Abstract
Background
The COVID-19 pandemic caused by SARS-COV-2 began in Wuhan, China in December 2019. Reports of COVID-19 with central (CNS) and peripheral nervous (PNS) system manifestations are emerging. In this systematic review, we compared and summarized the demographics, clinical features, Brighton criteria, immunological and laboratory findings with a focus on modified Erasmus GBS Outcome Score (mEGOS) in SARS-CoV-2 patients with GBS and its variants.
Methods
Based on PRISMA guidelines, we searched three databases (PubMed, Scopus, and Google Scholar) for studies on COVID-19 and GBS between December 1, 2019 to July 15, 2020. For descriptive analysis, we studied two groups with: 1) acute inflammatory demyelinating polyradiculoneuropathy (AIDP) variant, and 2) Non-AIDP/Other variants. We compared mEGOS scores for patients in both groups along with other key clinical features.
Results
Of the 50 GBS cases identified from 37 studies, 33 (66%) had acute inflammatory demyelinating polyradiculopolyneuropathy (AIDP) while 17 (34%) were of other (non-AIDP) variants. There mEGOS scores did not differ between AIDP patients and AMAN/AMSAN patients. Majority of the AIDP (66.7%) and AMAN/AMSAN (57.2%) patients belonged to Brighton level 1 indicating maximum diagnostic certainty.
Conclusion
To our knowledge, this is among the first reviews that includes GBS variants and the clinical prediction tool mEGOS for prognostication in COVID-19 patients. Further research is needed to assess whether IVIG is preferable over plasmapheresis in this population of GBS patients. It would also be crucial to follow these patients over time to identify the long-term disability as well as treatment outcomes.
Keywords: AIDP, AMSAN, Bickerstaff encephalitis, COVID-19, GBS, MFS, SARS-CoV-2
Abbreviations: nCov, Novel Coronavirus; AIDP, Acute inflammatory demyelinating polyneuropathy; AMSAN, Acute motor-sensory axonal neuropathy; AMAN, Acute motor axonal neuropathy; BFP, Bifacial weakness with paresthesias; BBE, Bickerstaff's brainstem encephalitis; MFS, Miller-Fisher syndrome; COVID-19, Coronavirus infectious disease-2019; GBS, Guillain-Barre Syndrome; MERS, Middle East Respiratory Syndrome; SARS-CoV-2, Severe Acute Respiratory Distress Syndrome coronavirus 2; IDSA/ATS, Infectious Disease Society of America/American Thoracic Society; HCQ, Hydroxychloroquine; PLEX, plasmapheresis; IVIG, Intravenous immunoglobulin; IL, Interleukin; mEGOS, Modified Erasmus GBS Outcome Score; IGOS, International GBS Outcome Study; EMG, Electromyography; MRI, Magnetic Resonance Imaging; CSF, Cerebrospinal fluid; HIV, Human immunodeficiency virus; RT-PCR, Reverse transcriptase polymerase chain reaction; WHO;, World Health Organization; MRC., Medical Research Council Scale for Muscle Strength
Highlights
-
•
Majority of GBS variants in COVID-19 patients were AIDP.
-
•
Most AIDP, AMAN/AMSAN patients exhibited Brighton level 1-maximum diagnostic certainty.
-
•
The mean latency between COVID-19 infection and GBS ranged between 11 and 13 days.
1. Introduction
The novel coronavirus disease-2019 (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was officially declared a pandemic on March 11, 2020 by the World Health Organization (WHO) due to its rapid spread worldwide [1]. Previous outbreaks of coronaviruses have included the severe acute respiratory syndrome (SARS) in 2002 and the Middle East respiratory syndrome (MERS) in 2012 [[1], [2], [3], [4]]. SARS-COV-2 novel coronavirus shares several common viral characteristics with SARS-CoV. Importantly, it has an even stronger affinity towards Angiotensin Converting Enzyme 2 (ACE2) receptor found in the human glial cells, neurons, respiratory epithelial and vascular endothelial cells [[5], [6], [7]]. Studies have found that the most frequent neurological manifestations among COVID-19 infected individuals are ischemic stroke, Guillain-Barré Syndrome (GBS) and encephalopathy due to ICU syndrome, cytokine storm with high fevers and ventilator use [8,9]. Similar neurological outcomes have been reported in previous coronavirus epidemics caused by SARS-CoV and MERS-CoV [[10], [11], [12]].
GBS is an acute immune mediated polyradiculoneuropathy that affects motor, sensory and autonomic nerves. It presents with a wide range of neurological manifestations, the most serious being rapidly progressive flaccid paralysis [[13], [14], [15], [16]]. The most severe manifestation leads to acute respiratory failure [[15], [16], [17]]. Overlap of respiratory paralysis in GBS and COVID-19 infection makes it critically important for the physicians to diagnose and manage GBS early in all patients of COVID-19, recognizing that respiratory compromise due to GBS may be rapidly progressive but treatable with a high success rate in COVID-19 patients [14,[18], [19], [20]].
The common variants of GBS are: Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) which is a motor sensory demyelinating disorder; and Acute motor axonal neuropathy (AMAN), and Acute motor and sensory axonal neuropathy (AMSAN), both of which are axonal disorders. Other rare variants of GBS include: Miller Fisher Syndrome (MFS), paraparetic GBS, pharyngeal-cervical-brachial weakness, bilateral facial palsy with paresthesia (BFP), Bickerstaff brainstem encephalitis (BBE) which can overlap with MFS, polyneuritis cranialis and acute autonomic neuropathy, which like acute pure sensory neuropathy has an uncertain relationship to other variants of GBS [17,21].
Although many microorganisms, viruses, bacteria and mycoplasma have been identified as triggers for GBS, including influenza, HIV, Zika virus, SARS and MERS, EBV, CMV, C. Jejuni [10,11,13,[21], [22], [23], [24], [25]]; new cases reported during the current pandemic have led to the recognition of GBS as a neurological complication of SARS-CoV-2, rather than being present coincidentally [5, [5,8,15,16,18,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. Early recognition and treatment with intravenous immunoglobulin (IVIG) or plasma exchange/plasmapheresis (PLEX), along with supportive care remains the mainstay of therapy [14,17,19].
Using a total of 37 case reports and case series comprising 50 patients with COVID-19 and GBS worldwide, we conducted a systematic review to compare and summarize the clinical characteristics and outcomes in SARS-CoV-2 patients with GBS and its variants. Our review focuses on comparing the reported distribution of variants of GBS among COVID-19 patients: AIDP and the other GBS variants (including AMSAN, AMAN, MFS, BFP, Polyneuritis cranialis). We examined clinical characteristics, electromyographic (EMG) findings, modified Erasmus GBS Outcome Score (mEGOS) which we calculated for the 24 cases of AIDP and 4 cases of AMAN/AMSAN where there was sufficient clinical information. We could not calculate it for the other 13 AIDP and AMAN/AMSAN cases and did not calculate it for MFS and BFP variant here it is not germane, using International Guillain-Barré Syndrome Outcome Study (IGOS) GBS prognosis tool [58], Brighton criteria which is used for confirmation of GBS diagnosis using clinical presentation, examination findings and diagnostic testing [59], treatment, severity, outcomes, imaging and laboratory findings. We further explored the case series and case reports on MERS and SARS associated neuromuscular manifestation and when possible, compared it to GBS in association with COVID-19 [[10], [11], [12]].
2. Methods
2.1. Study design
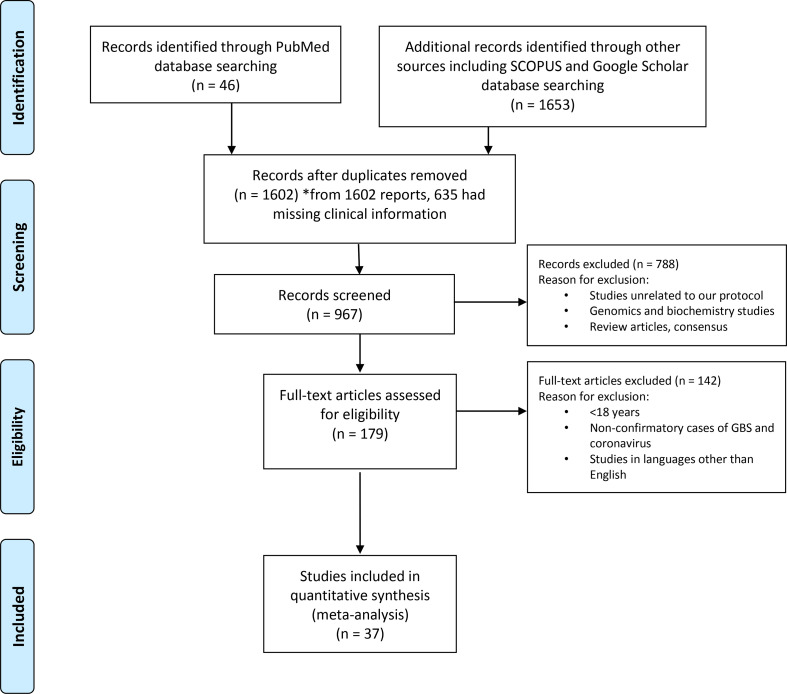
We conducted a thorough literature review in July 2020 using the terms “COVID-19 and GBS”, “SARS and GBS” and “MERS and GBS”. We searched PubMed, Google Scholar and Scopus databases for identifying case series and case reports published between December 1, 2019 to July 15, 2020 for COVID-19; January 01, 2002 to December 31, 2004 for SARS; January 1, 2012 to December 31, 2018 for MERS. Two reviewers independently conducted the search to identify the studies matching the keywords used. Studies describing the cases with SARS-CoV-2, SARS and MERS with GBS were included in the study (Fig. 1 ), while review articles and consensus statements were excluded from the analysis. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for the display of inclusions and exclusions [60]. Based on our search criteria, we found 1699 studies from PubMed, Google Scholar and Scopus. Duplicate studies, studies with missing clinical data, review articles and articles unrelated to our study objective were excluded and 179 full-text literatures were reviewed in accordance with our study objective. We included 40 studies for review that met our above-mentioned inclusion criteria, out of which 37 were of COVID-19 and GBS, 2 were of SARS and GBS and 1 was of MERS and GBS. We excluded studies on SARS and MERS from statistical analysis due to low sample size although we briefly describe these cases in Discussion. Therefore 37 studies of COVID-19 and GBS were reviewed for descriptive analysis (Figure-1).
Fig. 1.
PRISMA flow diagram of systemic review. The flow diagram depicts the flow of information through the different phases of the systematic review. It maps out the number of records identified, included and excluded, and the reasons for exclusions.
2.2. Inclusion criteria
The inclusion criteria for the published studies included: 1) Patient age ≥ 18 years; 2) COVID-19 diagnosis confirmed by RT-PCR nasopharyngeal or serum antibody test; 3) GBS confirmed through clinical presentation, and diagnostic tests inclusive of EMG and cerebrospinal fluid (CSF) studies.
2.3. Exclusion criteria
The exclusion criteria for the studies include: 1) Patient age < 18 years; 2) COVID-19 patients with diagnosis other than GBS such as myopathy, toxic induced polyneuropathy, critical illness polyneuropathy (CIP) and critical illness myopathy; 3) Duplicate studies which involved repetition of cases 4) Studies in languages other than English; 5) Exclusion of studies with GBS which did not have confirmatory diagnosis of COVID-19.
Furthermore, we excluded a patient from a case series study as the GBS variant was not described [44]. This resulted in a total of 50 cases from 37 studies as the final count for our review.
2.4. Quality assessment
The critical appraisal checklist for case reports provided by the Joanna Briggs Institute (JBI) was used to perform assessment of overall quality of case series and case reports.
2.5. Data acquisition
From the selected studies, we extracted the following variables for our analysis: study type, date of publication, country of case origin, age, gender, clinical presentation of GBS and its variants (including paraparesis/quadriparesis, cranial nerve deficits and diarrhea), diagnostic tests for SARS-CoV-2 infection in including RT-PCR nasopharyngeal and serum antibodies, latency between COVID-19 symptom onset and initial symptoms of GBS, severity of COVID-19 (based on IDSA/ATS criteria which includes either vasopressor use due to septic shock or requirement of mechanical ventilation [61], mEGOS scoring scale that we calculated based on clinical data reported in paper, treatments including standard commercially available IVIG, PLEX, chloroquine, hydroxychloroquine (HCQ), azithromycin, IL-6 blockers (tocilizumab), corticosteroids, cerebrospinal fluid (CSF) total protein levels, anti-ganglioside antibodies, imaging findings, EMG/NCS findings, Brighton electrophysiological criteria and mortality outcomes.
2.6. Data analysis
Pooled descriptive analyses were conducted to assess differences among two main types of GBS variants for all patients across the 37 case reports and case studies: 1) AIDP and 2) Others GBS variants (comprising of AMSAN, AMAN, BFP, MFS, Polyneuritis cranialis). We assessed the differences in two groups for the above-mentioned variables using chi-square test for categorical covariates and t-test for continuous covariates. Further, sub-analysis of the differences in frequencies and percentages was performed among three groups consisting of AIDP vs. AMSAN/AMAN vs. Others, using chi-square test. Stata v15 (Statacorp, College Station, TX) was used to conduct the analysis.
3. Results
A total of 50 patients with COVID-19 diagnosed with GBS were used for analyses from the 37 case reports and case series published in 13 different countries. Table 1 shows a detailed breakdown of studies with information on their respective country, type of study (case series/case report), number of patients in the study, age, gender, and type of GBS variant. Of the 50 cases, 12 were from Italy, 8 from the US, 6 each from Iran and Spain, 4 each from France and Switzerland, 3 from Germany, 2 from the UK, and 1 each from Austria, Canada, China, Morocco and Netherlands. Of all the 50 cases, 66% (n = 33) were of AIDP variant, 14% (n = 7) MFS variant, 12% (n = 6) AMSAN variant, 4% (n = 2) BFP variant, and 2% (n = 1) each of AMAN and polyneuritis cranialis variants.
Table 1.
Study origin, types, demographics and GBS variants.
| S. No. | Author | Country | Type of study | No. of patient | Mean age | Gender | GBS variant |
|---|---|---|---|---|---|---|---|
| 1 | D Ottaviani et al. [16] | Italy | Case Report | 1 | 66 | F | AIDP |
| 2 | Pfefferkorn et al. [46] | Germany | Case Report | 1 | 51 | M | AIDP |
| 3 | Scheidl et al. [39] | Germany | Case Report | 1 | 54 | F | AIDP |
| 4 | Hutchins et al. [47] | USA | Case Report | 1 | 21 | M | BFP |
| 5 | Arnaud et al. [48] | France | Case Report | 1 | 64 | M | AIDP |
| 6 | Su X.W.et al. [49] | USA | Case Report | 1 | 72 | M | AIDP |
| 7 | Riva et al. [37] | Italy | Letter to Editor | 1 | 60 | M | AIDP |
| 8 | Otmani et al. [31] | Morocco | Case Report | 1 | 70 | F | AMSAN |
| 9 | Camdessanche et al. [18] | France | Case Report | 1 | 64 | M | AIDP |
| 10 | Solomon et al. [28] | Spain | Case Report | 1 | 61 | M | BFP |
| 11 | Webb et al. [42] | UK | Case Report | 1 | 57 | M | AIDP |
| 12 | Assini et al. [15] | Italy | Case Report | 2 | 57.5 | 2 M | MFS, AMSAN |
| 13 | Toscano et al. [8] | Italy | Letter to Editor | 5 | 58.4 | 1 F, 4 M | 2 AIDP, 1 AMAN, 2 AMSAN |
| 14 | Dinkin et al. [44] | USA | Case Series | 2 | 53.5 | 1 F, 1 M | 1 MFS, 1 N/A |
| 15 | Gutierrez-Orti et al. [32] | Spain | Article | 2 | 44.5 | 2 M | 1 MFS, 1 Polyneuritis cranialis |
| 16 | Sedaghat et al. [40] | Iran | Case Report | 1 | 65 | M | AMSAN |
| 17 | Zhao et al. [43] | China | Letter to Editor | 1 | 61 | F | AIDP |
| 18 | Virani et al. [41] | USA | Case Report | 1 | 54 | M | AIDP |
| 19 | Alberti et al. [26] | Italy | Letter to Editor | 1 | 71 | M | AIDP |
| 20 | Padroni et al. [35] | Italy | Letter to Editor | 1 | 70 | F | AIDP |
| 21 | Coen et al. [30] | Switzerland | Letter to Editor | 1 | 70 | M | AIDP |
| 22 | Mozhdehipanah et al. [50] | Iran | Case Series | 3 | 53 | 1 M, 2F | 2 AIDP, 1 AMSAN |
| 23 | Tiet et al. [51] | UK | Case Report | 1 | 49 | M | AIDP |
| 24 | Ebrahimzadeh et al. [52] | Iran | Case Series | 2 | 55.5 | 2 M | 2 AIDP |
| 25 | Chan et al. [57] | USA | Case Series | 2 | 76 | 2 M | 2 AIDP |
| 26 | Rana et al. [36] | USA | Case Report | 1 | 54 | M | MFS |
| 27 | Bigaut et al. [27] | France | Scientific note | 2 | 56.5 | 1 M, 1F | 2 AIDP |
| 28 | Chan J et al. [29] | Canada | Case Report | 1 | 58 | M | AIDP |
| 29 | Helbok et al. [53] | Austria | Case Report | 1 | 68 | M | AIDP |
| 30 | Kilinc et al. [33] | Netherland | Case Report | 1 | 50 | M | AIDP |
| 31 | Lantos et al. [34] | USA | Case Report | 1 | 36 | M | MFS |
| 32 | Agustina et al. [45] | Switzer-land | Case Series | 3 | 58.6 | 3F | 3 AIDP |
| 33 | Reyes et al. [5] | Spain | Case Report | 1 | 51 | F | MFS |
| 34 | Sancho et al. [38] | Spain | Case Report | 1 | 56 | F | AIDP |
| 35 | Agosti et al. [54] | Italy | Case Report | 1 | 68 | M | AIDP |
| 36 | Lampe et al. [55] | Germany | Case Report | 1 | 65 | M | AIDP |
| 37 | Fernandes et al. [56] | Spain | Case Report | 1 | 64 | F | MFS |
M – Male; F – Female; AIDP- Acute Inflammatory demyelinating polyneuropathy; AMSAN - Acute motor and sensory axonal neuropathy; AMAN - Acute motor axonal neuropathy; BFP- Bifacial weakness with paresthesias; MFS - Miller-Fisher syndrome.
Table 2, Table 3 display the clinical characteristics of GBS and its variants. There was a significant difference in both the groups (AIDP vs. Non AIDP/Other GBS variants) with regards to age, p-value = 0.02 (Table 2). Furthermore, the age difference was not significant (p = 0.08) while comparing three variant groups (AIDP vs AMSAN/AMAN vs Others) (Table 3). There were 35 males and 15 females in the study. Seventy percent of the AIDP patients, 58% AMSAN/AMAN patients and 80% of the other variants were males.
Table 2.
Descriptive characteristics of cases with Guillain-Barre Syndrome with COVID-19 (n = 50) by variant subtype (AIDP vs. Non-AIDP/Othera)⁎.
| Characteristics | GBS subtype |
P-value | |
|---|---|---|---|
| AIDP n (%) |
Non-AIDP/other n (%) |
||
| Demographics | |||
| Number of patients | 33 (66.0) | 17 (34.0) | |
| Age, years (Mean ± SDbc) | 62 ± 9.9 | 52 ± 16.3 | 0.02⁎⁎ |
| Gender | 0.95 | ||
| Male | 23 (69.7) | 12 (70.6) | |
| Female | 10 (30.3) | 5 (29.4) | |
| Laboratory tests | |||
| RT-PCR Nasopharyngeal test (SARS-CoV-2) | 0.67 | ||
| Positive | 29 (90.6) | 16 (94.1) | |
| Negative | 3 (9.7) | 1 (5.9) | |
| Serological SARS-CoV-2 antibody test (confirmatory) | 6 (100.0) | 1 (100.0) | – |
| Antiganglioside antibody | 1 (4.8) GM2 IgM, IgG |
1 (9.1) GD1b-IgG |
0.63 |
| Mechanical ventilation | 1.00 | ||
| Ventilated | 20 (62.5) | 10 (62.5) | |
| Not ventilated | 12 (37.5) | 6 (37.5) | |
| Outcome | 0.74 | ||
| Survived | 27 (90.0) | 13 (86.7) | |
| Dead | 3 (10.0) | 2 (13.3) | |
| mEGOS score (Mean ± SD)d, e | 6.8 ± 3.8 | 8 ± 5.2 | 0.57 |
p < 0.05 indicates significant
Other includes following subtypes: AMSAN, AMAN, BFP, MFS
SD = Standard Deviation
CSF: Cerebrospinal fluid
mEGOS: Modified Erasmus GBS Outcome Score
mEGOS only calculated for AMSAN and AMAN in ‘Non-AIDP/Other’ Group
AIDP - Acute Inflammatory demyelinating polyneuropathy; AMSAN - Acute motor and sensory axonal neuropathy; AMAN - Acute motor axonal neuropathy; BFP - Bifacial weakness with paresthesias; MFS - Miller-Fisher syndrome; mEGOS – Modified Erasmus GBS outcome score, COVID-19 - Coronavirus infectious disease-2019; RT-PCR – Reverse transcriptase polymerase chain reaction; CSF - Cerebrospinal fluid.
Table 3.
Descriptive characteristics of cases with Guillain-Barre Syndrome with COVID-19 (n = 50) by variant subtype (AIDP vs. AMSAN/AMAN vs. Othersa)⁎.
| Characteristics | GBS subtype |
P-value | ||
|---|---|---|---|---|
| AIDP n (%) |
AMSAN/AMAN n (%) |
Others n (%) |
||
| Demographics | ||||
| Number of patients | 33 (66.0) | 7 (14) | 10 (20) | |
| Age, years (Mean ± SDb) | 62 ± 9.9 | 58 ± 17.3 | 48 ± 15.1 | 0.08 |
| Gender | 0.60 | |||
| Male | 23 (69.7) | 4 (57.14) | 8 (80) | |
| Female | 10 (30.3) | 3 (42.8) | 2 (20) | |
| Clinical presentation | ||||
| Ascending paralysis | 30 (90.9) | 6 (85.7) | 3 (30.0) | <0.001⁎⁎ |
| Paraparesis | 12 (36.4) | 2 (28.5) | 3 (17.7) | 0.28 |
| Quadriparesis | 16 (48.5) | 1(14.3) | 1 (10) | 0.04 |
| Quadriplegia | 2 (6.1) | 3 (42.8) | 0 (0.0) | 0.01 |
| Cranial Nerve III palsy | 0(0.00) | 0(0.00) | 6(60.0) | NA |
| Cranial Nerve VI palsy | 0(0.00) | 0(0.00) | 3(30.0) | NA |
| Cranial Nerve VII palsy | 16(48.5) | 3(42.9) | 5(50.0) | NA |
| Cranial Nerve X palsy | 4(12.2) | 1(14.3) | 1(10.0) | NA |
| Cranial Nerve XII palsy | 2(6.0) | 1(14.3) | 1(10.0) | NA |
| Preceding infection | ||||
| Diarrhea | 8 (24.2) | 0 (0.0) | 4 (40) | 0.16 |
| Duration between CoV infection and GBS presentation (Days, Mean ± SD) | 12.5 ± 7.7 | 11.1 ± 4.9 | 9.2 ± 6.0 | 0.34 |
| CSFc | ||||
| Protein, mg/dl (Mean ± SD) | 101 ± 61.6 | 103 ± 52.9 | 65.7 ± 23.7 | 0.06 |
| Albumino-cytological dissociation | 0.74 | |||
| Present | 26 (83.9) | 5 (83.3) | 5 (71.4) | |
| Absent | 5 (16.1) | 1 (16.7) | 2 (28.5) | |
| Brighton criteria | Total | |||
| Level 1 | 22 (66.7) | 4 (57.4) | 1 (10.0) | 27(55%) |
| Level 2 | 8 (24.2) | 3 (42.9) | 1(10.0) | 12(24%) |
| Level 3 | 2(6.1) | 0(0.0) | 0(0.0) | 2(4%) |
| Level 4 | 1(3.0) | 0(0.0) | 8(80.0) | 9(18%) |
| Total | 33(100.0) | 7(100.0) | 10(100.0) | 50(100%) |
p < 0.05 indicates significant.
Other includes following subtypes: BFP, MFS.
SD = Standard Deviation.
CSF: Cerebrospinal fluid.
AIDP - Acute Inflammatory demyelinating polyneuropathy; AMSAN - Acute motor and sensory axonal neuropathy; AMAN - Acute motor axonal neuropathy; BFP - Bifacial weakness with paresthesias; MFS - Miller-Fisher syndrome; mEGOS – Modified Erasmus GBS outcome score, COVID-19 - Coronavirus infectious disease-2019; CSF - Cerebrospinal fluid.
We explored the differences between groups with regards to laboratory testing for COVID-19 (Table 2). All the patients included in the study underwent confirmatory testing for diagnosis of COVID-19. Among the AIDP group, 29 cases had RT-PCR positive, 3 negative and 6 cases had SARS-CoV2 IgG positive. For the non-AIDP group, 16 patients tested positive with RT-PCR, one patient tested negative and SARS-CoV2 antibody was positive. Ganglioside antibody tests were reported for 28 patients, 17 patients in the AIDP group and 11 in Non-AIDP group. One patient in AIDP and one patient in non-AIDP group were positive for antiganglioside antibodies. Further, we also explored the mean mEGOS scores for AIDP patients and compared them with AMAN/AMSAN patients for the probability of walking independently after 6 months of admission. Mean mEGOS score for patients with AIDP variant (6.8 ± 3.8) was lower compared to AMSAN/AMAN (8 ± 5.2), p = 0.57 (Table 2).
Table 3 shows the pattern of symptomatology among three groups (AIDP, AMSAN/AMAN, and Others [BFP, MFS, and Polyneuritis Cranialis]). Although non-significant, a greater proportion of AIDP-GBS variant patients reported paraparesis (36.4%) and quadriparesis (48.5%) as compared to AMSAN/AMAN patients (28.5% paraparesis and 14.3% quadriparesis). There was a significantly greater proportion of patients with ascending paralysis in AIDP-GBS variant (90.9%) compared to AMSAN/AMAN-GBS variant patients (85.7%) and other variants (30%). Conversely, 48.5%, 12.2% and 6% patients of AIDP-GBS variant had CN VII palsy, CN IX and X palsy, respectively, compared to 42.9%, 14.3% and 14.3% of patients with AMSAN/AMAN-variant GBS (Table 3). A total of 8(24%) patients presented with diarrhea in the AIDP sub-group, whereas 4 cases with diarrhea (40%) were reported in other variants; no diarrhea was reported in AMSAN/AMAN variants sub-group (Table 3). The mean latency for the AIDP group was 12.5 ± 7.7, for AMSAN/AMAN was 11.1 ± 4.9 and for others was 9.2 ± 6.0 (0.34).
CSF protein levels were highest in the AMAN/AMSAN (103.1 + 52.9), and AIDP groups (100.5 + 61.5) and then other variants (65.7 + 23.7) but the differences were not significant. Further details of clinical characteristics are described in Table 3. Albuminocytological dissociation was present in 26 out of 31 AIDP patients (84%), 5 out of 6 patients in AMSAN/AMAN (83%) and 5 out of 7 patients with other variants (71%) (refer Table 3).
In our entire cohort, we found a total of 17 cases in which MRI imaging of brain and cranial nerves was reported. Among them, 6 (35%) had abnormal findings that included cranial nerve CN III, CN VI and CN VII enhancement. Apart from these, one case of leptomeningeal enhancement of brainstem and cervical spine was noted (24). MRI of the lumbosacral spine was also performed in 36% of the cases (18/50), of which 5 (27%) were found to have abnormal spine nerve root enhancement and the remaining 73% (13/18) had normal spine imaging.
Finally, we assessed whether the patients fulfilled the Brighton criteria for diagnosis of GBS. All the patients fulfilled the Brighton Criteria and were further divided into separate levels. Level 1 of Brighton criteria indicates the maximum diagnostic certainty while Level 4 indicates the least diagnostic certainty. We found that 66.7% (22/33) of AIDP patients belonged to Level 1 of the Brighton criteria, 24.2% (8/33) patients belonged to Level 2, 6% (2/33) patients belonged to Level 3 and 3% of the patients (1/33) belonged to Level 4. Among the 7 patients in the AMAN/AMSAN group, 4 patients (57.2%) belonged to Level 1 and 3 patients (42.8%) belonged to Level 2. Out of the 10 patients belonging to other variants, only 1 patient each (10% each) belonged to Level 1 and 2 respectively and 8 (80%) patients belonged to Level 4 indicating least diagnostic certainty (refer Table 3).
We also compared differences in treatments of GBS variant groups administered for both COVID-19 and GBS (refer Table 4 ). For COVID-19, this included use of HCQ, antivirals, IL-6R blockers and antibiotics. For GBS, it included use of IVIG and PLEX. Noticeably, a significantly greater proportion of patients with AIDP variant reported HCQ use, compared to patients with other variants (52.9% vs. 21.1%). For other treatments, no significant differences were found between the two groups. In our review, 44 patients received IVIG, out of which 30 (68%) had AIDP and 14 (32%) had other GBS variants. No data was available for treatment of the remaining patients. Out of all the patients who received IVIG, 10 patients (22.8%) had prolonged hospitalization at the time of reporting, 5 of whom still needed ICU care and only 5 had a fatal outcome among which 3 had AIDP and 2 patients had AMAN/AMSAN. In 3 cases (6.8%), outcomes were not reported. Recovery was observed in 31 patients (70.4%), of which, 21 had AIDP and 10 had other variants. However, this data is limited by lack of long term follow up.
Table 4.
Comparison of treatments used for GBS and COVID-19 by GBS variants.
| Management | AIDP n (%) |
Non-AIDP/ other variants n (%) |
p-Value |
|---|---|---|---|
| IVIG | 30 (90.9) | 14 (82.4) | 0.38 |
| Plasmapheresis (PLEX) | 6 (18.2) | 1 (5.9) | 0.24 |
| Hydroxychloroquine (HCQ) | 9 (52.9) | 7 (21.1) | 0.02* |
| Antivirals | 6 (35.3) | 7 (21.2) | 0.28 |
| IL-6 blocker | 1 (5.9) | 0 (0.0) | 0.16 |
| Antibiotics | 5 (29.4) | 3 (9.1) | 0.06 |
IVIG- Intravenous immunoglobulin; IL-6 – Interleukin 6; AIDP – Acute inflammatory demyelinating polyneuropathy.
Finally, Table 5 shows a detailed breakdown of the studies with respect to Brighton criteria, mEGOS scores and mEGOS score percentage probability of being unable to walk independently after 6 months of admission, EMG findings, to summarize our reported findings.
Table 5.
Electromyographic features mEGOS score, Brighton Criteria for COVID-19 and GBS and its variant.
| Author/country All studies from year (2020) |
⁎Time from neurological presentation to EMG | GBS subtypes based on original article | ⁎⁎mEGOS at day 7 of admission | Percentage ability to walk after 6 months | NCS findings consistent with one of the subtypes of GBS | ⁎⁎⁎Brighton Criteria Level of diagnostic Certainty (1–4) |
|---|---|---|---|---|---|---|
| Ottaviani D et al. [16] / Italy | 10 days | AIDP | 11 | 56% | Prolonged DL and slowed CV in tibial/peroneal nerves | 1 |
| Pfefferkorn T et al. [46] / Germany | 2 days | AIDP | No data available | No data available | Reported as demyelinating pattern, no EMG data available | 2 |
| Scheidl E et al. [39] / Germany | 10 days | AIDP | 1 | 2% | Prolonged distal latency but preserved CV in 1 nerve (peroneal) | 1 |
| Hutchins K.L et al. [47] / USA | 3 days | BFP | No data available | No data available | Slow peroneal and median nerve prolong DL and slow CV | 4 |
| Arnaud S et al. [48] / France | 5 days | AIDP | No data available | No data available | Slow CV in bilateral tibial and peroneal nerves | 1 |
| Su X.W.et al [49] / USA | 13 days | AIDP | 12 | 66% | Prolonged DL and slow CV in tibial and peroneal nerves | 1 |
| Riva N et al. [37] / Italy | 5 days | AIDP | 7 | 18% | There is conduction block and slow CV in peroneal and median nerves | 2 |
| Otmani H. EL et al. [31] / Morocco | 10 days | AMSAN | No data available | No data available | Reported as acute motor and sensory axonal neuropathy pattern, no EMG data available | 1 |
| Camdessanche J.P. et al. [18] / France | 5 days | AIDP | 11 | 56% | B/L tibial nerves distally with slow CV and prolonged DL | 1 |
| Caamaño D.S.J. et al. [28] / Spain | NA | BFP | NA | NA | EMG data not available | 4 |
| Webb S et al. [42] / U.K. | 3 days | AIDP | 8 | 25% | Prolonged DL and slowed CV in tibial/peroneal nerves | 1 |
| Assini A et al. [15] / Italy | NA | MFS | NA | NA | Reported as demyelinating pattern, no EMG data available | 4 |
| Assini A et al. [15] / Italy | NA | AMSAN | No data available | No data available | Reported as acute motor and sensory axonal neuropathy pattern, no EMG data available | 2 |
| Toscano G et al. [8] / Italy | 2 days | AMSAN | 11 | 56% | Reduced amplitudes in tibial/ulnar nerves (ulnar sensory) | 1 |
| Toscano G et al. [8] / Italy | 12 days | AMSAN | 0 | 1% | Tibial nerve with reduced amplitudes and mildly prolonged DL and ulnar sensory reduced amplitude | 1 |
| Toscano G et al. [8] / Italy | 1 day | AMAN | 10 | 45% | Tibial and ulnar motor nerves reduced amplitudes | 1 |
| Toscano G et al. [8] / Italy | 2 days | AIDP | No data available | No data available | Prolonged DL and slow CV in tibial nerve, prolonged F wave | 2 |
| Toscano G et al. [8] / Italy | 4 days | AIDP | 11 | 56% | Prolonged DL and slow CV in tibial nerve | 2 |
| Dinkin M et al. [44] / USA | NA | MFS | NA | NA | EMG data not available | 4 |
| Dinkin M et al. [44] / USA | NA | N/A | 2 | 2% | EMG data not available | 4 |
| Gutierrez-Orti C et al. [32] / Spain | NA | MFS | NA | NA | EMG data not available | 4 |
| Gutierrez-Orti C et al. [32] / Spain | NA | Polyneuritis Cranialis | NA | NA | EMG data not available | 4 |
| Sedaghat Z et al. [40] / Iran | 9 days | AMSAN | 11 | 56% | Prolonged CV and reduced amplitude in tibial nerves | 2 |
| Zhao H et al. [43] / China | 5 days | AIDP | 5 | 8% | Preserved CV only peroneal nerve has prolonged DL. | 1 |
| Virani A et al. [41] / USA | Not done | AIDP | 11 | 56% | EMG data not available | 3 |
| Alberti P et al. [26] / Italy | NA | AIDP | No data available | No data available | Slow CV and prolonged DL in peroneal nerve | 1 |
| Padroni M et al. [35] / Italy | 2 days | AIDP | 2 | 2% | Preserved CV only peroneal nerve has prolonged DL/Equivocal as only findings slow CV at median and ulnar nerve | 1 |
| Coen M et al. [30] / Switzerland | NA | AIDP | No data available | No data available | Reported as demyelinating pattern, no EMG data available | 1 |
| Mozhdehipanah H et al. [50] / Iran | 6 days | AIDP | 0 | 1% | Prolonged DL bilateral tibial and slow CV in tibial | 1 |
| Mozhdehipanah H et al. [50] / Iran | N/A | AMSAN | No data available | No data available | Fulfill criteria reduced amplitude intact DL and preserved CV- tibial/peroneal | 1 |
| Mozhdehipanah H et al. [50] / Iran | N/A | AIDP | 8 | 25% | Fulfill criteria prolong DL and slow CV in median ulnar, tibial, ulnar nerve | 1 |
| Tiet M.Y.et al. [51] / U.K. | N/A | AIDP | 10 | 45% | Fulfill criteria slow CV in median, prolong DL in median, ulnar, tibial | 1 |
| Ebrahimzadeh S.A. et al. [52] / Iran | 7 days | AIDP | 4 | 6% | Preserved CV | 1 |
| Ebrahimzadeh S.A. et al. [52] / Iran | 4 days | AIDP | 5 | 8% | Preserved CV | 2 |
| Chan M. et al. [57] / USA | Not done | AIDP | 2 | 2% | EMG not performed | 2 |
| Rana S. et al. [36] / USA | 21 days | MFS | NA | NA | Prolong DL in most nerves and slow CV | 2 |
| Bigaut K. et al. [27] / France | 9 days | AIDP | 2 | 2% | Reported as demyelinating pattern, no EMG data available | 1 |
| Bigaut K. et al. [27] / France | 7 days | AIDP | 6 | 12% | Reported as demyelinating pattern, no EMG data available | 1 |
| Chan J.L. et al. [29] / Canada | 6 days | AIDP | 1 | 2% | EMG data not available | 4 |
| Helbok R. et al. [53] / Austria | 3 days | AIDP | 8 | 25% | Preserved CV and with mildly prolonged DL- tibial/peroneal/ulnar | 1 |
| Kilinc D. et al. [33] / Netherlands | N/A | AIDP | No data available | No data available | EMG data not available | 2 |
| Lantos J.E. et al. [34] / USA | NA | MFS | NA | NA | EMG data not available | 4 |
| Lascano A.M. et al. [45] / Switzerland | N/A | AIDP | 11 | 56% | EMG data not available | 1 |
| Lascano A.M. et al. [45] /Switzerland | N/A | AIDP | No data available | No data available | EMG data not available | 2 |
| Lascano A.M. et al. [45] / Switzerland | N/A | AIDP | No data available | No data available | EMG data not available | 1 |
| Reyes-Bueno J.A. et al. [5] / Spain | 10 Days | MFS | NA | NA | EMG data not available | 1 |
| Sancho-Saldaña A et al. [38] / Spain | 11 days | AIDP | 10 | 45% | EMG data not available | 1 |
| Agosti E. et al. [54] / Italy | 4 days | AIDP | 8 | 25% | Prolong DL in tibial, peroneal, CV are preserved | 1 |
| Lampe A. et al. [55] / Germany | 2 Days | AIDP | No data available | No data available | EMG data not available | 1 |
| Fernández-Domínguez J. et al. [56] / Spain | NA | MFS | 2 | 2% | Criteria not fulfilled | 4 |
• case 2 was not included based on exclusion criteria of age.
Abbreviations
CV - Conduction Velocity.
DL - Distal Latency.
B/L - Bilateral.
mEGOS - Modified Erasmus GBS outcome score.
AIDP - Acute inflammatory demyelinating polyneuropathy.
MFS - Miller Fisher variant.
AMSAN - Acute motor sensory axonal neuropathy.
AMAN - Acute motor axonal neuropathy.
BFP - Bifacial weakness with paresthesias.
EMG - Electromyography.
Days elapsed between neurological onset.
Estimated mEGOS score based on clinical description.
Brighton Criteria Level of diagnostic Certainty (1–4): Level 1 highest diagnostic certainty to level 4 with lowest diagnostic certainty.
4. Discussion
In current analysis, we identified and reviewed a total of 50 cases of GBS with COVID-19 from 39 studies identified worldwide through different case series and reports [5,8,15,16,18,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. The cases were categorized into two groups for further statistical analysis, “AIDP” versus “Non-AIDP/Other variants” which included MFS, AMSAN, BFP, AMAN and Polyneuritis cranialis; and further into “AMAN/AMSAN” and “other variants” within the non-AIDP group for subanalysis of specific variables where indicated. The novel addition to our review was use of Brighton criteria for strength of diagnosis and employment of mEGOS score for prognosis on the appropriate GBS variants. GBS is a relatively rare disease of the peripheral nervous system (PNS) having an incidence of 1.6 /100,000 person-years [17]. Studies in COVID-19 patients have suggested a link between GBS and SARS-CoV-2. A large Italian study of 1200 patients admitted with SARS-CoV-2 reported an incidence of 0.42% for GBS, much higher than that for the general population [8]. Recent studies interestingly found GBS as one of the most frequent neurological manifestations of peripheral nervous system in COVID-19 patients [5,8,15,16,18,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]].
The most frequent GBS variant in association with COVID-19 in our analysis was AIDP, which is consistent with the literature in general [17] nearly 66% of GBS cases had AIDP. We found significant differences between AIDP vs. Non-AIDP/Other variants in age at onset; the mean age for AIDP was 62 ± 9.9, 58 ± 17.3 for AMAN/AMSAN and 48+/− 15.1 for MFP, BFP and Polyneuritis cranialis.
A latency period between the onset of the GBS symptoms and onset of COVID-19 has been reported in recent papers [8,14,40]. A prior study by Caress et al. showed an average latency of 11 days from the onset of COVID symptoms to the presentation of GBS [14]. The mean latency between COVID-19 infection and presentation of GBS between AIDP, AMSAN/AMAN and MFP, BFP and Polyneuritis cranialis groups analyzed in our review did not vary significantly and ranged between a duration of 11 to 13 days. There are reports of GBS in SARS-COV-2 positive individuals who were asymptomatic from the point of view COVID-19 [29,39]. Additionally, Zhao et al. also reported case where the latency period was recorded as 0 days since GBS-like neurological features preceded the diagnosis of COVID-19 [43]. This latency between the onset of COVID-19 manifestations and GBS symptoms provides clues to the pathogenesis of GBS in SARS-CoV-2 infection. The postinfectious mechanism of GBS is supported by the finding of autoantibodies that result from an immune response directed to an epitope of the infectious agent that then cross-reacts with a structurally similar component of peripheral nerve, resulting in delayed immune-mediated damage to the peripheral nerve [21,52]. This has been well demonstrated in several GBS variants as well as GM1 gangliosides IgG Ab with C. jejuni infection [62] and has been postulated with other infectious agents including Mycoplasma pneumoniae, H pylori and several viruses [10,11,13,[21], [22], [23], [24], [25]].
For GBS triggered by SARS-COV-2, it is hypothesized that the attachment of SARS-CoV-2 to cell surfaces is mediated by the viral spike (S) protein, which binds to angiotensin-converting enzyme 2 Receptor and also to gangliosides containing sialic acid residues, including the GalNAc residue of GM1 [7,14,20]. It has been suggested that cross-reactivity between the viral protein–associated gangliosides and peripheral nerve gangliosides as the result of molecular mimicry. In our review, we identified 28 patients in the entire cohort (56%) for whom ganglioside antibody tests were performed. Serum ganglioside antibodies were found to be positive in 2 cases (7%), one in each group (i.e., AIDP and Non-AIDP/Other variants). GD1b IgG antibodies were positive in the MFS subtype of GBS case whereas GM2 IgM, IgG was positive in AIDP variant [32,57]. Interestingly, a case reported by Lantos and colleagues had equivocal lab values of GM1 antibody [34].
Alternatively, the mechanism of nerve damage may be primarily facilitated by T-cell activation and release of inflammatory mediators by macrophages. A systematic evaluation of associations of ganglioside antibodies in GBS with COVID-19 will be needed before the mechanisms are clarified. A novel parainfectious mechanism for GBS mediated by the generalized, hyperinflammatory response that occurs with COVID-19 was suggested by some authors because the acute symptoms overlap with the onset of GBS and autoantibodies were not detected in their cases [8,43]. However, when all of the cases are considered, the clinical, antiganglioside testing and electrodiagnostic patterns are similar to those of typical GBS cases [14,29,50].
RT-PCR nasopharyngeal swab and serological antibody tests are currently standard and recommended for diagnosing SARS-CoV-2 infection [63]. In our review, out of a total cohort of 50 patients, 49 patients (98%) underwent nasopharyngeal RT-PCR test. A positive test was obtained in 45 patients (91%) and the rest 4 (9%) had a negative test result. The remaining 5 cases (10%) were diagnosed with COVID-19 with a confirmatory serum SARS-CoV-2 IgG antibody test [5,8,33,37,53] (Table 2). Interestingly, none of the reported patients had positive PCR for SARS-CoV-2 in the CSF. The absence of evidence of active infection when the patients have clinical GBS infection supports an immune-mediated mechanism is the most likely pathophysiology behind GBS associated with SARS-CoV-2. Whether this immune-mediated process results from molecular mimicry triggered in the peripheral immune system or results from release of PNS antigens by earlier asymptomatic damage by the virus leading to release of PNS into the peripheral immune system which responds by initiating an autoimmune process is not clear [15,16,37]. Indeed, different scenarios in different patients are possible.
In addition to the clinical evaluation, CSF protein elevation is a known critical biomarker which can be a useful tool to identify the disease severity and extent [64] .Additionally, mean CSF total protein levels were highest among patients with AMAN/AMSAN (103.1 ± 52.9) and AIDP-GBS (101 ± 61.6 mg/dl) variants. For our analysis, we considered CSF total protein of >45 mg/dl as elevated. Albumino-cytological dissociation was found in 36 patients (72%), of which 26 had AIDP (72%) and 10 had other variants (28%) (Table 2).
Modified Erasmus GBS Outcome score (mEGOS) is a key prognostic indicator that helps predict the long-term outcomes of patients based on their clinical presentation at day 7 of admission. Therefore, the higher the score, the greater probability of inability to walk independently at 6 months after admission. This score has been shown to be of significant predictive value in multiple cohort studies in GBS patients [65,66]. On further analysis, the mean mEGOS score for both groups of patients (AIDP vs AMSAN/AMAN) were compared, and did not show a significant difference. Mean mEGOS score for patients with AIDP (6.8 ± 3.8) was considerably lower compared to AMAN/AMSAN variants (8 ± 5.2) (Table 5). We also used the Brighton criteria to differentiate the certainty of classification of the reported variants of GBS [59]. The Brighton criteria is an important tool to evaluate patients using different features for confirmation of diagnosis of GBS and classification of its variants, including MFS. It assesses the patient's clinical presentation, exam findings, and diagnostic testing to help scoring levels 1–4 of diagnostic certainty (level 1 being the highest certainty). The criteria are key is assisting with diagnosis in low to high risk patients, as well as prompt diagnosis early on in the course of disease. It also helps in guiding different treatment options according to the patient's diagnosis.
All of the cases included in our analysis fulfilled the Brighton Criteria. Majority of the AIDP cases (66.6%) and the AMAN/AMSAN cases (57.1%) belonged to Level 1, marking the highest diagnostic certainty. While the majority of the patients belonging to the other variants (80%) were in Level 4 indicating the least diagnostic certainty (Table 3).
In our entire cohort, we found a total of 17 cases in which MRI imaging of brain and cranial nerves was reported. Among them, 6 (35%) had abnormal findings that included cranial nerve CN III, CN VI and CN VII enhancement [8,27,29,34,44,47]. Apart from these, one case of leptomeningeal enhancement of brainstem and cervical spine was noted [27]. MRI of the lumbosacral spine was also performed in 36% of the cases (18/50), of which 5 (27%) were found to have abnormal spine nerve root enhancement and the remaining 73% (13/18) had normal spine imaging [8,27,45,46].
There is still no specific treatment for COVID-19. However, at the time of reporting of some of the cases in this study there was a proposed approval by WHO for the use of HCQ which was later withdrawn, and antivirals like Ritonavir, Lopinavir, some of which were also proven to be ineffective against COVID-19 and IL-6 receptor (R) blockers such as Tocilizumab as needed [67,68]. Dexamethasone has proved useful in severely affected patients likely by inhibiting the destructive excess inflammatory response in these patients [69]. Our analysis also included the treatment given for COVID-19 in both AIDP vs Non-AIDP/other variants group. 38 patients (76%) in the entire cohort received some form treatment including antivirals (13/38, 34%), antibiotics (8/38, 21%), IL-6R blocker (1/38, 2.6%) or hydroxychloroquine (16/38, 42%). Furthermore, 11 patients who received antivirals also received IVIG, 13 patients who got HCQ, and 1 patient who received IL-6 blocker, also received IVIG therapy in combination (Table 4).
Standard management for GBS includes IVIG and PLEX [70,71]. In our review, we found 44 patients receiving IVIG 30 patients (68%) were in the AIDP group while 14 (32%) were in Non-AIDP/Other variants (Table 4). We further reviewed the number of patients receiving 0.4 g/kg/day x 5 days versus 2 g/kg IVIG administered over 5 days. Information about different IVIG regimens was not available in 14 cases (10 in the AIDP group and 4 in the Non-AIDP group). 14 patients (70%) in the AIDP group received 0.4 g/kg/day divided over 5 days, the other 6 patients (30%) received 2 g/kg IVIG regimen divided over 5 days. On the other hand, in the Non-AIDP /Other variants group, total patients on 0.4 g/kg and 2 g/kg IVIG regimen were 8 (80%) and 2 (20%) respectively. In total, out of 30 patients on IVIG in the entire cohort, 22 patients (73%) were on 0.4 g/kg dosage and 8 (27%) were on 2 g/kg dosage divided over 5 days.
In addition, 7 patients (14%) out of 50 underwent PLEX. Six cases (85%) were in the AIDP group while 1 case (15%) on PLEX was diagnosed with BFP included in the other variants group. Four patients (4/44, 9%) who were on IVIG also received PLEX.
Although IVIG has known association with thromboembolic adverse event, and SARS-COV-2 is associated with a pro-thrombotic state [72], none of the SARS-COV-2 GBS patients who received IVIG treatment developed thrombotic complications. Based on our review, we propose further studies to identify the consideration of IVIG and plasma-exchange as potential standardized treatment options for GBS in COVID-19 patients. While PLEX and IVIG have been shown to be equally effective for treatment of GBS, it would be interesting to compare the issue of side effects in this particular population of GBS patients. There is the potential for thrombotic events with IVIG which is prothrombotic and the potential for cardiovascular events with rapid fluid shifts in moderate and severe cases of COVID-19 [72]. The clinical manifestations of GBS are variable, with most cases having a mild clinical course and recovery with a good response to standard treatment with IVIG or PLEX. However, some cases have also had poor or fatal outcomes in GBS as per literature [17]. It is vital to understand the severity and mortality outcomes of COVID-19 associated peripheral nervous systems disorders; especially GBS, as respiratory failure can be a coinciding symptom of GBS and SARS-CoV-2 individually. Approximately 30% of the GBS patients have had poor outcomes secondary to the respiratory insufficiency [17]. In our review, 30 patients out of total 50 cases reviewed had severe COVID-19 (severity is based on the IDSA/ATS guidelines), classified as patients requiring mechanical intubation [61,73]. Out of the 30 severe cases, 20 (67%) were in the AIDP group while 10 (33%) were in the other groups. Information on intubation and mechanical ventilation were available in 48 cases. Information on outcomes were not available for 5 of the cases. (Table 2). Three AIDP and 2 of the other variants were fatal with a overall fatality rate of 11%. Of the patients who died, 3 (60%) were on combination therapy of 0.4 g/kg/day x 5 days IVIG, HCQ and antivirals; 1 (20%) was managed on antibiotics and IVIG (data not available) and the remaining 1 (20%) was treated with 0.4 g/kg/day x 5 days IVIG.
Given the similarities between SARS-CoV-2 and other coronaviruses, we reviewed papers describing neuromuscular complications in patients with MERS-CoV and SARS, two other severe coronaviral infectious outbreaks. We reviewed a total of 30 studies of SARS and found 2 relevant studies. A case series by Tsai L. et al. reviewed the neuromuscular findings in 4 patients with SARS-CoV infection. However, these patients were not included in our analysis as none of the patients could be confirmed to have GBS based on the diagnostic criteria. These patients were diagnosed as having neuropathy or myopathy and no albuminocytological dissociation was noted in their CSF findings [11]. Another excluded SARS-COV case series by Stainsby B. et al. reported 3 healthcare workers with SARS infection who developed neuropathy and myopathy [12]. We also reviewed a total of 45 studies of MERS and found 1 case series of interest. Kim and colleagues reported 4 patients it described a case of Bickerstaff brainstem encephalitis, a variant of GBS. This patient had ganglioside antibodies and CSF albuminocytological dissociation. While we reviewed this case, it could not be used for extensive comparative analysis due to lack of any other GBS cases in this report on MERS. The other patients were diagnosed with critical illness neuropathy and acute sensory neuropathy [10].
Although there are couple of recent literature published on COVID-19 and GBS, by Uncini et al. and Abu-Rumeileh et al. [73,74]; our review and analysis differ from these studies. Our review focuses on comparison of separate cohorts of different variants of GBS, i.e., AIDP v/s AMSAN/AMAN and others. In addition, we further analyzed the outcomes and severity (according to the ATS guidelines) in all the cases [61].The study by Abu-Rumeileh et al. has a greater number of cases in their review as pediatric GBS cases have also been included, while our study did not include pediatric population. Our study also differs from other studies, in terms of analyzing mEGOS scale, the use of the Brighton classification and also comparing these scale and classification between different variants of GBS [58,59]. Additionally, our study also includes a brief review of pathophysiology of COVID-19 and GBS, as well as the pathophysiology of the treatments for GBS and their correlation. Since both these studies are comparatively new and refer to a rapidly emerging pandemic, we did not discuss a comparison as our study is inclusive of the cases used in the prior studies and also focused on comparison of the different GBS variant cohorts.
The diagnosis of GBS in SARS-CoV-2 is especially challenging as symptoms such as shortness of breath and fatigue could be misinterpreted as being secondary to SARS-CoV-2 delaying the evaluation for GBS. Thus, it is highly advisable that physicians should promptly think about neuromuscular cause such as GBS in their differential when encountering SARS-CoV-2 patients even with minor initial clinical findings such as paresthesia, facial numbness or diplopia and ptosis. During this pandemic it is also useful to test for CoV-2 in patients with GBS who do not manifest clinical symptoms and signs of COVID-19 as there were such cases in our review [28,32,44].
Given the higher rates of requiring mechanical ventilation in SARS CoV-2 associated GBS patients, it is suggested by some that COVID-19 is a trigger for a rapidly progressing neuropathy [42] although some of the need for ventilator support may relate to lung damage from the infection itself. Successful management of GBS is dependent upon a high clinical index of suspicion and early diagnosis. It is important to differentiate GBS from viral myositis in COVID-19 patients complaining of paresthesia and mobility difficulties.
Our study had several strengths. This is among the first studies focused on comparing the clinical presentation, management and outcomes in COVID-19 patients who were diagnosed with GBS, highlighting on differences among the different variants of GBS. Additionally, we also focused on functional scoring of mEGOS GBS scale and Brighton classification.
Our study should be considered in light of several limitations. Cases included in this review were identified through a comprehensive search of databases using a systematic search strategy. However, despite the set criteria, there is a possibility of missing out new upcoming studies because of the evolving nature of the COVID-19 pandemic. However, substantial evidence on other neurological complications and manifestations of COVID-19 is emerging and sets a strong base for conducting this review. Second limitation associated with this systematic review is the concern that a disproportionate amount of atypical cases of GBS and other neurological disorders associated with COVID are more likely to be reported in case reports and series which can introduce a bias. With the rapidly growing evidence of COVID-19 and association with neurological disorders, case reports and series of atypical clinical GBS are more likely to be published and differences in variants and hence common symptomatology and management of GBS may be missed. Finally, because of the emerging nature of the pandemic, there are no suitable contemporary non-COVID-19 case studies from the institutions reporting the COVID-19 associated GBS variants, which would be the appropriate control for comparing the differences in clinical presentations, outcomes and pathophysiology. This can be a future indication from our study warranting further studies.
However, we consider our search comprehensive enough to capture all the relevant case series and reports. Third limitation is the possibility of limited external validity for the systematic review. Although we identified a full spectrum of studies worldwide, the differences in treatment modalities for COVID-19 in different parts of the world, including controversies surrounding HCQ use, the differences in treatments noted in this review should be cautiously interpreted. However, the protocols for GBS are standardized. Additionally, as another limitation, while mEGOS scores can be calculated for days 1 and 7, we considered mEGOS calculation only at day 7 of admission as Medical Research Council Scale for Muscle Strength (MRC) scores (required for mEGOS calculation were unavailable for majority of cases before day 7). It is also important to note that this score was calculated based on the clinical details reported in the cases included in our study and the score would be inaccurate if some pertinent clinical detail was not reported.
5. Conclusion
In this systematic review, we compared and summarized the clinical presentations, outcomes, and neurological complications in SARS-CoV-2 patients with GBS and its variants. It is to our knowledge, the only study which also includes GBS variants and the clinical prediction tool mEGOS for prognostication. Mean age in both the comparison group was greater than fifty and there were also greater proportion of males as compared to females. Lower range of mEGOS scores were the highlight of the GBS-AIDP cohort when comparing it with the AMAN/AMSAN, however, the values were not statistically significant. As a standardized management approach to GBS, nearly all the patients were treated with IVIG. Most of the patients had either full recovery or partial recovery, whereas five patients died. In our opinion, further studies are warranted to explore and compare the efficacy of various treatment modalities, especially IVIG and plasmapheresis, as the latter imposes significant stressors on an already fragile hemodynamic milieu of critically ill SARS-CoV-2 infected patients and increases the risk of exposure to health care workers caring for these patients, whereas IVIG, at least in theory, has prothrombotic potential in a disease that has frequent thrombotic complications including stroke and pulmonary infarction. It would also be important to follow these patients over time to learn more about long-term prognosis.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Data was extracted from the articles published in PUBMED, Google Scholar, Scopus. This will be provided on request.
Disclosures
Shitiz Sriwastava - Reports no disclosure.
Medha Tandon - Reports no disclosure.
Saurabh Kataria- Reports no disclosure.
Jenil Patel- Reports no disclosure.
Riddhi Patel - Reports no disclosure.
Abbas Jowkar - Reports no disclosure.
Maha Daimee - Reports no disclosure.
Preeti Jaiswal- Reports no disclosure.
Dr. Bernitsas has received grant support/ consulting fee from Roche/Genentech, Genzyme, EMD Serono, Biogen.
Dr. Lisak has received in the last 2 years participated as a speaker in meetings sponsored by and received consulting fees and/or grant support from: Alexion, Argenx, Ra Pharmaceuticals, Novartis, Mallinckrodt, Catalyst, Teva Pharmaceuticals, Genentech/Roche, Chugai, Medimmune, Janssen, GLG Consulting, Alpha Sites Consulting, Schlesinger Group Consulting, Slingshot Consulting, Health Sources, Adivo Associates, Smart Analyst, Clarivate. He served as Chair of Adjudication Committee for a MS Clinical Trial for MedDay (Biotin study). He is funded for R21 grant by NINDS Molecular Characterization of B Cell Exosomes in Multiple Sclerosis and as site PI for NINDS funded study of LP4/agrin Antibodies in double Seronegative Myasthenia Gravis. He has received publication royalties from Oxford University Press (Neuroimmunology, 2019) and Blackwell Wiley (International Neurology, 2016).
Author contribution statement
-
Conceptualization: Abbas Jowkar, Evanthia Bernitsas, Robert Lisak
Drafting the manuscript: Shitiz Sriwastava, Evanthia Bernitsas, Robert Lisak.
-
Data abstraction and data analysis: Saurabh Kataria, Jenil Patel, Riddhi Patel, Medha Tandon, Shitiz Sriwastava, Preeti Jaiswal
Editing and Final Draft: Evanthia Bernitsas, Robert Lisak.
Declaration of Competing Interest
None.
Acknowledgments
None.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeri N.C. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020 doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes-Bueno J.A. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baig, A.A.-O.X., et al., Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. (1948–7193 (Electronic)). [DOI] [PubMed]
- 8.Toscano G. Guillain-Barre syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020:1. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.E. Neurological complications during treatment of Middle East Respiratory Syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai L.-K. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 12.Stainsby B., Howitt S., Porr J. Neuromusculoskeletal disorders following SARS: a case series. J. Can. Chiropract. Assoc. 2011;55(1):32. [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs B.C. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 14.Caress, J.B., et al., COVID-19-associated Guillain-Barre Syndrome: the early pandemic experience. Muscle Nerve. [DOI] [PMC free article] [PubMed]
- 15.Andrea A. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol. Sci. 2020 doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottaviani D. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol. Sci. 2020:1. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Den Berg B. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014;10(8):469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 18.Camdessanche J.-P. COVID-19 may induce Guillain-Barré syndrome. Rev. Neurol. 2020 doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pike-Lee T., Li Y., Wolfe G. Neuromuscular complications in COVID-19: a review of the literature. RRNMF Neuromuscul. J. 2020;1(3):13–21. [Google Scholar]
- 20.Dalakas M.C. Guillain-Barré syndrome: The first documented COVID-19–triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurology. 2020:7(5). doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willison H.J., Jacobs B.C., Van Doorn P.A. Guillain-barre syndrome. Lancet. 2016;388(10045):717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 22.Haber P. Guillain-Barré syndrome following influenza vaccination. JAMA. 2004;292(20):2478–2481. doi: 10.1001/jama.292.20.2478. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd S.J. HIV positive patient with GBS-like syndrome. JMM Case Rep. 2017:4(8). doi: 10.1099/jmmcr.0.005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao-Lormeau V.-M. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees J.H. Campylobacter jejuni infection and Guillain–Barré syndrome. N. Engl. J. Med. 1995;333(21):1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 26.Alberti P. Guillain-Barre syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020:7(4). doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigaut K. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurology. 2020:7(5). doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caamaño D.S.J., Beato R.A. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan J.L., Ebadi H., Sarna J.R. Guillain-Barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can. J. Neurol. Sci. 2020:1–3. doi: 10.1017/cjn.2020.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coen M. Guillain-Barre syndrome as a complication of SARS-CoV-2 infection. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Otmani H. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev. Neurol. 2020 doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez-Ortiz C. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 33.Kilinc, D., et al., Guillain-Barré syndrome after SARS-CoV-2 infection. Eur. J. Neurol. [DOI] [PMC free article] [PubMed]
- 34.Lantos J.E., Strauss S.B., Lin E. COVID-19–associated miller fisher syndrome: MRI findings. Am. J. Neuroradiol. 2020;41(7):1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padroni M. Guillain-Barre syndrome following COVID-19: new infection, old complication? J. Neurol. 2020 doi: 10.1007/s00415-020-09849-6. (Germany) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana S. Novel coronavirus (COVID-19)-associated Guillain–Barré Syndrome: case report. J. Clin. Neuromuscul. Dis. 2020;21(4):240. doi: 10.1097/CND.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riva N. Post-infectious Guillain–Barré syndrome related to SARS-CoV-2 infection: a case report. J. Neurol. 2020:1. doi: 10.1007/s00415-020-09907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sancho-Saldaña A. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin. Med. (Lond.) 2020;20(4):e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheidl E. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J. Peripher. Nerv. Syst. 2020 doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virani A. IDCases. Elsevier Ltd; Netherlands: 2020. Guillain-Barre Syndrome associated with SARS-CoV-2 infection; p. e00771. (c) 2020 The Author(s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb S. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020;13(6) doi: 10.1136/bcr-2020-236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H. Guillain-Barré Syndrome Associated With SARS-CoV-2 Infection: Causality or Coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinkin M. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 45.Lascano A.M. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur. J. Neurol. 2020 doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfefferkorn T. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J. Neurol. 2020:1. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchins K.L. COVID-19–associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. Am. J. Neuroradiol. 2020;41(9):1707–1711. doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnaud S. Post SARS-CoV-2 Guillain-Barré syndrome. Clin. Neurophysiol. 2020 doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su X.W. SARS-CoV-2–associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020 doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mozhdehipanah H., Paybast S., Gorji R. Guillain–Barré syndrome as a neurological complication of COVID-19 infection: a case series and review of the literature. Int. Clin. Neurosci. J. 2020;7(3):156–161. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiet M.Y., AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13(7) doi: 10.1136/bcr-2020-236536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebrahimzadeh S.A., Ghoreishi A., Rahimian N. Guillain-Barré Syndrome associated with the coronavirus disease 2019 (COVID-19) Neurology. 2020 doi: 10.1212/CPJ.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helbok R. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur. J. Neurol. 2020 doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agosti E. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol. Sci. 2020:1–6. doi: 10.1007/s10072-020-04553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampe A. Guillain-Barré syndrome and SARS-CoV-2. Neurol. Res. Pract. 2020;2(1):19. doi: 10.1186/s42466-020-00066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernández-Domínguez J. Miller–Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19) J. Neurol. 2020:1. doi: 10.1007/s00415-020-09912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan M. A case series of Guillain-Barré Syndrome following Covid-19 infection in New York. Neurology. 2020 doi: 10.1212/CPJ.0000000000000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobs B.C. International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2017;22(2):68–76. doi: 10.1111/jns.12209. [DOI] [PubMed] [Google Scholar]
- 59.Fokke C. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(1):33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 60.Moher, D., et al., The, PRISMAG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med.. 6(7): (p. e1000097). [DOI] [PMC free article] [PubMed]
- 61.Metlay J.P. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs B.C. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann. Neurol. 1996;40(2):181–187. doi: 10.1002/ana.410400209. [DOI] [PubMed] [Google Scholar]
- 63.La Marca A. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. BioMed. Online. 2020 doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brettschneider J. Cerebrospinal fluid biomarkers in Guillain-Barré syndrome--where do we stand? J. Neurol. 2009;256(1):3–12. doi: 10.1007/s00415-009-0097-x. [DOI] [PubMed] [Google Scholar]
- 65.Yamagishi Y. Markers for Guillain-Barré syndrome with poor prognosis: a multi-center study. J. Peripher. Nerv. Syst. 2017;22(4):433–439. doi: 10.1111/jns.12234. [DOI] [PubMed] [Google Scholar]
- 66.Walgaard C. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology. 2011;76(11):968–975. doi: 10.1212/WNL.0b013e3182104407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pennica A. Clinical management of adult coronavirus infection disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. Comprehen. Clin. Med. 2020:1. doi: 10.1007/s42399-020-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson R.M., Vinetz J.M. British Medical Journal Publishing Group; 2020. Dexamethasone in the Management of Covid-19. [DOI] [PubMed] [Google Scholar]
- 70.Hughes R.A.C., Swan A.V., van Doorn P.A. Cochrane review: intravenous immunoglobulin for Guillain-Barré syndrome. Evid. Based Child Health. 2011;6(4):1176–1231. [Google Scholar]
- 71.Raphael J.C. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2012;7 doi: 10.1002/14651858.CD001798.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Marie I. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br. J. Dermatol. 2006;155(4):714–721. doi: 10.1111/j.1365-2133.2006.07390.x. [DOI] [PubMed] [Google Scholar]
- 73.Uncini A., Vallat J.-M., Jacobs B.C. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J. Neurol. Neurosurg. Psychiatry. 2020;91(10):1105. doi: 10.1136/jnnp-2020-324491. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Rumeileh S. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2020:1–38. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data was extracted from the articles published in PUBMED, Google Scholar, Scopus. This will be provided on request.