Regulation of immune function continues to be one of the most well-recognised extra-skeletal actions of vitamin D. In vitro data have shown that vitamin D modulates immune cells and induces immune tolerance, while in vivo data from animal studies and from vitamin D supplementation human studies have shown beneficial effects of vitamin D on immune function, particularly in the context of autoimmunity.1 In the present study, we examined whether vitamin D deficiency modulates the number of immune cells in COVID-19 patients.
This observational, single-centre study included consecutive COVID-19 intensive care unit (ICU) patients (N = 29) and consecutive patients hospitalised in a specialised non-ICU COVID-19 ward (N = 10) who were discharged from the hospital without being transferred to the ICU, from March 18th 2020 to May 25th 2020. The study was approved by the Hospital's Research Ethics Committee (129/19-3-2020), and all procedures carried out on patients were in compliance with the Helsinki Declaration. Informed written consent was obtained from all patients or patients' next-of-kin. Total 25-hydroxyvitamin D was measured on hospital admission using the electrochemiluminescence immunoassay method (Cobas E602, Roche Diagnostics International Ltd). Immune phenotyping was performed by flow cytometric analysis (Navios EX flow cytometer, Beckman Coulter).
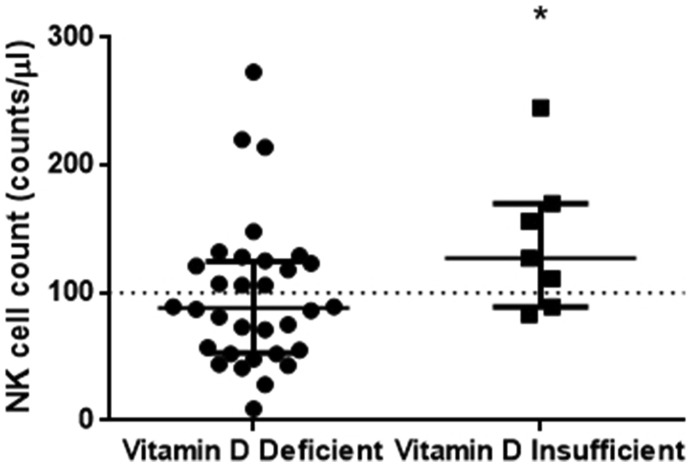
Vitamin D levels positively correlated with subpopulations of immune cells, namely, cytotoxic T cells (rs = 0.344, p = 0.032), natural killer (NK) cells (rs = 0.496, p = 0.001), NK-T cells (rs = 0.325, p = 0.044) and regulatory T cells (rs = 0.333, p = 0.038). With respect to all other clinical and laboratory parameters, vitamin D levels correlated only with albumin (rs = 0.387, p = 0.018). To further explore these associations, we divided our cohort into two groups based on their vitamin D levels; we classified them as vitamin D deficient (≤19.9 ng/ml, N = 32) and vitamin D insufficient (20-29.9 ng/ml, N = 7). Demographics, clinical and biochemical characteristics on hospital admission and important outcomes of the two patient groups are listed in Table 1 . As expected, hypertension was the most common comorbidity.2 The two groups differed only in the number of NK cells (Table 1 and Figure 1 ). Cytotoxic T cells, NK-T cells and regulatory T cells did not differ in the two groups. It should also be noted that the two patient groups did not differ with respect to hospital mortality or disease severity.
Table 1.
Patient characteristics on hospital admission stratified by vitamin D levels
| Parameters | Vitamin D |
p-value | |
|---|---|---|---|
| Deficient ≤19.9 ng/ml | Insufficient 20-29.9 ng/ml | ||
| Number of patients, N (%) | 32 (82) | 7 (18) | |
| Age (years), mean ± SD | 61 ± 14 | 62 ± 8 | 0.34 |
| Sex, N (%) | 0.62 | ||
| Male | 26 (81.2) | 5 (71.4) | |
| Female | 6 (18.8) | 2 (28.6) | |
| Days sick prior to admission, median (IQR) | 7 (5-10) | 6 (4-7) | 0.65 |
| Characteristics on admission | |||
| Vital signs | |||
| Heart rate (bpm), median (IQR) | 90 (82-105) | 84 (80-107) | 0.49 |
| Mean arterial pressure (mmHg), | |||
| median (IQR) | 78 (70-90) | 78 (73-89) | 0.75 |
| Temp (oC), mean ± SD | 37.5 ± 1.1 | 37.9 ± 1.5 | 0.35 |
| Respiratory rate (breaths/min), | |||
| mean ± SD | 24 ± 4 | 22 ± 2 | 0.21 |
| Comorbidities, N (%) | 21 (65.6) | 4 (57.1) | 0.69 |
| Hypertension | 14 | 4 | |
| COPD | 1 | 0 | |
| Hyperlipidaemia | 7 | 2 | |
| Diabetes | 6 | 0 | |
| CAD | 4 | 0 | |
| Asthma | 0 | 1 | |
| ICU vs ward admission, N (%) | 1.00 | ||
| ICU | 24 (75.0) | 5 (71.4) | |
| Ward | 8 (25.0) | 2 (28.6) | |
| ARDS, N (%) | 29 (90.6) | 5 (71.4) | 0.21 |
| PaO2/FiO2 (mmHg), mean ± SD | 206 ± 84 | 251 ± 130 | 0.28 |
| APACHE II score, mean ± SD | 14 ± 5 | 11 ± 7 | 0.28 |
| SOFA score, mean ± SD | 7 ± 3 | 7 ± 3 | 0.51 |
| High-sensitive troponin T | |||
| (ng/ml), median (IQR) | 21 (9-59) | 13 (6-16) | 0.19 |
| CKMB (IU/l), median (IQR) | 27 (17-34) | 29 (21-42) | 0.58 |
| Albumin (g/dl), mean ± SD | 3.4 ± 0.5 | 3.5 ± 0.7 | 0.76 |
| Lactate (mmol/l), mean ± SD | 1.18 ± 0.43 | 0.77 ± 0.25 | 0.12 |
| White blood cell count | |||
| (counts/μl), mean ± SD | 10579 ± 5095 | 9272 ± 4046 | 0.56 |
| Neutrophil cell count | |||
| (counts x 103/μl), median (IQR) | 8.56 (4.35-12.48) | 8.01 (4.54-10.47) | 0.75 |
| Lymphocyte cell count | |||
| (counts x 103/μl), median (IQR) | 1.09 (0.69-1.58) | 1.08 (0.70-1.48) | 0.92 |
| Neutrophil:Lymphocyte ratio, median (IQR) | 6.5 (4.5-12.0) | 7.1 (5.1-8.9) | 0.89 |
| T cells (counts/μl), median (IQR) | 608 (425-878) | 618 (271-847) | 0.64 |
| Helper T cell count | |||
| (counts/μl), median (IQR) | 381 (285-600) | 361 (187-593) | 0.35 |
| Cytotoxic T cell count | |||
| (counts/μl), median (IQR) | 161 (113-229) | 223 (70-240) | 0.80 |
| B cell count | |||
| (counts/μl), median (IQR) | 147 (97-220) | 105 (59-219) | 0.67 |
| NK cell count | |||
| (counts/μl), median (IQR) | 88 (53-125) | 127 (89-170) | 0.048∗ |
| NK-T cell count (counts/μl), | |||
| median (IQR) | 28 (16-49) | 38 (24-51) | 0.48 |
| Regulatory T cell count (counts/μl), | |||
| median (IQR) | 21 (12-31) | 24 (14-27) | 0.85 |
| CD4:CD8 ratio, mean ± SD | 3.16 ± 1.9 | 2.11 ± 0.44 | 0.16 |
| Outcomes | |||
| Hospital mortality, N (%) | 8 (25.0) | 1 (14.3) | 1.00 |
| Hospital stay (days), median (IQR) | 17 (12-30) | 10 (7-40) | 0.89 |
| Mechanical ventilation, N (%) | 21 (65.6) | 3 (42.9) | 0.40 |
∗p<0.05. Patients were categorised according to their vitamin D levels on admission; the two patient groups were vitamin D deficient (≤19.9 ng/ml) and insufficient (20-29.9 ng/ml). Data are expressed either as number of patients (N) and percentages of totals (%), mean ± SD or median (IQR), as appropriate. Two-group comparisons were performed using Student's t-test or non-parametric Mann-Whitney test for skewed data. Associations between qualitative variables were examined by Fisher's exact test. All characteristics were estimated within the first 48 hours post hospital admission. APACHE = Acute physiology and chronic health evaluation; ARDS = Acute respiratory distress syndrome; CAD= Coronary artery disease; CKMB= Creatine kinase myocardial band; COPD= Chronic obstructive pulmonary disease; ICU= Intensive care unit; NK= Natural killer; SOFA= Sequential organ failure assessment.
Figure 1.
Vitamin D deficiency and NK cell count. Vitamin D levels were measured in 39 COVID-19 patients on hospital admission (within 48 h). We subsequently divided our cohort into two groups based on their vitamin D levels; vitamin D deficient (≤19.9 ng/ml, N = 32) and vitamin D insufficient (20-29.9 ng/ml, N = 7). Non-parametric Mann-Whitney revealed a statistically significant relationship between the count of NK cells in vitamin D deficient and insufficient patients. Data are represented as scatter plots. Line in the middle, median value; lower and upper lines, 25th to 75th centiles; horizontal line, threshold for NK lymphopenia. ∗p <0.05. NK= Natural killer.
The beneficial effects of vitamin D on protective immunity are due in part to its effects on the innate immune system. In vitro studies have reported contradictory results on the role of vitamin D on NK cell function, but whether vitamin D induces or inhibits NK cell function in vivo remains unclear.3 NK cells are a type of cytotoxic lymphocytes that are critical to the innate immune system and secrete many cytokines and chemokines. Despite their vital role in viral infections, the contribution of NK cells in the immune defence against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has not yet been directly investigated. The reduced circulating NK cells and exhaustion in the peripheral blood of COVID-19 patients has been suggested to be directly responsible for the progression and severity of COVID-19.4, 5, 6 Another study demonstrated that in SARS-CoV-2 infection, NK cell number was significantly reduced; however, the cells were strongly activated, and this activation correlated with the development of severe disease.7
The immunomodulatory effects of vitamin D have prompted researchers to consider the preventive effect of vitamin D supplementation on SARS-CoV2 viral infection8 , 9. It has been suggested that vitamin D supplementation might boost the immune system of COVID-19 patients and reduce disease severity in vitamin D-deficient patients.10 Indeed, bolus vitamin D3 supplementation during or just prior to COVID-19 infection was associated in frail elderly with less severe COVID-19 and better survival rate.11
Vitamin D deficiency is a widespread situation and can cause abnormalities ranging from cardiovascular-related conditions to disturbances of the endothelial function and the immune system.12 In our pilot, single-centre, limited sample size study, we were able to demonstrate that vitamin D deficiency was associated with reduced numbers of NK cells; specifically, vitamin D deficient patients presented with mild NK lymphopenia (<100 cells/μl), while vitamin D insufficient patients had normal NK cell counts (≥100 cells/μl). This reported lymphopenia may obstruct the important cellular barrier during early viral infections in patients with vitamin D deficiency. A larger cohort of COVID-19 patients is required to study the correlation between vitamin D deficiency and NK lymphopenia and activation.
Declarations of interest
None.
Footnotes
Peer review under responsibility of Hellenic Society of Cardiology.
References
- 1.Bishop E., Ismailova A., Dimeloe S.K., Hewison M., White J.H. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR plus. 2020 Aug 22 doi: 10.1002/jbm4.10405. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachakis P.K., Tentolouris A., Tousoulis D., Tentolouris N. Current data on the cardiovascular effects of COVID-19. Hellenic J Cardiol HJC : HJC = Hellenike kardiologike epitheorese. 2020;61(1):46–48. doi: 10.1016/j.hjc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G.Y., Park C.Y., Cha K.S., Lee S.E., Pae M., Han S.N. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J Nutr Biochem. 2018;55:178–184. doi: 10.1016/j.jnutbio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggi E., Canonica G.W., Moretta L. COVID-19: Unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol. 2020;146(1):18–22. doi: 10.1016/j.jaci.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Market M., Angka L., Martel A.B., et al. Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maucourant C., Filipovic I., Ponzetta A., et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol. 2020;5(50) doi: 10.1126/sciimmunol.abd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaguarnera L. Vitamin D3 as Potential Treatment Adjuncts for COVID-19. Nutrients. 2020;12(11) doi: 10.3390/nu12113512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honardoost M., Ghavideldarestani M., Khamseh M.E. Role of vitamin D in pathogenesis and severity of COVID-19 infection. Arch Physiol Biochem. 2020:1–7. doi: 10.1080/13813455.2020.1792505. [DOI] [PubMed] [Google Scholar]
- 11.Annweiler C., Hanotte B., Grandin de l'Eprevier C., Sabatier J.M., Lafaie L., Célarier T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tousoulis D. Vitamin D deficiency and cardiovascular disease: Fact or fiction? Hellenic J Cardiol HJC : HJC = Hellenike kardiologike epitheorese. 2018;59(2):69–71. doi: 10.1016/j.hjc.2018.06.014. [DOI] [PubMed] [Google Scholar]