Abstract
Introduction
Persons with intellectual disability (ID) are at a higher risk of developing dementia than persons without ID, with an expected earlier onset. Assessment methods for the general population cannot be applied for persons with ID due to their pre-existing intellectual and functional impairments. As there is no agreed-upon measure to assess dementia in persons with ID, multiple instruments for this purpose have been developed and adapted in the past decades. This review aimed to identify all available informant-based instruments for the assessment of dementia in persons with ID, to evaluate and compare them according to their measurement properties, and to provide a recommendation for the most suitable instruments. Additionally, an overview of the amount and quality of research on these instruments will be provided.
Methods and analysis
This review will be conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. We will adhere to the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines and use a set of characteristics developed for assessment instruments for persons with ID, the Characteristics of Assessment Instruments for Psychiatric Disorders in Persons with Intellectual Developmental Disorders. Two comprehensive, systematic literature searches will be applied in 10 international databases, including ASSIA, CINAHL, Cochrane Library, ERIC, MEDLINE, PsycINFO, Scopus, Web of Science, OpenGrey and ProQuest Dissertations and Theses Global. Risk of bias and quality assessment will be done according to COSMIN guidelines. We will apply the modified Grading of Recommendations, Assessment, Development and Evaluation approach to rate the overall quality of the available evidence.
Ethics and dissemination
No ethics statement is needed for this study. The results will be submitted to a peer-reviewed journal and will be presented at international conferences.
Keywords: mental health, old age psychiatry, statistics & research methods, dementia
Strengths and limitations of this study.
This review follows the most up-to-date standards for conducting systematic reviews on assessment instruments, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Consensus-based Standards for the Selection of Health Measurement Instruments guidelines, and additionally uses the Characteristics of Assessment Instruments for Psychiatric Disorders in Persons with Intellectual Developmental Disorders, a system especially developed for evaluating assessment instruments for psychiatric disorders in persons with intellectual disability.
Two very comprehensive consecutive search strategies will be applied in a total of 10 international databases, including grey and unpublished literature.
We use no language restrictions to minimise language bias.
We include only informant-based instruments assessing dementia in our evaluation and exclude direct cognitive tests.
Due to expected heterogeneity in studies, a quantitative pooling of psychometric data will probably not be possible.
Introduction
Intellectual disability (ID) is characterised by limitations in intellectual functioning (IQ<70) and in adaptive behaviour originating in the developmental phase of an individual.1 It is also known as intellectual developmental disorder in the Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5)2 and Disorders of Intellectual Development in the 11th Revision of the International Classification of Diseases (ICD-11).3 Prevalence of ID is hard to establish since in many countries, no official records of persons with ID exist.4 In large meta-analyses and reviews, the worldwide prevalence of ID is estimated to range from 1.0% to 3.3%.5–7
Persons with ID are at the same or at higher risk of developing dementia than persons without ID.8–10 Yet, due to their limitations in intellectual functioning, it is often hard to recognise dementia in this population, especially at an early stage. Well-evaluated assessment and screening instruments for the general population, such as the frequently used Mini-Mental State Examination,11 are not suitable for persons with ID due to their pre-existing disabilities.12 13 Diagnostic overshadowing14 15 makes it difficult to distinguish symptoms linked to the pre-existing disability from symptoms caused by the onset of dementia. Additionally, the presentation of dementia in persons with ID can differ from the presentation in persons without ID, with behavioural symptoms and personality changes being more frequent and probably earlier in the course of the illness, especially in persons with Down syndrome (DS).16 17 To reliably detect dementia in persons with ID, it is recommended to compare a baseline assessment with periodic reassessments.18–20 Most dementia assessment methods for persons with ID rely on informant-based measures. The respondent of these instruments should be a person who knows the respective person with ID very well, for instance, a family member or care staff. In contrast to direct tests of cognitive functioning, informant-based instruments can be applied for all persons with ID, irrespective of their intellectual and functional capacity.
Early recognition of dementia is particularly important to start early interventions, to plan for the future and to get adequate support for family carers or care staff.21–23 Not being able to recognise early signs of dementia constitutes a disadvantage for persons with ID and contradicts the Convention on the Rights of Persons with Disabilities by the United Nations (UN-CRPD).24 Article 25 and 26 of the UN-CRPD require states parties to ensure that persons with disabilities can get the ‘highest attainable standard of health without discrimination on the basis of disability’.24
There are several tools and screening instruments in use for the early recognition of dementia in persons with ID.13 25 These instruments can be placed into one of three categories: medical tests (eg, fMRI and gene markers), direct cognitive tests and informant-based scales, which are also called observer-rated scales. In this review, we focus solely on informant-based scales, which include observer-reported outcome measures, as well as clinician-reported outcome measures.26
One systematic review found 114 instruments and four test-batteries that have been used to assess dementia in persons with ID. However, some of these instruments have never been designed or adapted to be used in persons with ID, or even to assess dementia.13 Although there are already some reviews summarising tools and screening instruments in use for assessing dementia in persons with ID,13 25 27 28 no systematic review on measurement properties using up-to-date guidelines for review conduction and psychometric evaluation has been conducted so far. We want to provide an inventory of available informant-based instruments and their measurement properties. This should help clinicians and research in choosing the adequate instrument for their respective purpose. Our review adds to the existing body of knowledge by using a very inclusive systematic search of the literature and, most importantly, by providing a systematic evaluation of informant-based dementia assessment instruments following up-to-date guidelines.
For each instrument, we will systematically summarise the amount and quality of available evaluation studies, depicting which measurement properties have been evaluated to what extent, and which measurement properties have not been sufficiently evaluated, yet.
The objectives of this systematic review were (1) to identify informant-based instruments suitable for the assessment of dementia in persons with ID, (2) to provide a systematic overview of descriptive aspects for each instrument (eg, respondent requirements and response format), (3) to provide a systematic overview of the amount and quality of available research for each instrument and each measurement property, and (4) to provide a recommendation for the most suitable instruments based on all information collected.
Methods and analysis
This review will be conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.29 The review protocol has been developed using the PRISMA guidelines for protocols (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols).30 31 We will adhere to the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines32 and complement them with a set of characteristics especially developed for assessment instruments for persons with ID, the Characteristics of Assessment Instruments for Psychiatric Disorders in Persons with Intellectual Developmental Disorders (CAPs-IDD).33 The systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020181773. If amendments to the protocol are needed, we will register these in PROSPERO, including date and rationale. In the final publication of our results, any amendments to the protocol will be depicted and explained.
Search strategy
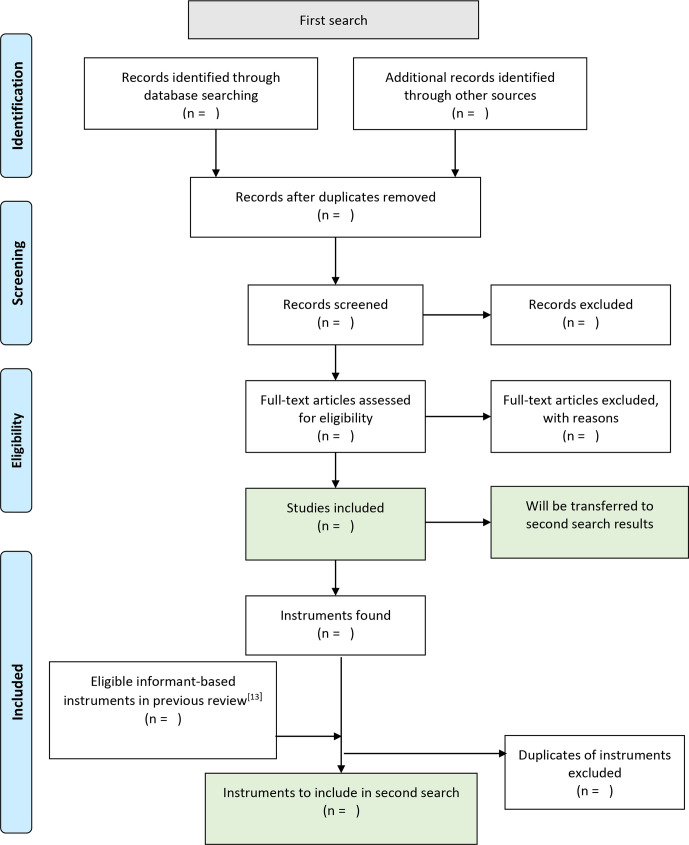
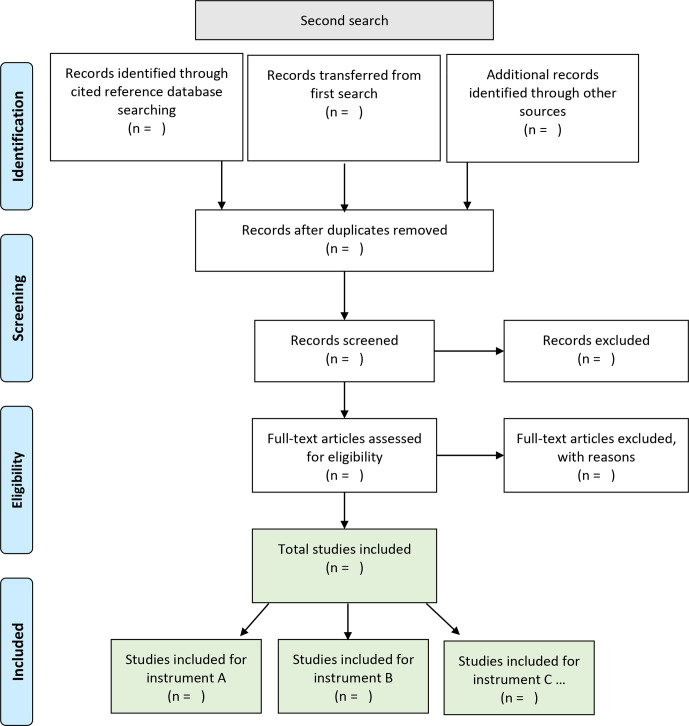
Two systematic searches will be applied consecutively and carried out between May 2020 and August 2020. The first search should provide an inventory of available informant-based assessment instruments for dementia in persons with ID. The goal of the second search is to locate evaluation studies for each instrument found in the first search. Figures 1 and 2 depict our search strategies using PRISMA flowcharts.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the first search.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the second search.
First search
To identify instruments, we will search in ten international electronic databases, including ASSIA, CINAHL, Cochrane Library, ERIC, MEDLINE, PsycINFO, Scopus, Web of Science, OpenGrey and ProQuest Dissertations and Theses Global. The search strategy is described in table 1 and depicted in detail in the online supplemental file. It will include various terms for the (1) output of interest, (2) construct of interest and (3) the specified population. As persons with DS are very prone to develop dementia, this subgroup of persons with ID is included in our search strategy. We will use a limit on the timespan of publication in the first search, not including publications before the year 2012. Instruments published up to the year of 2012 are summarised in a previous systematic review.13 This review used a very inclusive search strategy and listed all assessment instruments that have been used to assess dementia in persons with ID. We will examine the total of 114 dementia assessment instruments listed in the review of 2013 and include those instruments that are in line with our inclusion criteria.
Table 1.
Search strategy for the first search
| (1) Output | (2) Construct | (3) Population | |
| Search terms | Assessment instruments | Dementia | Intellectual disability |
| Synonyms | assessment; diagnostic; diagnosis; screening; instrument; tool; measurement; questionnaire; psychometrics; scale; interview | dementia; Alzheimer’s disease | Intellectual disability; learning disability; intellectual developmental disorder; trisomy 21, Down syndrome |
| Combined and truncated | assess* OR diagnosti* OR screen OR screening* OR instrument* OR tool* OR measure* OR questionnaire* OR psychometr* OR scale* OR interview* | dement* OR Alzheimer* | ((intellectual* OR learning) AND disab*) OR (intellectual* AND developmental* AND disorder*) OR trisom* 21 OR (down* AND syndrom*) |
| Example search string for Scopus | TITLE-ABS-KEY ((assess* OR diagnosti* OR screen OR screening* OR instrument* OR tool* OR measure* OR questionnaire* OR psychometr* OR scale* OR interview*) AND (dement* OR alzheimer*) AND (((intellectual* OR learning) AND disab*) OR (intellectual* AND developmental* AND disorder*) OR trisom* 21 OR (down* AND syndrom*))) AND PUBYEAR >2011 | ||
bmjopen-2020-040920supp001.pdf (23.8KB, pdf)
Inclusion criteria for the first search will be as follows: (1) studies need to focus on assessing dementia in persons with ID; (2) description of the development or evaluation of an informant-based instrument for the assessment of dementia; (3) and this instrument has to be especially developed or adapted for persons with ID. Exclusion criteria include (1) classification systems like ICD-11 and DSM-5, and (2) scales including dementia, but focusing on a broader spectrum of disorders for screening purposes or differential diagnosis, such as the Psychiatric Assessment Schedule for Adult with Developmental Disability.34
Second search
Once we have identified the instruments, we will conduct a search by citation strategy using the initial publications of each instrument as a reference point. This search strategy was chosen on the assumption that a paper evaluating an instrument would surely cite the initial publication of the respective instrument. The papers used as reference points will also be included in the further appraisal of the literature. For published papers, we will use five international databases allowing a search by citation strategy, including ERIC, PsycInfo, MEDLINE, Scopus and Web of Science. For published manuals not listed in at least one of the five databases, we will use Google Scholar. Additionally, all records fulfilling the inclusion and exclusion criteria of the first search will be transferred and examined in the second search.
The following inclusion criterion will be used in the second search: studies need to describe an evaluation of the respective instrument in persons with ID. Exclusion criteria comprise (1) the use of the respective instrument primarily for other investigations not related to an evaluation of the instrument or (2) or the study being a review on assessment instruments, not providing novel information.
To further include grey and unpublished literature in both searches, we will apply an invisible college approach, contacting authors in the field for information or articles on this topic, and we will follow up on meeting abstracts. Full texts of reviews on assessment instruments identified in the course of the two searches will be screened for possible further studies to include. References of papers meeting the inclusion criteria will be hand-searched. We will re-run both searches before the final analyses to include the most recent publications.
For study selection, one reviewer will exclude duplicates. All remaining records will be screened and reviewed for eligibility by two team members independently, that is, blinded to each other’s decisions. In the case of disagreement, dissonances will be discussed until agreement is reached. In the case of non-agreement, a third team member will be included in discussion.
Data extraction
The first search will result in a list of instruments. Data extracted will be the names of the instruments and information on their initial publications. In the second search, we will extract evaluation data of instruments, that is, measurement properties and characteristics as listed in the COSMIN checklists and the CAPs-IDD. For each characteristic/property extracted, we will record the study design and sample characteristics, including sample size, gender distribution, age distribution, aetiology of ID and country (language) in which the instrument was evaluated. We will include all studies, irrespective of their design.
The extraction of all relevant data will be done via standardised and piloted Excel spreadsheets (version 16.0) by two team members independently. In the case of disagreement, dissonances will be discussed until agreement is reached. In the case of non-agreement, a third team member will be included in discussion. If data necessary for coding are missing in a study, we will contact the respective study authors for this information.
Risk of bias and quality assessment
Quality and risk of bias will be assessed on study level (for each measurement property), on outcome level (for each assessment instrument) and on an aggregated outcome level, applying the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. We will combine the COSMIN checklists35–37 with the CAPs-IDD,33 a comprehensive tool specifically developed for the evaluation of assessment instruments for psychiatric disorders in persons with ID. The CAPs-IDD consists of two parts: (1) conceptual and measurement model (including descriptive aspects of instruments, eg, respondent requirements and theoretical foundation) and (2) psychometric properties. We will only use the first part as the second part is more comprehensively covered by the COSMIN checklists.
All ratings will be done by two reviewers independently. In the case of disagreement, dissonances will be discussed until agreement is reached. In the case of non-agreement, a third team member will be included in the discussion. Initial inter-rater agreement will be determined using percentage agreement calculated in R.38
As to publication bias, we assume that evaluation results not in favour of the respective instruments are likely to be under-reported. This may be partly due to evaluations being frequently done and published by the developers of the respective instrument. We will address this by including grey literature and by discussing this aspect in the interpretation of our results.
Strategy for data synthesis
A narrative synthesis will be conducted. Assessment instruments will be presented in a table along with descriptive aspects according to CAPs-IDD, and their measurement properties and quality ratings according to the COSMIN checklists. Quantitative data pooling will probably not be possible. This is due to an expected limited number of studies evaluating the same property (eg, internal consistency) for an instrument and an expected heterogeneity in the population studied (eg, severity of ID, persons with DS vs persons with ID of other aetiology). However, if applicable, we will calculate pooled estimates and 95% CIs using R.38
Analysis of subgroups
We define persons with DS/trisomy 21 as a special subgroup, as they are more often affected by Alzheimer’s dementia, with a suspected earlier onset.16 We will group instruments according to their intended use, and studies according to their participants in four clusters: (1) persons with ID, including persons with DS; (2) only persons with DS; (3) only persons with ID, not including DS; and (4) aetiology of ID not specified. For the fourth cluster, we will contact study authors to determine aetiology of ID in the respective sample or for the respective instrument. We will then allocate each study or instrument to the first three clusters according to the information provided by the authors. If no information is provided, the respective study or instrument remains in cluster 4.
Confidence in cumulative evidence
The modified GRADE approach as suggested by the COSMIN guidelines32 will be applied to grade the quality of the evidence.
Data management
We will use ZOTERO for saving records and managing and storing literature, including managing duplicates. For extracting data and recording decisions on quality ratings, we will use standardised and piloted Excel spreadsheets.
Patient and public involvement
This research was done without patient involvement due to limited resources.
Discussion
This review will summarise measurement properties of available informant-based assessment instruments for persons with ID and give an overview of the quality of each instrument and the quality of available evaluation studies. For each instrument, we will depict which psychometric properties are evaluated to what extent and which properties need further evaluation in future research. This will be the first systematic review of dementia assessment instruments for persons with ID using PRISMA and COSMIN guidelines, as well as applying the ID-specific criteria of the CAPs-IDD.
Our work will highlight gaps in research on these instruments, thus setting the ground for more effective research in the future. The results of this review will inform researchers and clinicians of the quality of available instruments to assess dementia in persons with ID and guide them in choosing an adequate instrument. This will hopefully contribute to an improvement of dementia assessment in persons with ID and a better, earlier and more adequate provision of healthcare services, as demanded by the UN-CRPD.24
Ethics and dissemination
No ethics statement is needed for this study. The results of this systematic review will be submitted for publication to a leading peer-reviewed journal, and presented at international conferences and congresses in the fields of ID, ageing and dementia.
Supplementary Material
Acknowledgments
Open access funding was provided by the University of Vienna.
Footnotes
Contributors: ELZ conceived the study, drafted the protocol and is the guarantor of the review. SK, IZ and FF contributed to the study design and drafting of the protocol. ELZ, SK and KW designed and tested the search strategy. FF and IZ tested quality rating tools and software options. All authors read and approved the final protocol.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not applicable as no datasets are generated and/or analysed for this study. No data were generated or analysed for this study.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Schalock RL, Borthwick-Duffy SA, Bradley VJ, et al. Intellectual disability: definition, classification, and systems of supports. 11th ed Washington, DC: American Association on Intellectual and Developmental Disabilities, 2010. [Google Scholar]
- 2.American Psychiatric Association Diagnostic and statistical manual of mental disorders. Washington, DC: Author, 2013. [Google Scholar]
- 3.World Health Organization International classification of diseases (11th revision), 2018. Available: https://icd.who.int/en
- 4.Holt G, Costello H, Bouras N, et al. BIOMED-MEROPE project: service provision for adults with intellectual disability: a European comparison. J Intellect Disabil Res 2000;44 (Pt 6):685–96. 10.1046/j.1365-2788.2000.00312.x [DOI] [PubMed] [Google Scholar]
- 5.Durkin M. The epidemiology of developmental disabilities in low-income countries. Ment Retard Dev Disabil Res Rev 2002;8:206–11. 10.1002/mrdd.10039 [DOI] [PubMed] [Google Scholar]
- 6.Maulik PK, Mascarenhas MN, Mathers CD, et al. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 2011;32:419–36. 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 7.Roeleveld N, Zielhuis GA, Gabreëls F. The prevalence of mental retardation: a critical review of recent literature. Dev Med Child Neurol 1997;39:125–32. 10.1111/j.1469-8749.1997.tb07395.x [DOI] [PubMed] [Google Scholar]
- 8.Strydom A, Hassiotis A, King M, et al. The relationship of dementia prevalence in older adults with intellectual disability (ID) to age and severity of ID. Psychol Med 2009;39:13–21. 10.1017/S0033291708003334 [DOI] [PubMed] [Google Scholar]
- 9.Strydom A, Chan T, King M, et al. Incidence of dementia in older adults with intellectual disabilities. Res Dev Disabil 2013;34:1881–5. 10.1016/j.ridd.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 10.Takenoshita S, Terada S, Kuwano R, et al. Prevalence of dementia in people with intellectual disabilities: cross-sectional study. Int J Geriatr Psychiatry 2020;35:414–22. 10.1002/gps.5258 [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 12.Burt DB. Issues in dementia assessment methods : Prasher VP, Neuropsychological assessment of dementia in down syndrome and intellectual disabilities. Cham, Switzerland: Springer, 2018. [Google Scholar]
- 13.Zeilinger EL, Stiehl KAM, Weber G. A systematic review on assessment instruments for dementia in persons with intellectual disabilities. Res Dev Disabil 2013;34:3962–77. 10.1016/j.ridd.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 14.Jopp DA, Keys CB. Diagnostic overshadowing reviewed and reconsidered. Am J Ment Retard 2001;106:416–33. [DOI] [PubMed] [Google Scholar]
- 15.Mason J, Scior K. ‘Diagnostic Overshadowing’ Amongst Clinicians Working with People with Intellectual Disabilities in the UK. J Appl Res Int Dis 2004;17:85–90. 10.1111/j.1360-2322.2004.00184.x [DOI] [Google Scholar]
- 16.Hartley D, Blumenthal T, Carrillo M, et al. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimers Dement 2015;11:700–9. 10.1016/j.jalz.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautarescu BA, Holland AJ, Zaman SH. The early presentation of dementia in people with Down syndrome: a systematic review of longitudinal studies. Neuropsychol Rev 2017;27:31–45. 10.1007/s11065-017-9341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aylward EH, Burt DB, Thorpe LU, et al. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res 1997;41 (Pt 2):152–64. 10.1111/j.1365-2788.1997.tb00692.x [DOI] [PubMed] [Google Scholar]
- 19.Deb S, McHugh R. Dementia among Persons with Down Syndrome : Urbano RC, International review of research in mental retardation. Academic Press, 2010: 221–55. [Google Scholar]
- 20.Kalsy S, Oliver C. The Assessment of Dementia in People with Intellectual Disabilities: Key Assessment Instruments. In: Assessing Adults with Intellectual Disabilities. John Wiley & Sons, Ltd 2008:207–19. [Google Scholar]
- 21.Heller T, Scott HM, Janicki MP, et al. Caregiving, intellectual disability, and dementia: report of the Summit Workgroup on caregiving and intellectual and developmental disabilities. Alzheimers Dement 2018;4:272–82. 10.1016/j.trci.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson L, Tang E, Taylor J-P. Dementia: timely diagnosis and early intervention. BMJ 2015;350:3029:h3029. 10.1136/bmj.h3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers A, MacDonald S. Psychosocial interventions for people with intellectual disabilities and dementia: a systematic review. J Appl Res Intellect Disabil 2020:1–17. [DOI] [PubMed] [Google Scholar]
- 24.United Nations Convention on the Rights of Persons with Disabilities. New York: United Nations, 2006. [Google Scholar]
- 25.McKenzie K, Metcalfe D, Murray G. A review of measures used in the screening, assessment and diagnosis of dementia in people with an intellectual disability. J Appl Res Intellect Disabil 2018;31:725–42. 10.1111/jar.12441 [DOI] [PubMed] [Google Scholar]
- 26.Walton MK, Powers JH, Hobart J, et al. Clinical outcome assessments: conceptual foundation-report of the ISPOR clinical outcomes assessment - emerging good practices for outcomes research task force. Value Health 2015;18:741–52. 10.1016/j.jval.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott‐King J, Shaw S, Bandelow S, et al. A critical literature review of the effectiveness of various instruments in the diagnosis of dementia in adults with intellectual disabilities. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 2016;4:126–48. 10.1016/j.dadm.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabbagh M, Edgin J. Clinical assessment of cognitive decline in adults with Down syndrome. Curr Alzheimer Res 2016;13:30–4. 10.2174/1567205012666150921095724 [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 32.Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1147–57. 10.1007/s11136-018-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeilinger EL, Nader IW, Brehmer-Rinderer B, et al. CAPs-IDD: characteristics of assessment instruments for psychiatric disorders in persons with intellectual developmental disorders. J Intellect Disabil Res 2013;57:737–46. 10.1111/jir.12003 [DOI] [PubMed] [Google Scholar]
- 34.Moss S, Patel P, Prosser H, et al. Psychiatric morbidity in older people with moderate and severe learning disability. I: development and reliability of the patient interview (PAS-ADD). Br J Psychiatry 1993;163:471–80. 10.1192/bjp.163.4.471 [DOI] [PubMed] [Google Scholar]
- 35.Mokkink LB, Prinsen C, Patrick DL, et al. COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs) - user manual. Amsterdam, The Netherlands: VU University Medical Center, 2018. [Google Scholar]
- 36.Terwee CB, Prinsen CA, Chiarotto A, et al. COSMIN methodology for assessing the content validity of PROMs – user manual. Amsterdam, The Netherlands: VU University Medical Center, 2018. [Google Scholar]
- 37.Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1171–9. 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. https://www.R-project.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040920supp001.pdf (23.8KB, pdf)