Abstract
Allergic asthma is a chronic airway inflammatory disorder triggered by inhalant allergens. Interleukin (IL)-4 and IL-13 play a main role in the generation of T helper cell type 2 (Th2) immune response, induction of immunoglobulin E (IgE) synthesis and persistence of airway inflammation. The aim of the present study was to investigate the influence of Dermatophagoides pteronyssinus allergen Der p 1, the major allergen of house dust mite, on the synthesis of IL-4 and IL-13 by monocyte-derived dendritic cells (DCs) and naive CD4+ T cells cocultured with DCs, as well as their role in the production of serum IgE, in house dust mite (HDM) allergic patients. Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood of patients allergic to HDM and healthy donors and incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 to generate immature DCs. The obtained cells were stimulated for 24 h with Der p 1 to induce DC maturation, washed, and afterwards cocultured for 24 h with autologous naive CD4+ T cells. Culture supernatants were harvested for IL-4, IL-13 and IFN-γ level measurements. DCs stimulation with Der p 1 induced higher synthesis of IL-4 and IL-13 in HDM allergic patients, compared to healthy donors. The allergic group showed significant correlation between IL-13 production by Der p 1-pulsed DCs, and total serum IgE and IL-4 production of the same cells and Der p-specific IgE. To conclude, IL-4 and IL-13 are critically related to the regulation of serum IgE production in patients with allergic asthma. The relevance of these two cytokines in the pathophysiology of Th2 asthma endotype makes them an appropriate target in its management.
Keywords: allergic asthma, CD4+ T cells, dendritic cells, Ig E, interleukin-4, interleukin-13
Introduction
Allergic asthma is a chronic airway inflammatory disorder characterized by reversible airway obstruction and bronchial hyperreactivity (1). Experimental evidence shows that the main mediators of allergic inflammation are T helper cell type 2 (Th2) cells and their cytokines. The stimulation of Th2 cells enhances expression of interleukin (IL)-4, IL-5 and IL-13, the key cytokines responsible for the allergic immune response by induction of airway inflammation, immunoglobulin E (IgE) production and eosinophilia (2,3). The role of these cytokines was confirmed by in vivo functional assays on animal models (4). Because the presence of IL-4 is absolutely required in the differentiation process of naive CD4+ T cells into Th2 cells, this is considered the critical immunoregulatory cytokine implicated in the Th2 immune response. IgE synthesis in naive B lymphocytes is preferentially stimulated by IL-4 and IL-13 and inhibited by interferon (IFN)-γ (5). A close correlation was found between production of serum IgE and the development of allergic asthma. Allergic rhinitis seems to be independent of total IgE level, but there is an association with cutaneous reactivity to aeroallergens (6,7). Both IL-4 and IL-13 promote acute inflammatory processes, but it was shown that IL-4 can induce lung inflammation even in the absence of IL-13, and the stimulatory role of IL-4 is not inhibited by IL-13 antagonists (8). It has been suggested that IL-13 alone may be sufficient to initiate eosinophilic airway infiltration through its ability to stimulate chemokine expression. In addition to eosinophilic inflammation and mucus hypersecretion, IL-13 also induces bronchial hyperreactivity (9).
Some authors have demonstrated the ability of IL-13 to downregulate the synthesis of proinflammatory cytokines by macrophages and monocytes and to induce the expression of MHC II on antigen-presenting cells (10). On the other hand, IL-4 has a stimulatory effect on the function of CD8+ T cells. One study observed that IL-4-deficient mice, that generally have undetectable IgE levels, can still produce them after certain antigenic stimulations or infections (11). It was postulated that IL-4 induces the switch of IgM production to IgE by differentiating B cells. This cytokine is countered by IFN-γ, which can inhibit the immunoglobulin class switch (12). IFN-γ also inhibits allergic eosinophilia and airway hyperreactivity (13). It was demonstrated that a decreased production of IFN-γ in neonates is an important risk factor for the subsequent development of any atopic disease (14). There are conflicting data demonstrating that elevated IFN-γ is significantly correlated with allergic disorders (15).
IL-4, IL-5 and IL-13 are also involved in the generation and maintenance of chronic inflammation found in other atopic diseases, such as atopic dermatitis (AD), in a similar manner. In addition, they have a role in downregulation of the expression of various genes that control integrity and function of the skin barrier (16,17). Dupilumab, recently approved for the therapy of adult patients with moderate to severe AD, is a fully human monoclonal antibody directed against IL-4 receptor α, with an inhibitory effect on IL-4 and IL-13 signaling (18). There are ongoing studies which demonstrate that patients who undergo this treatment present a significantly downregulation of markers associated with T-cell activation and eosinophils, and suppressed serum total IgE from baseline (19,20). In 2018, dupilumab was also approved by the US Food and Drug Administration as an add-on maintenance treatment in patients aged 12 years and older, with moderate to severe asthma and eosinophilic phenotype or with oral corticosteroid-dependent asthma. Studies in patients who received dupilumab have shown a significant decrease in severe exacerbations and better asthma control (21).
Although many studies have shown the involvement of IL-4 and IL-13 in IgE production, their specific role in inducing and maintaining IgE synthesis is not well clarified. The purpose of the present study was to investigate the influence of Der p 1, the major allergen of house dust mite (HDM) Dermatophagoides pteronyssinus, on IL-4 and IL-13 synthesis by monocyte-derived dendritic cells (DCs) and naive CD4+ T cells cocultured with DCs, as well as their role in production of serum IgE, in HDM allergic patients.
Materials and methods
Ethics approval and consent to participate
All peripheral blood samples were obtained after signing informed consent expounded under an approved protocol by the Ethics in Scientific Research Commission of the County Emergency Clinical Hospital ‘Pius Brinzeu’ Timisoara, which complies with Romanian laws (95/2006, article L67 and article 28, chapter VIII 904/2006) and with EU GCP Directives [2005/28/EC (22), International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (23)] and the Declaration of Helsinki (Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects) (24).
Study groups
The present study included 9 HDM allergic patients (APs), aged between 19 and 60 years (mean age 32.67±3.95 years), who presented between October 2017 and March 2018 at the Allergy Outpatient Department of the Timisoara Infectious Diseases and Pneumology Hospital. The APs fulfilled the diagnostic criteria for allergic rhinitis and allergic asthma, according to ARIA and GINA guidelines, respectively (25,26). For the control group, 7 healthy donors (HDs) with no personal history of allergic or respiratory diseases and normal lung function, aged between 22 and 59 years (mean age 36.32±14.87 years), were selected. HDM allergy was diagnosed based on highly positive skin prick test for Dermatophagoides pteronyssinus antigens (wheal of ≥3 mm ± pseudopods), increased total serum IgE levels (1,165.92±1,097.52 IU/ml) and positive Der p-specific IgE (23.26±12.73 IU/ml). In the control group, the skin prick test to HDM and Der p-specific IgE were negative, with normal total serum IgE levels (Table I). All participants enrolled in one of the two groups did not use any antihistaminic and corticosteroid drugs in the last 30 days before entering the study and any other medication was discontinued 12 h before biological sampling.
Table I.
Characteristics of the two study groups.
| APs | HDs | |
|---|---|---|
| Number | 9 | 7 |
| Sex (male/female) | 6/3 | 3/4 |
| Age (years) | 32.67±3.95 | 36.32±14.87 |
| Total IgE (IU/ml) | 1,165.92±1,097.52 | 62.83±55.12 |
| Der p-specific IgE (IU/ml) | 23.26±12.73 | <0.35 |
Data are expressed as mean ± SD. APs, allergic patients; HDs, healthy donors; IgE, immunoglobulin E.
Evaluation of serum IgE and interleukin levels
The peripheral venous blood collected from the subjects of the study was analyzed for the level of IL-4, IL-13, IFN-γ, Der p-specific IgE, and total IgE using a specific ELISA technique (R&D Systems) in accordance with the manufacturer's instructions. For the three cytokines, the sensitivity of detection was <0.11, 32 and 8 pg/ml, respectively. Der p-specific IgE levels higher than 0.35 IU/ml were considered as positive. In the healthy group, the reference range of total IgE was up to 150 IU/ml.
Isolation and culture of cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral venous blood by density Ficoll gradient centrifugation (1,500 x g, 15 min). The cells at the interface were harvested and washed three times in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). After the centrifugation (600 x g, 5 min) purified PBMCs were resuspended (5x106 cells/ml) in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin and 1% L-glutamine, and incubated for adhesion into 6-well culture plates at 37˚C and 5% CO2 for 90 min. In order to generate immature DCs, adherent cells were washed and subsequently cultured for the next 7 days in the same medium as previously mentioned by adding granulocyte-macrophage colony-stimulating factor (GM-CSF) (1,000 U/ml) and IL-4 (400 U/ml) (R&D Systems). These immature DCs were maturated by incubation for 24 h with 10 ng/ml Der p 1 (GenWay Biotech, Inc.).
Immunoassay of cytokine level
After 24 h, the supernatant from Der p 1-pulsed DCs was harvested and the levels of IL-4, IL-13 and IFN-γ were determined with ELISA kits using Eli-pairs (R&D Systems) in accordance with the manufacturer's instructions. The sensitivity of cytokine detection was <0.11, 32 and 8 pg/ml, respectively.
Coculture of DCs with autologous T cells
Autologous naive CD4+ T cells were isolated from fresh heparin-anticoagulated peripheral blood by depletion of non-T helper cells and memory CD4+ T cells from PBMCs, using a negative separation by an indirect immunomagnetic labelling system with a cocktail of biotin-conjugated monoclonal antibodies (Miltenyi Biotech) following the manufacturer's instructions.
DCs previously matured with Der p 1 for 24 h were washed and cocultured with autologous naive CD4+ T cells at a ratio of 1:10 for 24 h.
Immunoassay of cytokine levels
As we described above, the supernatant was harvested and IL-4, IL-13 and IFN-γ production was assayed by the same specific ELISA kits (R&D Systems) in accordance with the manufacturer's instructions, with detection sensitivity of <0.11, 32 and 8 pg/ml, respectively.
Statistical analysis
Data were analyzed with SPSS v. 20.0 software (IBM Corp.). Apart from descriptive statistics for the numerical results (mean, standard error), statistical significance of the difference observed in the DC response in allergic patients vs. healthy donors was determined using paired t-test. To investigate the relationship between the serological outcome and the DC response to allergenic stimulation, a multivariable regression analysis was conducted. For the statistical tests, the 0.05 (i.e. 5%) two-tailed level of significance was considered, while values close to 0.1 (i.e. 1%) were also discussed.
Results
Serum cytokine profile
The serum cytokine profile of the two groups of study is shown in Table II. It was observed that the concentration of serum IL-4 was extremely significantly higher in the AP group when compared with the healthy donors (HDs) (P<0.001). It should be noted that the levels of this cytokine were on average 4.75 times higher in the APs than in the control group. This was not observed for IL-13 and IFN-γ (P>0.05) (Table II and Fig. 1).
Table II.
Serum cytokines levels in the two groups of study.
| Cytokine levels (pg/ml) | APs | HDs | P-value |
|---|---|---|---|
| IL-4 | 0.038±0.023 | 0.008±0.006 | <0.001 |
| IL-13 | 19.433±9.680 | 20.569±2.861 | >0.05 |
| IFN-γ | 5.523±0.917 | 6.695±0.864 | >0.05 |
Data are expressed as mean ± SD. APs, allergic patients; HDs, healthy donors; IL, interleukin; IFN, interferon.
Figure 1.
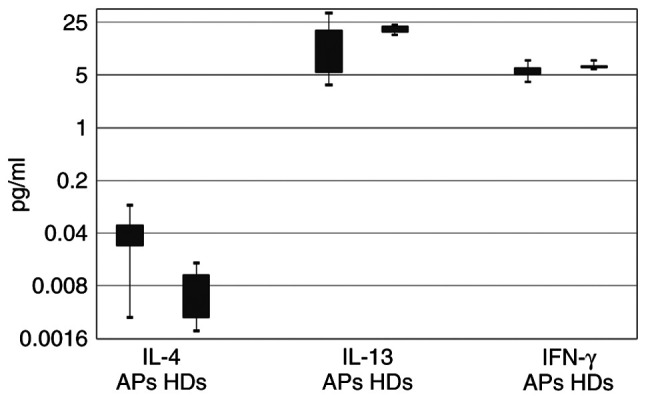
Serum cytokine (IL-4, IL-13 and IFN-γ) levels in allergic patients (APs) and healthy donors (HDs). IL, interleukin; IFN, interferon.
Cytokine production by stimulated DCs
In the HDM allergic patients, the production of IL-4 and IL-13 by Der p 1 pulsed-DCs generated from PBMCs was higher in comparison with the HDs, but did not differ significantly (P>0.05). In contrast, IFN-γ amounts were very high, but comparable in both groups (Table III).
Table III.
Cytokine production by stimulated DCs.
| Cytokine production (pg/ml) | APs | HDs | P-value |
|---|---|---|---|
| IL-4 | 14.120±12.546 | 9.458±8.936 | >0.05 |
| IL-13 | 10.180±12.208 | 7.538±8.632 | >0.05 |
| IFN-γ | 319.637±628.179 | 423.723±345.283 | >0.05 |
Data are expressed as mean ± SD. DCs, dendritic cells; APs, allergic patients; HDs, healthy donors; IL, interleukin; IFN, interferon.
Cytokine production by autologous T cells in coculture with DCs
Coculture of autologous T cells with Der p 1-pulsed DCs induced significantly higher production of IL-4 (P<0.05) and extremely significant lower synthesis of IFN-γ (P<0.001) in the AP group compared with the HDs. We found no statistically significant difference in the IL-13 level between the two groups (Table IV).
Table IV.
Cytokine production by autologous T cells in coculture with DCs.
| Cytokine production (pg/ml) | APs | HDs | P-value |
|---|---|---|---|
| IL-4 | 5.576±8.061 | 2.863±5.428 | <0.05 |
| IL-13 | 8.177±7.795 | 7.842±6.588 | >0.05 |
| IFN-γ | 72.116±180.559 | 184.581±121.392 | <0.001 |
Data are expressed as mean ± SD. DCs, dendritic cells; APs, allergic patients; HDs, healthy donors; IL, interleukin; IFN, interferon.
Correlation between levels of serum cytokines and total or Der p-specific IgE in the AP group
There was no significant correlation between IL-4, IL-13 and IFN-γ serum levels and production of total or Der p-specific IgE (Table V).
Table V.
Correlation between the levels of serum cytokine and total or Der p-specific IgE in the AP group.
| IgE | Cytokines | P-value | Regressional model R-square, ANOVA, P-value |
|---|---|---|---|
| Total IgE | IL-4 | 0.409 | r=0.413 |
| IL-13 | 0.669 | F=4.933; df=3,21; P=0.146 | |
| IFN-γ | 0.567 | ||
| Der p-spec IgE | IL-4 | 0.359 | r=0.311 |
| IL-13 | 0.727 | F=2.260; df=3,15; P=0.123 | |
| IFN-γ | 0.223 |
AP, allergic patient; IgE, immunoglobulin E; IL, interleukin; IFN, interferon.
Correlation between cytokine synthesis by Der p 1-pulsed DCs and total or Der p-specific IgE
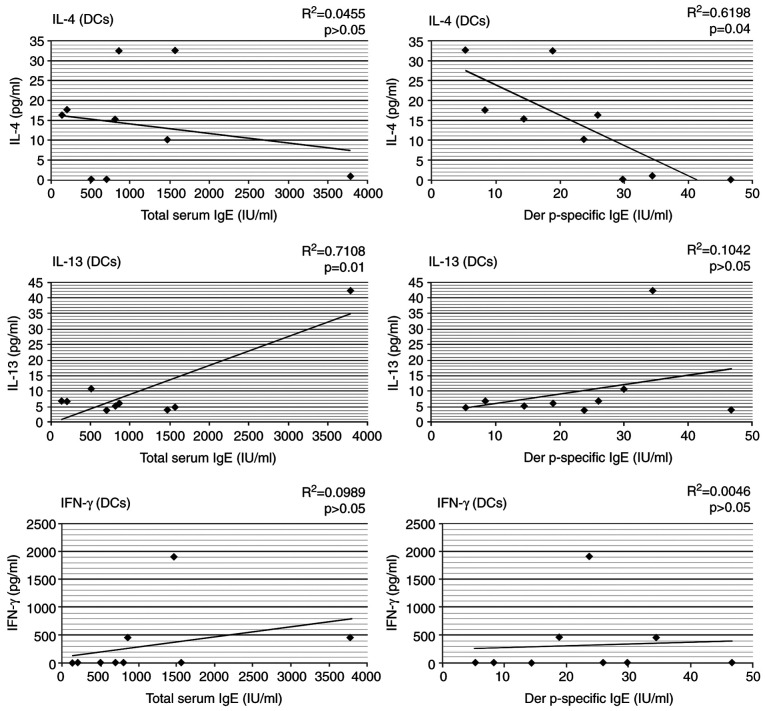
We observed that Der p 1-pulsed DCs produced levels of IL-4 that were significantly correlated with Der p-specific IgE serum levels in the HDM allergic patients (r=0.619, P=0.04). Although the production of IL-13 was comparable with that of IL-4, this was significantly correlated only with total IgE levels (r=0.71, P=0.01) and appeared not to be related to the specific IgE sensitization; we have found no correlation between IFN-γ levels and total serum IgE, nor Der p-specific IgE (Fig. 2).
Figure 2.
Correlation between cytokine (IL-4, IL-13 and IFN-γ) synthesis by stimulated-DCs and total or Der p-specific IgE in the AP group. AP, allergic patient; IL, interleukin; IFN, interferon; DCs, dendritic cells; IgE, immunoglobulin E.
Correlation between cytokine synthesis by autologous T cells and total or Der p-specific IgE
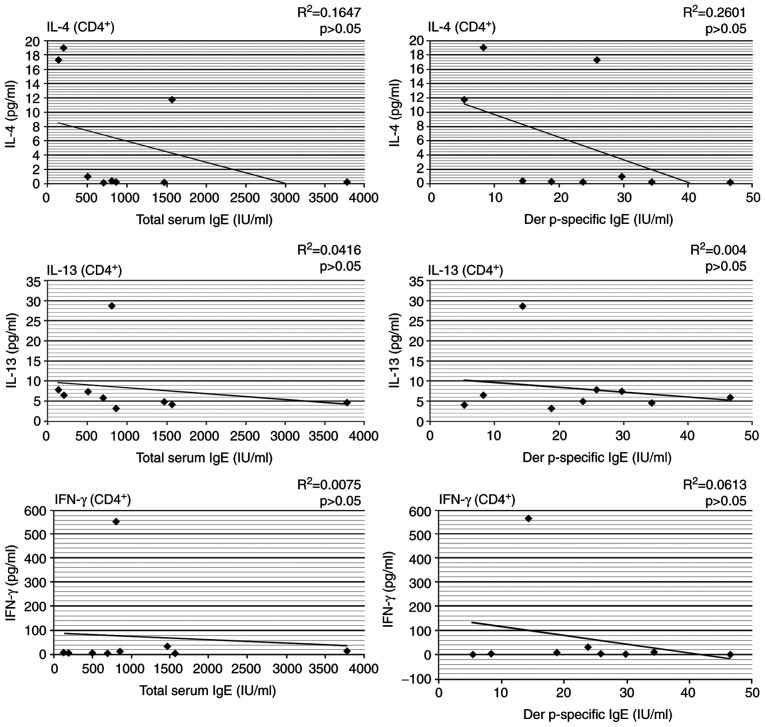
The production of IL-4, IL-13 and IFN-γ by autologous T cells in coculture with Der p 1-pulsed DCs was not significantly correlated with total or Der p-specific IgE levels in the HDM allergic patients (Fig. 3).
Figure 3.
Correlation between cytokine (IL-4, IL-13 and IFN-γ) synthesis by autologous T cells and total or Der p-specific IgE in the AP group. AP, allergic patient; IL, interleukin; IFN, interferon; IgE, immunoglobulin E.
Discussion
Allergic diseases are a group of immunoglobulin E (IgE)-dependent inflammatory disorders associated with the presence of the T helper cell type 2 (Th2) cell subpopulation and specific cytokine secretion. The major role in the development of the allergic reactions is played by the activation of CD4+ T cells. The main cytokines involved in the inflammatory process, which have a critical role in the Th2 immune response are interleukin (IL)-4, IL-5, IL-13 and IL-10. The cellular and humoral factors that contribute to the development of atopic diseases are complemented in a significant manner by exposure to the environment and the genetic background of the individual (27-29).
IL-4 is a key element in the differentiation and stimulation of B cells with increased IgE production. High amounts of IL-4 are associated with elevated IgE levels (30,31). The expression of the gene for IL-4 in peripheral blood mononuclear cells (PBMCs) is also correlated with the serum IgE level, supporting their synthesis in an IL-4-dependent manner (32). IL-13 regulates IgE production similarly to IL-4, but does not influence Th2 cell differentiation (33). Both IL-4 and IL-13 promote inflammatory processes, determining structural changes in the airways.
The present study observed extremely significantly higher IL-4 serum levels in allergic patients, in comparison with the healthy population, but this could not be correlated with total or Der p-specific IgE levels. No significant differences were identified for serum IL-13 and IFN-γ levels between the two groups and no relationship was found between these cytokines and IgE levels. Similar observations regarding serum IL-13 levels were also made in other studies (34,35). The importance of IL-4 in the pathogenesis of the allergic immune response was confirmed by the significant correlation between IL-4 synthesis by Der p 1-pulsed dendritic cells (DCs) and serum levels of Der p-specific IgE in the house dust mite (HDM) allergic patients. These data suggest that the role of IL-4 is more relevant than that of IL-13 in the stimulation of specific IgE production.
A very significant correlation was also identified between total IgE amounts and the levels of IL-13 produced by Der p 1-pulsed DCs. These findings confirm the main role of this cytokine in inducing total IgE production.
Most studies conclude that IL-4 is the cytokine more responsible for the initiation and IL-13 for the effector phase of Th2 allergic response (36). Both IL-13 and IL-4 have synergistic effects with tumor necrosis factor (TNF)-α and IL-5 on eosinophil activation (37,38). We did not observe any correlation between synthesis of IL-4 and IL-13 by autologous T cells and total or Der p-specific IgE levels.
There are data suggesting that IL-4 and IL-13 partly share the same receptor and signaling pathways, and, as we discussed previously, it was demonstrated that both of them are involved in IgE synthesis and eosinophil activation. Because of this, these cytokines appear as the most suitable targets to treat Th2-mediated forms of asthma, but some recent controversial observations have shown that neither IL-4 nor IL-13 are absolutely necessary for the production of IgE by B lymphocytes, or for the activation of IgE-dependent mast cells (39). Based on these findings, different therapeutic strategies such as anti-IL-13 agents (lebrikizunab and tralokinumab), and anti-IL-4 agents (dupilumab and pascolizumab) have been tested, but from all of these agents, dupilumab has been recognized as having the best therapeutic response. This leads to the conclusion that only certain endotypes of allergic asthma respond to these types of treatments and supports the hypothesis of an additional pathway of IgE synthesis (40,41).
It seems that IL-4 and IL-13 receptors are unregulated by the decrease in IFN-γ level (42). IFN-γ induces IL-12 production by DCs and macrophages, favoring a Th1 immune response. This cytokine also induces a specific inhibition of IL-4-induced IgE synthesis by B cells (43). In HDM allergic patients we observed that autologous T cells cocultured with Der p 1-pulsed DCs synthesized lower amounts of IFN-γ compared with the control group, thereby allowing IL-4 to induce the allergen-specific response. Decreasing IFN-γ level is associated with an increase in IgE production, but there is no correlation between them.
Genetic studies have identified a correlation between the expression of IL-4 gene and the serum IgE level in untreated asthmatic patients, but not in steroid-dependent patients, due to gene suppression. Correlations between IL-13 mRNA expression and the severity of allergic asthma have also been observed, suggesting the regulatory function of this cytokine in the disease development (44,45). A significant observation for the role of IL-13 was provided by Brusselle et al (46). They found that the blocking of IL-13 activity by intratracheal administration of soluble IL-13 receptor in a mouse model of asthma significantly reduced bronchial hyperreactivity and mucus production, but did not influence IgE production and eosinophil level.
Some authors have reported that the CD8+ T cell implication in allergic immune response is comparable with CD4+ T cells (47). It was observed that peripheral blood CD8+ T cells in asthma patients produced significantly higher amounts of IL-4 than those in healthy patients (48,49). From this point of view, it is considered that this type of cells warrants the same attention regarding its role in the allergic cascade, but further clarifications are needed.
In summary, our findings suggest the crucial importance of IL-4 and IL-13 in inducing the Th2 immune response, but their specific place in the inflammatory process is different and needs further confirmation regarding the certain role played by each of them in allergic asthma.
Acknowledgements
Not applicable.
Funding
This research was supported through the project, ‘Innovative Strategies for Prevention, Diagnosis and Therapy of Ragweed Pollen-Induced Respiratory Diseases’, ID P_37_747, SMIS code 103663, funded under Competitiveness Operational Programme 2014-2020.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LDC and LH contributed equally to the present study. LDC and LH conceptualized the experiments, developed the methodology, performed the experiments, analyzed and interpreted the data, and prepared the original draft of the manuscript. CP provided the resources and supervised the experiments and analysis of the data. LDC, LH and CP reviewed and edited the final manuscript. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
All peripheral blood samples were obtained after signing informed consent expounded under an approved protocol by the Ethics in Scientific Research Commission of the County Emergency Clinical Hospital ‘Pius Brinzeu’ Timisoara, which complies with Romanian laws (95/2006, article L67 and article 28, chapter VIII 904/2006) and with EU GCP Directives [2OOS/28/EC, International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)] and the Declaration of Helsinki (Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–464. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 2.Olsson A, Cagnoni F, Dignetti P, Melioli G, Canonica GW. Low concentrations of cytokines produced by allergen-stimulated peripheral blood mononuclear cells have potent effects on nasal polyp-derived fibroblasts. Clin Exp Immunol. 2003;132:254–260. doi: 10.1046/j.1365-2249.2003.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leru PM. Eosinophilic disorders: Evaluation of current classification and diagnostic criteria, proposal of a practical diagnostic algorithm. Clin Transl Allergy. 2019;9(36) doi: 10.1186/s13601-019-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 5.Tang M, Kemp A, Varigos G. IL-4 and interferon-γ production in children with atopic disease. Clin Exp Immunol. 1993;92:120–124. doi: 10.1111/j.1365-2249.1993.tb05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agache I, Doros IC, Leru PM, Bucur I, Poenaru M, Sarafoleanu C. MP-AzeFlu provides rapid and effective allergic rhinitis control: Results of a non-interventional study in Romania. Rhinology. 2018;56:33–41. doi: 10.4193/Rhin16.278. [DOI] [PubMed] [Google Scholar]
- 7.Leru PM, Eftimie AM, Thibaudon M. First allergenic pollen monitoring in Bucharest and results of three years collaboration with European aerobiology specialists. Rom J Intern Med. 2017;56:27–33. doi: 10.1515/rjim-2017-0033. [DOI] [PubMed] [Google Scholar]
- 8.Perkins C, Wills-Karp E, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagawa H, Sone S, Haku T, Mizuno K, Yano S, Ohmoto Y, Ogura T. Contrasting effect of interleukin-13 on interleukin-l receptor agonist and proinflammatory cytokme production by human alveolar makrophages. Am J Respir Cell Mol Biol. 1995;12:71–76. doi: 10.1165/ajrcmb.12.1.7811472. [DOI] [PubMed] [Google Scholar]
- 11.Morawetz RA, Gabriele L, Rizzo LV, Noben-Trauth N, Kühn R, Rajewsky K, Müller W, Doherty TM, Finkelman F, Coffman RL, Morse HC III. Interleukin (IL)-4-independent immunoglobulin class switch to immunoglobulin (Ig)E in the mouse. J Exp Med. 1996;184:1651–1661. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngoc LP, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 13.Coyle AJ, Tsuyuki S, Bertrand C, Huang S, Aguet M, Alkan SS, Anderson GP. Mice lacking the IFN-gamma receptor have impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T cells to exhibit a Th2 cytokine profile. J Immunol. 1996;156:2680–2685. [PubMed] [Google Scholar]
- 14.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–985. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 15.Ten Hacken NH, Oosterhoff Y, Kauffman HF, Guevarra L, Satoh T, Tollerud DJ, Postma DS. Elevated serum interferon-gamma in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. Eur Respir J. 1998;11:312–316. doi: 10.1183/09031936.98.11020312. [DOI] [PubMed] [Google Scholar]
- 16.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139 (Suppl 4):S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon I, Ilie MA, Draghici C, Voiculescu VM, Căruntu C, Boda D, Zurac S. The impact of lifestyle factors on evolution of atopic dermatitis: An alternative approach. Exp Ther Med. 2019;17:1078–1084. doi: 10.3892/etm.2018.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton JD, Suárez-Fariñas M, Dhingra N, Cardinale I, Li X, Kostic A, Ming JE, Radin AR, Krueger JG, Graham N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293–1300. doi: 10.1016/j.jaci.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton J, Hamon S, Simpson E, Chaudhry U, Swanson B, Zhang R, Graham N, Pirozzi G, Ardeleanu M, Rizova E. 372 The effect of dupilumab on biomarkers in a randomized phase 2b clinical trial in adults with moderate-to-severe atopic dermatitis. J Invest Dermatol. 2016;136: (Suppl 2)(S224) [Google Scholar]
- 20.Eshtiaghi P, Gooderham MJ. Dupilumab: An evidence-based review of its potential in the treatment of atopic dermatitis. Core Evid. 2018;13:13–20. doi: 10.2147/CE.S133661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 22.Verheugen G. Commission directive 2005/28/EC laying down principles and guidelines for good clinical practice as regards investigational medicinal products for human use, as well as the requirements for authorization of the manufacturing or importation of such products. Off J Eur Union. 2005;91:13–19. [Google Scholar]
- 23. doi: 10.1111/j.1365-2125.1994.tb05705.x. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Retrieved from https://www.ema.europa.eu/en/partners-networks/international-activities/multilateral-organisations-initiatives/international-council-harmonisation-technical-requirements-registration-pharmaceuticals-human-use#ich-guidelines-and-technical-requirements-section. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects. JAMA. 1997;277:925–926. World Medical Association Declaration of Helsinki. doi:10.1001/jama.1997.03540350075038. [PubMed] [Google Scholar]
- 25.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 26. Global strategy for asthma management and prevention. Global Initiative for Asthma (GINA), 2018. URL: http://www.ginasthma.org. [Google Scholar]
- 27.Pawankar R. Inflammatory mechanisms in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2007;7:1–4. doi: 10.1097/ACI.0b013e3280145347. [DOI] [PubMed] [Google Scholar]
- 28.Leru PM, Deleanu DM. Romanian allergology in the actual European context. Rom J Intern Med. 2015;53:111–117. doi: 10.1515/rjim-2015-0015. [DOI] [PubMed] [Google Scholar]
- 29.Leru PM, Eftimie AM, Anton VF, Thibaudon M. Five-year data on pollen monitoring, distribution and health impact of allergenic plants in Bucharest and the Southeastern region of Romania. Medicina (Kaunas) 2019;55(140) doi: 10.3390/medicina55050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frieri M. Advances in the understanding of allergic asthma. Allergy Asthma Proc. 2007;28:614–619. doi: 10.2500/aap.2007.28.2952. [DOI] [PubMed] [Google Scholar]
- 31.Leru PM. Drug allergies in primary care practice in Romania: A questionnaire-based survey. Allergy Asthma Clin Immunol. 2014;10(16) doi: 10.1186/1710-1492-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavakkol Afshari J, Farid Hosseini R, Hosseini Farahabadi S, Heydarian F, Boskabady MH, Khoshnavaz R, Razavi A, Ghayoor Karimiani E, Ghasemi G. Association of the expression of IL-4 and IL-13 genes, IL-4 and IgE serum levels with allergic asthma. Iran J Allergy Asthma Immunol. 2007;6:67–72. [PubMed] [Google Scholar]
- 33.Boyton RJ, Altamann DM. Asthma: New developments in cytokine regulation. Clin Exp Immunol. 2004;136:13–14. doi: 10.1111/j.1365-2249.2004.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davoodi P, Mahesh PA, Holla AD, Vijayakumar GS, Jayaraj BS, Chandrashekara S, Ramachandra NB. Serum levels of interleukin-13 and interferon-gamma from adult patients with asthma in Mysore. Cytokine. 2012;60:431–437. doi: 10.1016/j.cyto.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Ceylan E, Bulut S, Yılmaz M, Örün H, Karadağ F, Ömürlü İK, Kırdar S, Karul A. The levels of serum biomarkers in stable asthma patients with comorbidities. Iran J Allergy Asthma Immunol. 2019;18:27–37. [PubMed] [Google Scholar]
- 36.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 37.Luttmann W, Matthiesen T, Matthys H, Virchow JC Jr. Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. Am J Respir Cell Mol Biol. 1999;20:474–480. doi: 10.1165/ajrcmb.20.3.3326. [DOI] [PubMed] [Google Scholar]
- 38.Leru PM. Eosinophilia and hypereosinophilic disorders-update on etiopathogeny, classification and clinical approach. Rom J Intern Med. 2015;53:289–295. doi: 10.1515/rjim-2015-0049. [DOI] [PubMed] [Google Scholar]
- 39.Fish SC, Donaldson DD, Goldman SJ, Williams CM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol. 2005;174:7716–7724. doi: 10.4049/jimmunol.174.12.7716. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 41.Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170:122–131. doi: 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- 42.Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol. 2000;115:81–87. doi: 10.1046/j.1523-1747.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 43.Morse HC III, McCarty T, Giese NA, Taddesse-Heath L, Grusby MJ. STAT6-deficient mice exhibit normal induction of murine AIDS and expression of immunoglobulin E following infection with LP-BM5 murine leukemia viruses. J Virol. 1999;73:7093–7095. doi: 10.1128/JVI.73.8.7093-7095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antczak A, Domańska-Senderowska D, Górski P, Pastuszak-Lewandoska D, Nielepkowicz-Goździńska A, Szewczyk K, Kurmanowska Z, Kiszałkiewicz J, Brzeziańska-Lasota E. Analysis of changes in expression of IL-4/IL-13/STAT6 pathway and correlation with the selected clinical parameters in patients with atopic asthma. Int J Immunopathol Pharmacol. 2016;29:195–204. doi: 10.1177/0394632015623794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland SK, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117:269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 47.Billiau A, Dijkmans R. Interferon-gamma: Mechanism of action and therapeutic potential. Biochem Pharmacol. 1990;40:1433–1439. doi: 10.1016/0006-2952(90)90437-p. [DOI] [PubMed] [Google Scholar]
- 48.Cho SH, Stanciu LA, Begishivili T, Bates PJ, Holgate ST, Johnston SL. Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin Exp Allergy. 2002;32:427–433. doi: 10.1046/j.1365-2222.2002.01281.x. [DOI] [PubMed] [Google Scholar]
- 49.Stanciu LA, Shute J, Holgate ST, Djukanović R. Production of IL-8 and IL-4 by positively and negatively selected CD4+ and CD8+ human T cells following a four-step cell separation method including magnetic cell sorting (MACS) J Immunol Methods. 1996;189:107–115. doi: 10.1016/0022-1759(95)00240-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.