Abstract
Due to lack of uniform diagnostic criteria, gastrointestinal (GI) complications in critically ill occur with variable frequency,1 and overall incidence of such complications seems to be less in children compared to adults. Major risk factors are use of catecholamines, sedatives, and muscle relaxants in patients with shock. GI dysmotility in critically ill patients is the main reason behind abdominal distension, increased gastric residual volume, and constipation. GI bleeding is described in about 10% of patients with critical illness with about 1.6% have clinically significant bleeding, particularly in patients with coagulopathy, respiratory failure, or PRISM scores >10.2 In this review, the most common GI issues encountered in children will be discussed as mentioned earlier. In addition management of acute GI bleeding will also be discussed.
How to cite this article: Khilnani P, Rawal N, Singha C. Gastrointestinal Issues in Critically Ill Children. Indian J Crit Care Med 2020;24(Suppl 4):S201–S204.
Keywords: Constipation, Critically ill children, Dysmotility, Feeding intolerance, Gastrointestinal bleeding
Most commonly encountered gastrointestinal (GI) issues in critically ill children will be discussed:
Feeding intolerance
Dysmotility
Constipation
Gastrointestinal bleeding
Feeding Intolerance, Dysmotility, and Constipation
Gut plays an important role in the maintenance of health. At baseline, the intestinal epithelium absorbs nutrients and provides mucosal immunity. Critical illness is a well-known cause associated with potential worsening multiple organ dysfunction syndrome (MODS). Apoptosis (programmed cell death) ensues in the epithelium, proliferation decreases, and cellular migration slows down. Significant gut barrier dysfunction occurs due to altered tight junction and intestinal hyperpermeability. Mucus layer gets damaged that normally separates the contents of the intestinal lumen from the epithelium. Gut microbiome becomes a pathobiome with virulent commensal bacteria. Toxins enter the circulation via both portal blood flow and mesenteric lymph channels, leading to distant organ damage.3
Although enteral feeding is the most preferred route of delivery, due to varied physiological changes in the gut during an acute illness, enteral feeding tolerance can be a challenge. Feeding intolerance has been a loosely defined arbitrary term but frequently used as a reason for holding or stopping feeds.4
Due to lack of a precise definition of feeding, intolerance research in this field makes the comparison of studies difficult and meta-analysis impossible.
Dysmotility in the critically ill child causes delayed gastric emptying (GE) or gastroparesis. It has been reported in up to 50% of critically ill children. Potential for aspiration, ventilator-associated pneumonia, inadequate enteral nutrition, and resulting poor efficacy of enteral medications results in poor patient outcomes.5
Gastric dysmotility in a critically ill child may accompany gastroesophageal reflux (GER),6 with high risk for aspiration and ventilator-associated pneumonia (VAP) in mechanically ventilated patients.7 In a prospective study involving a cohort of critically ill children, enteral nutrition (EN) was found to be associated with higher risk of VAP.8 Delayed GE often leads to delayed initiation or interruption of EN resulting in poor intake of nutrients. In a prospective study of critically ill mechanically ventilated children, only one-third got 66.6% of recommend enteral nutrition by seventh day of PICU admission.9 Inadequate nutrition resulting in malnutrition is well documented to be associated with poor outcomes with prolonged need for mechanical ventilation, longer PICU stay, multiorgan dysfunction, and increased mortality.10–12 Bacterial overgrowth in a malnourished child with altered gut integrity and bacterial translocation can lead to sepsis.13,14 Gastric residual volume measurement may at times be inaccurate. Gastric emptying nuclear scans, to evaluate the motility of the gastric antrum, has been frequently used to evaluate gastroparesis but might be difficult to perform in a critically ill child.
To summarize, current therapies for gastric dysmotility are limited, empirical at best, not based on strong evidence, and need to be further investigated. At authors institution, abdominal girth and bowel sounds in combination with gastric residual volumes are commonly used regarding decisions to continue or hold feeds.
Constipation is common in critically ill children and defined as no bowel movement for 3 days. It has been seen more frequently in patients’ receiving midazolam, fentanyl, muscle relaxants, and inotropic support. In one pediatric study, it was found to be common in postsurgical, older, and obese children and ones with fecal continence (p value < 0.01). Patients with constipation had higher severity (PIM2: Pediatric Index of Mortality 2) scores and started nutrition later and with a lower volumes (p value < 0.01). Overall management included enemas and/or oral laxatives.15 Major independent risk factors are body weight, PIM2 clinical severity score, ICU admission after surgery, and the need for vasoconstrictor therapy.
In summary feeding intolerance, dysmotility, and constipation are interrelated problems frequently encountered in PICU due to multiple factors, such as clinical severity of illness and gut barrier dysfunction via alterations to the tight junction, resulting in intestinal hyperpermeability as well as associated therapies such as vasoconstrictor therapy, and overall management mainly remains empirical.
Gastrointestinal Bleeding
GI bleeding is associated with risk factors such as respiratory failure, coagulopathy, or PRISM score >10.16 Pathophysiology is complex and begins with vasoconstriction and ischemia, eventually leading to bleeding that results from stress ulcerations, called stress ulcer-related bleeding (SURB). Upper GI bleeding (UGIB) has different pathophysiological mechanism, for example, in acid reflux–related esophagitis. It has also been documented that acid suppression does not prevent UGIB or SURB. Stress ulcers are caused by decreased mucosal blood flow and reperfusion injury and less related to chronic acid secretion-related peptic ulcers. Exact pathophysiology is not fully understood.17
Gut Prophylaxis
Most critically ill children empirically receive gastric acid suppressants in an attempt to reduce the risk of stress-induced ulcers. However, the quantity and quality of the evidence supporting their use remains scant. Acid suppression therapy in critically ill patients can be questioned, since it is not clear that critical patients produce acid in shock.
Skillman demonstrated as early as 1970 that there was a 72% reduction in acid secretion in hemorrhagic shock. Acid suppression may not be beneficial and have no effect to treat or to prevent stress ulcers. Mucosal ischemia, on other hand, is a well-known cause of UGIB in critically ill children, and it is therefore important to restore mucosal perfusion as early as possible. Although early reversal of shock with fluids, inotropes, and vasopressors may improve gut perfusion, there is not enough evidence to support vasodilators targeted to improve splanchnic circulation, since there is associated risk of hypotension.18 Proton pump inhibitors (PPI) have some evidence in prevention of gastrointestinal bleeding; however, PPI use has also been associated with increased risk of developing pneumonia when compared to sucralfate. Evaluation for coagulopathy is equally important in critically ill children and should be addressed simultaneously.
UGIB in intensive care patients needs an overall good clinical care with a special attention to circulation, oxygenation hemoglobin level, and coagulopathy. Mostly, these measures are enough, and endoscopic and surgical intervention may not be required.18,19
Recently published Surviving Sepsis Pediatric Guidelines 202020 for critically ill children with septic shock or other sepsis-associated organ dysfunction were published. These guidelines do not recommend the routine use of stress ulcer prophylaxis, except for high-risk patients, but few high-risk patients may indeed benefit from stress ulcer prophylaxis, since studies have supported benefit of stress ulcer prophylaxis when baseline rate of clinically important bleeding is approximately 13%. Additionally, feeding should not be withheld solely based on vasoactive inotropic therapy, and feedings are notcontra indicated if escalating doses of inotropes have been stopped or the weaning of inotropic therapy has already begun after achievement of hemodynamic stability. Enteral route for feeding should be preferred preferably through a nasogastric tube, rather than a post-pyloric feeding tube, and if no enteral feedings are established by seventh day, then parenteral nutrition may be considered. Routine measurement of gastric residual volume is not recommended. Routine use of prokinetic agents for the treatment of feeding intolerance is also not recommended. Routine use of special lipid emulsions in parenteral nutrition is discouraged. In addition, no recommendation was given for or against early low-calorie trophic enteral feeds before establishing full feeds vs full enteral feeds early.
Management of Children with Acute Gastrointestinal Bleeding
Massive GI bleeding, though uncommon due to critical illness, it is still important that a proper diagnostic approach be followed for all cases. Main modalities of diagnosis of upper GI bleeding include fiberoptic endoscopy and double-contrast radiography. Selective abdominal arteriography and surgery are rarely required to make a diagnosis, since the introduction of fiberoptic endoscopy.
The main advantage of fiberoptic endoscopy in upper GI bleeding include direct visualization of lesions, which remain undetected by conventional contrast radiography and application of therapeutic modalities, such as sclerotherapy, band ligation, hemoclip placement as well as laser coagulation and intralesional administration of vasoconstrictors such as adrenaline and epinephrine.
In the pre-endoscopic era, 20% to 50% of the bleeding sites remained unidentified, whereas most authors using fiberoptic endoscopes now report identification of more than 80% of the bleeding sites.21,22 Although the diagnostic capabilities of the procedure are recognized, obtaining a more precise diagnosis in previous studies did not seem to influence the requirements for blood transfusion or to decrease the mortality rate of upper GI bleeding.23 These conclusions may be challenged, however, as more experience is gained with the use of sclerotherapy, heater probes, and lasers in the pediatric population. Emergency endoscopy is usually reserved for the patient who continues to bleed at a life-threatening rate and in whom a precise etiologic diagnosis will lead to specific endoscopic or surgical therapy. Depending on the nature of bleeding, a colonoscopy might also be required in addition to other studies such Meckel's scan. In patients where the source of bleeding cannot be identified with the help of either upper GI endoscopy and/or a colonoscopy, video capsule endoscopy (VCE) is a very effective way to visualize the small bowel and identify the source of bleeding. However, VCE has a disadvantage of being only a diagnostic study, as no therapeutic interventions can be made. Once the source of obscure GI bleeding has been identified, further treatment options include a laparotomy or a push enteroscopy. In addition, any patient with a significant bleeding episode resulting in a hematocrit lower than 30 and a normal endoscopy should undergo radiography to rule out a gastric or duodenal duplication, which may not be appreciated on routine endoscopy.
Intravascular infusion of vasoactive agents has been well described in managing GI bleeding. Vasopressin has been the agent of choice. Continuous intravenous infusion is just as effective as intra-arterial.24 Increasing data in children have shown somatostatin to be as effective as vasopressin with fewer complications in cirrhotic patients with variceal hemorrhage.25
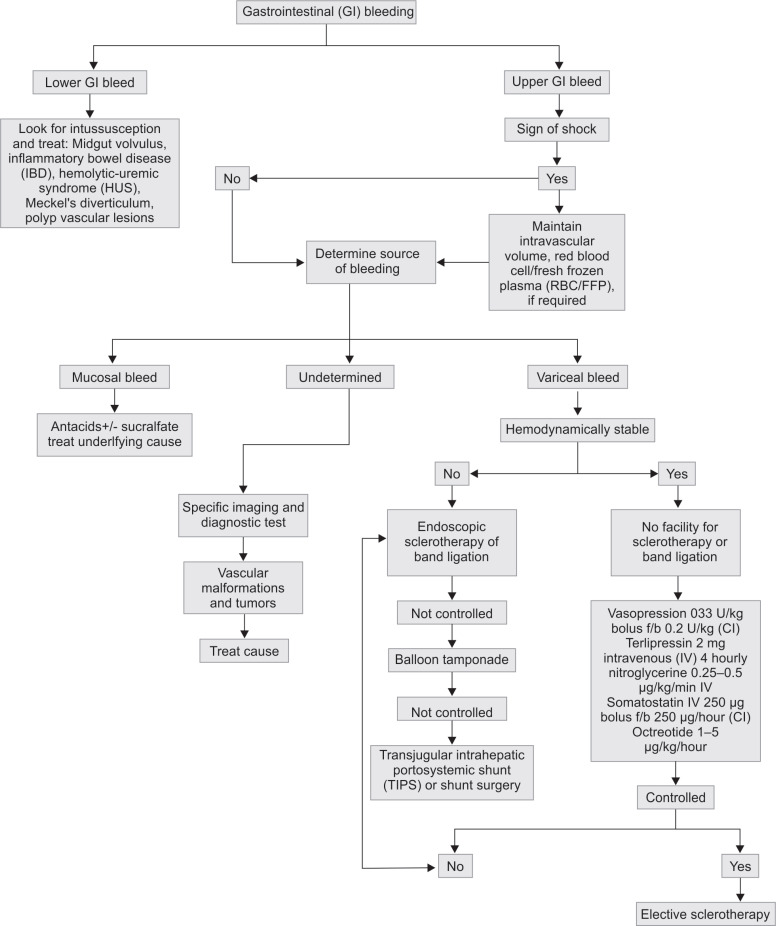
Most children with acute hemorrhage secondary to peptic ulcer disease or a mucosal lesion will stop bleeding with any therapy that raises gastric pH to greater than 5. Rare etiologies such as dieulafoy lesion, where in a large tortuous arteriole bleeds rapidly, should also be kept as possible reason for bleeding. It can be found anywhere in the GI tract but is most commonly found in the stomach. Treatment of bleeding esophageal varices has included vasopressin, as mentioned above, balloon tamponade, and sclerotherapy. Flowchart 1 shows a general flow diagram of management of any patient with GI bleeding, and a detailed description of all modalities of treatment is beyond the scope of this review.
Flowchart 1.
Gastrointestinal (GI) bleeding: a flowchart for diagnosis and management (CI: confidence interval)
Summary
Risk factors for digestive tract complications such as dysmotility, constipation, and feeding intolerance are shock, severity of illness, and the administration of drugs (catecholamines, sedatives, and muscle relaxants).
Overall management mainly remains empirical with some evidence although low to moderate quality.
Despite no evidence, gastric residual volume is still used for clinical bedside decisions about enteral feeding and feeding tolerance; however, recent sepsis guidelines for pediatrics do not recommend a routine measurement of gastric residual volume.
Respiratory failure, coagulopathy, or PRISM score >10 do increase the risk of GI bleeding, emphasizing the need for good critical care to address those issues promptly.
Majority of GI bleeding in the pediatric ICU can be managed medically and surgical intervention is rarely required. Massive GI bleeding though rare can be life-threatening requiring prompt management with fluid resuscitation and close monitoring in the pediatric intensive care unit (PICU), blood transfusion correction of coagulopathy, and efforts directed at source identification and control of bleeding.
Advances in endoscopy and radiology as well as new therapeutic modalities allow more accurate identification and treatment of the source of bleeding.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.López-Herce J. Gastrointestinal complications in critically ill patients: what differs between adults and children? Curr Opin Clin Nutr Metab Care. 2009;12(2):180–185. doi: 10.1097/MCO.0b013e3283218285. DOI: PMID: 19202390. [DOI] [PubMed] [Google Scholar]
- 2.Vergara ACZ, Sotelo MAP, Pérez IST. What's new in preventing pediatric gastrointestinal bleeding in critically ill patients? J Pediatr Care. 2019;5(3):1. doi: 10.36648/2471-805X.5.3.10003. DOI: [DOI] [Google Scholar]
- 3.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019;7:17. doi: 10.1186/s40560-019-0372-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tume LN, Valla FV. A review of feeding intolerance in critically ill children. Eur J Pediatr. 2018;177(11):1675–1683. doi: 10.1007/s00431-018-3229-4. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Martinez EE, Douglas K, Nurko S, Mehta NM. Gastric dysmotility in critically ill children: pathophysiology, diagnosis and management. Pediatr Crit Care Med. 2015;16(9):828–836. doi: 10.1097/PCC.0000000000000493. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waseem S, Islam S, Kahn G, Moshiree B, Talley NJ. Spectrum of gastroparesis in children. J Pediatr Gastroenterol Nutr. 2012;55(2):166–172. doi: 10.1097/MPG.0b013e31824cf06e. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Inglis TJ, Sherratt MJ, Sproat LJ, Gibson JS, Hawkey PM. Gastroduodenal dysfunction and bacterial colonisation of the ventilated lung. Lancet. 1993;341(8850):911–913. doi: 10.1016/0140-6736(93)91208-4. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123(4):1108–1115. doi: 10.1542/peds.2008-1211. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Martinez EE, Bechard LJ, Mehta NM. Nutrition algorithms and bedside nutrient delivery practices in pediatric intensive care units: an international multicenter cohort study. Nutr Clin Pract. 2014;29(3):360–367. doi: 10.1177/0884533614530762. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Hulst JM, van Goudoever JB, Zimmermann LJ, Hop WC, Albers MJ, Tibboel D, et al. The effect of cumulative energy and protein deficiency on anthropometric parameters in a pediatric ICU population. Clin Nutr. 2004;23(6):1381–1389. doi: 10.1016/j.clnu.2004.05.006. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Briassoulis G, Zavras N, Hatzis T. Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition. 2001;17(7–8):548–557. doi: 10.1016/s0899-9007(01)00578-0. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children—an international multicenter cohort study. Crit Care Med. 2012;40(7):2204–2211. doi: 10.1097/CCM.0b013e31824e18a8. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000;95(11):3044–3052. doi: 10.1111/j.1572-0241.2000.03176.x. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42(3):431–435. doi: 10.1136/gut.42.3.431. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López J, Botrán M, García A, González R, Solana MJ, Urbano J, et al. Constipation in the critically ill child: frequency and related factors. J Pediatr. 2015;167(4):857–861.e1. doi: 10.1016/j.jpeds.2015.06.046. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Chaïbou M, Tucci M, Dugas MA, Farrell CA, Proulx F, Lacroix J. Clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit: a prospective study. Pediatrics. 1998;102(4 Pt 1):933–938. doi: 10.1542/peds.102.4.933. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Jensen MM, Marker S, Do HQ, Perner A, Møller MH. Stress ulcer prophylaxis in critically ill children: protocol for a systematic review. Acta Anaesthesiol Scand. 2019;63(7):966–972. doi: 10.1111/aas.13361. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Scarpignato C, Gatta L, Zullo A SIF-AIGO-FIMMG Group, Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. doi: 10.1186/s12916-016-0718-z. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krag M, Perner A, Wetterslev J, Wise MP, Hylander Møller M. Stress ulcer prophylaxis vs placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40(1):11–22. doi: 10.1007/s00134-013-3125-3. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Executive summary: surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(Suppl 1:):1–9. doi: 10.1007/s00134-019-05877-7. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Ament ME, Berquist WE, Vargas J, Perisic V. Fiberoptic upper intestinal endoscopy in infants and children. Pediatr Clin North Am. 1988;35(1):141–155. doi: 10.1016/s0031-3955(16)36404-5. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Hyams JS, Leichtner AM, Schwartz AN. Recent advances in diagnosis and treatment of gastrointestinal hemorrhage in infants and children. J Pediatr. 1985;106(1):1–9. doi: 10.1016/s0022-3476(85)80455-8. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Erickson RA, Glick ME. Why have controlled trials failed to demonstrate a benefit of esophagogastroduodenoscopy in acute upper gastrointestinal bleeding? A probability model analysis. Dig Dis Sci. 1986;31(7):760–768. doi: 10.1007/BF01296455. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Chojkier M, Groszmann RJ, Atterbury CE, Bar-Meir S, Blei AT, Frankel J, et al. A controlled comparison of continuous intra-arterial and intravenous infusions of vasopressin in hemorrhage from esophageal varices. Gastroenterology. 1979;77(3):540–546. doi: 10.1016/0016-5085(79)90020-9. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Kravetz D, Bosch J, Terés J, Bruix J, Rimola A, Rodés J. Comparison of intravenous somatostatin and vasopressin infusions in treatment of acute variceal hemorrhage. Hepatology. 1984;4(3):442–446. doi: 10.1002/hep.1840040315. DOI: [DOI] [PubMed] [Google Scholar]