ||
|---|---|---|---|---|
|
|
|
|||
| Cases/Controls | VE (95% CI) | Cases/Controls | VE (95% CI) | |
| Dose Number | ||||
| Dose 1 | 223/635 | 68% (45%–82%) | 64/240 | 96% (67%–99%) |
| Dose 2 | 239/832 | 78% (66%–85%) | 99/735 | 80% (68%–88%) |
| Dose 3 | 354/2117 | 80% (74%–84%) | NA | NA |
|
| ||||
| Season | ||||
| 2012 | 67/916 | 76% (58%–86%) | 15/298 | 73% (11%–92%) |
| 2013 | 287/1201 | 80% (73%–85%) | 84/437 | 83% (70%–90%) |
|
| ||||
| Clinical Setting | ||||
| Inpatient | 96/433 | 83% (71%–90%) | 27/148 | 84% (53%–94%) |
| ED | 258/1684 | 77% (69%–83%) | 72/587 | 79% (63%–87%) |
|
| ||||
| Year of Life | ||||
| 1 | 32/398 | 91% (78%–96%) | 20/209 | 82% (52%–93%) |
| 2 | 73/591 | 82% (69%–89%) | 30/305 | 86% (68%–94%) |
| 3 | 78/368 | 88% (78%–93%) | 31/142 | 80% (51%–92%) |
| 4 | 50/241 | 76% (51%–88%) | 14/57 | 58% (−64%–89%) |
| 5 | 44/208 | 60% (16%–81%) | Φ | Φ |
| 6–7 | 77/296 | 69% (43%–84%) | Φ | Φ |
|
| ||||
| Predominant Genotype | ||||
| G1, P[8] | 11/2117 | 89% (55%–97%) | Φ | Φ |
| G2, P[4] | 21/2117 | 87% (65%–95%) | 20/735 | 53% (−26%–82%) |
| G3, P[8] | 58/2117 | 80% (64%–89%) | 24/735 | 88% (70%–95%) |
| G12, P[8] | 249/2117 | 78% (71%–84%) | 50/735 | 82% (66%–91%) |
|
| ||||
| Ethnicity | ||||
| Hispanic | 130/909 | 72% (57%–81%) | 21/175 | 81% (46%–93%) |
| Non-Hispanic | 222/1206 | 81% (74%–87%) | 78/558 | 80% (65%–88%) |
ф = Insufficient RV1 coverage/subjects.
Exact odds ratio (95% CI).
Abbreviations: CI, confidence interval; ED, emergency department; NA, not applicable; VE, vaccine effectiveness.
Stratified Analyses of Vaccine Effectiveness by Clinical Setting, Season, and Predominant Rotavirus Genotype
RV5 and RV1 were similarly effective in preventing hospitalizations due to rotavirus gastroenteritis (83% [CI, 71%–90%] and 84% [CI, 53%–94%], respectively, P = .96) and rotavirus-associated ED visits (77% [CI, 69%–83%] and 79% [CI, 63%–87%], respectively, P = .79) (Table 2).
For RV5 and RV1 vaccines, VE was slightly higher in 2013 (80% [CI, 73%–85%] and 83% [CI, 70%–90%], respectively), compared with 2012 (76% [CI, 58%–86%] and 73% [CI, 11%–92%], respectively), although the differences by year were not statistically significant. Of note, the year 2013 corresponded with the biennial “rotavirus peak” season observed through national surveillance systems to have increased rotavirus incidence [21] and was when 82% of our analyzed rotavirus-positive cases occurred.
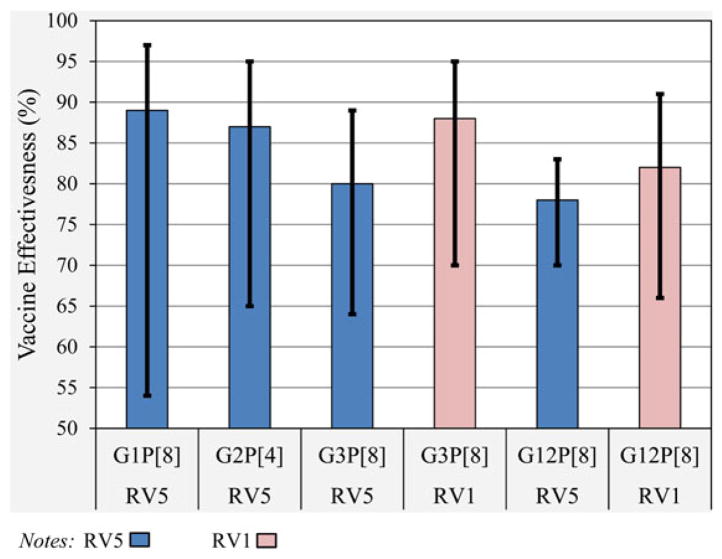
The 4 predominant rotavirus genotypes observed during the study period were G1P[8] (2.5%), G2P[4] (9.5%), G3P[8] (18.9%), and the most commonly observed strain, G12P[8] (69.1%). Genotype-specific RV5 VE estimates ranged from 78% (CI, 71%–84%) for G12P[8] to 89% (CI, 55%–97%) for G1P[8], each with statistically significant and overlapping 95% CIs (Figure 2). Significant RV1 VE estimates for G3P[8] and G12P[8] were 88% (CI, 70%–95%) and 82% (CI, 66%–91%), respectively. Inadequate sample size precluded comparisons of RV1 VE for other strains.
Figure 2.
RV5 and RV1 vaccine effectiveness by predominant rotavirus strain, 2012–13 (hospitalizations and emergency department visits).
Stratified Analyses of Vaccine Effectiveness by Age, Ethnicity and Modified-Vesikari Severity Score
Statistically significant VE was observed to the seventh birthday (ie, through the seventh year of life) for RV5 and to the third birthday (ie, through the third year of life) for RV1 (Table 2). These differences in duration of VE are due to the fact that RV1 was licensed approximately 2 years later in time than RV5, affecting vaccination coverage and corresponding study power for older age groups for RV1 analyses. For RV5, VE was highest during the first (91% [CI, 78%–96%]) and third years of life (88% [CI, 78%–93%]), whereas RV1 VE was highest during the second year of life (86% [CI, 68%–94%]). We compared our current age-specific VE results with published active surveillance studies using a similar protocol, and these comparisons demonstrate relatively consistent VE estimates for the first 3 years of life (Supplementary Figure).
RV5 and RV1 each provided significant protection to children of Hispanic and non-Hispanic ethnicity, and there was no statistically significant difference in vaccine performance by Hispanic ethnicity. The RV5 VE estimate for Hispanic children was 72% (CI, 57%–81%) compared with non-Hispanic children (81%; CI, 74%–87%) (P = .233), and the RV1 VE estimate for Hispanic children was 81% (CI, 46%–93%) compared with non-Hispanic children (80%; CI, 65%–88%) (P = .949).
For children having full clinical data and who received a complete course of either vaccine, VE estimates against rotavirus infections categorized as mild, moderate, and severe were 67% (CI, 48%–79%), 78% (CI, 70%–85%), and 84% (CI, 71%–92%), respectively.
DISCUSSION
Since the US licensure of RV5 and RV1 rotavirus vaccines, the long-term persistence of vaccine-induced immunity and the degree to which these vaccines protect against genotypically heterologous rotavirus strains have been of keen interest to pediatricians, parents, vaccinologists, and health policy makers. Our data confirm that RV5 and RV1 vaccines each provide a lasting, broadly heterologous protection against rotavirus gastro-enteritis amid geographically diverse rotavirus strains. Notably, we found no statistically significant difference in vaccine-specific effectiveness for RV5 and RV1 among children receiving all recommended vaccine doses.
Statistically significant rotavirus VE was observed through the seventh year of life for RV5 and through the third year of life for RV1. Protection was significant against all of the predominant circulating rotavirus strains in the United States, including rotavirus genotype G12 P[8] which has emerged internationally as a commonly circulating strain [22] and whose viral protein (VP)-7 (glycoprotein G12) is not included in either vaccine [23]. Our 2012–2013 results are similar to the 2010–2011 estimates [8] using a similar methodology but having a much more robust sample size, confirming the consistency of these genotypic-specific VE results over time. Using a validated severity score assessment, we found that a full course of rotavirus vaccination was most protective against rotavirus gastroenteritis infections that were classified as severe, as expected, but we also noted broad, significant protection against moderate and even mild illnesses.
Pre-licensure longitudinal studies showed that the severity of rotavirus infections was most acute at the youngest ages, but that subsequent exposures throughout childhood would result in rotavirus episodes of decreasing severity until infections often became largely asymptomatic [24]. In response to this natural progression of immunity, the development of both current rotavirus vaccines was conceptualized to immunologically mimic an early exposure to rotavirus without causing symptomatic infection. Our data demonstrate that strong, long-term rotavirus vaccine protection persists through several post-vaccination years of life and, importantly, does not appear to displace severe rotavirus infections to later in childhood.
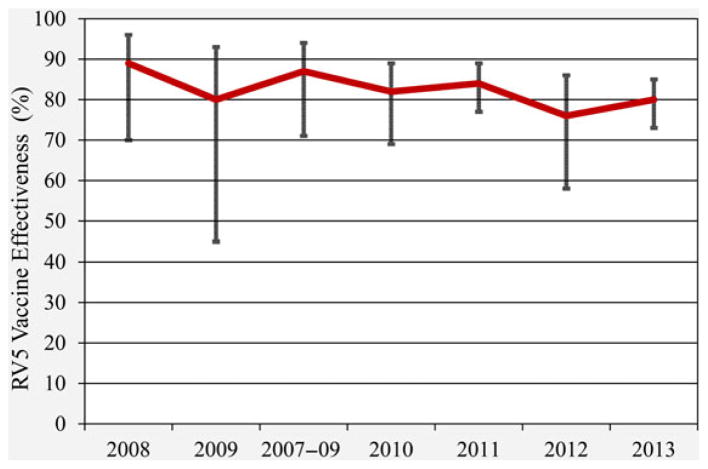
We did not observe a statistically significant difference in VE for full courses of RV5 and RV1 in a direct comparison of data over a 4-year time period. We directly compared our 2012–2013 VE data for complete courses of RV5 and RV1 with those published data from 2010 to 2011 [8] obtained from the identical 7 medical institutions using similar protocol NVSN methodologies. For a full course of RV5 and RV1, VE did not statistically differ over time for either vaccine (P = .261 and P = .513, respectively). No statistical difference in VE was observed between fully vaccinated children in their second year of life from the published 2010–2011 study period and in their third year of life from our current 2012–2013 results, for either RV5 or RV1 (P = .848 and VE = P = .551, respectively). This finding is consistent with that of a prospective follow-up of Finnish Extension Study clinical trial participants showing significant reductions in rotavirus test-positive hospitalizations and ED visits for a period of at least 3.1 years following the last RV5 dose [25]. Comparing our 2012–2013 results from other similarly constructed studies [6–8, 26] which analyzed VE for subjects enrolled from 2007 through 2011 (Figure 3), RV5 trends appear stable over time (VE range: 76%–89%, with mean annual variation = 4.2%). These VE estimates remain similar despite the increasing median ages of subjects in these studies over time. We observed that unvaccinated rotavirus cases were older (median age = 36 months) than those cases who had been vaccinated (median age = 30 months). A similar long-term comparison was not possible for RV1 due to its later US licensure and lower vaccine coverage during these prior years.
Figure 3.
RV5 vaccine effectiveness 2007–2013. Amalgamated results from active surveillance studies using a similar research protocol for evaluating vaccine effectiveness. Notes: (2008) Boom JA, et al Pediatrics 2010. (2009) Boom JA, et al Pediatr Infect Dis J 2010. (2007–09) Staat MA, et al Pediatrics 2011. (2010) Payne DC, et al (1) Clin Infect Dis 2013. (2011) Payne DC, et al (1) Clin Infect Dis 2013. (2012) Payne DC, et al (2) Clin Infect Dis 2015. (2013) Payne DC, et al (2) Clin Infect Dis 2015.
Our study is important in refining the understanding of how currently US-licensed rotavirus vaccines perform in “real world” settings, with broad geographic and demographic diversity, actively obtained enrollment, verified vaccination status, laboratory-confirmed rotavirus case classification, and large sample sizes. In particular, we are able to report a more robust and complete picture of post-licensure RV1 vaccine performance among US children and have found the performance profile of RV5 and RV1 to be similar across many stratified subject and secular characteristics.
Limitations to our study include that unvaccinated controls may be selectively less representative of the source population of cases as the proportion of overall rotavirus vaccine coverage increases. Age differences existed between the subjects included in the RV5 and RV1 analytical datasets. We employed several methods to reduce this potential confounding, including the restriction of eligible subjects to those at least 8 months old, adjustments for year and month of birth in our regression analyses, and stratification of our results by age. Assessing RV1 VE beyond the third year of life was hindered by small sample sizes for the older ages. Our finding that the RV1 dose 1 point estimate is higher than that for dose 2 is likely due to the smaller dose 1 sample size. Although our reported VE estimates fulfill the a priori definition of statistical significance, this smaller sample size affects the precision of the estimate. Nonetheless, our overall study power to detect statistical significance was improved from our previously published 2010–2011 estimates [8], especially for our RV1 analyses which included 67% more test-positive rotavirus cases and over 400% more eligible controls.
In conclusion, our US rotavirus VE estimates for 2012–2013 continue to support the theme that RV5 and RV1 rotavirus vaccines perform consistently well, now several years following licensure. In this large, geographically and demographically diverse sample of US children, we observed that each rotavirus vaccine provided a lasting, and broadly heterologous protection against rotavirus gastroenteritis.
Supplementary Material
Acknowledgments
Financial support. This work was funded by a cooperative agreement by the US CDC (IP11-010).
Footnotes
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Potential conflicts of interest. M. A. S. received past research funding from Merck Research Laboratories, Inc., and current funding from GlaxoSmithKline, Inc., and served on the Rotavirus Advisory Board for Merck and Co. and for GlaxoSmithKline, Inc. D. I. B. received research funding from GlaxoSmithKline; Merck & Co., Inc., and Wyeth Laboratories; Patent on GlaxoSmithKline Rotavirus Vaccine (RV1).
References
- 1.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 2.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastro-enteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX 4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–43. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2009;58:1–26. [PubMed] [Google Scholar]
- 6.Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J. 2010;29:1133–5. doi: 10.1097/INF.0b013e3181ed18ab. [DOI] [PubMed] [Google Scholar]
- 7.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128:e267–75. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 8.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis. 2013;57:13–20. doi: 10.1093/cid/cit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132:e25–33. doi: 10.1542/peds.2012-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–71. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 11.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 suppl):S30–4. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 12.Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29:489–94. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- 13.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 suppl):S56–60. doi: 10.1097/INF.0b013e3181fefdc0. [DOI] [PubMed] [Google Scholar]
- 14.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011;53:245–53. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 15.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122:1235–43. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 16.Payne DC, Szilagyi P, Staat MA, et al. Secular variation in US rotavirus disease rates and serotypes – Implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J. 2009;28:948–53. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 17.Hull JJ, Teel EN, Kerin TK, et al. United States rotavirus strain surveillance from 2005 to 2008. Pediatr Infect Dis J. 2011;30:S42–7. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- 18.Russka T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–67. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 19.Wikswo ME, Payne DC, Parashar UD. Validation of a modified Vesikari Score in US children with gastroenteritis. 11th International Rotavirus Symposium; New Delhi. September 2014. [Google Scholar]
- 20.World Health Organization. Handbook: IMCI Integrated Management of Childhood Illness. 2005. pp. 27–9. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination: 2000–2014. MMWR. 2015;64:337–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman M, Matthijnssens J, Yang X, et al. Evolutionary history and global spread of the emerging G12 human rotaviruses. J Virol. 2007;81:2382–90. doi: 10.1128/JVI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mijatovic-Rustempasic S, Teel EN, Kerin TK, et al. Genetic analysis of G12P[8] rotaviruses detected in the largest US G12 genotype outbreak on record. Infect Genet Evolution. 2014;21:214–9. doi: 10.1016/j.meegid.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velazquez R, Calva JJ, Lourdes Guerrero M, et al. Cohort study of rotavirus serotype patterns in symptomatic and asymptomatic infections in Mexican children. Pediatr Infect Dis J. 1993;12:54–61. doi: 10.1097/00006454-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Vesikari T, Karnoven A, Ferrante SA, et al. Efficacy of the pentavalent rotavirus vaccine, RotaTeq, in Finnish infants up to 3 years of age: The Finnish Extension Study. Eur J Pediatr. 2010;169:1379–86. doi: 10.1007/s00431-010-1242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199–207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.