Abstract
Background
Emerging clinical evidence has shown that patients with the novel coronavirus disease-2019 (COVID-19) have complications that include venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE). The prevalence of VTE in patients hospitalized with COVID-19 is unclear.
Methods
Eligible studies on COVID-19 were collected from PubMed, Web of Science, and Embase. Patient characteristics and information were extracted for three categories of patients: consecutive, ICU, and non-ICU group. All PEs and DVTs were diagnosed by computed tomographic pulmonary arteriography and duplex ultrasound examination, respectively. A subgroup analysis of testing strategies in ICU and non-ICU patients for PE and DVT was also performed.
Results
Forty clinical studies involving 7966 patients hospitalized with COVID-19 were included. Pooled VTE prevalence was 13% in consecutive patients (95% confidence interval [CI], 0.05-0.24; I2 = 97%), 7% in non-ICU patients (95% CI, 0.01-0.18; I2 = 93%), and 31% in ICU patients (95% CI, 0.22-0.42; I2 = 91%). ICU patients had the highest prevalence of PE among the three groups (17% [95% CI, 0.12-0.23] vs 8% in consecutive patients [95% CI, 0.04-0.13], 4% in non-ICU patients [95% CI, 0.01-0.08]). ICU patients also had the highest DVT prevalence (25% [95% CI, 0.14-0.37] vs 7% in consecutive patients [95% CI, 0.03-0.14], and 7% in non-ICU [95% CI, 0.02-0.14]). The subgroup analysis showed a three-fold improvement in the PE and DVT detection rates in both ICU and non-ICU patients with COVID-19 when the screening test for VTE was applied. In the settings of screening tests for VTE, ICU patients have a significantly higher prevalence of PE (37% vs 10%; P < .0001) and DVT (40% vs 12%; P = .0065) compared with non-ICU patients.
Conclusions
VTE is common in patients hospitalized with COVID-19, especially among ICU patients. Screening tests for PE and DVT may significantly improve detection rates in both ICU and non-ICU patients with COVID-19 than tests based on clinical suspicion.
Keywords: COVID-19, Venous thromboembolism, Deep vein thrombosis, Pulmonary embolism, Meta-analysis
Article Highlights.
-
•
Type of Research: Systematic review and meta-analysis
-
•
Key Findings: One in 10 consecutive patients hospitalized with the novel coronavirus disease-2019 (COVID-19) may have venous thromboembolism (VTE). In COVID-19 patients screened for VTE, patients in the intensive care unit (ICU) have a significantly higher prevalence of pulmonary embolism (PE) and deep vein thrombosis (DVT) than non-ICU patients. Testing for VTE based on clinical suspicion for non-ICU patients with COVID-19 is standard management, whereas for ICU patients with COVID-19, screening tests for PE should be considered despite the lack of usual clinical manifestations of PE.
-
•
Take Home Message: VTE is common in patients hospitalized with COVID-19. The screening test strategy may significantly improve detection rates of PE and deep vein thrombosis in patients hospitalized with COVID-19 than tests based on clinical suspicion.
The novel coronavirus disease-2019 (COVID-19) is contagious pneumonia caused by infection with severe acute respiratory disease novel coronavirus 2. The COVID-19 pandemic has caused thousands of deaths worldwide since its outbreak in December 2019.1 The number of confirmed COVID-19 cases as of June 13 exceeded 7.5 million, with more than 400,000 confirmed deaths.2 To date, the exact and detailed pathogenesis of COVID-19 has remained unclear, and there is no particularly effective medication for COVID-19 treatment. A clinical study suggested that coagulopathy may be prevalent in patients with COVID-19, and that an elevated d-dimer may be associated with poor clinical outcomes.3 Venous thromboembolism (VTE), mainly consisting of deep vein thrombosis (DVT), and pulmonary embolism (PE), may have a relatively high prevalence in patients with COVID-19.4
According to recently published recommendations and guidelines, the efficacy of routine screening for VTE in patients with COVID-19 is uncertain, and testing for the diagnosis of VTE should be based on the clinical index of suspicion.5 This factor may partly contribute to the uncertainty regarding the prevalence of VTE in patients with COVID-19 with different disease severity. Therefore, the understanding of the prevalence of VTE, PE, and DVT in specific groups of patients with COVID-19 can be helpful. This study's objective was to identify the prevalence of VTE, PE, and DVT in consecutive hospitalized patients, intensive care unit (ICU) patients, and non-ICU patients with COVID-19 by a meta-analysis based on currently available clinical studies.
Methods
Search strategy
This systematic review followed the PRISMA guidelines.6 , 7 Searching of the literature was conducted from December 1, 2019, to August 27, 2020, in the PubMed, Web of Science, and Embase database by Z.R. and S.N. The search strategy is presented in the Supplementary Table (online only). The inclusion criteria were patients hospitalized with COVID-19 with reported events of DVT, PE, or VTE, and all PEs and DVTs were diagnosed by computed tomographic pulmonary arteriography and duplex ultrasound examination, respectively. Only English publications were included. The exclusion criteria were clinical studies on specific groups of patients with COVID-19, including pregnant patients, HIV patients, pediatric patients, deceased patients, outpatients with COVID-19, and other selected groups of patients. Studies reporting fewer than 20 cases were also excluded. In selecting the literature, Z.R. and X.D. would independently assess the qualification of studies according to exclusion and inclusion criteria. L.N. will independently decide whether to include or exclude a study if there is discordance.
Data extraction
Information regarding author, study location, study design, demographics, and the sample size was collected from the selected literature by X.W. and B.M. The prevalence of DVT, PE, or VTE was defined as the percentage of DVT, PE, or VTE events among the total number of patients with COVID-19. DVT, PE, and VTE events were obtained from the included studies. If the study did not report the number of VTE events, we added DVT and PE events together as the number of VTE events. Information on whether VTE was diagnosed by screening or testing based on clinical suspicion was also collected for each patient.
Subsequently, we divided the patients with confirmed COVID-19 into groups for analysis based on patient state: ICU patients, non-ICU patients, and consecutive patients. ICU patients were defined as continuously enrolled patients treated in an ICU; non-ICU patients as continuously enrolled patients treated in a general ward; and consecutive patients as continuously enrolled hospitalized patients during the study period (all-comers). By these definitions, consecutive hospitalized patients are the combination of ICU and non-ICU patients in this study. Whether patients with COVID-19 were diagnosed with VTE by routine screening tests or tests based on clinical suspicion was recoded and extracted for subgroup analysis.
Quality assessment
The Joanna Briggs Institute's critical appraisal checklist tool for a prevalence study was used for quality assessment of the included studies.8 The checklist includes nine questions with four categories of answers: yes, no, unclear, and not applicable for insufficient data. Yes is scored as 1, and no is scored as 0, with a total quality score ranging from 0 to 9. Quality assessment was carried out by independent reviewers (L.N. and X.D.).
Data analysis
We used the meta (version 4.12-0) and forrestplot (version 1.9) packages in R (version 3.6.2; The R Foundation, Vienna, Austria) to perform the meta-analysis. The Shapiro-Wilk test was introduced as a normality test, and log transformation was used. Prevalence with a 95% confidence interval (95% CI) was used. A random-effects model was developed for significant heterogeneity (P < .10 or I2 > 50%), and a fixed-effects model was used for non-significant heterogeneity (P > .10 or I2 < 50%). A funnel plot and the Egger test were used for assessment of publication bias. A two-sided P value of less than .05 was regarded as statistically significant. Pooled results for the prevalence of VTE, PE, and DVT in each group were analyzed, and a subgroup analysis of screening or testing upon indication of symptoms for VTE, PE, and DVT was conducted.
Results
Study selection and summary of studies
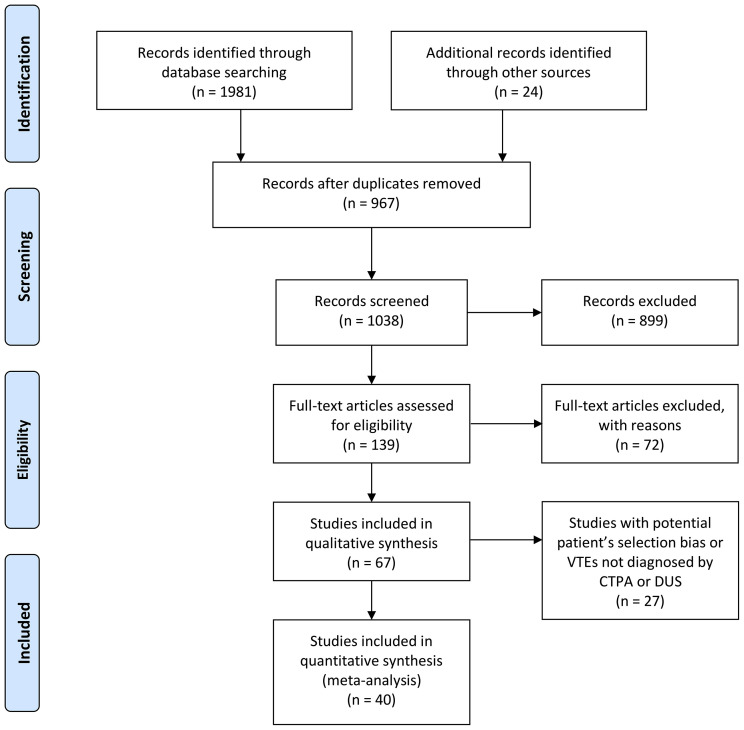
Systematic searches obtained a total of 2005 citations. After excluding duplicates and applying the inclusion and exclusion criteria, 40 articles remained and met the inclusion criteria for quality appraisal after the full-text examination (Fig 1 ).4 , 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 In the quality assessment, 31 articles received a score of 8 out of 9, and 9 studies received a score of 9 out of 9.
Fig 1.
The PRISMA flowchart. CTPA, Computed tomographic pulmonary arteriography; DUS, duplex ultrasound.
Thirty-four retrospective studies and six prospective studies were included in this meta-analysis. Data for 7966 patients with COVID-19 were collected, including 4222 American patients, 616 Asian patients, and 3128 European patients. Male patients accounted for 61.0% (2225/3648) of the included patients. Regarding comorbidities, 46.1% of patients (1191/2582) had hypertension and 21.4% (604/2828) had diabetes mellitus. The malignancy rate was 8.5% (274/3230). Only 4.0% of patients (100/2510) had prior VTE history. Twenty-eight studies and 77.0% of patients (1868/2427) had been treated with prophylactic or therapeutic anticoagulation for the prevention of VTE (Table ).
Table.
Summary of 40 studies for meta-analysis
| Study | Country | Study type | Sample size (n) | Age, years | Male (%) | Hypertension (%) | Diabetes mellitus (%) | Cancer (%) | ICU (%) | Intubation (%) | Prior VTE (%) | Prophylaxis/therapeutic anticoagulation (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consecutive | ||||||||||||
| Artifoni et al9 | France | Retrospective | 71 | 64 (46-75)a | 43 (61) | 29 (41) | 14 (20) | 4 (6) | 5 (7) | 13 (18) | NR | 71 (100) |
| Bavaro et al10 | Italy | Retrospective | 20 | 62 (56-80)a | 8 (40) | 11 (55) | 3 (15) | 2 (10) | 6 (30) | 6 (30) | NR | 17 (85) |
| Barrett et al11 | United States | Retrospective | 100 | 65a | 61 (61) | 53 (53) | NR | NR | NR | NR | NR | NR |
| Grillet et al12,b | France | Retrospective | 100 | 66 ± 13 | 70 (70) | NR | 20 (20) | 20 (20) | 39 (39) | 34 (34) | NR | NR |
| Koleilat et al13 | United States | Retrospective | 3404 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lodigiani et al14,b | Italy | Retrospective | 388 | 66 (55-75)a | 264 (68) | 183 (47) | 88 (23) | 25 (6) | 61 (16) | NR | 12 (3) | NR |
| Mei et al15 | China | Retrospective | 256 | 56 (0.5-87)c | 131 (51) | 60 (23) | 46 (18) | 4 (2) | NR | 45 (18) | 0 (0) | NR |
| Mestre-Gómez et al16 | Spain | Retrospective | 452 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Middeldorp et al17,b | The Netherlands | Retrospective | 198 | 61 ± 14 | 130 (66) | NR | NR | 7 (4) | 75 (38) | NR | 11 (6) | NR |
| Moll et al18,b | United States | Retrospective | 210 | 62 ± 16 | 101 (48) | 125 (60) | 70 (33) | 40 (19) | 102 (49) | 86 (41) | 9 (4) | 190 (91) |
| Poyiadji et al19 | United States | Retrospective | 328 | NR | 150 (46) | 197 (60) | 126 (38) | 45 (14) | 82 (25) | 55 (17) | 26 (8) | 122 (37) |
| Stoneham et al20 | United Kingdom | Retrospective | 274 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Zhang et al21 | China | Prospective | 143 | 63 ± 14 | 74 (52) | 46 (32) | 26 (18) | 7 (5) | 15 (10) | NR | 1 (1) | 53 (37) |
| Non-ICU | ||||||||||||
| Cattaneo et al22 | Italy | Retrospective | 64 | 70 (58-77.5)a | 35 | NR | NR | 7 | 0 (0) | NR | 0 (0) | NR |
| Demelo-Rodríguez et al23 | Spain | Prospective | 156 | 68.1 ± 14.5 | 102 (65) | NR | NR | 16 (10) | 0 (0) | NR | 2 (1) | NR |
| Giorgi-Pierfranceschi et al24 | Italy | Retrospective | 66 | 71.5 ± 11 | 46 (70) | 22 (33) | 9 (13) | 6 (9) | 0 (0) | 0 (0) | 1 (1) | 62 (94) |
| Grillet et al12,b | France | Retrospective | 61 | NR | NR | NR | NR | NR | 0 (0) | NR | NR | NR |
| Le Jeune et al25 | France | Retrospective | 42 | 65 ± 19 | 23 (55) | 20 (48) | 13 (31) | 3 (7) | 0 (0) | 0 (0) | NR | 42 (100) |
| Lodigiani et al14,b | Italy | Retrospective | 327 | 68 (55-77)a | 215 (66) | 156 (48) | 77 (24) | 23 (7) | 0 (0) | NR | 12 (4) | NR |
| Middeldorp et al17,b | The Netherlands | Retrospective | 123 | 60 ± 16 | 72 (59) | NR | NR | 4 (3) | 0 (0) | 0 (0) | 9 (7) | NR |
| Moll et al18,b | United States | Retrospective | 108 | 60 ± 17 | 42 (39) | 56 (52) | 31 (29) | NR | 0 (0) | NR | 5 (5) | 91 (84) |
| Pizzolo et al26 | Italy | Retrospective | 43 | 66 (28-96)c | 29 (67) | 23 (53.5) | 6 (14) | 4 (9) | 0 (0) | NR | 0 (0) | 43 (100) |
| Santoliquido et al27 | Italy | Retrospective | 84 | 67.6 ± 13.5 | 61 (73) | 45 (54) | 18 (21) | 14 (17) | 0 (0) | 0 (0) | 3 (4) | 84 (100) |
| ICU | ||||||||||||
| Alharthy et al28 | United States | Prospective | 89 | 43 (32-54)a | 74 (84) | 45 (51) | 40 (45) | NR | 89 (100) | 74 (84) | NR | 89 (100) |
| Chen et al29 | China | Retrospective | 88 | 63 (55- 71)a | 54 (61) | 31 (35) | 9 (10) | 5 (6) | 88 (100) | NR | NR | 88 (100) |
| Desborough et al30 | United Kingdom | Retrospective | 66 | 59 (49-66) | 48 (73) | 30 (45) | 27 (41) | 5 (8) | 66 (100) | 52 (79) | 5 (8) | 66 (100) |
| Devreese et al31 | Belgium | Retrospective | 31 | 63 (38-82)c | 28 (90) | NR | 8 (26) | 6 (19) | 31 (100) | 26 (84) | 1 (3) | 31 (100) |
| Grandmaison et al32 | Switzerland | Retrospective | 29 | 66 (37-79)c | 18 (64) | NR | NR | 2 (7) | 29 (100) | NR | 2 (7) | 29 (100) |
| Grillet et al12,b | France | Retrospective | 39 | NR | NR | NR | NR | NR | 39 (100) | NR | NR | NR |
| Helms et al33 | France | Prospective | 150 | 63 (53-71)a | 122 (81) | NR | 30 (20) | 9 (6) | 150 (100) | NR | 8 (5) | 150 (100) |
| Hippensteel et al34 | United States | Retrospective | 91 | NR | 53 (58) | NR | 28 (31) | 3 (3) | 91 (100) | NR | NR | 0 (0) |
| Ierardi et al35 | Italy | Retrospective | 234 | 62 (7-98)c | 70 (30) | 93 (40) | 40 (17) | 26 (11) | 234 (100) | NR | NR | 234 (100) |
| Klok et al4,36 | The Netherlands | Retrospective | 184 | 64 ± 12 | 139 (76) | NR | NR | 5 (2.7) | 184 (100) | NR | 17 (9) | 184 (100) |
| Llitjos et al37 | France | Retrospective | 26 | 68 (52-75)a | 20 (77) | 22 (85) | NR | 0 (0) | 26 (100) | 26 (100) | 1 (4) | 26 (100) |
| Lodigiani et al14,b | Italy | Retrospective | 61 | 61 (55-69)a | 61 (100) | 27 (44) | 11 (18) | 2 (3) | 61 (100) | NR | 0 (0) | NR |
| Longchamp et al38 | Switzerland | Retrospective | 25 | 68 ± 11 | 16 (64) | 10 (40) | 1 (4) | 2 (8) | 25 (100) | 23 (92) | 0 (0) | 25 (100) |
| Middeldorp et al17,b | The Netherlands | Retrospective | 75 | 62 ± 10 | 58 (77) | NR | NR | 3 (4) | 75 (100) | 75 (100) | 2 (3) | NR |
| Moll et al18,b | United States | Retrospective | 102 | 64.6 ± 14.9 | 59 (58) | 69 (68) | 39 (38) | NR | 102 (100) | NR | 4 (4) | 99 (97) |
| Nahum et al39 | France | Prospective | 34 | 62.2 ± 8.6 | 25 (78) | 13 (38) | 15 (44) | 1 (3) | 34 (100) | 34 (100) | NR | 34 (100) |
| Patel et al40 | United Kingdom | Retrospective | 39 | 53 (29-79)c | 32 (82) | 15 (39) | 8 (21) | NR | 39 (100) | 39 (100) | NR | 39 (100) |
| Pavoni et al41 | Italy | Retrospective | 40 | 61 ± 13 | 24 (60) | 16 (40) | 16 (40) | NR | 40 (100) | NR | NR | NR |
| Poissy et al42 | France | Retrospective | 107 | NR | NR | NR | NR | NR | 107 (100) | NR | NR | NR |
| Ren et al43 | China | Retrospective | 48 | 70 (62-80)a | 26 (54) | 19 (40) | 13 (27) | NR | 48 (100) | 47 (98) | NR | 1 (2) |
| Stessel et al44 | Belgium | Retrospective | 46 | 70 (62-76)a | 34 (74) | 29 (63) | 14 (30) | NR | 48 (100) | NR | NR | 0 (0) |
| Taccone et al45 | Belgium | Retrospective | 40 | 61 (57-66) | 28 (70) | 28 (70) | 5 (13) | 5 (13) | 40 (100) | 40 (100) | NR | 40 (100) |
| Thomas et al46 | United Kingdom | Retrospective | 63 | NR | 44 (70) | NR | NR | 1 (2) | 63 (100) | 52 (83) | 1 (2) | 63 (100) |
| Voicu et al47 | France | Prospective | 56 | NR | 42 (75) | 26 (46) | 25 (45) | NR | 56 (100) | 56 (100) | NR | 49 (88) |
| Zhang et al48 | China | Retrospective | 81 | NR | NR | NR | NR | NR | 81 (100) | NR | NR | 36 (44) |
ICU, Intensive care unit; NR, not reported; VTE, venous thromboembolism.
Median (interquartile range); only the median age was provided in Berger et al.
Clinical study containing consecutive patients, ICU patients, and non-ICU patients.
Median (range).
In this meta-analysis, we categorized all patients into three groups: consecutive patients (13 studies; 5944 patients), non-ICU patients (10 studies; 1074 patients), and ICU patients (25 studies; 1844 patients). There were four included studies that contained consecutive patients, ICU patients, and non-ICU patients.12 , 14 , 17 , 18 In total, 16 studies underwent screening tests for VTE. There were six studies in which patients were routinely screened for DVT by duplex ultrasound examination; computed tomographic pulmonary arteriography was performed only for suspected PE. Another 17 studies use imaging tests only for symptomatic or suspected VTEs. One study did not report a screening test strategy for VTE.
Outcome of meta-analysis
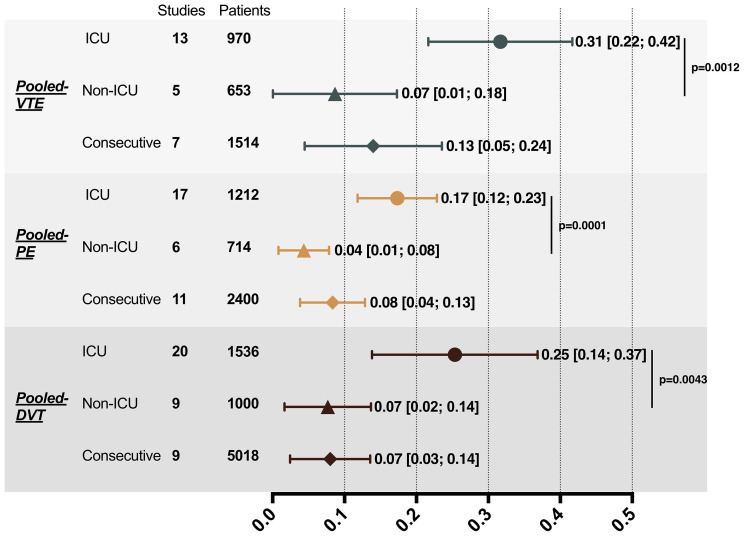
All meta-analyses were performed by random-effects models. The pooled prevalence of VTE, PE, and DVT for consecutive hospitalized patients were 13% (95% CI, 0.05-0.24), 8% (95% CI, 0.04-0.13), and 7% (95% CI, 0.03-0.14), respectively. ICU patients had a pooled VTE prevalence of 31% (95% CI, 0.22-0.42), which was significantly higher than non-ICU patients (prevalence of 7%; 95% CI, 0.01-0.18; P = .0012). Compared with non-ICU patients, ICU patients also had three-fold higher pooled prevalence of PE (17% [95% CI, 0.12-0.23] vs 4% [95% CI, 0.01-0.08]; P = .0001) and DVT (25% [95% CI, 0.14-0.37] vs 7% [95% CI, 0.02-0.14]; P = .0043; Fig 2 ).
Fig 2.
Pooled results of VTE, PE, and DVT prevalence in patients with COVID-19. DVT, Deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolic event.
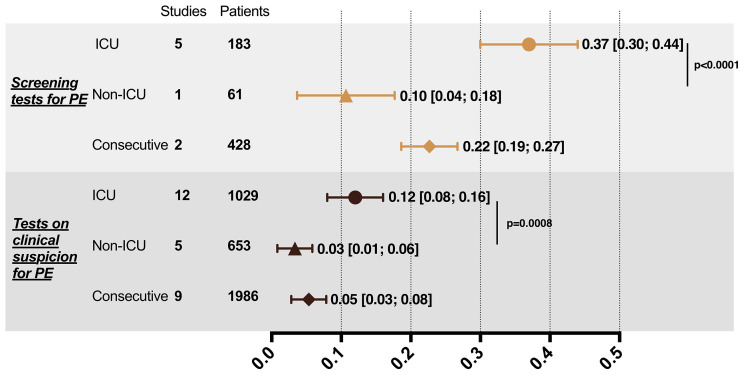
In the subgroup analysis, the prevalence of PE and DVT obtained from screening or testing on clinical suspicion was compared. For ICU patients, the PE prevalence for screening (prevalence of 37%; 95% CI, 0.30-0.44) was significantly higher than that for testing on clinical suspicion (prevalence of 12%; 95% CI, 0.08-0.16) (P < .0001). Screening for DVT in ICU patients also significantly improved the detection rate compared with testing on clinical suspicion (40% vs 11%; P = .0069). Similarly, consecutive patients with COVID-19 showed a significant difference in detection rate by two testing strategies for the diagnosis of PE (22% vs 5%; P < .0001) and DVT (33% vs 2%; P = .0010). As for non-ICU patients, DVT screening test increased the detection rate (12% vs 1%; P = .0007). However, the difference in the detection of PE by different test strategies in non-ICU patients did not reach significance (10% vs 3%; P = .0530). Despite testing strategies, ICU patients had a significantly higher prevalence of PE and DVT than non-ICU patients (Fig 3 ).
Fig 3.
Subgroup analysis of prevalence of DVT and PE in patients with COVID-19 by different test strategies. A, Subgroup analysis of PE. B, Subgroup analysis of DVT. DVT, Deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism.
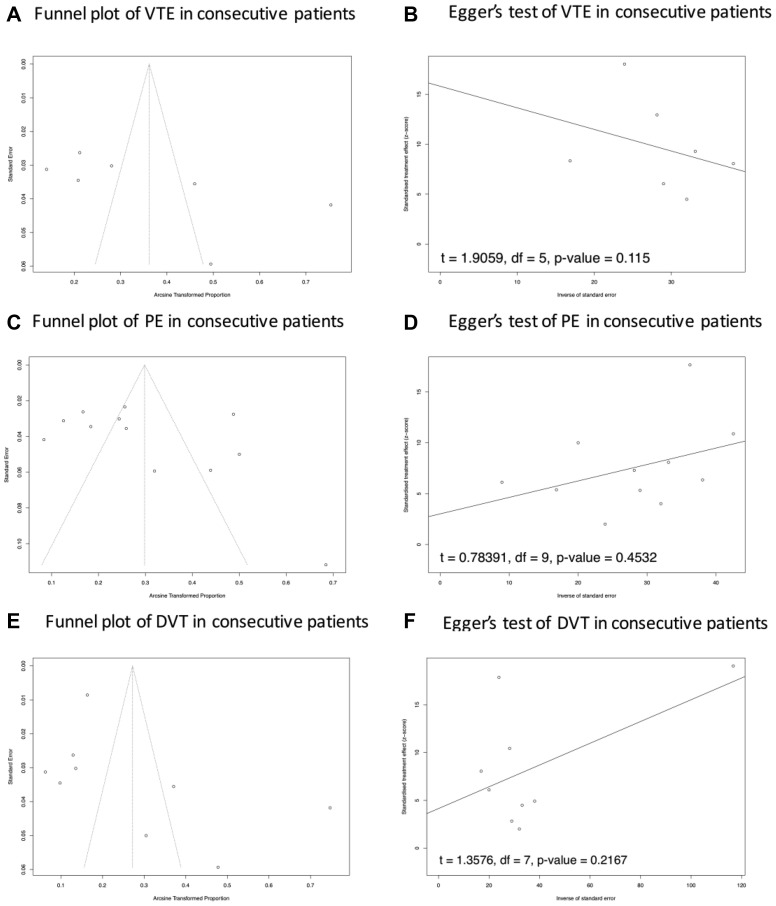
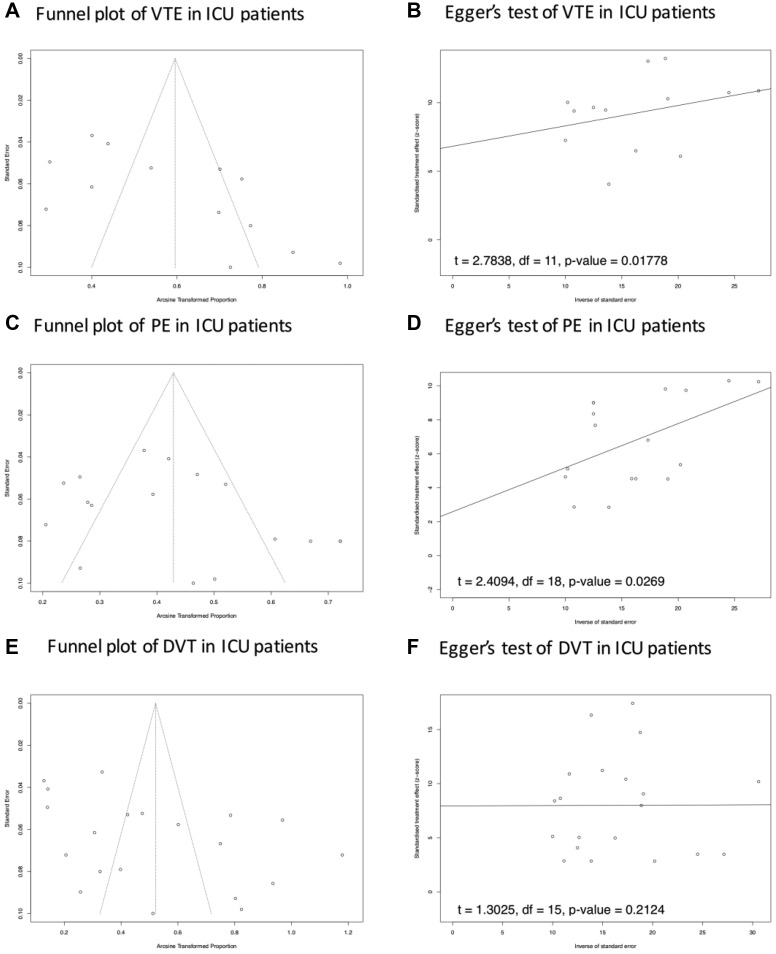
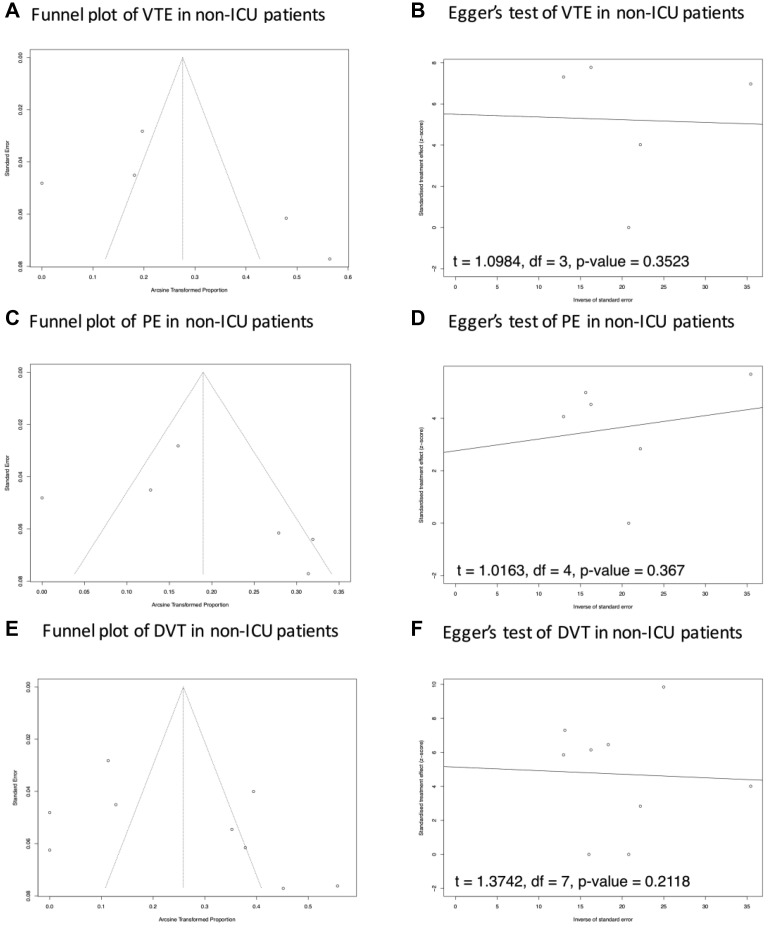
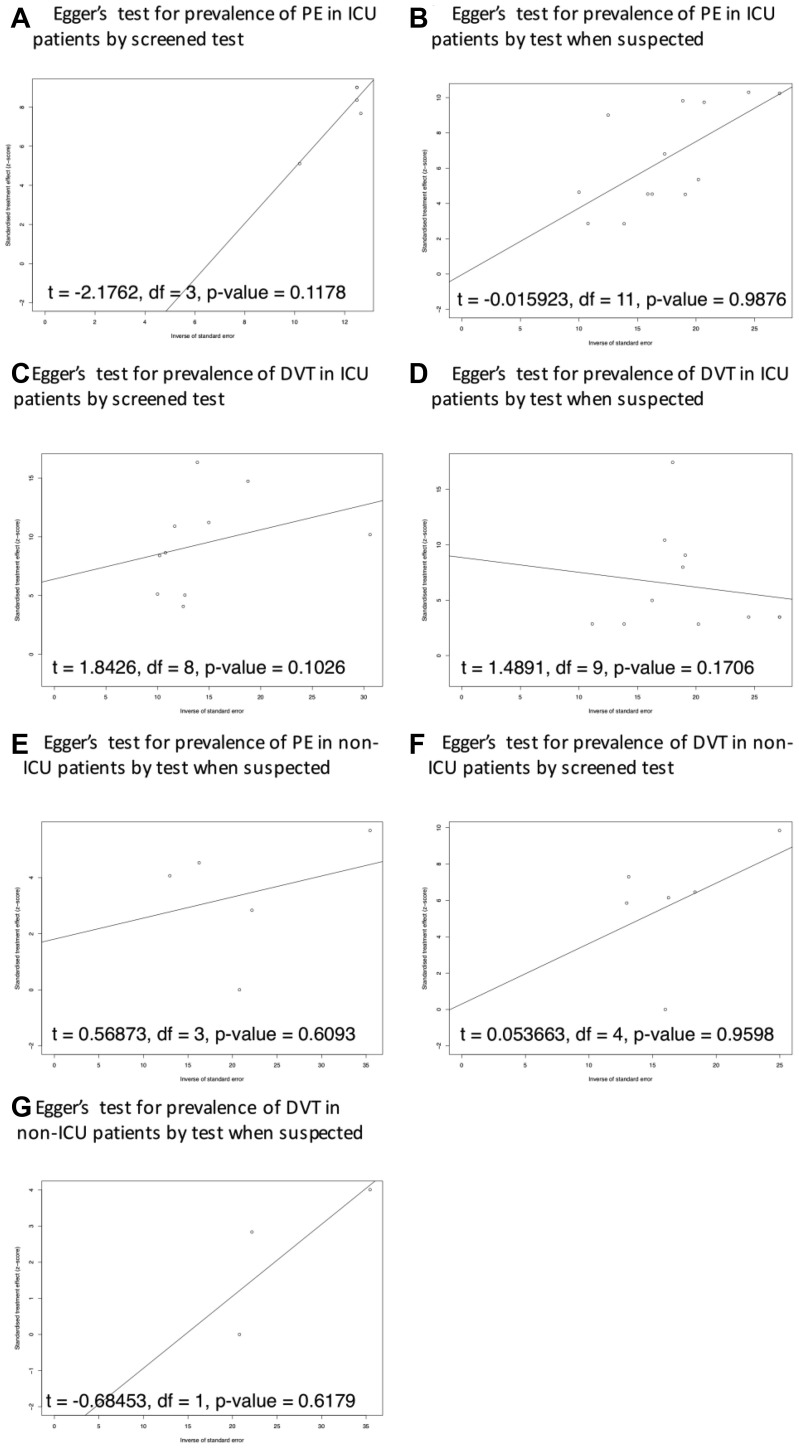
The results of the funnel plot and Egger's test showed a potential publication bias in the prevalence of VTE and PE in ICU patients. No potential publication bias of VTE, PE, and DVT was reported in consecutive patients and non-ICU patients (Supplementary Fig 1 (online only), Supplementary Fig 2 (online only), Supplementary Fig 3 (online only), online only). In the setting of subgroup analysis of PE and DVT testing strategy, only one study reported a screening test strategy for PE for non-ICU patients, so this topic was not suitable for this analysis. There was no potential publication bias reported in the Egger's test of PE and DVT prevalence in both ICU and non-ICU patients (Supplementary Fig 4, online only).
Supplementary Fig 1 (online only).
Publication bias analysis of VTE, DVT, and PE in consecutive patients. A, C and E, Potential asymmetry of trials' distribution, which indicates a lack of relevant trials with high quality included in the comparisons. The results of Egger's test presented in B, D and F suggested potential publication bias in the results of PE, DVT, and VTE prevelance of consecutive patients. DVT, Deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Supplementary Fig 2 (online only).
Publication bias analysis of VTE, DVT, and PE in ICU patients. In (A, C and E) Potential asymmetry of trials' distribution, which indicates a lack of relevant trials with high quality included in the comparisons. The results of the Egger's test in B, D and F suggested potential publication bias in the results of DVT prevalence in ICU patients, and no publication bias of PE and VTE prevalence in ICU patients. DVT, Deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism.
Supplementary Fig 3 (online only).
Publication bias analysis of VTE, DVT, and PE in non-ICU patients. In (A, C and E) Potential asymmetry of trials' distribution, which indicates a lack of relevant trials with high quality included in the comparisons. The results of the Egger's test in B, D and F suggested potential publication bias in the results of PE, DVT, and VTE prevalence in non-ICU patients. DVT, Deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism.
Supplementary Fig 4 (online only).
Publication bias analysis of DVT and PE diagnosed by different test strategy. DVT, Deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism.
Discussion
Accumulating clinical studies and reviews have suggested that patients with COVID-19 can be complicated with coagulopathy, and prothrombotic characteristics may be associated with a high risk of VTE.49 One study identified increased d-dimer as a risk factor for a poor prognosis in patients with COVID-19.50 Another study showed that anticoagulant treatment with heparin was associated with decreased mortality in patients with severe COVID-19 with coagulopathy and a sepsis-induced coagulopathy score of 4 or higher or a d-dimer of more than six-fold of the upper limit of normal.51 The current understanding of COVID-19 pathogenesis, including excessive systematic inflammation, platelet activation, endothelial dysfunction, and stasis, could also explain the high risk of VTE.52 Nevertheless, the detailed pathogenesis of VTE in patients with COVID-19 remains unknown.
In a recently published meta-analysis, Hasan et al53 reported a prevalence of VTE of 31% in ICU patients with COVID-19 despite anticoagulation. According to Chi et al54 and Porfidia et al,55 the prevalence of VTE in hospitalized patients was 23.9% to 26.0%. The present meta-analysis is the updated report of the prevalence of VTE in patients with COVID-19, which includes a greater number of continuously enrolled patients compared with previous studies. Our study included 5944 continuously enrolled, consecutive patients hospitalized with COVID-19, likely a fair representation of the real-world incidence of VTE among these patients. According to the definition of this study, consecutive hospitalized patients may consist of patients with COVID-19 who are moderately, severely, or critically ill. Because thrombosis and VTEs may strongly correlate with the severity of COVID-19, the prevalence of consecutive patients may vary vastly among studies and be influenced by the different testing strategies for the diagnosis of VTE.34 In comparison, non-ICU patients with COVID-19 included patients treated in general wards, mostly without acute respiratory distress syndrome and invasive mechanical ventilation, and therefore, may have a lower pooled prevalence of VTE, PE, and DVT than ICU patients with COVID-19.
The present study demonstrated a significantly higher prevalence of VTE, PE, and DVT in ICU patients with COVID-19 compared with non-ICU patient group, which is consistent with previous studies. In this study, the pooled PE prevalence of ICU patients was 17%. Similarly, Poissy et al42 reported a notably higher PE prevalence in ICU patients with COVID-19 compared with other ICU patients and ICU patients with influenza. In their center, the PE prevalence was 20.6% in ICU patients with COVID-19 (22/107), compared with 6.1% in other ICU patients (12/196) and 7.5% in ICU patients with influenza (7.5%) during the same period. Similarly, there was a significant difference in the DVT prevalence between ICU and non-ICU patients in this study, which is in line with previous reports.49
Although unclear, the discrepancy between the PE and DVT prevalence in ICU patients with COVID-19 may be attributed mainly to the pathogenesis of COVID-19 compared with that of other diseases. Among patients treated in an ICU, general risk factors for VTE include advanced age, prior VTE, a history of cancer, prolonged immobilization, obesity, pregnancy, trauma, spinal cord injury, recent surgery, and stroke.56 , 57 Approximately one-fifth of all-cause PE cases were reported to be isolated PE without the presence of DVT.58 This proportion certainly seems to be higher in the setting of COVID-19, according to current clinical studies. The suspected PE may be “pulmonary thrombi,” despite the similar clinical manifestations to acute PE. If so, the pulmonary thrombi in patients with COVID-19 probably result from excessive vascular damage, viral infection, and severe inflammation.22 Invasive mechanical ventilation and acute respiratory distress syndrome may also contribute to pulmonary venous thrombosis; patients treated in a general ward were shown to have a low incidence of VTE.59 Nevertheless, autopsy and postmortem reports of patients with COVID-19 described certain cases in which thrombi were derived from the deep veins of the lower extremities and caused death.60 Despite the pathogenic mechanisms of DVT-associated PE or pulmonary venous thrombosis in patients with COVID-19, emerging clinical evidence has shown that VTE is associated with poor clinical outcomes and should be addressed with greater caution.50
Another strength of this study is the analysis of different test strategies for VTE in patients with COVID-19. A recent guideline recommended standard-of-care objective testing to diagnose VTE based on the clinical index of suspicion, whereas routine screening for VTE by bedside Doppler ultrasound imaging or elevated d-dimer was not recommended.5 In this meta-analysis, screening tests in ICU patients with COVID-19 showed a nearly three-fold increase in PE and DVT detection rates compared with testing based on a clinical index of suspicion. In non-ICU patients, there was also a significant increase of the detection rate of DVT by screening tests. However, the results show no significant difference in the PE detection rate in non-ICU patients. The discrepancy in the publication bias of pooled results and subgroup analysis further suggested the potentially underestimated prevalence of VTE by different test strategies, especially in ICU patients. Considering the more severe condition, higher mortality rate, and a greater prevalence of VTE in ICU patients, screening for VTEs may be acceptable for ICU patients. Still, whether screening test for VTE in patients with COVID-19 necessary is worthy of further study.
There are limitations to this meta-analysis. First, the patients with COVID-19 enrolled from different centers may have varied extensively in terms of disease severity and comorbidities, which may induce heterogeneity and a potential publication bias of the pooled results. Second, a potential bias may have existed by under-reporting VTE diagnoses, DVT, and PE based on the different strategies applied in various centers. Third, some patients may have undergone prophylactic or therapeutic anticoagulation, which may influence the prevalence of VTE. Finally, included studies that were retrospective in nature were also inherently limited. Therefore, the results were limited by low-quality evidence owing to heterogeneity and the potential risk of bias. A prospective study with a larger sample size and justified measurement strategies is warranted.
Conclusions
VTE events are common in patients hospitalized with COVID-19, especially in ICU patients. Screening tests for PE and DVT may significantly improve detection rates in both ICU and non-ICU patients with COVID-19 than tests based on clinical suspicion. Owing to the low-quality evidence of this study, a prospective study with larger sample size and justified measurement strategies is warranted.
Author contributions
Conception and design: RZ, CL
Analysis and interpretation: RZ, LN, XD
Data collection: RZ, XW, BM, SN, CL
Writing the article: RZ
Critical revision of the article: LN, XD, XW, BM, SN, CL
Final approval of the article: RZ, LN, XD, XW, BM, SN, CL
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: CL
Footnotes
Author conflict of interest: none.
Additional material for this article may be found online at www.jvsvenous.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only).
Supplementary Table (online only).
Search strategy of meta-analysis
| PUBMED | ||
|---|---|---|
| #1 | "COVID-19"[Title/Abstract] OR "Coronaviruses"[Title/Abstract] OR "SARS-CoV-2"[Title/Abstract] | 47,628 |
| #2 | "venous thromboembolic"[Title/Abstract] OR "thromboembolic events"[Title/Abstract] OR "thrombosi∗"[Title/Abstract] OR "emboli∗"[Title/Abstract] OR "VTE"[Title/Abstract] | 259,752 |
| #3 | "pulmonary embolism"[Title/Abstract] OR "pulmonary emboli∗"[Title/Abstract] | 38,108 |
| #4 | "deep vein thrombosis"[Title/Abstract] OR "DVT"[Title/Abstract] | 21,514 |
| #5 | "thromboembolic event"[Title/Abstract] | 1,506 |
| #6 | #2 OR #3 OR #4 OR #5 | 261,063 |
| #7 | #6 AND #1 | 850 |
| EMBASE | ||
| #1 | 'covid-19'/exp OR 'covid-19' | 45,190 |
| #2 | 'coronaviruses' OR coronaviruses | 4,097 |
| #3 | 'sars-cov-2'/exp OR 'sars cov 2' | 17,580 |
| #4 | #1 OR #2 OR #3 | 49,941 |
| #5 | 'venous thromboembolic':ti,ab OR 'thromboembolic events':ti,ab OR 'thrombosi∗':ti,ab OR 'emboli∗':ti,ab OR 'vte':ti,ab | 377,720 |
| #6 | 'pulmonary embolism':ti,ab OR 'pulmonary emboli∗':ti,ab | 56,916 |
| #7 | 'deep vein thrombosis':ti,ab OR 'dvt':ti,ab | 35,300 |
| #8 | 'thromboembolic event':ti,ab | 2,619 |
| #9 | #5 OR #6 OR #7 OR #8 | 381,379 |
| #10 | #3 AND #9 | 700 |
| Web of Science | ||
| #1 | TI = (COVID-19 OR Coronaviruses OR SARS-CoV-2) OR AB = (COVID-19 OR Coronaviruses OR SARS-CoV-2) | 41,372 |
| #2 | TI = (venous thromboembolic OR thromboembolic events OR thrombosi∗ OR emboli∗ OR VTE) OR AB = (venous thromboembolic OR thromboembolic events OR thrombosi∗ OR emboli∗ OR VTE) | 314,100 |
| #3 | TI = (pulmonary embolism OR pulmonary emboli∗) OR AB = (pulmonary embolism OR pulmonary emboli∗) | 55,852 |
| #4 | TI = (deep vein thrombosis OR DVT) OR AB = (deep vein thrombosis OR DVT) | 26,409 |
| #5 | TI = thromboembolic event OR AB = thromboembolic event | 12,354 |
| #6 | #2 OR #3 OR #4 OR #5 | 315,194 |
| #7 | #1 AND #6 | 431 |
The search was conducted from December 1, 2019, to August 27, 2020, in Pubmed, Web of Science, and Embase database.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus disease (COVID-19) pandemic. www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at:
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JBI The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews 2017. https://joannabriggs.org/ebp Available at:
- 9.Artifoni M., Danic G., Gautier G., Gicquel P., Boutoille D., Raffi F. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavaro D.F., Poliseno M., Scardapane A., Belati A., De Gennaro N., Stabile Ianora A.A. Occurrence of acute pulmonary embolism in COVID-19-a case series. Int J Infect Dis. 2020;98:225–226. doi: 10.1016/j.ijid.2020.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett T.J., Lee A., Xia Y., Lin L.H., Black M., Cotzia P. Biomarkers of platelet activity and vascular health associate with thrombosis and mortality in patients with COVID-19. Circ Res. 2020;127:945–947. doi: 10.1161/CIRCRESAHA.120.317803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koleilat I., Galen B., Choinski K., Hatch A.N., Jones D.B., Billett H. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2020 Jun 25 doi: 10.1016/j.jvsv.2020.06.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei F., Fan J., Yuan J., Liang Z., Wang K., Sun J. Comparison of venous thromboembolism risks between COVID-19 pneumonia and community-acquired pneumonia patients. Arterioscler Thromb Vasc Biol. 2020;40:2332–2337. doi: 10.1161/ATVBAHA.120.314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestre-Gómez B., Lorente-Ramos R.M., Rogado J., Franco-Moreno A., Obispo B., Salazar-Chiriboga D. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. 2020 June 29 doi: 10.1007/s11239-020-02190-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moll M., Zon R.L., Sylvester K.W., Chen E.C., Cheng V., Connell N.T. VTE in ICU patients with COVID-19. Chest. 2020;158:2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K. Acute pulmonary embolism and COVID-19. Radiology. 2020;297:E335–E338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoneham S.M., Milne K.M., Nuttal E., Frew G.H., Sturrock B.R., Sivaloganathan H. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med (Lond) 2020;20:e76–e81. doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macías M., Toledo-Samaniego N. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi-Pierfranceschi M., Paoletti O., Pan A., De Gennaro F., Nardecchia A.L., Morandini R. Prevalence of asymptomatic deep vein thrombosis in patients hospitalized with SARS-CoV-2 pneumonia: a cross-sectional study. Intern Emerg Med. 2020;15:1425–1433. doi: 10.1007/s11739-020-02472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Jeune S., Suhl J., Benainous R., Minvielle F., Purser C., Foudi F. High prevalence of early asymptomatic venous thromboembolism in anticoagulated COVID-19 patients hospitalized in general wards. J Thromb Thrombolysis. 2020 Aug 18 doi: 10.1007/s11239-020-02246-w. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzolo F., Rigoni A.M., De Marchi S., Friso S., Tinazzi E., Sartori G. Deep vein thrombosis in SARS-CoV-2 pneumonia-affected patients within standard care units: exploring a submerged portion of the iceberg. Thromb Res. 2020;194:216–219. doi: 10.1016/j.thromres.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porfidia A., Santoliquido A., Cammá G., Porceddu E., Pola R. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18:31110–31111. doi: 10.1111/jth.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alharthy A., Faqihi F., Abuhamdah M., Noor A., Naseem N., Balhamar A. Prospective longitudinal evaluation of point-of-care lung ultrasound in critically ill patients with severe COVID-19 pneumonia. J Ultrasound Med. 2020 Aug 14 doi: 10.1002/jum.15417. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S., Zhang D., Zheng T., Yu Y., Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis. 2020 Jun 30 doi: 10.1007/s11239-020-02181-w. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandmaison G., Andrey A., Périard D., Engelberger R.P., Carrel G., Doll S. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;4:e113–e115. doi: 10.1055/s-0040-1713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hippensteel J.A., Burnham E.L., Jolley S.E. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol. 2020;190:e134–e137. doi: 10.1111/bjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ierardi A.M., Coppola A., Fusco S., Stellato E., Aliberti S., Andrisani M.C. Early detection of deep vein thrombosis in patients with coronavirus disease 2019: who to screen and who not to with Doppler ultrasound? J Ultrasound. 2020 Aug 18 doi: 10.1007/s40477-020-00515-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llitjos J.-F., Leclerc M., Chochois C., Monsallier J.-M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longchamp A., Longchamp J., Manzocchi-Besson S., Whiting L., Haller C., Jeanneret S. Venous thromboembolism in critically ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842–847. doi: 10.1002/rth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;1:e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel B.V., Arachchillage D.J., Ridge C.A., Bianchi P., Doyle J.F., Garfield B. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 43.Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142:181–183. doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 44.Stessel B., Vanvuchelen C., Bruckers L., Geebelen L., Callebaut I., Vandenbrande J. Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. Thromb Res. 2020;194:209–215. doi: 10.1016/j.thromres.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taccone F.S., Gevenois P.A., Peluso L., Pletchette Z., Lheureux O., Brasseur A. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48:e1087–e1090. doi: 10.1097/CCM.0000000000004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voicu S., Bonnin P., Stepanian A., Chousterman B.G., Le Gall A., Malissin I. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020;76:480–482. doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P., Qu Y., Tu J., Cao W., Hai N., Li S. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID-19 and treatment with low molecular weight heparin. J Clin Ultrasound. 2020;48:522–526. doi: 10.1002/jcu.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: Preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8:694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasan S.S., Radford S., Kow C.S., Zaidi S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J Thromb Thrombolysis. 2020;50:814–821. doi: 10.1007/s11239-020-02235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med. 2020;9:2489. doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dagadaki O., Birbas K., Mariolis T., Baltopoulos G., Myrianthefs P. Necessity of the periodical ultrasound assessment of the peripheral venous system in intensive care unit patients. Ultrasound Med Biol. 2019;45:367–373. doi: 10.1016/j.ultrasmedbio.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Costanzo L., Palumbo F.P., Ardita G., Antignani P.L., Arosio E., Failla G. Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J Vasc Surg Venous Lymphat Disord. 2020;8:711–716. doi: 10.1016/j.jvsv.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palareti G., Antonucci E., Dentali F., Mastroiacovo D., Mumoli N., Pengo V. Patients with isolated pulmonary embolism in comparison to those with deep venous thrombosis. Differences in characteristics and clinical evolution. Eur J Intern Med. 2019;69:64–70. doi: 10.1016/j.ejim.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Criel M., Falter M., Jaeken J., Van Kerrebroeck M., Lefere I., Meylaerts L. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur Respir J. 2020;56:2001201. doi: 10.1183/13993003.01201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]