Abstract
Purpose
In NSABP B-41, pathologic complete response (pCR) was associated with prolonged survival among women with HER2-positive operable breast cancer treated with neoadjuvant chemotherapy and lapatinib, trastuzumab, or the combination. We used a large human breast cancer gene expression panel to select candidate prognostic biomarkers for pCR among women treated with trastuzumab in NSABP B-41.
Patients and Methods
Eligible patients had a baseline pre-adjuvant treatment core biopsy sample, known pCR status, and no withdrawal of consent. We analyzed extracted RNA using the human nCounter® Breast Cancer 360™ gene expression panel. Gene counts were normalized to housekeeping genes and transformed into logarithmic scale with base two. To screen for candidate genes and meta-gene signatures prognostic of pCR, we used univariate logistic regression. Variable selection was done by multivariable logistic regression with lasso regularization.
Results
Analyses of data from 130 patients revealed that a composite of gene expression from 19 genes and one gene signature appeared to predict pCR in women with HER2-positive early- stage breast cancer undergoing neoadjuvant chemotherapy with trastuzumab-containing regimens. The identified genes are involved in important pathways such as epithelial-mesenchymal transition, adhesion and migration, estrogen receptor signaling, DNA damage and repair, apoptosis, and proliferation. The AUC from a 10-fold cross validation on predicting pCR, with these 20 genomic markers in a logistic regression model, was 0.73.
Conclusions
The expression level of ERBB2, ESR1, and few other genomic markers was highly predictive of pCR after trastuzumab-containing regimens. These findings need to be validated and calibrated in future studies.
Keywords: Neoadjuvant, breast cancer, genomic, biomarker, trastuzumab
Introduction
Neoadjuvant treatment in patients with HER2-positive breast cancer has been an excellent model for studying highly effective HER2-targeted therapy. Several studies have shown that tumors that are HER2-enriched (HER2E), as assessed by Prediction Analysis of Microarray 50 (PAM50) intrinsic subtypes, are associated with a higher pathologic complete response (pCR) rate (1–6). One of these studies has correlated this finding with a trend for increased event-free survival (EFS) (2). An analysis of baseline tumor samples from two clinical trials found that combined analysis of HER2E subtype and ERBB2 mRNA into a single assay identified tumors with high responsiveness to HER2-targeted therapy (7).
The Cancer Genome Atlas (TCGA) molecular analyses of primary breast cancer tumors (8) have provided the foundation for numerous attempts to define other genes and gene signatures that are prognostic for pCR. In the Cancer and Leukemia Group B (CALGB) 40601 study, multivariable models revealed an association between pCR and ESR1 and ERBB2 gene expression, p53 (TP53) mutation signature, and immunoglobulin G signature, but no association with intrinsic subtype signature and clinical levels of estrogen receptor (ER) or HER2 (1). In all treatment arms of the NeoALTTO study, high ERBB2/HER2 expression and low ESR1 expression were significantly associated with higher pCR rates; whereas only in the combination arm, high expression of immune gene signatures was positively associated with pCR and high expression of the stroma gene signatures was negatively associated with pCR (4). In a subsequent genomic analysis of samples from CALGB 40601, tumor genetics (mutations, DNA copy number alterations), tumor mRNA subtype (HER-E, luminal), and immune microenvironment (B-cell features) were independent predictors of pCR in patients treated with regimens containing trastuzumab and paclitaxel (9).
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-41 open-label, phase 3 randomized neoadjuvant trial compared trastuzumab, lapatinib and the combination in HER2-positive, operable breast cancer patients treated with concurrent standard neoadjuvant chemotherapy (10). From 2007 to 2011, 529 patients were enrolled. The results showed no statistically significant differences in pCR rate and EFS, but patients whose tumors achieved a pCR had a better outcome than those who did not (10, 11). Similar to other investigators, we previously reported that patients with HER2E tumors achieved a higher pCR rate, especially in the trastuzumab-containing arms of NASBP B-41 (12).
In the present, more detailed evaluation of the NSABP B-41 trastuzumab-containing arms, we have used a large human breast cancer gene expression panel to find other candidate biomarkers prognostic of pCR in women with early-stage HER2-positive, operable breast cancer treated with neoadjuvant chemotherapy combined with single or dual HER2-targeted therapy. The primary objectives of the present analysis were to determine the prognostic utility of these genomic signatures for pCR and their predictive ability for treatment benefit from the addition of lapatinib to trastuzumab in this patient population. A secondary objective was to determine the utility of genes and gene signatures in predicting EFS. This report adheres to REMARK criteria (Supplementary Table S1) (13).
Materials and Methods
Study design and patients
The NSABP B-41 study design and eligibility criteria have been previously reported (10). All patients received neoadjuvant chemotherapy with four cycles of standard doxorubicin and cyclophosphamide (AC) followed by four cycles of weekly paclitaxel. Concurrent with the weekly paclitaxel, patients were randomized to receive either weekly trastuzumab, daily lapatinib, or both lapatinib and trastuzumab until surgery. The study’s primary endpoint was pCR in the breast and lymph (nodes, defined as absence of any invasive component in the resected breast specimen and absence of cancer in all resected lymph nodes after neoadjuvant therapy, (ypT0/Tis ypN0) (14). One secondary endpoint was EFS, defined as time from randomization to the first occurrence of local, regional, or distant recurrence, contralateral breast cancer, second primary cancer, or death from any cause. Also, a comparison was undertaken between pre- and post-treatment biopsy paired samples in patients treated with trastuzumab with or without lapatinib. During the B-41 trial, tumor specimens were collected and processed at local sites. Patients eligible for this correlative science study had to have a baseline core biopsy sample, known pCR status and no withdrawal of consent. A study protocol with objectives and statistical analysis plan for this correlative science project was submitted to the NSABP Foundation for the permission to use these tissue samples prior to further sample process and assay. At entry to the B-41 trial, all participants signed informed consent and allowed their tissue samples to be used in the future for the purpose of the study, including the development of molecular predictors of pCR.
RNA isolation
For each patient, the NSABP Department of Pathology produced serial 10 μM sections from selected formalin-fixed paraffin-embedded (FFPE) tumor samples and sent anonymized, matched unstained slides as well as hematoxylin and eosin (H&E) stained slides to the Genomics and Epigenomics Shared Resource (GESR) at Georgetown University Medical Center (GUMC). At the GUMC Histopathology and Tissue Shared Resource (HTSR), the samples underwent pathological examination to confirm diagnosis and identify malignant tissue. Using the matching H&E slides as templates, tumor-containing areas were macrodissected from the unstained slides and processed for RNA isolation.
After deparaffinization, the macrodissected tissues were processed using the Roche High Pure FFPET RNA Isolation Kit (Roche Molecular Systems, Pleasanton, CA). The concentration of the extracted RNA was estimated by ultraviolet-visible spectrophotometry (NanoDrop 1000 spectrophotometer; Thermo Fisher Scientific, Waltham, MA) to ensure sample purity (optical density 260/280 nm ratio 1.7–2.5). We assessed RNA quality using the Agilent RNA 6000 Nano Kit with the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA) and the degree of RNA integrity with the Agilent 2100 Expert Software, as previously described (12).
Gene expression profiling
From each sample, 100 ng of RNA was hybridized to the human nCounter® Breast Cancer 360™ (BC360) gene expression panel (NanoString Technologies, Seattle, WA), and processed on the nCounter® SPRINT Profiler (NanoString Technologies, Seattle, WA) according to manufacturer protocols. The BC360 panel includes 776 individual genes, including 18 housekeeping genes for normalization, and 40 gene signatures associated with breast cancer signaling pathways and biological processes (15–18). Our analyses of BC360 data focus primarily on the 758 unique, non-housekeeping genes, and the 40 gene signatures. The nCounter® SPRINT Profiler system generates simultaneous, multiplexed digital measurements (i.e., reporter-code-counts) of the relative abundance of mRNA transcripts using sequence-specific probes; a reporter probe tagged with a target-specific, four-color, six-position fluorescent barcode; and a capture probe to immobilize the complex for data collection (19, 20). The reporter-code-count files for each patient sample were forwarded to NanoString Technologies (Seattle, WA) for analysis. The raw count data were log2-transformed and normalized to housekeeping genes. These data were used to calculate the PAM50 subtype calls and BC360 single and meta-gene signature scores for each sample using proprietary algorithms.
Statistical analysis
To determine if the included patient samples were representative, we used the Pearson’s Chi-square test to compare treatment and stratification factors (i.e., age, clinical nodal status, clinical tumor size, estrogen receptor (ER) and progesterone receptor status (PR) between the included and excluded patient populations. The primary analyses focused on patient samples from the trastuzumab-containing regimens because the lapatinib-alone arm had a lower pCR rate than the trastuzumab-containing arms (10). Also, the outcomes from the trastuzumab-containing arms would be more relevant to inform the present clinical management of patients with early stage HER2-positive breast cancer.
In the primary analyses, we initially screened candidate gene signatures and individual genes that were prognostic of pCR among patients treated with trastuzumab-based regimens. The BC360 analysis includes 34 carefully curated meta-gene signatures and select single genes. For each of the BC360 meta-gene signatures or single gene expression other than the PAM50 output of basal-like, HER2-enriched, luminal A, and luminal B subtype scores, we used univariate logistic regression model to obtain the P value and odds ratio (OR) associated with pCR. These signatures were subsequently ranked based on the P values. We then applied the Benjamini-Hochberg procedure to identify candidate signatures for pCR with the false discovery rate (FDR) controlled at 0.1 (21). We used the Holm’s step-down procedure to identify gene signatures statistically significantly prognostic for pCR with a familywise error rate (FWER) controlled at 0.05 (22). Similarly, we obtained a list of candidate genes among the 758 genes on the BC360 panel with FDR controlled at 0.1. We entered the selected genes and gene signatures as predictors in a multivariate logistic regression model and applied the lasso regularization to select a final multivariate model for the prognosis of pCR. To assess the performance of this multivariate model, we used the area under the receiver operating characteristic (ROC) curve from 10-fold cross validation.
We compared the prognostic utility of the following conventional clinical factors: treatment (trastuzumab plus lapatinib plus chemotherapy, trastuzumab plus chemotherapy), ER (positive, negative), clinical nodal status (positive, negative), clinical tumor size (in cm), and tumor grade (well, moderate, poor) to the prognostic utility of known genes and gene signatures: ESR1, ERBB2, and Tumor Inflammation Signature (TIS). We then combined significant clinical factors and genomic markers in a multivariate logistic regression and evaluated whether clinical factors or genomic markers supplement each other in the prognosis of pCR among trastuzumab-treated patients. To compare these models, we used the likelihood ratio test between the nested model and a sub-model, and the Akaike information criterion (AIC) between non-nested models (23). Logistic regression models with treatment, individual gene expression, and their interaction as covariates were used to screen for potential predictive genetic markers for the benefit of the addition of lapatinib to trastuzumab among patients treated with trastuzumab-containing regimens.
In the wheel plots that describe expression profiles of each sample, signature scores were mapped to the empirical distribution of the calibrated breast invasive cohort data in TCGA using quantile normalization. Paired t-test was used to compare scores from pre- and post-treatment.
Results
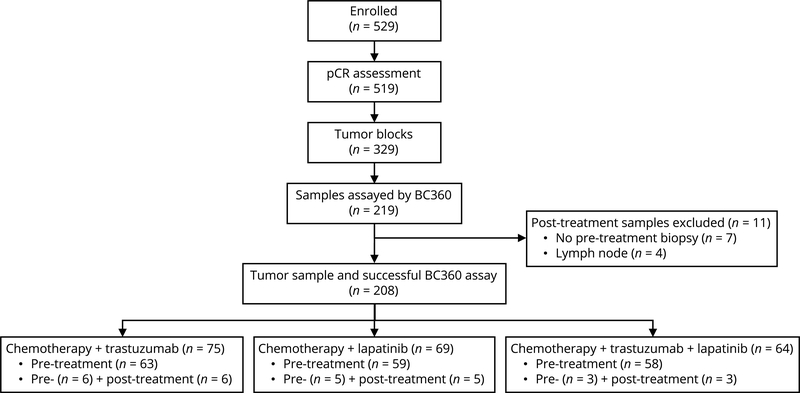
A total of 219 tissue samples from 202 patients enrolled in NSABP B-41 were available, including 194 baseline samples from core biopsy prior to neoadjuvant therapy and 25 samples from surgical specimens after neoadjuvant therapy (21 from breast and four from lymph node material). All analyses were based on data from the 194 patients with core biopsy breast tissue samples prior to neoadjuvant therapy. Their median follow-up time was 5.2 years. As shown in Fig. 1, 69 patients had been randomized to neoadjuvant chemotherapy plus trastuzumab, 64 to chemotherapy plus lapatinib, and 61 to chemotherapy plus trastuzumab and lapatinib. Supplementary Fig. S1 shows the heatmap of mean expression levels of the BC360 single gene and meta-gene signatures across the 194 samples. Comparison of treatment and stratification factors between the patients included and not included in the analyses revealed no statistically significant differences, with P values of the Pearson’s Chi-square test ranging from 0.1 to 0.87 (Supplementary Table S2).
Figure 1.
NSABP B-41 patient sample flowchart
Selection of biomarkers for pCR
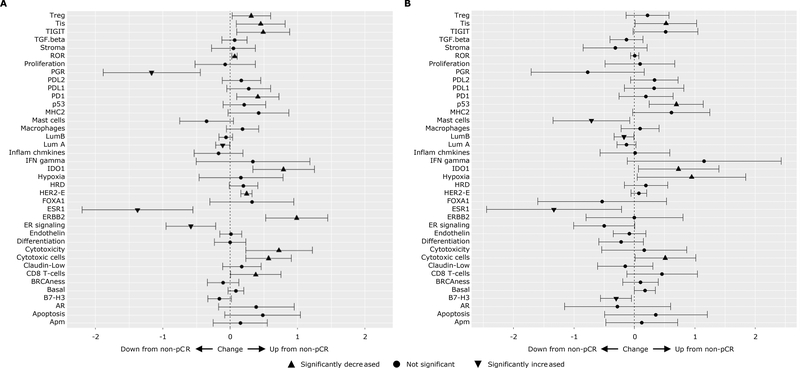
In exploratory analyses of the gene signatures measured by BC360, we compared the mean gene expression differences between patients who did or did not achieve pCR following treatment with trastuzumab-containing (Fig. 2A) or lapatinib-only (Fig. 2B) regimens. Among patients randomized to trastuzumab-containing regimens, TIS, p53, hypoxia, and basal-like meta-gene signatures, and IDO1 single gene were overexpressed in the pCR group; mast cell abundance, luminal B, ER signaling meta-gene signatures, and B7-H3 (CD276), ESR1 single genes were overexpressed in the non-pCR group. When analyzing all 194 patients there were significant differences between patients with or without pCR in the features where the confidence intervals did not include 0. (Supplementary Table S3). For example, on average, the expression level of ESR1 was lower in patients with pCR than that in patients who did not achieve pCR while the opposite was the case for IDO1 and ERBB2 single genes or cytotoxic cell and TIS meta-gene signatures. There was no interaction between pCR status and treatment when comparing trastuzumab-containing arms versus the lapatinib only arm in all genes or signatures except ERBB (Unadjusted p=0.025, Supplementary Table S4).
Figure 2.
Differences in mean gene expression and 95% confidence intervals between patients with and without pCR. (A) patients treated with trastuzumab or trastuzumab + lapatinib. (B) patients treated with lapatinib alone
In the primary analyses, we focused on data from 130 patients on trastuzumab-containing regimens. Applying the Benjamini-Hochberg procedure on results from univariate logistic regression models on the prognosis of pCR, we identified 10 candidate gene signatures out of 34 evaluated with FDR controlled at 0.1 (Table 1). ERBB2 and IDO1 were the top candidates with adjusted P values < 0.05. Among these, cytotoxic cells, cytotoxicity, and TIS signatures as well as IDO1, PD1 (PDCD1), and TIGIT single genes were highly correlated, with coefficients ranging from 0.55 to 0.93 (data not shown). The correlation coefficient between IDO1 and TIS was 0.91. Using the Benjamini-Hochberg procedure, we also identified 38 genes among the 758 individual, non-housekeeping genes on the BC360 panel, with FDR controlled at 0.1 (Supplementary Table S5). Among these 38 genes, ERBB2, IDO1, ESR1 and PGR were also among the selected genes we identified in the primary analyses. In total, we found 44 individual genes or gene signatures whose expression levels were potentially prognostic for pCR.
Table 1.
Selected 10 gene signatures in prediction of pCR from univariate analysis on 34 meta-gene signatures (shown in bold) or single gene expression (shown in italics) from the BC360 panel among patients on trastuzumab-containing regimens (n = 130)
| Signatures | OR (95% CI) | P | Adjusted P |
|---|---|---|---|
| ERBB2 | 1.73 (1.30, 2.31) | 0.00016 | 0.005 |
| IDO1 | 1.58 (1.19, 2.09) | 0.0014 | 0.05 |
| ESR1 | 0.79 (0.68, 0.91) | 0.0018 | 0.06 |
| Cytotoxic cells | 1.83 (1.25, 2.68) | 0.0019 | 0.06 |
| PGR | 0.76 (0.64, 0.91) | 0.0027 | 0.08 |
| ER signaling | 0.6 (0.43, 0.84) | 0.0029 | 0.08 |
| Cytotoxicity | 1.43 (1.11, 1.85) | 0.006 | 0.17 |
| PD1 | 1.67 (1.12, 2.51) | 0.013 | 0.35 |
| TIS | 1.53 (1.08, 2.17) | 0.016 | 0.42 |
| TIGIT | 1.47 (1.07, 2.03) | 0.017 | 0.43 |
With these 44 biomarkers as prognostic factors, we performed multivariate logistic regression models and applied lasso regularization for model selection to narrow the list to 19 individual genes and one gene signature (Table 2). These selected genes are involved in important pathways such as epithelial-mesenchymal transition (HEMK1, GRB7, ERBB2, TMPRSS4), adhesion and migration (ITGB6, COL27A1, NRCAM), ER signaling (ELOVL2, IFT140, MAPT), DNA damage and repair (NPEPPS, PRKDC), apoptosis (BCL2), and proliferation (TFDP1). The AUC from a 10-fold cross validation on predicting pCR, with these 20 genetic markers in a logistic regression model, was 0.73 (Supplementary Fig. S2).
Table 2.
The selected list of 19 genes and one gene signature from the multivariate logistic regression model to predict pCR in patients on trastuzumab-containing regimens after lasso regularization (n = 130)
| Pathways | Selected Genes or Gene Signature |
|---|---|
| Epithelial-mesenchymal transition | HEMK1, GRB7, ERBB2, TMPRSS4 |
| Adhesion and migration | ITGB6, COL27A1, NRCAM |
| JAK-STAT | SOCS2 |
| Hedgehog | LRP2 |
| ER signaling | ELOVL2, IFT140, MAPT |
| DNA damage and repair | NPEPPS, PRKDC |
| MAPK | DUSP6, PRKCB |
| Apoptosis | BCL2 |
| Proliferation | TFDP1 |
| Multiple pathway | MYCN |
| Cytotoxicity pathway | GZMA, GZMB, GZMH, PRF1, GNLY |
Similar univariate analyses were also performed to screen for potential prognostic markers for pCR among patients who were on the lapatinib-only regimen. With FDR controlled at 0.1, none of the gene signatures or genes were selected as candidate prognostic markers. The odds ratios, their 95% confidence intervals and unadjusted p-values for the top five meta-gene signatures and top 10 genes are presented in Supplementary Table S6. Only IFT140, ZNF205 and TCEAL1 appeared in the list of top genes for trastuzumab-containing regimens in Supplementary Table S5.
Clinical and genetic predictors of pCR
Table 3 summarizes the results from four multivariate logistics regression models for predicting pCR. In model 1, which investigated the predictive value of clinical factors (ER status, clinical nodal status, clinical tumor size, tumor grade), only ER status was a statistically significant predictor of pCR (OR = 0.46, 95% CI = 0.22–0.99; P = 0.05). In model 2, which tested the prognostic value of two known genes (ESR1, ERBB2) and a signature (TIS), both ERBB2 (OR = 1.74, 95% CI = 1.27–2.38; P < 0.001) and TIS (OR = 1.75, 95% CI = 1.18–2.59; P =0.006) were strong predictors, and ESR1 (OR = 0.87, 95% CI = 0.73–1.02, P = 0.09) was a marginal predictor. In model 3, which tested the combined predictive value of ER and the three genes in model 2, there was no evidence that ER status (P = 0.24) provided any independent information in predicting pCR beyond the three genetic markers. ESR1 was differentially expressed across ER status with respective mean and standard deviations of −5.0 and 0.88 among patients with ER-negative tumors, and −1.1 and 1.79 among patients with ER-positive tumors. In model 4, HER2 subtype (vs other subtypes) replaced ERBB2 in model 2. Model 4 is a better fitted model than model 2 in predicting pCR because the AIC for model 4 is 146.8, which is smaller than that for model 2, 157.4.
Table 3.
Multivariate logistic regression models for predicting pCR with clinical factors, genetic markers and combination of clinical factors and genetic markers (n = 130)
| Variables | OR (95% CI) | P |
|---|---|---|
| Model 1: Prediction with clinical factors | ||
| Trastuzumab + lapatinib (vs. trastuzumab-alone) | 1.0 (0.47, 2.10) | 0.99 |
| ER (positive vs negative) | 0.46 (0.22, 0.99) | 0.05 |
| Node (positive vs negative) | 0.60 (0.29, 1.27) | 0.18 |
| Clinical tumor size (cm) | 1.09 (0.92, 1.29) | 0.34 |
| Grade (moderate vs well) | 0.83 (0.28, 2.50) | 0.74 |
| Grade (poor vs well) | 0.75 (0.28, 2.03) | 0.57 |
| Model 2: Prediction with 3 genetic markers | ||
| ESR1 | 0.87 (0.73, 1.02) | 0.09 |
| ERBB2 | 1.74 (1.27, 2.38) | <0.001 |
| TIS | 1.75 (1.18, 2.59) | 0.006 |
| Model 3: Prediction with ER and 3 genetic markers | ||
| ER (positive vs. negative) | 2.33 (0.57, 9.5) | 0.24 |
| ESR1 | 0.75 (0.55, 1.01) | 0.06 |
| ERBB2 | 1.71 (1.24, 2.35) | 0.001 |
| TIS | 1.79 (1.20, 2.67) | 0.004 |
| Model 4: Prediction with HER2 subtype and 2 other genetic markers | ||
| HER2 subtype (vs Other subtypes) | 10.99 (3.72, 32.45) | <0.001 |
| ESR1 | 0.87 (0.73, 1.04) | 0.12 |
| TIS | 1.54 (1.04, 2.27) | 0.03 |
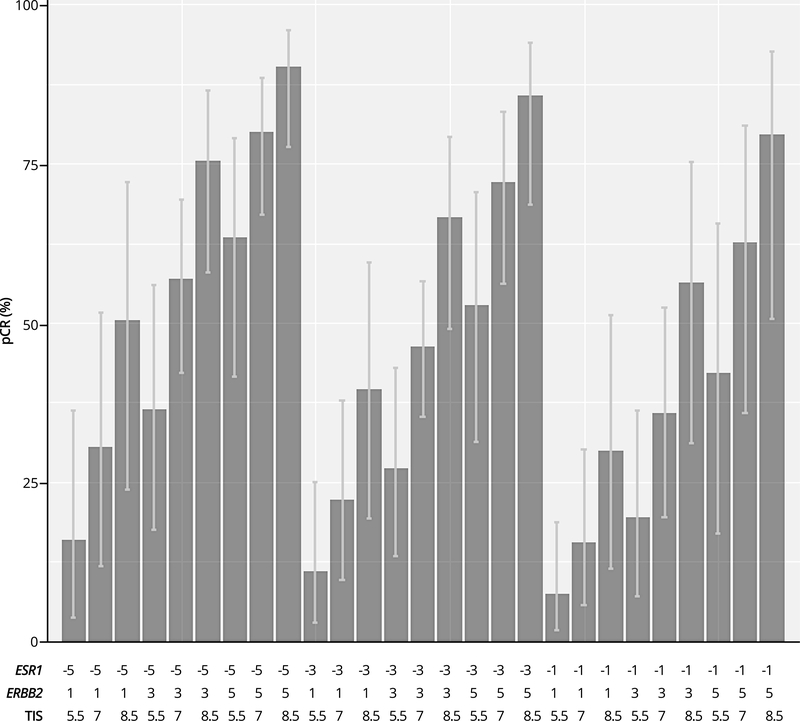
In order to illustrate how the results of model 2 could be used in clinical practice, we selected expression levels of the three genetic markers over their dynamic ranges: ESR1 (from −5, −2, 1), ERBB2 (from 1, 3, 5), and TIS (from 5.5, 7, 8.5). Fig. 3 shows the predicted chance of pCR for patients with various gene expression profiles for HER2-positive breast cancer treated with neoadjuvant chemotherapy plus a trastuzumab-containing regimen.
Figure 3.
Predicted chance of pCR and its confidence interval across various combinations in expression levels of ESR1, ERBB2 and TIS in patients on trastuzumab-containing regimens (n = 130)
Predictive utility of pCR with genetic markers
In the parent NSABP B-41 study, patients randomized to trastuzumab plus lapatinib had a higher pCR rate than those randomized to trastuzumab-alone (60.2% vs. 49.4%, P = 0.056) (10). In the present analysis, we used a logistic regression model for predicting pCR with treatment (trastuzumab plus lapatinib vs. trastuzumab), individual genes or signatures, and their interaction, to screen for potential predictive markers for benefit from the addition of lapatinib among the 758 genes and 34 signatures on the BC360 panel. We were not able to identify any candidate predictive markers with FDR controlled at 0.1 (data not shown). The top three genes on the list were CCL21 (unadjusted P = 0.007), PRLR1 (PRLR) (unadjusted P = 0.015), and PTGDS (unadjusted P = 0.03); the top three BC360 single or meta-gene signatures were p53 meta-gene signature (unadjusted p=0.08), PD1 single gene expression (unadjusted p=0.13) and apoptosis meta-gene signature (unadjusted p=0.15). TIS did not predict treatment benefit in pCR from the additional lapatinib (unadjusted p=0.19).
ERBB2 gene expression was a statistically significant predictor of pCR among the 130 patients treated with trastuzumab-containing regimen. To study the prognostic utility of ERBB2 in patients on the lapatinib-only arm, we used a logistic regression model with Pspline for a flexible characterization of the dose-response relationship. The model showed that ERBB2 was not prognostic of pCR in patients on lapatinib-only regimen (P = 1).
Prognosis of EFS
The total number of EFS events were 33 (9 in trastuzumab-alone, 12 in lapatinib-alone and 12 in the combination arm.) Using data from the 130 patients treated with trastuzumab-containing regimens, we used univariate Cox proportional hazards models to study the prognostic utility of individual genes on EFS. None of the 758 individual genes identified using the BC360 panel were promising when FDR was controlled at 0.1. The volcano plot (Supplementary Fig. S3) shows the strength of the relationships between the candidate genes and EFS, along with their unadjusted P values.
Paired samples
For 14 of 194 patients, we had gene expression data from paired core biopsy and resected tissue samples. In an analysis of paired samples from the nine patients treated with trastuzumab-containing regimens, the expression levels in pre- and post-treatment samples were strongly correlated for 26% of the 758 non-housekeeping genes on the BC360 panel, with Pearson correlation coefficient > 0.8. Paired t-test identified differential expression levels among many genes; the box plots in Supplementary Fig. S4 show the differences for the top 10 differentially expressed genes. The wheel plots of selected gene signatures in pre- and post-treatment paired samples for two representative patients who did not achieve a pCR are shown in Supplementary Fig. S5. The patterns of signatures are similar in pre- and post-pairs, as expected.
Discussion
In the present exploratory analysis of the NSABP B-41 trastuzumab-containing arms, we used a large human breast cancer gene expression panel to define candidate biomarkers prognostic for pCR in women with early-stage HER2-positive operable breast cancer treated with neoadjuvant chemotherapy combined with single or dual HER2 targeted therapy. We found that TIS, p53, hypoxia, and basal-like signatures and IDO1 single gene expression were overexpressed in the pCR group; mast cell abundance, luminal B, ER signaling signatures and B7-H3, ESR1 single gene expression were overexpressed in the non-pCR group. After a variable selection procedure to control the correlation among candidate genomic markers, the combination of 19 genes and one gene signature predicted pCR in women with HER2-positive early-stage breast cancer treated with regimens containing trastuzumab alone or trastuzumab and lapatinib. These markers included pathways such as epithelial-mesenchymal transition (HEMK1, GRB7, ERBB2, TMPRSS4), adhesion and migration (ITGB6, COL27A1, NRCAM), ER signaling (ELOVL2, ESR1, IFT140, MAPT), DNA damage and repair (NPEPPS, PRKDC), apoptosis (BCL2), and proliferation (TFDP1).
Furthermore, our data show that ESR1 expression is a more powerful predictor of pCR than the clinical ER status. ERBB2, whose amplification defines HER2-positivity, is a natural and established quantitative predictor of pCR in patients undergoing targeted therapy for HER2-positive disease (1, 4). TIS is an 18-gene signature for the pathways associated with a suppressed adaptive immune response (24–26). In our analyses, TIS was overexpressed in patients who had a pCR while undergoing treatment with trastuzumab-containing regimens. Based on a multivariate logistic regression model with ESR1, ERBB2, and TIS, we could potentially predict the pCR for patients with HER2-positive breast cancer across a large variety of genomic profiles. The predictive pCR values and their confidence intervals could be used by patients and treating physicians to help decide if trastuzumab-containing systemic therapies would be an appropriate option with the estimated chance for achieving pCR.
Although pCR is not an established surrogate marker for long-term clinical outcomes, it is well known that patients who achieve pCR have a better prognosis than those without pCR (27–29). Using data from 11,955 patients with breast cancer treated with neoadjuvant therapies in 12 international trials, Cortazar, et al., showed that the HR for EFS between patients with pCR and without pCR was 0.49 (95% CI 0.33–0.71) in the hormone-receptor–positive, HER2-negative subgroup and 0.39 (95% CI 0.31–0.50) in the HER2-positive subgroup (30). In the NeoALLTO study, the 3-year EFS was 86% for patients with pCR and 72% for patients without pCR (HR = 0.38, 95% CI 0.22–0.63) (31). Therefore, it is important to identify prognostic markers of pCR for patients with HER2-positive tumors being treated with neoadjuvant trastuzumab-containing regimens. Among the conventional clinical factors, only ER status has been found to be associated with pCR in clinical trials (10, 11, 27, 28, 32, 33). The development of genomic markers for pCR has become an urgent need in the pursuit of personalized medicine in clinical practice.
Unlike the NSABP B-41 study (10), in the NeoALTTO study neoadjuvant therapy consisted solely of paclitaxel and HER2 targeted therapy (trastuzumab, lapatinib, or the combination) and then anthracycline-containing therapy after surgery (34). The addition of anthracyclines prior to surgery in the NSABP B-41 study might explain some of the differences in the findings between the two studies. Our current study support an RNA sequencing analysis of samples from the NeoALTTO study which showed that ESR1 mRNA levels were more predictive of pCR than ER protein levels measured by immunohistochemistry (4). Di Cosimo and colleagues (35) profiled RNA from 226 pretreatment tumor biopsies from the NeoALTTO study to evaluate a trastuzumab risk (TRAR) prediction model based on 41 genes associated with early relapse (36). These authors reported that patients benefiting the most from trastuzumab treatment had tumors with higher levels of CD8 immune cells, higher ERBB2 expression, and lower ESR1 expression (35). Confirming their findings, our analysis of NSABP B-41 samples identified ERBB2, ESR1, and TIS (which includes the CD8 gene) as a powerful panel of signatures that are ready for implementation in management of patients with HER2-positive breast cancer.
In our study, ERBB2 gene expression was highly predictive of pCR for trastuzumab-containing regimens but not for lapatinib-only regimens. This differs from other studies which do show benefit from lapatinib to trastuzumab either with endocrine therapy in the HER2-E group (6, 37) or NeoALTTO where the immune signature was significantly associated with pCR in the combination group (4). We did not find any genes or gene signatures to be predictive of EFS possibly because of the small sample size and limited number of events in our study. Future analyses are planned to combine data sets from several similar neoadjuvant trials to predict both benefit of adding lapatinib to trastuzumab and long-term outcomes.
The immune system has been shown to play a prognostic role in HER-positive breast cancer (38) and to modulate response to targeted HER2 therapies (39). The data presented here add to these studies and also identify two signatures that could reach significance as potential biomarkers in a larger dataset. We found the overexpression of IDO1 to be associated with patients who achieved a pCR and TIS to be a predictor of pCR in patients treated with trastuzumab-containing regimens in NSABP B-41. IDO1 is an immunoregulatory enzyme involved in immunosuppression (40) after an inflammatory response and has been shown to be present in breast cancer (41) in both the HER2 positive tumor and immune cells. However, IDO1 gene expression, induced by IFN, is a general marker of inflammation and good response (17, 25). GeoMx™ Digital Spatial Profiling (42) is a novel technology that can more deeply explore immune interactions using quantitation of protein targets in space. The immune biomarkers distinguished with the gene expression panel along with additional immune biomarkers for responsiveness to targeted therapies in patients with HER2-positive breast cancer could be evaluated more deeply with spatial profiling (42, 43).
The serial monitoring of circulating tumor DNA (ctDNA) is another novel approach to elucidate biomarkers that predict sensitivity or resistance to neoadjuvant treatment. Recently, McDonald and colleagues tested ctDNA targeted digital sequencing (TARDIS) using personalized patient-specific mutations in the neoadjuvant setting to evaluate residual disease (44). This study found that patients with a pCR had lower levels of ctDNA compared with patients who did not have a pCR. Combining these different assay approaches to biomarker discovery could provide innovative tools to personalize therapy for women with HER2-positive breast cancer.
This biomarker study was prospectively designed to investigate the prognostic genomic markers from the NanoString Technologies nCounter® Breast Cancer 360™ Panel using FFPE tumor samples from patients on a randomized clinical trial. Participants of this biomarker study were similar to the others in patient characteristics. Quality RNA and associated annotations were preserved in these tissue samples. The panel of 776 genes under investigation was expertly curated in that the panel covered 23 key breast cancer pathways and processes. Statistical analyses followed standard approaches on adjusting for multiple testing and variable selection. However, our sample size is limited to 194 overall and 130 for analyses on trastuzumab-related questions. Interactions among genomic markers in predicting pCR could not be fully studied. Recently developed statistical methods on identifying individualized treatment rules based on biomarkers have yet to be explored (45). Bulk gene expression was a strong approach to evaluate many different areas of biology with a small sample, however, some heterogeneity of disease may not be fully captured. Other methods of detection of residual disease or predictors of outcome such as ctDNA or digital spatial profiling combined with somatic genomic data will be important to further validate the studies.
The results of this study not only confirmed previous findings on the prognostic utility of ERBB2 and ESR1 gene expression, but also provided an additional list of 17 genes and one signature that jointly predicted pCR in HER2-positive patients treated with trastuzumab-containing regimens. It was illustrated how the prognostic power of these markers could be unleashed using a model based on ERBB2, ESR1 and TIS. Our results need to be further calibrated in other existing and future studies.
Supplementary Material
Statement of Translational Relevance.
The development of biomarkers for pathologic complete response (pCR) has become an urgent need in the pursuit of personalized medicine in clinical practice. The NSABP B-41 clinical trial compared trastuzumab, lapatinib, and the combination given with concurrent chemotherapy until surgery for HER2-positive, operable breast cancer. Using expression data of the NanoString Technologies nCounter® Breast Cancer 360™ panel from available tissue samples, we screened potential prognostic biomarkers for pCR among women treated with trastuzumab in NSABP B-41. We found that the combination of 19 genes and one meta-gene signature predicted pCR in women with HER2-positive early-stage breast cancer treated with regimens containing trastuzumab alone or trastuzumab and lapatinib. Upon further validation, our data would help patients and treating physicians to decide if trastuzumab-containing systemic therapies would be an appropriate treatment option.
Acknowledgements
The authors thank the patients, investigators, and NSABP Foundation staff for their contributions, Phillips Gilmore Oncology Communications (Philadelphia, USA) for providing technical assistance, and T. Blaise Springfield for assistance with figures, tables, and article submission.
Financial Support: This study was partially supported by the Alexandr Savchuk Foundation, Breast Cancer Research Foundation, GlaxoSmithKline, NSABP Foundation, and the Genomics & Epigenomics Shared Resource and Histopathology & Tissue Shared Resource at the Georgetown Lombardi Comprehensive Cancer Center (P30CA051008, PI: Weiner from the National Cancer Institute). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the National Institutes of Health.
Footnotes
Conflict of Interest Disclosure Statement: S.M. Swain declares remuneration from Bristol-Myers Squibb, Caris Life Sciences, Daiichi-Sankyo, Molecular Templates, Eli Lilly & Co., Silverback Therapeutics, Genentech/Roche, NanoString Technologies, Novartis, AstraZeneca for participation on IDMC; and support for third-party writing assistance funded in kind by Roche for other manuscripts; consultant/advisory role with Cardinal Health, Daiichi-Sankyo, Eli Lilly & Co., Genentech/Roche, Genomic Health, Inivata, Peiris Pharmaceuticals, and Tocagen; and funding from the Aleksandr Savchuk Foundation, Breast Cancer Research Foundation, and Genentech. G. Tang declares consultant/ advisory role with Georgetown University. H.A. Brauer declares employment with NanoString Technologies. P.C. Lucas declares consultant/ advisory role with Bayer/Loxo Pharmaceuticals and stock ownership in Amgen (AMGN). A. Robidoux declares consultant/ advisory role with AstraZeneca and Apobiologix and funding from Merck. Y. Ren declares employment with NanoString Technologies. C.E. Geyer declares remuneration from Abbvie, Daiichi-Sankyo, Genentech/Roche and funding from GlaxoSmithKlein. P. Rastogi declares renumeration from AstraZeneca, Genentech/Roche, and Eli Lilly & Co. E.P. Mamounas declares consultant/advisory role with Biotheranostics, Daiichi-Sankyo, Genentech/Roche, Genomic Health, and Merck. N. Wolmark declares funding from the Breast Cancer Research Foundation. All remaining authors declare no potential conflicts of interest.
References
- 1.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 2016;34(6):542–9 doi 10.1200/jco.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prat A, Bianchini G, Thomas M, Belousov A, Cheang MC, Koehler A, et al. Research-based PAM50 subtype predictor identifies higher responses and improved survival outcomes in HER2-positive breast cancer in the NOAH study. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20(2):511–21 doi 10.1158/1078-0432.Ccr-13-0239. [DOI] [PubMed] [Google Scholar]
- 3.Dieci MV, Prat A, Tagliafico E, Pare L, Ficarra G, Bisagni G, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Annals of oncology : official journal of the European Society for Medical Oncology 2016;27(10):1867–73 doi 10.1093/annonc/mdw262. [DOI] [PubMed] [Google Scholar]
- 4.Fumagalli D, Venet D, Ignatiadis M, Azim HA Jr., Maetens M, Rothe F, et al. RNA sequencing to predict response to neoadjuvant anti-HER2 therapy: a secondary analysis of the NeoALTTO randomized clinical trial. JAMA oncology 2017;3(2):227–34 doi 10.1001/jamaoncol.2016.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesurf R, Griffith OL, Griffith M, Hundal J, Trani L, Watson MA, et al. Genomic characterization of HER2-positive breast cancer and response to neoadjuvant trastuzumab and chemotherapy-results from the ACOSOG Z1041 (Alliance) trial. Annals of oncology : official journal of the European Society for Medical Oncology 2017;28(5):1070–7 doi 10.1093/annonc/mdx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llombart-Cussac A, Cortes J, Pare L, Galvan P, Bermejo B, Martinez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. The Lancet Oncology 2017;18(4):545–54 doi 10.1016/s1470-2045(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 7.Prat A, Pascual T, De Angelis C, Gutierrez C, Llombart-Cussac A, Wang T, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. Journal of the National Cancer Institute 2019:pii: djz042. doi: 10.1093/jnci/djz042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70 doi 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanioka M, Fan C, Parker JS, Hoadley KA, Hu Z, Li Y, et al. Integrated analysis of RNA and DNA from the phase III trial CALGB 40601 identifies predictors of response to trastuzumab-based neoadjuvant chemotherapy in HER2-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(21):5292–304 doi 10.1158/1078-0432.Ccr-17-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robidoux A, Tang G, Rastogi P, Geyer CE Jr., Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. The Lancet Oncology 2013;14(12):1183–92 doi 10.1016/s1470-2045(13)70411-x. [DOI] [PubMed] [Google Scholar]
- 11.Robidoux A, Tang G, Rastogi P, Geyer CE, Azar CA, Atkins JN, et al. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: 5-year outcomes of NSABP protocol B-41. J Clin Oncol 2016;34(15 Suppl):Abstr 501 doi 10.1200/JCO.2016.34.15_suppl.501. [DOI] [PubMed] [Google Scholar]
- 12.Swain SM, Tang G, Lucas PC, Robidoux A, Goerlitz D, Harris BT, et al. Pathologic complete response and outcomes by intrinsic subtypes in NSABP B-41, a randomized neoadjuvant trial of chemotherapy with trastuzumab, lapatinib, or the combination. Breast cancer research and treatment 2019;178(2):389–99 doi 10.1007/s10549-019-05398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). Journal of the National Cancer Institute 2005;97(16):1180–4 doi 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 14.FDA. 2014. 12/3/2019. Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. U.S. Food & Drug Administration; <https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathologic-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use-endpoint>. Accessed 2019 12/3/2019. [Google Scholar]
- 15.Pascual T, Gonzalez-Farre B, Teixidó C, Oleaga L, Oses G, Ganau S, et al. Significant clinical activity of olaparib in a somatic BRCA1-mutated triple-negative breast cancer with brain metastasis. JCO Precis Oncol 2019(3):1–6 doi 10.1200/PO.19.00012. [DOI] [PubMed] [Google Scholar]
- 16.Schroth W, Hoppe R, Büttner F, Winter S, Kandabarau S, Kumbrink J, et al. Gene expression signatures for the prediction of endocrine treatment outcome in early-stage luminal breast cancer patients. Cancer Res 2019;79(13 Suppl):Abstr 464 doi 10.1158/1538-7445.AM2019-464. [DOI] [Google Scholar]
- 17.Chumsri S, Asleh K, Brauer HA, Hinerfeld D, Kachergus JM, Lauttia S, et al. Effects of immune architecture on response to adjuvant capecitabine in triple-negative breast cancer (FinXX trial). J Clin Oncol 2019;37(15 Suppl):Abstr 3142 doi 10.1200/JCO.2019.37.15_suppl.3142. [DOI] [Google Scholar]
- 18.NanoString. 2019 11/12/2019. nCounter® Breast Cancer 360™ Panel website. NanoString Technologies, Inc; <https://www.nanostring.com/products/gene-expression-panels/gene-expression-panels-overview/ncounter-breast-cancer-360-panel>. Accessed 2019 11/12/2019. [Google Scholar]
- 19.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature biotechnology 2008;26(3):317–25 doi 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 20.Reis PP, Waldron L, Goswami RS, Xu W, Xuan Y, Perez-Ordonez B, et al. mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC biotechnology 2011;11:46 doi 10.1186/1472-6750-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300. [Google Scholar]
- 22.Hommel G A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;75(2):383–6 doi 10.1093/biomet/75.2.383. [DOI] [Google Scholar]
- 23.Akaike H A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19(6):716–23 doi 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 24.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. The Journal of clinical investigation 2017;127(8):2930–40 doi 10.1172/jci91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danaher P, Warren S, Lu R, Samayoa J, Sullivan A, Pekker I, et al. Pan-cancer adaptive immune resistance as defined by the Tumor Inflammation Signature (TIS): results from The Cancer Genome Atlas (TCGA). Journal for immunotherapy of cancer 2018;6(1):63 doi 10.1186/s40425-018-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019;37(4):318–27 doi 10.1200/jco.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 27.Bear HD, Anderson S, Smith RE, Geyer CE Jr., Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol 2006;24(13):2019–27 doi 10.1200/jco.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 28.Bear HD, Tang G, Rastogi P, Geyer CE Jr., Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. The New England journal of medicine 2012;366(4):310–20 doi 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30(15):1796–804 doi 10.1200/jco.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 30.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 2014;384(9938):164–72 doi 10.1016/s0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 31.de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. The Lancet Oncology 2014;15(10):1137–46 doi 10.1016/s1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 32.Bear HD, Tang G, Rastogi P, Geyer CE Jr., Liu Q, Robidoux A, et al. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial. The Lancet Oncology 2015;16(9):1037–48 doi 10.1016/s1470-2045(15)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdel-Razeq H, Marei L, Saadeh SS, Abdulelah H, Abu-Nasser M, Salam M, et al. From clinical trials to clinical practice: outcome of NSABP-B27 neoadjuvant chemotherapy regimen for high-risk early-stage breast cancer. Breast cancer research and treatment 2017;165(3):771–7 doi 10.1007/s10549-017-4359-5. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England) 2012;379(9816):633–40 doi 10.1016/s0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Cosimo S, Triulzi T, Pizzamiglio S, De Cecco L, de Azambuja E, Fumagalli D, et al. The 41-gene classifier TRAR predicts response of HER2 positive breast cancer patients in the NeoALTTO study. European journal of cancer (Oxford, England : 1990) 2019;118:1–9 doi 10.1016/j.ejca.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Triulzi T, De Cecco L, Sandri M, Prat A, Giussani M, Paolini B, et al. Whole-transcriptome analysis links trastuzumab sensitivity of breast tumors to both HER2 dependence and immune cell infiltration. Oncotarget 2015;6(29):28173–82 doi 10.18632/oncotarget.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prat A, Cheang MCU, Galván P, Nuciforo P, Paré L, Adamo B, et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor–Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA oncology 2016;2(10):1287–94 doi 10.1001/jamaoncol.2016.0922. [DOI] [PubMed] [Google Scholar]
- 38.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. The Lancet Oncology 2014;15(2):e58–68 doi 10.1016/s1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 39.Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. Journal for immunotherapy of cancer 2019;7(1):90 doi 10.1186/s40425-019-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front Immunol 2018;9(151) doi 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol 2018;31(10):1513–22 doi 10.1038/s41379-018-0061-3. [DOI] [PubMed] [Google Scholar]
- 42.Toki MI, Merritt CR, Wong PF, Smithy JW, Kluger HM, Syrigos KN, et al. High-plex predictive marker discovery for melanoma immunotherapy treated patients using Digital Spatial Profiling. Clinical cancer research : an official journal of the American Association for Cancer Research 2019:clincanres.0104.2019 doi 10.1158/1078-0432.CCR-19-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decalf J, Albert ML, Ziai J. New tools for pathology: a user’s review of a highly multiplexed method for in situ analysis of protein and RNA expression in tissue. J Pathol 2019;247(5):650–61 doi 10.1002/path.5223. [DOI] [PubMed] [Google Scholar]
- 44.McDonald BR, Contente-Cuomo T, Sammut SJ, Odenheimer-Bergman A, Ernst B, Perdigones N, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Science translational medicine 2019;11(504):pii: eaax7392 doi 10.1126/scitranslmed.aax7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Zeng D, Rush AJ, Kosorok MR. Estimating Individualized Treatment Rules Using Outcome Weighted Learning. J Am Stat Assoc 2012;107(499):1106–18 doi 10.1080/01621459.2012.695674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.