Abstract
Objectives:
To investigate the clinical efficacy and tolerability of the combination of bevacizumab (B) and erlotinib (E) compared to sorafenib (S) as first-line treatment for patients with advanced hepatocellular carcinoma (HCC).
Methods:
A total of 90 patients with advanced HCC, Child-Pugh class A– B7 cirrhosis, and no prior systemic therapy were randomly assigned (1: 1) to receive either 10 mg/kg B intravenously every 14 days and 150 mg E orally daily (n = 47) (B+E) or 400 mg S orally twice daily (n = 43). The primary endpoint was overall survival (OS). Secondary endpoints included event-free survival (EFS), objective response rate based on Response Evaluation Criteria in Solid Tumors (RECIST 1.1), time to progression, and safety and tolerability.
Results:
The median OS was 8.55 months (95% CI: 7.00–13.9) for patients treated with B+E and 8.55 months (95% CI: 5.69–12.2) for patients receiving S. The hazard ratio (HR) for OS was 0.92 (95% CI: 0.57–1.47). The median EFS was 4.37 months (95% CI: 2.99–7.36) for patients receiving B+E and 2.76 months (95% CI: 1.84–4.80) for patients receiving S. The HR for EFS was 0.67 (95% CI: 0.42–1.07; p = 0.09), favoring B+E over S. When OS was assessed among patients who were Child-Pugh class A, the median OS was 11.4 months (95% CI: 7.5–15.7) for patients treated with B+E (n = 39) and 10.26 months (95% CI: 5.9–13.0) for patients treated with S (n = 38) (HR = 0.88; 95% CI: 0.53–1.46).
Conclusions:
There was no difference in efficacy between the B+E and S arms, although the safety and tolerability profile tended to favor B+E over S based on competing risk analysis.
Clinical trial No. NCT00881751.
Keywords: Hepatocellular carcinoma, Hepatoma, Clinical trial, Sorafenib, Bevacizumab, Erlotinib, Vascular endothelial growth factor, Epidermal growth factor, Targeted therapies
Introduction
Hepatocellular carcinoma (HCC) is a common tumor worldwide, and among the few malignancies for which both the incidence and the death rate continue to rise [1–4]. The multitargeted tyrosine kinase inhibitor (TKI) sorafenib (S) was the first systemic therapy to prolong the survival of advanced HCC patients [5] and is approved in the first-line setting, while regorafenib, a similar oral TKI, is approved for second-line treatment of advanced HCC [6]. Since chronic hepatic inflammation, cirrhosis, liver regeneration, and vascular invasion are common in HCC [7], substantial clinical research has focused on targeting oncogenic signaling pathways related to inflammatory cytokine expression, growth factor upregulation, and angiogenesis [8–14].
Increased growth factor expression, including hepatocyte, epidermal (EGF), vascular endothelial (VEGF), insulin-like, platelet-derived, and transforming growth factors [15], have been implicated in hepatocarcinogenesis [15–19]. HCC is a highly vascular tumor that commonly invades adjacent blood vessels [20]. Overexpression of VEGF has been observed in HCC cell lines and tumors, as well as in the serum of patients with HCC [21–23]. Elevated expression of VEGF in the serum and tumors of patients with HCC has been linked with HCC tumor grade, vascular invasion, disease recurrence, and poor disease-free and overall survival (OS) [9, 20, 24, 25]. The EGF receptor (EGFR) signaling pathway is commonly activated in liver disease and HCC [26–31] and EGFR plays a key role in hepatic regeneration triggered by acute liver injury [32, 33]. EGFR overexpression has been identified in 40–70% of HCCs and has been linked to tumorigenesis, but its precise role in malignant progression is poorly understood [30, 32, 34, 35]. However, EGFR activating mutations in exons 18–21 are rare in HCC [28, 36].
S is a multitargeted TKI with activity against Raf kinases via the Raf/MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) pathway, VEGF, platelet-derived growth factor, and c-Kit [37, 38]. The anticancer activity of S results from a dual inhibitory effect on angiogenesis and tumor cell proliferation [38, 39]. S is currently the only first-line systemic therapy approved for the treatment of patients with advanced HCC based on results from the pivotal SHARP trial [5]; thus, additional effective and tolerable treatment options are needed for these patients.
Bevacizumab (B) is a monoclonal antibody that binds the circulating ligand of the transmembrane VEGF receptor [40–42]. Erlotinib (E) is a TKI that inhibits EGFR signal transduction [43]. There is a strong scientific rationale for evaluating the combination of B+E in advanced HCC, because the two agents target different pathways that are both important in hepatocarcinogenesis [11, 44, 45]. Preclinical studies in xenograft models of HCC and other tumor types have demonstrated that the combination of B+E results in greater efficacy than either agent alone [46–49]. Published data from several single-arm clinical trials (Table 1) suggest a clinical benefit from B+E in HCC, which provided the justification for this randomized phase II study comparing B+E to S.
Table 1.
Phase II trials of bevacizumab plus erlotinib in advanced HCC
| Study | Patient population | Sample size, n | Outcome |
||
|---|---|---|---|---|---|
| mOS, months | mPFS, months | RR, % | |||
| First line for advanced HCC | |||||
| Govindarajan et al. | Child-Pugh A | 21 | 8.3 | ||
| Philip et al. | Child-Pugh A 74% | 27 | 9.5 | ||
| Child-Pugh B 26% | |||||
| Hsu et al. | Child-Pugh A | 51 | 10.7 | 2.9 | |
| Second line for advanced HCC | |||||
| Thomas et al. | Child-Pugh A 87% | 40 | 15.6 | 9.0 | 25 |
| Child-Pugh B 13% | |||||
| Yau et al. | Child-Pugh A | 10 | 4.37 | ||
| Kaseb et al. | Child-Pugh A 86% | 59 | 13.7 | 7.2 | 24 |
| Child-Pugh B 14% | |||||
| Kaseb et al. | Child-Pugh A 98% | 44 | 9.9 | ||
| Child-Pugh B 2% | |||||
HCC, hepatocellular carcinoma; mOS, median overall survival; mPFS, median progression-free survival; RR, response rate.
Subjects and Methods
Study Population
Eligible patients had advanced HCC defined as: not amenable to transplantation, resection, or liver-directed therapy, or progressed after prior surgery or liver-directed therapy, with Child-Pugh class A–B7 liver function [50, 51], no prior systemic therapy, a Cancer Liver Italian Program (CLIP) [52–54] score ≤5, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2, platelets ≥75,000/mm3, total bilirubin ≤2.0× ULN, and transaminases ≤5× ULN. Patients with fibrolamellar HCC and prior liver transplantation were excluded. Prior surgery, local ablation, transarterial hepatic artery embolization, and trans-arterial chemoembolization or radioembolization were allowed; any prior therapy had to have been completed ≥28 days prior to study entry.
Eligibility criteria also included no uncontrolled or significant cardiovascular disease, including: a history of stroke or transient ischemic attack within 6 months; a history of arterial thrombotic events of any type within the previous 6 months; and significant or symptomatic vascular disease (e.g., aortic aneurysm, aortic dissection, or peripheral vascular disease) within 6 months. As determined by the treating investigator, patients must have had well-controlled blood pressure, defined as systolic blood pressure < 150 mm Hg and/or diastolic blood pressure < 100 mm Hg, for the majority of measurements.
Patients with a history of Common Terminology Criteria for Adverse Events (CTCAE) grade 3 bleeding esophageal or gastric varices within the previous 2 months were excluded unless they had undergone banding or sclerotherapy and there had been no evidence of bleeding for 2 months. All patients at risk for varices were screened (using either esophagogastroduodenoscopy or capsule endoscopy) unless screening had been performed within the prior 2 years and the patients were receiving medical prophylaxis for variceal bleeding. If varices were identified at screening that required intervention (banding), patients were not eligible until the varices were adequately treated [55]. Patients with gastric varices were not eligible.
Patients with a history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within 6 months prior to registration were ineligible. Patients were also ineligible if they had a serious, non-healing wound, active ulcer, or untreated bone fracture; had a history of allergy to B, E, S, or related compounds; or had undergone a major surgical procedure or open biopsy, or had had a significant traumatic injury within 28 days prior to registration, or anticipated a need for a major surgical procedure during the course of the study.
Trial Design and Treatment
This was an investigator-initiated, industry-sponsored, open-label, randomized phase II first-line systemic therapy trial conducted at six sites throughout the USA. The primary objective of this study was to estimate clinical efficacy outcomes of patients treated with B+E and patients treated with S. OS was the primary objective; however, the trial was not designed to perform a hypothesis test for OS comparing the two groups, due to insufficient power. The goal was to estimate the degree of difference between the two arms to inform the design of a potential phase III trial. Most patients with HCC have underlying liver disease, which can complicate treatment of their cancer. In order to assess whether patients withdrew from the study due to drug-related toxicity and/or other clinical events related to liver disease and not necessarily to tumor progression, it was decided to incorporate a competing risk approach into the data analysis.
Based on the results seen in previous single-arm trials of B+E (Table 1), it was expected and of interest that there was a difference in OS between the B+E and the S arm, favoring the B+E arm with a hazard ratio (HR) of approximately 0.67 based on median OS times in the B+E and S arms of 15 and 10 months, respectively, in these trials. Forty-five patients in each arm were deemed sufficient to achieve precision in the estimation of median OS in each arm and for the estimated HR. If the true HR was 0.67, then the expected width of the 95% confidence interval (CI) would be 1.57, and 38% of the 95% CIs would exclude 1. Given that this was not a randomized phase III trial, we would not require a sample size that allowed 80% or more 95% CIs to exclude 1 if the true HR were 0.67. The primary objective was to estimate the HR for OS with B+E versus S with its 95% CI for a sample size of 90 evaluable patients. Secondary endpoints included event-free survival (EFS), safety and toxicity, and response rate (RR). All randomized patients who received at least one dose of the study drug(s) were considered evaluable.
The patients were randomized 1: 1 to receive 400 mg S orally twice daily, continuously, or 10 mg/kg B IV every 14 days and 150 mg E orally daily, continuously. Clinic visits for patients in both study arms were conducted weekly during the first cycle, and biweekly thereafter. The treatment cycles lasted 28 days. Treatment crossover was not allowed.
Outcomes and Assessments
The patients in each investigational arm underwent restaging evaluations every 8 weeks (2 cycles). All abdominal imaging was performed using a four-phase “liver protocol” image capture technique defined as using multislice spiral CT to obtain images during the precontrast, hepatic arterial, portal-venous, and delayed phases of intravenous contrast enhancement. The patients continued therapy until documentation of progressive disease due to RECIST version 1.1 [56] intolerable toxicity, withdrawal of patient consent, or other events. Progressive disease necessitating patient withdrawal was determined by the investigator and confirmed by the diagnostic imaging collaborator at each site as well as by central radiologic review. The patients were followed up for survival every 3 months for 1 year following treatment discontinuation. In the subsequent years, those patients who were enrolled or reconsented to be followed up for survival were contacted every 6 months.
OS was defined as the number of months from the date of randomization to the date of the patient’s death from any cause. Secondary endpoints included EFS, time to progression, RR, and toxicity and tolerability. EFS was defined as the time from randomization to any of the following four types of event: (1) progression, (2) withdrawal due to excessive toxicity, (3) another clinical event requiring withdrawal from the study, or (4) death from another cause (i.e., not progression of HCC). EFS was analyzed using the same approaches as described above for OS. Time to progression is defined as the time from initiation of therapy until documented disease progression, with deaths from other causes censored at the time of death.
Statistical Analysis
Kaplan-Meier curves were used to display OS and EFS distributions in the two treatment groups, and to examine the impact of several factors important to HCC, including ECOG PS, Barcelona Clinic Liver Cancer (BCLC) stage [57–59], and Child-Pugh class. Survival curves were compared with log-rank tests. The precision of median OS was calculated using Greenwood’s formula. HRs and their 95% CIs for OS were estimated using the Cox proportional hazards model. The proportional hazards assumption was tested using graphical approaches, and it appeared to be met.
Comparisons of continuous variables across treatment groups were made with two-sample t tests; comparisons of categorical variables were made with Fisher’s exact test. RRs and toxicity rates were estimated with exact 95% CIs. A competing risks approach was used to analyze time to progression, where deaths from non-HCC causes and discontinuation due to adverse events (AEs) were considered competing risks. Specifically, patients were (a) censored if they neither had died nor had disease progression by the end of the study; (b) treated as having progression if they had disease progression prior to death or the end of the study, or if death was due to disease; (c) treated as having died from other cause if they had died from a cause unrelated to the disease and prior to another event; and (d) treated as having discontinued treatment due to an AE if they were removed from the study due to a study-related AE. Cumulative incidence [60] was calculated for each class of event and graphically displayed. Cumulative risk regression [61] was used to calculate HRs comparing risk rates and their 95% CIs. Wald tests were used for testing the significance of the HRs.
Results
Patients
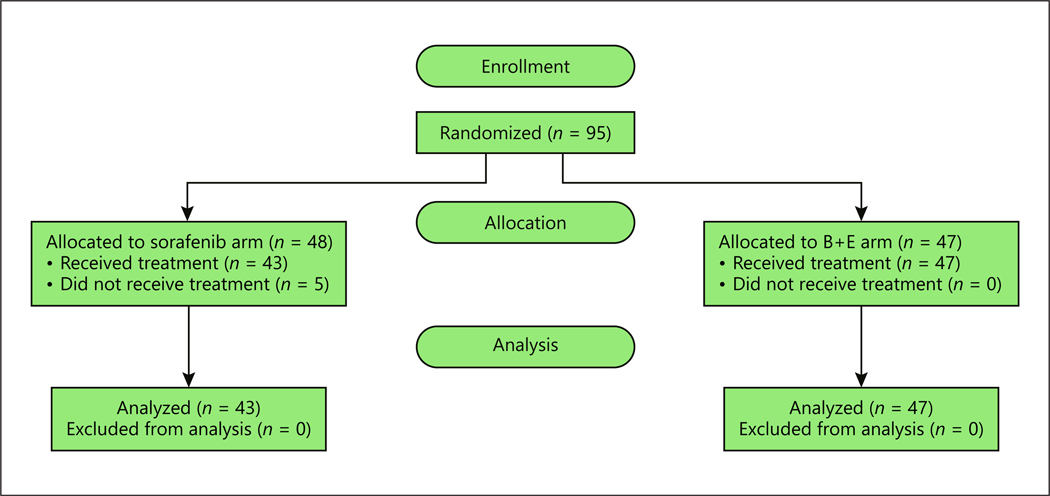
A total of 95 patients were registered and randomized, 5 patients withdrew, and 90 patients received at least 1 dose of the study drug and were evaluable (Fig. 1). The patient characteristics are summarized in Table 2.
Fig. 1.
Enrollment summary.
Table 2.
Distribution of clinical and demographic characteristics of the patients (N = 90)
| Characteristic | S (n = 43) | B+E (n = 47) | p value1 |
|---|---|---|---|
| Age, years | 0.72 | ||
| Median | 61 | 61 | |
| Range | 44–81 | 43–82 | |
| ECOG performance status | 0.44 | ||
| 0 | 17 (40) | 15 (32) | |
| 1 | 25 (58) | 32 (68) | |
| 2 | 1 (2) | 0 | |
| Child-Pugh class [8, 9] | 0.56 | ||
| A | 38 (88) | 39 (83) | |
| B7 | 5 (12) | 8 (17) | |
| CLIP score [10, 11] | 0.81 | ||
| 0 | 4 (9) | 7 (15) | |
| 1 | 10 (23) | 10 (21) | |
| 2 | 17 (40) | 16 (34) | |
| 3 | 9 (21) | 8 (17) | |
| 4–5 | 3 (7) | 6 (13) | |
| Race | 0.27 | ||
| White | 31 (72) | 28 (60) | |
| Other | 12 (28) | 19 (40) | |
| BCLC stage [12, 13] | 0.40 | ||
| A | 4 (9) | 1 (2) | |
| B | 11 (26) | 14 (30) | |
| C | 28 (65) | 32 (68) | |
| Prior treatment | |||
| Resection | 5 (11) | 7 (15) | 0.76 |
| Ablation | 5 (11) | 1 (2) | 0.10 |
| Transarterial intrahepatic therapy | 15 (35) | 13 (27) | 0.50 |
| Tumor characteristics | |||
| Extrahepatic spread | 11 (25) | 19 (40) | 0.18 |
| Gross vascular invasion | 11 (25) | 8 (17) | 0.44 |
Values are presented as n (%) unless specified otherwise. S, sorafenib; B+E, bevacizumab plus erlotinib.
p values based on two-sample t test for continuous variables; Fisher’s exact test for categorical variables.
Efficacy
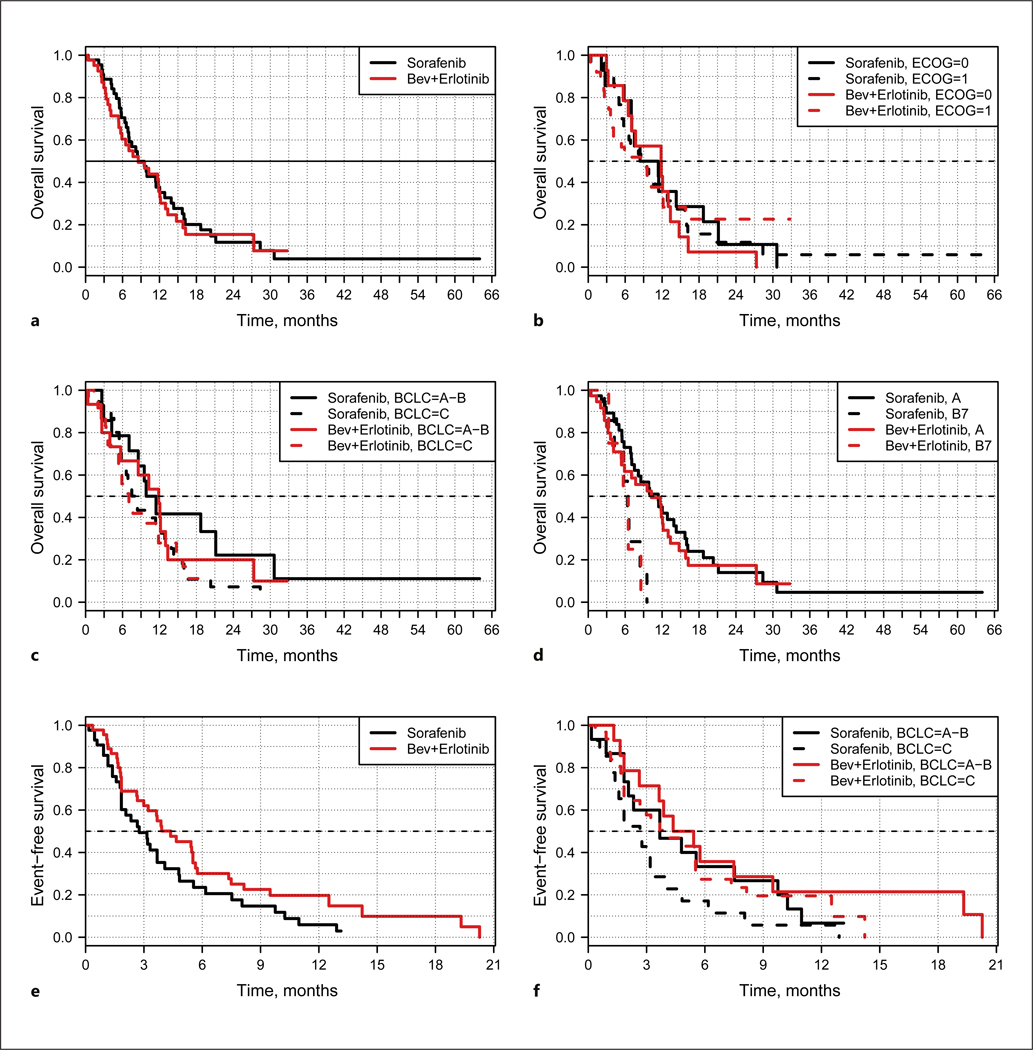
The efficacy results are summarized in Table 3 and Figure 2. Investigator assessment, confirmed by the diagnostic imaging collaborator at each institution and by centralized blinded radiology review, was used in determining tumor response and progression. The median OS of the patients treated with B+E and those treated with S were essentially the same. The 12-month survival was 37% (95% CI: 25–55) among patients treated with B+E, and 35% (95% CI: 22–55) among patients treated with S. The HR for OS was 0.92 (95% CI: 0.57–1.47). The objective RR for B+E was 15% (95% CI: 6.2–28) and that for S was 9% (95% CI: 2.6–22). OS did not differ between the B+E and the S arm based on ECOG PS or BCLC stage A or B versus BCLC stage C.
Table 3.
Efficacy summary
| Study arm | Response rate, % | mOS (HR = 0.92; 95% CI: 0.57–1.47) | mEFS, months (HR = 0.67; 95% CI: 0.42–1.07) | mOS Child-Pugh class A patients (HR = 0.88; 95% CI: 0.53–1.46) (S, n = 38; B+E, n = 39) | ||
|---|---|---|---|---|---|---|
| median, months | 12-month, % | median, months | 12-month, % | |||
| S (n = 43) | 9 (2.6–22) | 8.6 (5.7–12.2) | 35 (22–55) | 2.76 (1.84–4.80) | 10.26 (5.9–13.0) | 40 (26–61) |
| B+E (n = 47) |
15 (6.2–28) | 8.6 (7.0–13.9) | 37 (25–55) | 4.37 (2.99–7.36) | 11.4 (7.5–15.7) | 45 (31–65) |
Values in parentheses are 95% CI. mOS, median overall survival; mEFS, median event-free survival; S, sorafenib; B+E, bevacizumab plus erlotinib.
Fig. 2.
Survival summary. a Overall survival. b Overall survival by ECOG status. c Overall survival by BCLC stage. d Overall survival Child-Pugh class A versus B. e Event-free survival. f Event-free survival by BCLC stage. The p value for testing overall survival was 0.73 using the Cox proportional hazards model; however, a hypothesis test for overall survival comparing the two groups was not included in the study design due to insufficient power. Bev, bevacizumab.
Since most other randomized HCC trials included only patients with Child-Pugh class A liver function [5, 62–65], OS was also assessed for the subgroup of patients in this study who were Child-Pugh class A versus B7 (Fig. 2d). The median OS of the patients treated with B+E (n = 39) was 11.4 months (95% CI: 7.5–15.7) and that of the patients treated with S (n = 38) was 10.26 months (95% CI: 5.9–13.0) (median OS in the SHARP trial: 10.7 months). The 12-month survival among the Child-Pugh class A patients was 45% (95% CI: 31–65) with B+E and 40% (95% CI: 26–61) with S (HR = 0.88; 95% CI: 0.53–1.46) (1-year survival in the SHARP trial: 44%), thus the trial was negative for any difference in OS based on overlapping CIs for the HR.
Median EFS (Fig. 2e, f) was 4.37 months (95% CI: 2.99–7.36) among all patients treated with B+E and 2.76 months (95% CI: 1.84–4.80) among the patients treated with S. The HR for EFS was 0.67 (95% CI: 0.42–1.07; p = 0.09). These data suggest that the patients in the B+E arm were able to stay on therapy longer than those in the S arm, even if the difference was not statistically significant.
Safety and Tolerability
The causes and grades of the 25 most common AEs in each arm are summarized in Table 4. Table 5 summarizes the safety and tolerability data by treatment arm. The overall number of grade 1–4 AEs (serious AEs [SAEs]) in the B+E arm was higher than the number in the S arm; however, the AE rate (where the rate is SAE or AE number/number of cycles of treatment administered) was slightly higher in the S arm than in the B+E arm. All SAEs including investigator-assessed causality are described for B+E and S in online supplementary Tables 1 and 2 (see www.karger.com/doi/10.1159/000485384 for all online suppl. material). Of note, the incidence of grade 3 or 4 hemorrhage was higher in the B+E arm (n = 9) than in the S arm (n = 2), which can likely be attributed to B.
Table 4.
Safety and tolerability
| AE type | S arm |
B+E arm |
||||
|---|---|---|---|---|---|---|
| grade |
total | grade |
total | |||
| 1–2 | 3–4 | 1–2 | 3–4 | |||
| Fatigue | 25 | 8 | 33 | 30 | 3 | 33 |
| Diarrhea | 26 | 4 | 30 | 37 | 4 | 41 |
| Nausea | 16 | 2 | 18 | 24 | 0 | 24 |
| Anorexia | 16 | 1 | 17 | 26 | 2 | 28 |
| Weight loss | 17 | 0 | 17 | 25 | 0 | 25 |
| Platelets decreased | 15 | 2 | 17 | 16 | 1 | 17 |
| Hand-foot skin reaction | 12 | 4 | 16 | 0 | 0 | 0 |
| Dry skin | 16 | 0 | 16 | 24 | 0 | 24 |
| Pruritus | 0 | 0 | 0 | 22 | 1 | 23 |
| Vomiting | 14 | 1 | 15 | 0 | 0 | 0 |
| Acne | 0 | 0 | 0 | 18 | 9 | 27 |
| Limb edema | 13 | 1 | 14 | 0 | 0 | 0 |
| Dysgeusia | 9 | 0 | 9 | 21 | 0 | 21 |
| Epistaxis | 0 | 0 | 0 | 21 | 0 | 21 |
| Hypertension | 11 | 3 | 14 | 17 | 4 | 21 |
| Weakness | 12 | 2 | 14 | 0 | 0 | 0 |
| AST increased | 9 | 4 | 13 | 20 | 5 | 25 |
| Bilirubin increased | 9 | 3 | 12 | 16 | 3 | 19 |
| Hemoglobin decreased | 9 | 3 | 12 | 17 | 3 | 20 |
| Hyponatremia | 6 | 6 | 12 | 11 | 4 | 15 |
| Dizziness | 11 | 0 | 11 | 0 | 0 | 0 |
| ALT increased | 9 | 2 | 11 | 16 | 1 | 17 |
| Rash | 11 | 0 | 11 | 16 | 0 | 16 |
| Back pain | 9 | 1 | 10 | 0 | 0 | 0 |
| Abdominal pain | 8 | 2 | 10 | 17 | 7 | 24 |
| Fever | 0 | 0 | 0 | 14 | 1 | 15 |
| Dry mouth | 0 | 0 | 0 | 13 | 1 | 14 |
| Chills | 0 | 0 | 0 | 13 | 1 | 14 |
| Proteinuria | 0 | 0 | 0 | 13 | 1 | 14 |
| Hypoalbuminemia | 0 | 0 | 0 | 13 | 1 | 14 |
| Constipation | 10 | 0 | 10 | 0 | 0 | 0 |
| Alopecia | 7 | 0 | 7 | 0 | 0 | 0 |
| Alkaline phosphatase increased | 6 | 2 | 8 | 14 | 4 | 18 |
| Total | 306 | 51 | 357 | 460 | 56 | 516 |
The worst grade experienced per patient is included in the table. For example, if a patient had grade 3 diarrhea and then grade 4 diarrhea, the grade 4 diarrhea would be included in the table and the grade 3 diarrhea would not. This table includes the 25 most common AEs in each arm (complete AE information included in online suppl. Tables S1 and S2). AE, adverse event; S, sorafenib; B+E, bevacizumab plus erlotinib.
Table 5.
Summary of the safety and tolerability data for the treatment arms
| Event | S (n = 43) | AE rate1 | B+E (n = 47) | AE rate1 |
|---|---|---|---|---|
| Number of cycles2 initiated per arm | 184 | 287 | ||
| Median number of cycles initiated | 3 | 5 | ||
| Total number of grade 1–2 AEs per arm | 306 | 1.6 | 461 | 1.6 |
| Total number of grade 3–4 AEs per arm | 51 | 0.27 | 56 | 0.19 |
| Total number of SAEs in treatment arm | 30 | 0.163 | 40 | 0.139 |
AE, adverse event; SAE, severe AE; S, sorafenib; B+E, bevacizumab plus erlotinib.
The AE rate is the total number of events/total number of cycles.
One cycle = 28 days.
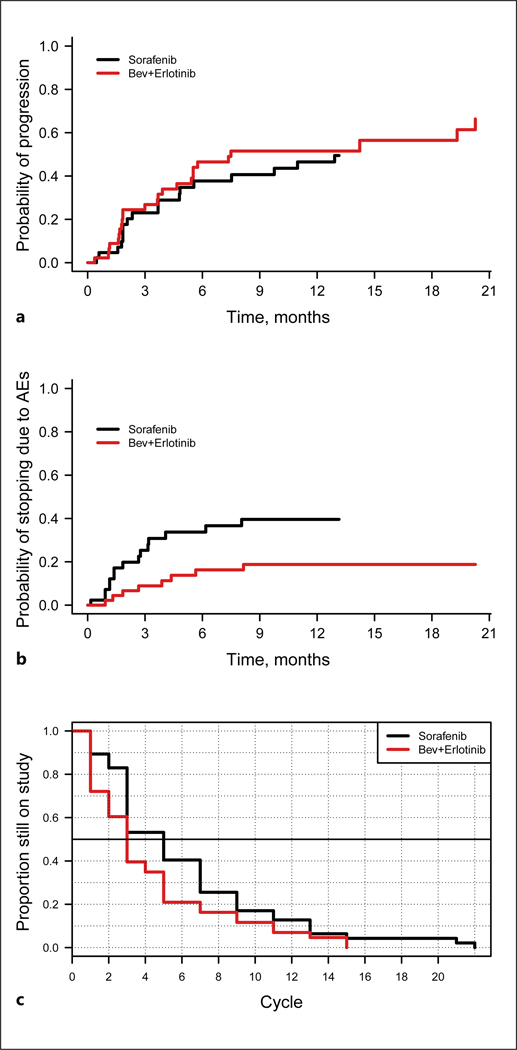
The results of the competing risk analysis are summarized in Figure 3. A competing risk is an event that either hinders the observation of the event of interest, which in this study was survival, or modifies the chance that this event occurs. The cumulative incidence of progression based on the competing risk analysis was slightly higher in the B+E arm (HR = 1.32; p = 0.35) (Fig. 3a). The cumulative incidence rates of death from other causes and of other clinical events were comparable in the two groups (results not shown).
Fig. 3.
Competing risk analysis. a Progression as a reason for discontinuation. HR = 1.32 (p = 0.35). b Toxicity as a reason for discontinuation. HR = 0.40 (p = 0.03). c Time on treatment. This plot shows the number of cycles administered in each study arm. The data show that the sorafenib (S) patients discontinued treatment sooner than the bevacizumab plus erlotinib (B+E) patients. Of the 43 evaluable patients in the S arm, 15 (35%) received only one cycle of treatment. In the B+E arm, 6 of the 47 evaluable patients (13%) received only one cycle. The difference in the curves is significant (p = 0.02) by a log-rank test. Bev, bevacizumab; AEs, adverse events.
Figure 3b shows that the S arm had a statistically significantly higher rate of treatment discontinuation due to toxicity than the B+E arm, with an HR of 0.40 (p = 0.03) favoring B+E. Figure 3c shows the number of cycles administered and the time to treatment discontinuation in the two study arms. The patients in the B+E arm stayed on treatment longer than those in the S arm: 87% of the patients in the B+E arm received more than one cycle of treatment, compared with 65% of the patients in the S arm (Fig. 3c; p = 0.02). Taken together, the competing risk analysis showed that the B+E regimen was generally better tolerated by the patients in this study than the S regimen, and the patients in the two arms had similar rates of progression.
Discussion
Although this was a negative study that did not meet its primary endpoint of demonstrating significant improvement in median OS for patients treated with B+E despite the dual targeting of important pathways in HCC, the results are nonetheless informative. The outcomes are confounded in part by the inclusion of Child-Pugh class B patients in this study, who are well known to have shorter OS than Child-Pugh class A patients [66–68]. This is confirmed by the finding that the median OS for the S arm of 8.6 months (95% CI: 5.7–12.2) is lower than that reported in several other randomized trials where only Child-Pugh class A patients were included [5, 62–65, 69]. The median OS of the Child-Pugh class A patients treated with S in this trial (10.26 months) is essentially the same as the median OS of 10.7 months reported in the pivotal SHARP trial [5]. Although the current study had a randomized, open-label, phase II design and its results cannot be compared directly to the results of other trials, the outcomes for the S arm are generally consistent with those seen in other studies.
This study suggests that the combination of B+E has some efficacy compared to S in patients with advanced HCC based on the RR of 15 versus 9%, EFS of 4.37 versus 2.76 months, and median OS of 11.4 versus 10.26 months when the data for Child-Pugh class A patients only are analyzed, although there was no statistically significant difference in any endpoint. It is acknowledged that the magnitude of the difference in OS based on the analysis of Child-Pugh class A patients only is not significant. However, the B+E regimen was better tolerated by the patients than was the S regimen, as evidenced by slightly lower AE and SAE rates as well as a statistically significant difference in the number of treatment cycles the patients were able to receive and the longer time on treatment. The competing risk analysis showed that the patients in the S arm were more likely to discontinue treatment due to toxicity than were the patients in the B+E arm (HR = 0.40).
Given strong preclinical rationale supporting the use of growth factor-targeting agents in HCC, the clinical efficacy of B+E should have been more impressive. Unfortunately, as yet, translating promising preclinical data into significant clinical benefits for HCC patients has been disappointing across multiple drugs, drug combinations, and trial designs [62–64, 70, 71]. It is certainly possible that in this trial, OS was confounded by postprogression therapy, although these data were not captured. The trial design and its potential for success were likely hampered by an ambitious primary endpoint based on singlearm, single-institution trials, which commonly overestimate clinical benefits [72–74].
The long history of negative clinical trials of “targeted” agents in HCC underscores the urgent need for better identification of the key driving carcinogenic mechanisms in HCC that are prognostic, predictive, and “actionable.” An identification of such validated targets has been lacking in all trials of growth factor inhibitors in HCC [75, 76]. While targeting signaling pathways themselves has always held appeal in anticancer drug development, the underlying genetic alterations that lead to deregulation of signaling pathways may represent better biotargets than growth factor expression itself [77]. Given the complexity and heterogeneity of HCC, unraveling the pattern of genomic alterations that are intrinsic to the liver itself, versus those due to hepatocarcinogenesis and the surrounding inflammatory milieu, is pivotal to identifying systemic therapies that will further improve patient outcome.
Supplementary Material
Acknowledgments
Funding Sources
Funding was provided by Genentech, a member of the Roche Group, and was supported in part by the Clinical Trials Office and Biostatistics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313) and by the Gastrointestinal Malignancies SmartState® Center of Economic Excellence.
Statement of Ethics
The study was approved by the institutional review boards at the Medical University of South Carolina and each participating institution, and was performed in accordance with the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines, and applicable local regulatory requirements and laws. All patients provided written informed consent.
Footnotes
Disclosure Statement
All authors report no potential conflicts of interest.
References
- 1.Zhu AX: Current status of hepatocellular carcinoma in the United States. Chin Clin Oncol 2013; 2: 45. [DOI] [PubMed] [Google Scholar]
- 2.Maillard E: Epidemiology, natural history and pathogenesis of hepatocellular carcinoma (in French). Cancer Radiother 2011; 15: 3–6. [DOI] [PubMed] [Google Scholar]
- 3.Yuen MF, Hou JL, Chutaputti A; Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma: Hepatocellular carcinoma in the Asia Pacific region. J Gastroenterol Hepatol 2009; 24: 346–353. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, et al. : Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Zhang L: Liver regeneration microenvironment of hepatocellular carcinoma for prevention and therapy. Oncotarget 2017; 8: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Siddiq A, Emdad L, et al. : Insulin-like growth factor-binding protein-7 (IGFBP7): a promising gene therapeutic for hepatocellular carcinoma (HCC). Mol Ther 2013; 21: 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Pang R, Poon RT: Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett 2006; 242: 151–167. [DOI] [PubMed] [Google Scholar]
- 10.Pang RW, Joh JW, Johnson PJ, et al. : Biology of hepatocellular carcinoma. Ann Surg Oncol 2008; 15: 962–971. [DOI] [PubMed] [Google Scholar]
- 11.Pang RW, Poon RT: From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology 2007; 72(suppl 1): 30–44. [DOI] [PubMed] [Google Scholar]
- 12.Giordano S, Columbano A: Met as a therapeutic target in HCC: facts and hopes. J Hepatol 2014; 60: 442–452. [DOI] [PubMed] [Google Scholar]
- 13.Nalesnik MA, Michalopoulos GK: Growth factor pathways in development and progression of hepatocellular carcinoma. Front Biosci (Schol Ed) 2012; 4: 1487–1515. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva A, Llovet JM: Targeted therapies for hepatocellular carcinoma. Gastroenterology 2011; 140: 1410–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr AW, Toole K, Mathew J, et al. : Transforming growth factor-α expression is altered during experimental hepatocarcinogenesis. J Pathol 1996; 179: 276–282. [DOI] [PubMed] [Google Scholar]
- 16.Whittaker S, Marais R, Zhu AX: The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010; 29: 4989–5005. [DOI] [PubMed] [Google Scholar]
- 17.Höpfner M, Schuppan D, Scherübl H: Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol 2008; 14: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moradpour D, Blum HE: Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2005; 17: 477–483. [DOI] [PubMed] [Google Scholar]
- 19.Wang YP, Huang LY, Sun WM, et al. : Insulin receptor tyrosine kinase substrate activates EGFR/ERK signalling pathway and promotes cell proliferation of hepatocellular carcinoma. Cancer Lett 2013; 337: 96–106. [DOI] [PubMed] [Google Scholar]
- 20.Yang ZF, Poon RT: Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008; 291: 721–734. [DOI] [PubMed] [Google Scholar]
- 21.Okano H, Shiraki K, Yamanaka Y, et al. : Functional expression of a proliferation-related ligand in hepatocellular carcinoma and its implications for neovascularization. World J Gastroenterol 2005; 11: 4650–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mise M, Arii S, Higashituji H, et al. : Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology 1996; 23: 455–464. [DOI] [PubMed] [Google Scholar]
- 23.Aucejo F, Kim R, Zein N, et al. : Vascular endothelial growth factor receptor 2 expression in non-tumorous cirrhotic liver is higher when hepatoma is beyond Milan criteria. Liver Transpl 2009; 15: 169–176. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Ng IO, Lau C, et al. : Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg 2001; 233: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon RT, Lau CP, Cheung ST, et al. : Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res 2003; 63: 3121–3126. [PubMed] [Google Scholar]
- 26.Cioca A, Cimpean A, Ceausu R, et al. : Crosstalk between EGFR and p53 in hepatocellular carcinoma. Asian Pac J Cancer Prev 2014; 15: 8069–8073. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs BC, Fujii T, Dorfman JD, et al. : Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res 2008; 68: 2391–2399. [DOI] [PubMed] [Google Scholar]
- 28.Nault JC, Zucman-Rossi J: Genetics of hepatobiliary carcinogenesis. Semin Liver Dis 2011; 31: 173–187. [DOI] [PubMed] [Google Scholar]
- 29.Su YH, Ng KF, Yu MC, et al. : Impact of epidermal growth factor receptor protein and gene alteration on Taiwanese hepatocellular carcinomas. J Gastroenterol Hepatol 2015; 30: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 30.Urtasun R, Latasa MU, Demartis MI, et al. : Connective tissue growth factor autocriny in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology 2011; 54: 2149–2158. [DOI] [PubMed] [Google Scholar]
- 31.Bassullu N, Turkmen I, Dayangac M, et al. : The predictive and prognostic significance of c-erb-B2, EGFR, PTEN, mTOR, PI3K, p27, and ERCC1 expression in hepatocellular carcinoma. Hepat Mon 2012; 12:e7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berasain C, Nicou A, Garcia-Irigoyen O, et al. : Epidermal growth factor receptor signaling in hepatocellular carcinoma: inflammatory activation and a new intracellular regulatory mechanism. Dig Dis 2012; 30: 524–531. [DOI] [PubMed] [Google Scholar]
- 33.Wang LJ, Bai L, Su D, et al. : Proinflammatory conditions promote hepatocellular carcinoma onset and progression via activation of Wnt and EGFR signaling pathways. Mol Cell Biochem 2013; 381: 173–181. [DOI] [PubMed] [Google Scholar]
- 34.Berasain C, Castillo J, Prieto J, et al. : New molecular targets for hepatocellular carcinoma: the ErbB1 signaling system. Liver Int 2007; 27: 174–185. [DOI] [PubMed] [Google Scholar]
- 35.Luo X, Xie H, Long X, et al. : EGFRvIII mediates hepatocellular carcinoma cell invasion by promoting S100 calcium binding protein A11 expression. PLoS One 2013; 8:e83332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su MC, Lien HC, Jeng YM: Absence of epidermal growth factor receptor exon 18–21 mutation in hepatocellular carcinoma. Cancer Lett 2005; 224: 117–121. [DOI] [PubMed] [Google Scholar]
- 37.Xu M, Zheng YL, Xie XY, et al. : Sorafenib blocks the HIF-1α/VEGFA pathway, inhibits tumor invasion, and induces apoptosis in hepatoma cells. DNA Cell Biol 2014; 33: 275–281. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Cao Y, Chen C, et al. : Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/ PRF/5. Cancer Res 2006; 66: 11851–11858. [DOI] [PubMed] [Google Scholar]
- 39.Cervello M, Bachvarov D, Lampiasi N, et al. : Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle 2012; 11: 2843–2855. [DOI] [PubMed] [Google Scholar]
- 40.Grothey A, Ellis LM: Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J 2008; 14: 170–177. [DOI] [PubMed] [Google Scholar]
- 41.Grothey A, Galanis E: Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol 2009; 6: 507–518. [DOI] [PubMed] [Google Scholar]
- 42.Fang P, Hu JH, Cheng ZG, et al. : Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II trials. PLoS One 2012; 7:e49717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zalcman G: EGFR pathway and mechanism of action of tyrosine kinase inhibitors (in French). Rev Pneumol Clin 2007; 63(pt 2): 2S5–2S6. [PubMed] [Google Scholar]
- 44.Marijon H, Faivre S, Raymond E: Targeted therapies in hepatocellular carcinomas: recent results and future development (in French). Bull Cancer 2009; 96: 553–561. [DOI] [PubMed] [Google Scholar]
- 45.Furuse J: Growth factors as therapeutic targets in HCC. Crit Rev Oncol Hematol 2008; 67: 8–15. [DOI] [PubMed] [Google Scholar]
- 46.Sandler A, Herbst R: Combining targeted agents: blocking the epidermal growth factor and vascular endothelial growth factor pathways. Clin Cancer Res 2006; 12: 4421s–4425s. [DOI] [PubMed] [Google Scholar]
- 47.Press MF, Lenz HJ: EGFR, HER2 and VEGF pathways: validated targets for cancer treatment. Drugs 2007; 67: 2045–2075. [DOI] [PubMed] [Google Scholar]
- 48.Herbst RS, Johnson DH, Mininberg E, et al. : Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005; 23: 2544–2555. [DOI] [PubMed] [Google Scholar]
- 49.Bozec A, Sudaka A, Fischel JL, et al. : Combined effects of bevacizumab with erlotinib and irradiation: a preclinical study on a head and neck cancer orthotopic model. Br J Cancer 2008; 99: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Child CG, Turcotte JG: Surgery and portal hypertension. Major Probl Clin Surg 1964; 1: 1–85. [PubMed] [Google Scholar]
- 51.Pugh RN, Murray-Lyon IM, Dawson JL, et al. : Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 52.Toyoda H, Kumada T, Kiriyama S, et al. : Comparison of the usefulness of three staging systems for hepatocellular carcinoma (CLIP, BCLC, and JIS) in Japan. Am J Gastroenterol 2005; 100: 1764–1771. [DOI] [PubMed] [Google Scholar]
- 53.Farinati F, Rinaldi M, Gianni S, et al. : How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer 2000; 89: 2266–2273. [PubMed] [Google Scholar]
- 54.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000; 31: 840–845. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. : Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46: 922–938. [DOI] [PubMed] [Google Scholar]
- 56.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 57.Kitai S, Kudo M, Izumi N, et al. : Validation of three staging systems for hepatocellular carcinoma (JIS score, biomarker-combined JIS score and BCLC system) in 4,649 cases from a Japanese nationwide survey. Dig Dis 2014; 32: 717–724. [DOI] [PubMed] [Google Scholar]
- 58.Vitale A, Saracino E, Boccagni P, et al. : Validation of the BCLC prognostic system in surgical hepatocellular cancer patients. Transplant Proc 2009; 41: 1260–1263. [DOI] [PubMed] [Google Scholar]
- 59.Llovet JM: Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40: 225–235. [DOI] [PubMed] [Google Scholar]
- 60.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 61.Fine JP, Gray RJ: A proportional hazards model for the distribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 62.Zhu AX, Rosmorduc O, Evans TR, et al. : SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015; 33: 559–566. [DOI] [PubMed] [Google Scholar]
- 63.Cainap C, Qin S, Huang WT, et al. : Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015; 33: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson PJ, Qin S, Park JW, et al. : Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013; 31: 3517–3524. [DOI] [PubMed] [Google Scholar]
- 65.Cheng AL, Kang YK, Lin DY, et al. : Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013; 31: 4067–4075. [DOI] [PubMed] [Google Scholar]
- 66.Cabibbo G, Maida M, Genco C, et al. : Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. World J Hepatol 2012; 4: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta N, Fidelman N, Sarkar M, et al. : Factors associated with outcomes and response to therapy in patients with infiltrative hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013; 11: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greten TF, Papendorf F, Bleck JS, et al. : Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005; 92: 1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruix J, Raoul JL, Sherman M, et al. : Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012; 57: 821–829. [DOI] [PubMed] [Google Scholar]
- 70.Llovet JM, Decaens T, Raoul JL, et al. : Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013; 31: 3509–3516. [DOI] [PubMed] [Google Scholar]
- 71.Worns MA, Schuchmann M, Duber C, et al. : Sunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatment. Oncology 2010; 79: 85–92. [DOI] [PubMed] [Google Scholar]
- 72.Estey E, Hoth D, Simon R, et al. : Therapeutic response in phase I trials of antineoplastic agents. Cancer Treat Rep 1986; 70: 1105–1115. [PubMed] [Google Scholar]
- 73.Kass N, Taylor H, Fogarty L, et al. : Purpose and benefits of early phase cancer trials: what do oncologists say? What do patients hear? J Empir Res Hum Res Ethics 2008; 3: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kotecki N, Penel N, Adenis A, et al. : Predictive value of clinical judgment of tumour progression in phase II trials. PLoS One 2012; 7: e52638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thillai K, Ross P, Sarker D: Molecularly targeted therapy for advanced hepatocellular carcinoma – a drug development crisis? World J Gastrointest Oncol 2016; 8: 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duffy AG, Ulahannan SV, Cao L, et al. : A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib. United European Gastroenterol J 2015; 3: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bild AH, Yao G, Chang JT, et al. : Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006; 439: 353–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.