Abstract
Ewing sarcoma (ES) is thought to arise from mesenchymal stem cells and is the second most common bone sarcoma in pediatric patients and young adults. Given the dismal overall outcomes and very intensive therapies used, there is an urgent need to explore and develop alternative treatment modalities including immunotherapies. In this article, we provide an overview of ES biology, features of ES tumor microenvironment (TME) and review various tumor-associated antigens that can be targeted with immune-based approaches including cancer vaccines, monoclonal antibodies, T cell receptor-transduced T cells, and chimeric antigen receptor T cells. We highlight key reasons for the limited efficacy of various immunotherapeutic approaches for the treatment of ES to date. These factors include absence of human leukocyte antigen class I molecules from the tumor tissue, lack of an ideal surface antigen, and immunosuppressive TME due to the presence of myeloid-derived suppressor cells, F2 fibrocytes, and M2-like macrophages. Lastly, we offer insights into strategies for novel therapeutics development in ES. These strategies include the development of gene-modified T cell receptor T cells against cancer–testis antigen such as XAGE-1, surface target discovery through detailed profiling of ES surface proteome, and combinatorial approaches. In summary, we provide state-of-the-art science in ES tumor immunology and immunotherapy, with rationale and recommendations for future therapeutics development.
Keywords: immunotherapy, t-lymphocytes, immunotherapy, adoptive, vaccination, receptors, chimeric antigen
Background
Ewing sarcoma (ES) is thought to arise from mesenchymal stem cells in pediatric patients.1 2 It is the second most common osseous sarcoma in pediatric patients and young adults.3 ES can present as conventional ES or extraosseous ES, and it is now grouped under the undifferentiated small round cell sarcomas of bone and soft tissue.4 ES belongs histologically to the group of small round blue cell tumors that are composed of scattered small tumor cells with a high nucleus/cytoplasm ratio. They have finely dispersed chromatin and are arranged in sheets with occasional rosettes and varying degrees of neuroectodermal differentiation along with areas of necrosis.3 5 6 The neoplastic cells commonly harbor a translocation, which occurs between the central exons of the ES breakpoint region 1 (EWSR1 or EWS) gene on chromosome 22 to the central exons of an erythroblast transformation specific (ETS) family gene such as the Friend leukemia integration 1 (FLI1) on chromosome 11. This leads to the translocation t(11;22) and the gene product EWS–FLI1, which characterizes ES. The second most common translocation occurs between EWSR1 and another member of the ETS family, namely, ETS-related gene (ERG; chromosome 21), leading to t(21;22).
EWS–FLI1 is a chimeric protein that has been demonstrated to lead to tumorigenesis and is crucial to maintaining the malignant phenotype in ES.7 EWS–FLI1 acts as a transcription factor, in which case the EWS portion (which belongs to the RNA binding TET family), contributes to the transactivation domain, and the FLI1 portion (which is a gene of the ETS-transcription family) contributes to the DNA binding domain.6 8
EWS–FLI1 t(11;22)(q24;q12) is the most common translocation, seen in 85% of patients with ES tumors. Two EWS–FLI1 fusions have been described: exon 7 of EWS to exon 6 of FLI1 and exon 7 of EWS to exon 5 of FLI1, referred as type 1 and type 2 fusions, respectively. Alternative translocations such as t(21;22 22;12) resulting in EWS–ERG fusion are seen in about 10%–15% of cases.5 8–10 Approximately 1%–5% of cases show translocations involving fusion of EWS gene and other members of ETS family of transcription factors. This leads to translocations such as EWS and ETS variant 1 (t(2;22)(p22;q12)), EWS and E1AF (ETS variant 4 – ETV4/E1A enhancer binding protein) (t(17;22)(q21;q12)), and EWS and fifth Ewing variant (FEV) (t(2;22)(q33;q12)).9 11 12 Ewing-like sarcomas consist of a group of undifferentiated round cell sarcomas that resemble classic ES from a morphological standpoint but lack the hallmark EWSR1–ETS fusion.4 6 13 These tumors were historically classified as ES or unclassified round cell sarcomas; however, with improved molecular diagnostics, these have now been better characterized.14 To date, four main types of Ewing-like sarcoma have been described, and these include BCL6 corepressor (BCOR)-rearranged sarcomas, capicua transcriptional repressor (CIC)-rearranged sarcomas, sarcomas that harbor a fusion between EWSR1 and a non-ETS family member gene, and unclassified round cell sarcomas.6 13 14 Screening based on whole genome sequencing indicated a CIC–DUX fusion presence in up to 66% of EWSR-1 negative Ewing-like tumors.15–17 They overall tend to present at an older age and even in adults up to the fourth decade of life. Most of the data available for these tumors in regards to treatment and prognosis is based on retrospective reviews; however, except for the unclassified undifferentiated small round cell sarcoma, they are treated with classical ES chemotherapy and are thought to have a worse prognosis.6
Only 25% of patients with metastatic/recurrent classic ES can be cured by currently available multimodal treatments that include systemic chemotherapy combined with local control either through surgery or radiation.18 Molecularly targeted therapies are being explored for relapsed ES. An attractive target is the EWS–FLI1 fusion protein given that it is only found in tumor cells. This confers specificity to targeted approaches, and preclinical data have demonstrated that the deletion of EWS–FLI1 fusion protein resulted in ES cell.19–21 However, there is not a drug available that can directly inhibit the fusion protein.22
There has been some promise with strategies targeted to inactivate or reduce the expression or function of the EWS–FLI1 oncoprotein; some of these approaches include inhibitory oligonucleotides and small-molecule inhibitors that can disrupt its transcriptional complex.22 23 Moreover, these inhibitory oligonucleotides have been designed for ES to bind to certain sequences coding for the EWS–FLI1 fusion protein in preclinical models and has led to decreased expression of the fusion protein and subsequently lead to a reduction in tumor growth.24 25 Unfortunately, these have not been successfully translated into the clinic.26
Alternatively, a small-molecule inhibitor of RNA helicase A, YK-4–279, has shown in vitro activity against ES,27 and it competes against RNA helicase A’s specific binding site on the EWS–FLI1 protein, which is needed for it to function.28 29 TK216 is an analog of YK-4–279, which is being tested in a phase Ib clinical trial (NCT02657005). TK216 has shown favorable interim results, including a deep and sustained clinical response reported for one of the patients treated at the highest exposure dose regimen and is currently recruiting patients for an expansion cohort that will further evaluate the recommended phase 2 dose regimen of TK216 in combination with vincristine for patients with relapsed or refractory ES.
Lysine-specific histone demethylase 1 (LSD1) is a histone demethylase required for EWS/FLI1 mediated oncogenesis. Sankar et al30 showed that LSD1 inhibitors block growth and survival of multiple ES patient-derived cell lines. The LSD1 inhibitor SP-3577 is being evaluated in a phase I study in patients with relapsed or refractory ES. Preliminary data show that SP-3577 is well tolerated with a promising PK profile (NCT03600649).
Poly (ADP-ribose) polymerase 1 (PARP1) interacts with the EWS–FLI1 protein, and they create a positive feedback loop that aids with transcriptional activation, which can be disrupted by the use of PARP inhibition.22 31 This finding, along with the high response rate to PARP inhibition preclinically31 and its safety,32 has led to an ongoing phase I trial testing a PARP inhibitor, olaparib, in combination with temozolamide in adult patients with recurrent ES following failure of prior chemotherapy (NCT01858168).33
Alternative antineoplastic approaches include histone deacetylase inhibitors that work by reversing EWS–FLI1 mediated histone deacetylation as well as decreasing EWS–FLI1 mRNA and protein levels, inhibiting cell proliferation as well as by inducing tumor necrosis factor related apoptosis-inducing ligand (TRAIL) dependent apoptosis of ES cells.22 34–38 Additional approaches being explored focus on targeting downstream signaling molecules that are driven by the EWS–FLI1 fusion protein.39–42
In conclusion, given the dismal outcomes and very intensive therapies used upfront in the treatment of ES, there is a need to explore other treatment modalities in order to attempt to increase response and survival rates and to decrease the toxicities associated with currently available treatments. In this review article, we will focus on immunotherapeutic approaches for classical ES.
The immune microenvironment of ES
Cellular immunotherapies using engineered T cells have shown impressive clinical activity in hematologic cancers such as B cell malignancies.43 Unfortunately, it has proven difficult to translate these successes to other types of cancer, particularly solid tumors. This might be based on the fact that the immediate microenvironment of solid tumors is characterized by a number of additional factors undermining an effective antitumor immune response.44
CD4+ and CD8+ T cells from the peripheral blood and bone marrow (BM) of patients with ES show an exhausted phenotype characterized by a more pronounced programmed cell death 1 (PD-1) expression when compared with healthy donors. The T cells expressing PD-1 are dysfunctional in both antigen-specific proliferation and cytokine production, particularly when interacting with PD-1 ligand (PD-1L).45
Myeloid-derived suppressor cells (MDSCs) have been described as a group of immature monocytic and granulocytic cells (figure 1), which suppress immune responses through several different mechanisms including nutrient depletion, oxidative stress, and activation of regulatory T cells (Treg). MDSCs accumulate in blood, lymphoid tissues and in the tumor microenvironment as the tumor burden increases, whereas they are only rarely found in healthy donors.46–48 Importantly, it has been demonstrated that MDSCs are also able to inhibit chimeric antigen receptor (CAR) T cells targeting different types of sarcoma.49
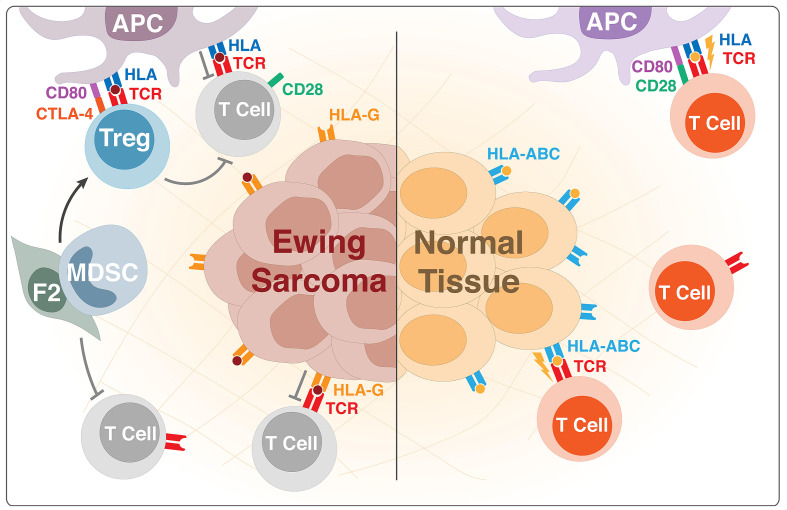
Figure 1.
Immunosuppression in the Ewing sarcoma tumor microenvironment. Low expression of human leukocyte antigen (HLA)-A, B, C on Ewing sarcoma cells prevents recognition of tumor-associated antigens by antigen presenting cells and effector T cells, while high expression of HLA-G actively suppresses tumor-specific T cells. Tregs also function to dampen the antitumoral T cell response, namely through production of suppressive cytokines and binding of CD80 on antigen presenting cells (APCs). The binding of CD80 on APCs by Treg CTLA-4 prevents CD80-CD28 costimulation of T cells, resulting in T cell anergy. The presence and activity of intratumural Tregs is further augmented by cytokines produced by F2 fibrocytes and myeloid-derived suppressor cells (MDSCs). Similarly to Tregs, F2 fibrocytes and other MDSCs also produce cytokines that dampen the cytotoxic T cell response. HLA, human leukocyte antigen; TCR, T cell receptor; Tregs, regulatory T cells.
In addition to the previous mechanisms, a cancer-driven expansion of immunosuppressive fibrocytes in patients with ES has been described (figure 1). Zhang et al50 were the first to describe this novel type of fibrocytes, a subset of MDSC with immunosuppressive properties. The F2 fibrocytes express CD45+CD34+ human leukocyte antigen (HLA)-DR+ and have the ability to induce angiogenesis and contribute to an immunosuppressive environment. These cells are capable of producing extracellular matrix proteins and can induce angiogenesis, and rather than acting as antigen presenting cells, they mediate immune suppression through their expression of indoleamine oxidase. In patients with metastatic cancer the expansion of F2 fibrocytes correlates with a shift toward a Th2 phenotype and enhanced tumor growth via induction of angiogenesis as well as increased immunosuppression.50
Immune-inhibitory ligands and soluble agents in the tumor microenvironment have also been found to cause immune tolerance among T cells. HLA-G is a non-classical major histocompatibility complex (MHC) class I molecule (figure 1), and Spurny et al51 reported that up to 34% of ES tumor samples evidenced HLA-G expression. Importantly, the presence of HLA-G can lead to direct inhibition of natural killer (NK) and T cells as well as induction and expansion of MDSCs.
Tumor-infiltrating lymphocytes (TILs)
Across different types of cancer, TILs are often found within the malignant tissue, reflecting a potential immune response against the tumor. Accordingly, in a pooled meta-analysis looking at a variety of tumor types, CD3+ TILs as well as CD8+ TILs had a positive effect on survival.52 Overall, ES does not seem to be a tumor type rich in TILs,53 and most groups did not observe a prognostic effect of tumor infiltration by CD8+ T cells.53 54 Accordingly, PD-L1 expression by tumor cells, as a mechanism mediating peripheral tolerance, is relatively low53 55 and does not seem to have a prognostic relevance in ES.53 54
Interestingly, tumor antigen-specific TILs in the tumor tissue are in principle capable of recognizing ES cells; however, deficient HLA expression on the sarcoma cells protects them from being killed.56 The expression of HLA is important for the recognition of tumors by tumor-reactive T cells (figure 1) and, accordingly, loss of HLA will affect a tumor’s susceptibility to a variety of cell-mediated immunotherapies.57 Berghuis et al58 evaluated HLA class I and class II expression in 67 ES tumors by immunofluorescence. Remarkably, they observed complete or partial absence of HLA class I expression in 79% of the ES tissue samples. Lung metastases consistently lacked HLA class I, and longitudinally tumors demonstrated a tendency towards decreased expression on disease progression. Yabe et al59 showed that ES patients with reduced HLA class I expression in the tumor tissue evidence a significantly poorer survival. They also demonstrated that the extent of CD8+ T cell infiltration of the ES tumor tissue is closely associated with the expression levels of HLA class I, which could explain why ES tumor tissue characteristically only shows a low content of TILs.
Highlighting a different mechanism behind the reduced migration of T cells into the ES tumor tissue, one study found that in ES tissue samples, expression levels of several Th1-type, interferon-gamma (IFNγ)-inducible chemokines such as CXCL9, CXCL10, and CCL5, correlated positively with numbers of infiltrating CD8+ T cells expressing the corresponding chemokine receptors. Importantly, in this study, comparably high levels of tumor infiltrating CD8+ T cells were associated with a better overall survival (OS).60
Checkpoint inhibitors
Immune checkpoint inhibitors, such as monoclonal antibodies directed against PD-1 or PD-L1, have shown clinical efficacy in a variety of solid tumors. However, a clinical trial investigating PD-1 checkpoint inhibitor pembrolizumab in adults with ES did not result in significant clinical activity. This was attributed to a low mutational burden and lack of PD-L1 expression in ES tumors.61 In addition to the low mutational burden and a resulting lack of high-affinity neoepitopes in ES,62–64 the lack of potentially tumor-reactive T cells in the tumor tissue and HLA loss, as outlined previously, could also play a role. Machado et al53 have recently published on the prognostic significance of PD-1 and PD-L1 in Ewing Sarcoma Family of Tumors (ESFTs) and did not find any statistically significance on EFS or OS based on PD-1 expression. Up to 26% of tumor cells expressed PD-L1 and suggest that it may have a role on survival. They also report and review the variance in expression that different authors have reported on checkpoint molecule expression in ESFTs and attribute that this might be due to differences in immunohistochemical staining and antibodies used for this.53 Despite some successes in other adult tumors particularly melanoma,65 there is still a need for further in vivo and clinical trials to explore the benefit of checkpoint inhibition in ES, and there are ongoing trials investigating their use in adults.53 66 As outlined further, there are alternative pathways that have been explored in ES as immunotherapeutic targets, in addition to checkpoint inhibition.
Chemokines
The chemokine network has become recognized as a contributor to a broad spectrum of physiological and pathological processes including malignancies as part of their role as an essential mediator of directional cell migration in inflammation and homing of the immune system.67 The levels of proinflammatory chemokines such as C-X-C motif ligand 9, 10 and 5 (CXCL9, CXCL10 and CXCL5), which are T cell chemoattractants, had been found to positively correlate with the amount of infiltrating CD8+ T cells.60 Chemokine C-C motif ligand 21 (CCL21) is another potent T cell chemoattractant, which acts via its receptor chemokine (C-C motif) receptor 7 (CCR7) or in combination with CXCL9 and CXCL10.68 69 Dendritic cell (DC) provoked T cell responses may be increased secondary to CCL21, and this can lead to more efficient antitumor immune responses.70 71 The use of CCL21 as a immunotherapeutic approach has been successful, and a trial that used DCs expressing CCL21 demonstrated better results when compared with the use of CCL21 along in non-small cell lung cancer.72 Given the immunogenic role of CCL21, Sand et al73 analyzed the CCL21 expression in both ES cell lines and in 18 primary therapy-naïve ES samples. This was done by analysis of RNA expression levels of CCL21. These RNA levels were then correlated with the number of infiltrating T cells as well as with the CD4+/CD8+ T cell ratio found in the ES samples.73 Sand et al found that the CD4+/CD8+ T cell ratio had an inverse correlation with the CCL21 expression level and that elevated CCL21 expression levels were associated with improved survival in patients. These findings suggest that therapy-naïve patients with ES could be tested for CCL21 levels to be used as a prognostic marker as well as a potential role for the use of this cytokine in antitumor immunity.73 Importantly, a reversed CD4+/CD8+ T cell ratio has been previously reported to be a predictor of improved outcome in other malignancies.74 75
The CXCR4-CXCL12 axis (chemokine receptor CXCR4 and its ligand CXCL12) has been reported to play critical roles in tumor progression, promotion of tumor cell proliferation, survival, metastatic processes, and angiogenesis.76–79 Lungs and BM are organs that have high levels of CXCL12 and are frequent sites of metastasis in ES. Elevated CXCR4 gene expression has recently been associated with a metastatic phenotype in ES,80 and CXCL12 has been shown to lead to neovascularization and ES tumor growth in a mouse xenograft model.81 Berguis et al demonstrated an expression level-dependent negative prognostic impact of CXCR4 protein expression in therapy-naïve ES samples. These findings point to a role of the CXCR4-CXCL12 axis promotion of ES cell growth.60 82 The same authors also showed that CXCL12 induced proliferation of ES cell lines expressing high levels of CXCR4 and that this could be inhibited by CXCR4-antagonist AMD3100 while AMD3100 alone did not inhibit spontaneous cell proliferation. These findings suggest that there is a predominant role for paracrine nature of signaling (stroma-derived CXCL12) rather than autocrine signaling (tumor cell-derived CXCL12).60 Several CXCR4 antagonists are being evaluated in clinical trials in solid tumors82 83 after having demonstrated antineoplastic activity in preclinical and animal tumor models.84 Though the disruption of the CXCR4-CXCL12 via a CXCR4 antagonist, as proposed by Berguis et al and supported by Krook et al,85 provides a rationale for exploring the use of CXCR4-targeted therapies for ES, more recent data suggest that AMD3100 might actually increase ES cell viability and proliferation in vitro,86 and further investigation is needed in this area before this target can be introduced into the clinic.
Transforming growth factor (TGF)-β coreceptor endoglin, an endothelial cell marker, is expressed by tumor cells, and its expression correlates with tumor cell plasticity in ES. ES cells with reduced endoglin levels show a reduced tumor growth in vivo. At last some of these effects seem to be mediated by the immunomodulating properties of this cytokine impacting the influx of immune cells, expression of HLA and induction of microvasculature.87 88
The search for tumor targets: intracellular antigens
The identification of appropriate tumor antigens is a prerequisite for any immunotherapeutic approach. Tumor-specific antigens, even if not expressed as proteins on the cell surface, can still potentially represent attractive targets if presented as peptides in an HLA context to endogenous or adoptively transferred T cells specific for the same antigen (figure 2). In ES, low or negative HLA class I surface expression theoretically prevents the recognition of intracellular antigens by tumor-specific cytotoxic T lymphocytes (CTL). However, HLA expression is highly inducible by inflammatory cytokines such as IFNγ, and these cytokines could play an important role as combination partners for future immunotherapy trials for ES.89
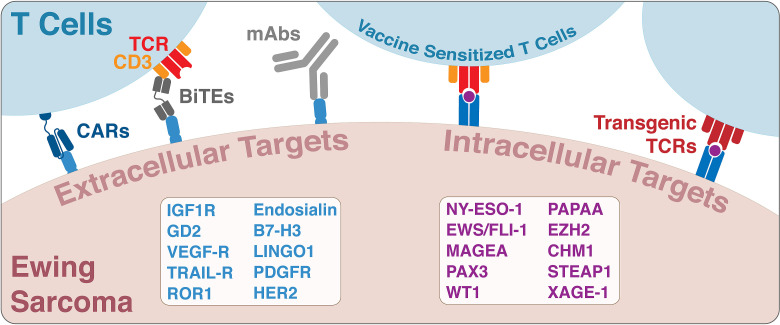
Figure 2.
Targets for cancer immunotherapies in Ewing sarcoma. Extracellular targets are natively expressed on the surface of Ewing sarcoma cells and can be targeted by both cellular and non-cellular immunotherapies. These therapies include CAR T cells, monoclonal antibodies (mAbs) and bispecific T cell engagers. In contrast, intracellular targets require presentation of naturally processed peptides in an HLA context and cellular immunotherapies such as transgenic T cell receptor (TCR) T cells, cancer vaccine or autologous tumor-infiltrating lymphocytes. CAR, chimeric antigen receptor; TCR, T cell receptor.
Wilms tumor 1 (WT1) is a transcriptional regulatory protein that is overexpressed in a wide variety of hematologic malignancies and solid tumors including sarcomas.90 McCarty et al91 demonstrated that WT1 is upregulated by hypoxia in ES cells in vitro, and that in these cells, WT1 is a direct positive regulator of vascular endothelial growth factor (VEGF) expression. Later, the same group showed that WT1 is actually a key mediator of tumor angiogenesis in ES.92 The fact that WT1 has an important biological function in ES indicates that it could represent an attractive target because it would prevent the tumor cells from downregulating its expression under the selective pressure of a T cell-mediated immune response.
As explained previously, ES is characterized by a pathognomonic chromosomal translocation that generates the EWS–FLI1 chimeric transcription factor; however, the transcriptional targets of EWS–FLI1 that are essential for tumorigenicity are incompletely defined. In a very recent study, Gallegos et al93 found that EWS–FLI1 modulates the expression of a certain class of immunogenic tumor-associated genes, so-called cancer-testis (CT) antigens. Among CT antigens, the expression of which is typically restricted to germ cells and cancer cells, fetal and adult testis expressed 1 (FATE1) was found to be the most robustly induced in ES. Importantly, the authors also showed that EWS–FLI1-induced FATE1 is required for survival and anchorage-independent growth of ES cells. These data indicated that EWS–FLI1 directly activates the expression of the CT antigen FATE1 as a means of supporting ES sarcoma tumor cell survival.93
Performing a database homology search CT antigen XAGE-1 was found to be expressed in ES.94 Later, Liu et al found XAGE-1 to be expressed in 7/8 ES cell lines and in 4/9 ES patient samples. Among normal tissues, XAGE-1 was very strongly expressed in testis with minimal expression in lung tissue and peripheral blood lymphocytes.95 A different group later confirmed these findings and found XAGE-1 expression in 3/9 ES patient samples and no expression in any normal tissues other than testis and placenta.96
Jacobs et al used quantitative real-time PCR to measure the expression of eight MAGE genes and of genes NY-ESO-1 and GAGE-1, 2, eight in nine in different pediatric solid tumors including 18 ESs. Overall, ES showed a comparably infrequent and low expression of CT antigens. However, MAGE-A6 was still detected in 39% of patients, followed by MAGE-A3 in 28%, MAGE-A4 and MAGE-A10 in 22%, and MAGE-C2 and GAGE-1/2/8 in 11%, respectively.97 In a different study, microarray datasets from ES and normal tissues were used to identify new ES-associated CT antigens and lipase I (LIPI) was a CT antigen found to be highly specific for ES. Importantly, CTL specific for two LIPI-derived peptides were able to lyse HLA-A2+ ES cells in vitro.98
Altvater and coauthors asked whether the CT antigens expressed in ES were capable of eliciting spontaneous immune responses in the patients. To this end, they screened normal donors and patients for antigen-specific T cells using libraries of overlapping peptides. Ex vivo, only a minority of patients evidenced detectable T cell responses against tumor antigens STEAP1, XAGE1 and PRAME. They were able to induce cytotoxic T cells specific for the tumor-associated antigens by in vitro priming using professional antigen-presenting cells; however, the T cells generated did not recognize the respective naturally processed antigen.45
Cancer vaccines for ES
Immunization of patients using peptides, full-length proteins, or tumor cell lysates with or without certain adjuvants is potentially able to induce T cell responses against ES-associated antigens (figure 2). Via their T cell receptor (TCR), these tumor antigen-specific T cells will then potentially be able to recognize the same antigen in form of a processed peptide presented in an appropriate HLA context on the surface of the tumor cell.
Some studies have investigated peptide vaccine approaches for ES in a preclinical setting. The transcription factor PAX3, for example, is expressed during early embryogenesis and in multiple cancer types including ES. Rodeberg et al99 used MHC peptide binding algorithms to predict potential HLA-A*02:01-restricted PAX3 epitopes. They found that one peptide and its modified version were capable of inducing antigen-specific CTLs. The respective CTLs were able to lyse peptide-pulsed, HLA-A*02:01-expressing target cells and PAX3-positive ES tumor cell lines that had naturally processed the antigen.
ES-specific CD4+ T cells were induced using a peptide library covering the EWS–FLI1 fusion. The HLA class II-restricted T cell epitope identified could potentially be used for vaccination strategies or even adoptive cellular therapies using TCR-transduced T cells.100 More recently, a novel, HLA-A2-restricted peptide epitope of the EWS–FLI1 fusion protein was identified, and the induced CD8+ effector T cells were able to specifically secrete IFN-γ and lyse the EWS–FLI1-positive HLA-matched cells tumor cell lines. In addition, treatment of mice using DCs pulsed with the EWS–FLI1 epitope led to the rejection of ES in vivo.101
A number of clinical trials have been performed investigating different types of vaccines and adjuvants in patients with ES (table 1). DCs pulsed with peptides derived from the EWS–FLI1 fusion protein were used in some of the initial clinical trials. Small series of patients with ES received DC pulsed with peptides derived from the EWS–FLI1 fusion protein concomitant with continuous intravenous recombinant human interleukin (IL)-2. Toxicity was limited to IL-2-related effects and was generally mild; however, following vaccination, all patients showed relatively rapid clinical progression.102 In another clinical trial targeting tumor-specific fusion proteins, patients with translocation-positive, recurrent or metastatic ES or alveolar rhabdomyosarcoma underwent prechemotherapy cell harvest. A total of 30 of these patients received an influenza vaccine as a control as well as unmodified autologous T cells and DC pulsed with peptides derived from tumor-specific translocation breakpoints. Interestingly, all immunotherapy recipients generated influenza-specific immune responses; however, immune responses to the translocation breakpoint peptides occurred only in ~40% of all patients indicating a comparably poor immunogenicity of the neoantigens.103
Table 1.
Clinical vaccination studies in Ewing sarcoma
| Status | Phase | Type of vaccine | Antigen | Trial number |
| R | III | TC transfected with rhGM-CSF/RNAi bi-shRNAfurin+temozolimide | Autologous tumor cells | NCT03495921 |
| C | I | DC+adjuvant | NY-ESO-1, MAGEA1, MAGEA3 | NCT01241162 |
| C | III | DC+autologous T cells | EWS/FLI-1 | NCT00001566 |
| C | I | TC transfected with rhGM-CSF/RNAi bi-shRNAfurin | Autologous tumor cells | NCT01061840 |
| C | I | Antigen presenting cells (APC)+IL-2±autologous T cells | EWS/FLI-1 | NCT00001564 |
| C | I/II | DC+IL-7+autologous T cells | Tumor cell lysate | NCT00923351 |
| C | I | DC+decitabine | NY-ESO-1, MAGEA1 and MAGEA3 | NCT01241162 |
| C | II | TC transfected with rhGM-CSF/RNAi bi-shRNAfurin+temozolimide | Autologous tumor cells | NCT01241162 |
| C | I | Racotumomab anti-idiotype antibody | – | NCT01598454 |
| C | I | Peptide+adjuvant | MAGEA12 | NCT00020267 |
C, completed; DC, dendritic cells; IL, interleukin; R, recruiting; rhGM-CSF, recombinant human granulocyte macrophage-colony stimulating factor; TC, tumor cells.
Autologous tumor cells potentially contain a variety of tumor-associated proteins and have been used as a source of antigen in a number of clinical vaccination studies in ES. Tumor lysate-pulsed DCs were evaluated in a phase I trial children with relapsed solid malignancies who had failed standard therapies. Fifteen patients, including two patients with ES, were enrolled with 10 patients completing all three vaccinations. No significant toxicities were observed, and three out of seven patients developed measurable antitumor T cell responses. Both patients with ES showed progressive disease; however, one patient with fibrosarcoma experienced significant regression of multiple metastatic sites and five patients showed stable disease.104 As part of a comparable clinical vaccination protocol, immature DCs were generated from the peripheral blood monocytes from five children with refractory solid tumors. The DCs were then pulsed with tumor lysates or, in the case of the two patients with ES, peptides designed to include the junction region of the fusion protein. Pulsed DCs were administered subcutaneously every 1 or 2 weeks without any toxicity. In one of the patients with ES, the residual tumor disappeared following autologous peripheral blood stem cell transplantation and DC therapy, and a complete remission was maintained for 77 months.105 In another recent study, patients with different pediatric sarcomas received adjuvant immunotherapy following antineoplastic therapy. The immunotherapy consisted of autologous lymphocytes, DC pulsed with autologous tumor cell lysate±recombinant human IL-7. A total of 43 patients were enrolled and 29 patients, including 20 patients with ES, received the immunotherapy. The regimen was well tolerated, and T cell responses to autologous tumor lysate were detected in 62% of immunotherapy recipients. Survival seemed to be better in patients who had received the immunotherapy and in those with detectable T cell responses.106
The FANG (or Vigil) immunotherapy comprises autologous tumor cells transfected with a plasmid expressing recombinant human granulocyte macrophage-colony stimulating factor and bifunctional short hairpin RNA against furin to induce release of tumor antigen and immunization, DC recruitment, activation and enhanced migration to local lymph nodes, and reversion of immune tolerance by blocking furin activation of endogenous TGFβ1 and TGFβ2. In a phase I study in patients with ES, the treatment was well tolerated, elicited a tumor-specific systemic immune response, and was associated with an objective response in one out of 12 patients.107 The same group subsequently reported long-term outcomes for the 12 patients and an additional four patients following immunization with Vigil. The 16 ES patients were compared with 14 contemporaneous patients with advanced ES who fulfilled the same inclusion criteria and had undergone a similar surgical procedure. The patients who had received the vaccine showed a 1-year survival of 73% compared with 23% in the group of patients treated with conventional therapy. In addition, there seemed to be a 17.2 months OS advantage in the experimental group.108 However, one has to keep in mind that this was a small non-randomized trial and that the control group may not be representative of the typical ES patient population, where a 5-year OS is historically closer to 30%, instead of the exceedingly poor 1-year OS of 23% in the Vigil control group.109 Vigil is now being investigated in an ongoing phase III randomized study of intradermal autologous Vigil immunotherapy in combination with irinotecan and temozolomide versus combination therapy alone in patients with metastatic ES, refractory/intolerant or recurrent to one prior line of chemotherapy (NCT03495921).
Oncolytic viruses
Oncolytic viruses are used to immunologically target cancer cells based on their: (1) proinflammatory characteristics and (2) their cytolytic properties potentially leading to the release of target antigens and the subsequent induction of antitumor immunity.110–114 A direct mechanism of action includes virus replication-associated necrosis or oncolysis.115 116 The tumor-associated vasculature can also be targeted by oncolytic viruses that lead to necrosis of the neoplastic cells.117 118 In pediatrics, there have been a few clinical trials using different strains of oncolytic viruses in patients with solid tumors, that have included patients with ES and have demonstrated that intratumoral administration of oncolytic viruses is safe in children; however, objective responses were not observed in these small cohorts.111 119 Moreover, the first clinical trial using herpes virus simplex-1 oncolytic virus intravenously administered to pediatric patients (NCT009311931) demonstrated that there were no dose-limiting toxicities; however, none of the patients had an objective clinical response. The authors proposed to continue to explore this virus at higher doses and potentially in combination with other antineoplastic therapies.120 Denton et al121 have explored targeting the immune microenvironment in xenograft mouse models with ES in order to enhance oncolytic herpes virus virotherapy. A regimen to deplete tumor macrophages from the tumor microenvironment was used prior to administration of the oncolytic virus. The regimens used were liposomal clodronate and trabectedin with the goal of reducing tumor macrophages, given that the M2-macrophages have been found to suppress the host antitumor and antiviral immune response.122–125 Trabectedin is a DNA intercalating agent that disrupts EWS/FLI1 transcription, and in some models, it resulted in single-agent antineoplastic activity,126–129 and in addition to inhibiting EWS/FLI-mediated tumor progression, trabectedin has been found to deplete TRAILR2+ tumor leukocytes (includingmacrophages and MDSCs).130–132 However, Denton et al121 did not observe any efficacy of trabectedin alone in A673 or TC71, ES cell lines, despite administering doses that had previously shown to decrease EWS/FLI1 expression. Both agents were found to enhance the oncolytic viral activity through suppressing macrophages and suppression of MDSCs.121 This response in animal models poses an attractive therapeutic approach to be considered in a clinical trial setting.
Adoptive cellular immunotherapies: TCR-transducted T cells
Compared with cancer vaccines that aim at eliciting a T cell-mediated immune response in the patient, the adoptive transfer of tumor-reactive T cells allows for the selection of T cells that recognize the tumor and an in vitro expansion of the effector cells (figure 2). A proof of principle was provided by Zhang et al, who showed in a xenograft model of ES that the adoptive transfer of tumor-reactive, anti-CD3/4-1BBL expanded T cells controlled primary growth and prevented metastasis of autologous tumors, while anti-CD3/anti-CD28-activated CD8+ T cells did not.133
The isolation and expansion of autologous tumor-reactive T cells can be difficult, inefficient, and labor-intensive and, therefore, the focus has shifted to more efficient ways of producing larger numbers of tumor-reactive T cells for the adoptive transfer into cancer patients. One way to do this is to isolate TCRs from T cells with proven tumor reactivity and to use these TCRs to transduce polyclonal autologous T cells followed by expansion and adoptive transfer into patients whose tumors express the given tumor antigen.
Pregnancy-associated plasma protein-A (PAPPA), also known as pappalysin, is overexpressed and required for proliferation in ES. In a recent study, CD8+ T cells targeting PAPPA were generated from HLA-A*02:01+ healthy donors by priming with peptide-loaded DC. The respective TCRs were identified and retrovirally TCR-transduced CD8+ T cells demonstrated specific reactivity toward HLA-A*02:01+/PAPPA+ ES cell lines. In a xenograft model, tumors of treated mice showed infiltration by transgenic T cells and tumor growth in mice with xenografted ES was significantly reduced after treatment with PAPPA TCR transgenic T cells.134
Allo-restricted CD8+ T cells against antigens such as homolog 2 (EZH2), and chondromodulin-I (CHM1) have been generated in vitro135; however, the therapeutic relevance of these cells is questionable due to due to high complexity in production with low cell numbers and rapid T cell exhaustion. In order to overcome these obstacles and to facilitate off-the-shelf ES-specific T cells, Blaeschke et al generated HLA-A*02:01-restricted TCR transgenic T cells directed against antigen CHM1 by retroviral transduction. The transduced T cells recognized CHM1-positive ES cell lines in vitro and, when coinjected with ES cells in Rag2−/−ɣc−/− mice, CHM1-specific TCR-transgenic T cells significantly inhibited the formation of lung and liver metastases.136 The same group also examined the potential of allo-restricted CD8+ T cells directed against the ES-associated antigen 6-transmembrane epithelial antigen of the prostate 1 (STEAP1). Following repeated stimulation with STEAP1 peptide using DC, allo-restricted HLA-A*02:01+ CD8+ T cells were expanded, and TCRs were identified. Transgenic T cells specifically recognized STEAP1+ target cells in vitro and inhibited the growth of STEAP1-expressing HLA-A0201+ ES cells in vivo.137
Three refractory HLA-A2+ ES patients were treated with HLA-A0201/CHM1-specific allorepertoire-derived haplodisparate CD8+ T cells. The TCR-transduced T cells were well tolerated without any signs of graft-versus-host disease (GvHD). In vitro, the HLA-A0201/CHM1-specific allorestricted CD8+ T cells were capable of killing all patient-derived ES cell lines. Two of the patients showed slow progression of their disease and one patient with BM involvement showed partial metastatic regression associated with T cell homing to the involved lesions. Interestingly, the CHM1 TCR transgenic T cells persisted in the patient’s BM for weeks.138 However, the successful application of TCR-based T cell targeting in ES will rely in the identification of combinatorial strategies that improve antitumor immunity, for example, by rescuing HLA downregulation.
The search for tumor targets: surface antigens
Cell surface antigens can potentially be used for the design of CAR T cell approaches, monoclonal antibodies, bispecific T cell engager, and other types of targeted immunotherapies (figure 2). The identification of surface antigens specifically expressed on ES cells (table 2) would, therefore, have enormous therapeutic potential. Monoclonal antibodies directly targeting antigens expressed on the surface of ES cells (figure 1) have been evaluated in clinical trials (table 3). Town et al139 recently described the expression of surface antigen LINGO1 on ES. LINGO1 was expressed on over 90% of ES tumors and treatment with an antibody–drug conjugate targeting LINGO1 resulted in the efficient killing of ES cells in vitro. However, while otherwise showing a highly restricted expression in healthy tissues, LINGO1 could also be detected in the central nervous system, currently prohibiting its use as a therapeutic target.
Table 2.
Surface proteins expressed on Ewing sarcoma (ES)
| Surface receptor | Expression (% of ES cases) | Expression in normal tissues | References |
| LINGO1 | 91 | Neuronal tissue. | 139 |
| CD99 | 90 | Testis, gastric mucosa, prostate, hematopoietic tissues and leukocytes. | 202 |
| Insulin-like growth factor (IGF) receptor | 90 | Brain, GI tract, lungs, endocrine tissues, muscle and pancreas. | 140 144 |
| GD2 | 40–90 | Cerebellum and peripheral nerve tumors. | 180 203 |
| B7-H3 (CD276) | 100 | Testis, endocrine tissues, GI tract and lungs. | 204 |
| Endosialin | 33 | Fibroblasts and pericytes in endometrium, synovium, bone marrow, salivary gland, thyroid gland, thymus, lymph nodes and spleen. | 174 |
| STEAP-1 | 62 | Bladder, prostate, brain and lung. | 205 |
| ROR1 | 100 | B cells and adipose tissue. | 198 |
| TRAIL-R | 100 | Monocytes, macrophages, natural killer cells, T cells and B cells. | 163 166 |
| CXCR4 | 82 | Low to absent. | 73 82 206 |
| Neuropeptide Y receptor Y1 | 81 | Central nervous system, kidney and gastrointestinal tract. | 207 208 |
| c-kit (CD117) | 60 | Mast cells, hematopoietic stem cells, interstitial cells of Cajal, melanocytes and germ cells. | 139 209 |
| NOTCH receptor | 97 | Lymphocytes, adipocytes, hematopoetic cells, thyroid and adipocytes. | 139 210 211 |
Table 3.
Clinical trials with monoclonal antibodies in Ewing sarcoma
| Status | Phase | Name of monoclonocal antibody | Target antigen | Trial number |
| C | III | Ganitumab+chemotherapy | IGF-R1 | NCT02306161 |
| C | II | Cixutumumab | IGF-R1 | NCT00668148 |
| C | I/II | Figitumumab | IGF-R1 | NCT00560235 |
| C | II | Robatumumab | IGF-R1 | NCT00617890 |
| C | II | R1507 | IGF-R1 | NCT00642941 |
| C | I | Dalotuzumab | IGF-R1 | NCT01431547 |
| C | I | BIIB022 | IGF-R1 | NCT00555724 |
| C | II | Bevacizumab+VCT | VEGF-R | NCT00516295 |
| T | I | Lexatumumab | TRAIL-R | NCT00428272 |
| C | I | Ontuxizumab | Endosialin | NCT01748721 |
| C | I | Enoblituzumab | B7-H3 | NCT02982941 |
| C | I | Hu14. 18K322A | GD2 | NCT00743496 |
C, completed; R, recruiting; T, terminated.
Type I insulin-like growth factor receptor (IGF-1R) has been found to be expressed on a wide range of solid and hematologic malignancies.140 141 As a potential tumor antigen, IGF-1R has been implicated to be necessary for the transformation capacity of certain oncogenes,142 and the binding of IGF-1 to its receptor, IGF-1R, was found to initiate a cascade of events that affect protein turnover, leading to mitogenic and differentiating effects on the majority of cell types. The IGF-1R-mediated signaling pathway has been found to be constantly active in preclinical models of ES, which also suggests a role for it in the oncogenesis of ES.140 143–145 Inhibition of IGF-1R has been shown to result in reduced tumor growth in vitro and in vivo through the inhibition of the migration of ES cells.144 146 147 These findings as well as its cell surface expression have rendered IGF-1R a potential immunotherapeutic target in ES.
The use of a monoclonal antibody against IGF-1R in patients with refractory ES resulted in an overall response rate of 10%–14% and a median progression free survival of less than 2 years.148–150 A randomized phase III trial lead by the Children’s Oncology group (COG) studied the use of ganitumab, a monoclonal antibody against IGF-1R as part of the upfront therapy for metastatic ES along with conventional chemotherapy (NCT02306161).151 Unfortunately, the study was closed in the spring of 2019, and ganitumab was discontinued in patients who had been randomized to the ganitumab arm based on a lack of benefit as well as the potential for increased toxicities such as pneumonitis. Several other monoclonal antibodies targeting IGF-1R have been explored including robatumumab, cixutumumab and figitumumab, all with limited clinical efficacy in ES.22
The tyrosine kinase-like orphan receptor 1 (ROR1) has been demonstrated to harbor a key role in tumor cell migration and invasiveness in ES, and it was initially thought that its presence on healthy adults tissues was limited to B cell precursors and adipose tissue,152–155 making it a desirable immunotherapeutic target. However, there has been some conflicting data, as it was later demonstrated that ROR1 is expressed in normal lung tissue. Treatment of immune-deficient NOD scid gamma (NSG) mice with multiple myeloma with anti-ROR1 CAR T cells led to an accumulation of the effector cells with the pulmonary tissue where they caused vasculitis and interstitial pneumonia.156 Balakrishnan et al157 also found ROR1 expression in several normal tissues and stated that this expression in other healthy tissues raises concerns for on-target off-tumor toxicities. Nevertheless, other investigators found anti-ROR1 CAR T cells to be safe in in preclinical animal models including primates158 159 and have found it to be overall effective and safe and hypothesize that it might be due to the specificity of the single chain variable fragment (scFv) antibody used.160–162
As a member of the tumor necrosis factor related apoptosis receptors, TRAIL is involved in cell apoptosis and immunosurveillance. TRAIL-R2 is widely expressed on pediatric sarcomas such as ES and rhabdomyosarcoma and has been found to lead to activations of the extrinsic apoptosis pathway in ES cell lines.163–165 The fact that its expression on healthy tissues is limited166 makes it an attractive surface receptor to target, and a monoclonal antibody named lexatumumab has been studied in pediatric solid tumors; however, objective responses were very rare in the ES population.167
Surface protein CD99 is overexpressed in ES and frequently used as part of diagnostic work-up of a ‘small round blue cell tumor’. At first glance, CD99 is an enticing target to use for immunotherapies; however, it is also expressed in several healthy tissues, particularly on hematologic cells, making it a less desirable target due to potential toxicities that could arise.168
Another immune-based approach that has been explored is the inhibition of VEGF with bevacizumab, which is a monoclonal antibody targeting VEGF receptor. This approach led to reduced cell growth and tumor vessel density in the preclinical setting.169–171 It has recently been tested through COG in a phase II clinical trial for relapsed patients. This trial is comparing salvage chemotherapy with vincristine, cyclophosphamide and topotecan with or without the addition of bevacizumab (NCT00516295).
Olaratumab is an antiplatelet-derived growth factor receptor (PDGFR) antibody that is currently being tested in combination with gemcitabine and docetaxel for relapsed and refractory soft tissue sarcomas (NCT02659020).172 Cell surface glycoprotein endosialin is found on mural cells, myofibroblasts, as well as a variety of pediatric solid tumors including ES, rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and neuroblastoma.173–176 Endosialin is in charge of promoting tumor cell growth and neovascular formation via the platelet-derived growth factor (PDGF) pathway.177 A humanized monoclonal antibody targeting endosialin named ontuxizumab can block PDGF signaling and tumor stroma organization in vitro.178 A recent phase I clinical trial of single agents ontuxizumab in relapsed or refractory pediatric solid tumors showed that ontuxizumab was well tolerated, however there were no objective responses in the four patients with ES enrolled (NCT01748721).179
Gangliosides such as GD2 and GD3 are surface antigens that are expressed by many pediatric solid tumors, such as neuroblastoma, osteosarcoma, and rhabdomyosarcoma.180 Targeting of these using anti-GD2 monoclonal antibodies has shown clinical efficacy in neuroblastoma181 182; however, their use in pediatric sarcomas is still being studied in clinical trials.65
Recently, genomic studies have highlighted that the landscape of cancer is heterogenous and complex183–185 and the potential role it plays in treatment response and prognosis.184 186 187 The intratumoral heterogeneity, characterized by the presence of multiple cancer cell phenotypes within a single neoplasia, can render certain antineoplastic approaches ineffective.188 189 Intratumoral heterogeneity can be driven by different mechanisms, which include cancer plasticity as well as clonal evolution and selection.190
Taking this concept into a clinical perspective is the use of monoclonal antibodies, which target a specific sequence of an antigen that is specifically expressed on a tumor with the goal of induced tumor cell death.191 These overall have different response rates and a potential reason for this is that fact that these monoclonal antibodies are very specific and so they only recognize one specific epitope of the surface antigen.192 This fact renders any other isoform of the epitope unrecognizable by the monoclonal antibodies. These different isoforms of the epitope of the target tumor antigens can result from mutations.191 Recently, Bühnemann et al193 combined single cell imaging data from tissue and incorporated these into a high dimensional feature distribution and a cross-validated random survival forest in order to generate a pipeline for the discovery of prognostic classifiers in ES leading to an unbiased analysis of subpopulations of cells that are heterogenous in a tumor. Analyses like this seem particularly useful when these may have a disproportionate contribution to clinical outcomes in patient cohorts.
In addition to their use as immunotherapeutic targets surface antigens can potentially be used as diagnostic markers.194 Accordingly, targeted imaging has been used to aid with ensuring tumor-free surgical margins. This form of imaging uses overexpressed tumor-associated membrane proteins to visualize tumors.194 The near infrared fluorescence imaging has been explored for targeted imaging, and it provides an optical contrast between tumor and its surrounding healthy tissue in preclinical tumor types and is an attractive approach to better delineate soft tissue involvement of ES during surgery. For ES surface membranes, proteins such as LINGO1, CD99, NOTCH1, CXCR4, c-Kit and NYPRY1 (table 2) have been proposed as potential targets.194
Adoptive cellular immunotherapies: CAR T cells
T cells engineered to express CARs can be used to effectively target tumor cells. CARs combine a binding domain against a surface antigen with signaling domains inducing T cell activation. The adoptive transfer of CAR T cells targeting CD19 has resulted in impressive overall response rates and durable responses in patients with different B cell lymphomas.43 195–197
The clinical success of engineered T cells in the treatment of hematologic malignancies has proven difficult to translate into the immunotherapy of solid tumors. However, CAR T cell approaches are under investigation for different solid tumors including ES, and performing a database search, we were able to identify two clinical CAR T cell trials (NCT03356782 and NCT03618381) currently enrolling patients with ES.
CAR T cells targeting IGF-1R and tyrosine kinase-like orphan receptor 1 (ROR1) have been explored in the preclinical setting in ES. Huang et al198 demonstrated that both IGF-1R and ROR1 were highly expressed in sarcoma cell lines including ES. CAR T cells targeting IGF1R or ROR1 were cytotoxic against sarcoma cells and the adoptive transfer of IGF1R and ROR1 CAR T cells significantly reduced tumor growth in pre-established, systemically disseminated and localized osteosarcoma xenograft models. However, both types of CAR T cells have not yet made it into the clinic for ES, and this is likely secondary to potential toxicities. In general, a key problem in the development of effective CAR T cell therapies for solid tumors such as ES remains the expression of the antigen on healthy tissues leading to on-target off-tumor toxicities. At this time, ROR1 is being evaluated as a target in clinical trials with CAR T cells in patients with breast and lung cancer, as well as in hematological malignancies (NCT02194374 and NCT02706392)161 162; however, there are currently no trials using this target in ES.
One study found that 20% of ES express tumor antigen GD2 and T cells engineered to express a third-generation GD2-CAR incorporating CD28, OX40, and CD3z signaling domains mediated efficient lysis of both GD2+ sarcoma and neuroblastoma cell lines in vitro. However, in xenograft models, GD2 CAR T cells had no antitumor effect against GD2+ sarcoma, despite effectively controlling GD2+ neuroblastoma. The investigators observed that pediatric sarcoma xenografts, but not neuroblastoma xenografts, induced large populations of MDSC that inhibited human CAR T cell responses in vitro. Importantly, treatment of sarcoma-bearing mice with all-trans retinoic acid (ATRA) largely eradicated monocytic MDSCs and diminished the suppressive capacity of granulocytic MDSCs. Consequently, combined CAR T cell plus ATRA treatment significantly improved antitumor efficacy against sarcoma xenografts. The authors concluded that coadministration of retinoids may enhance the clinical efficacy of CAR therapies targeting solid tumors.49
Instead of T cells, Kailayangiri and coauthors used CARs to enhance the activity of NK cells against ES in a tumor antigen-specific manner. Expression of CARs directed against the ganglioside antigen GD2 in activated NK cells enhanced their responses to GD2+ ES cells in vitro and overcame resistance of individual cell lines to NK cell lysis. However, adoptive transfer of GD2-specific CAR gene-modified NK cells failed to eliminate GD2-expressing ES xenografts. Interestingly, post-treatment intratumoral upregulation of the immunosuppressive ligand HLA-G seemed to be responsible for tumor immune escape, suggesting that HLA-G needs to be targeted in order to enhance the efficacy of NK CAR cells and possibly also other cellular immunotherapies.199
Recently, results on one of the very few clinical trials using CAR T cells in ES were reported. In a phase I/II clinical study, patients with recurrent/refractory human epidermal growth factor receptor 2 (HER2)-positive sarcoma received escalating doses of T cells expressing an HER2-specific CAR with a CD28 signaling domain. A total of 19 patients, including one patient with ES, were enrolled. HER2-CAR T cell infusions were well tolerated with no dose-limiting toxicity. Of the 17 evaluable patients, most showed progression of their disease but four had stable disease for 12 weeks–14 months. The patient with ES, who was among the patients receiving the highest dose level, did not show a clinical response.200
Conclusions and perspectives
The identification of a specifically expressed antigen is a prerequisite for any immunotherapy directly targeting malignant cells. Given the superior efficacy of CAR T cells and bispecific T cell engager approaches as well as the broad applicability of monoclonal antibodies, the identification of a surface antigen specifically, frequently and homogenously expressed on the tumor cells of ES patients, would have enormous clinical implications. Unfortunately, no surface antigen with all the mentioned qualities has been identified in the case of ES, and future studies should perform a detailed and thorough profiling of the ES surface proteome.
Tumor antigens specifically expressed in the intracellular compartment of the ES tumor cells represent an alternative to surface antigens and can be targeted by T cells recognizing peptide antigens in an appropriate HLA context. One very obvious target antigen would be peptides derived from the pathognomonic chromosomal translocation generating the EWS–FLI1 chimeric transcription factor. A first step in the development of vaccine-based approaches or TCR-transduced T cells targeting EWS–FLI1 would be the identification of naturally produced and presented peptide epitopes. Unfortunately, it has previously been shown that naturally occurring EWS–FLI1 peptides induce only weak CTL activity against ES cells reference. In contrast, peptides with modified anchor residues induced T cells that showed potent CTL killing of ES cells presenting endogenous (native) peptides. Accordingly, the adoptive transfer of CTL specific for the modified peptides resulted in enhanced survival of mice with established ES.201 Similarly, EWS–FLI1-specific TCRs with improved affinity could be generated for the transduction of adoptively transferred T cells.
As an alternative to the EWS–FLI1 chimeric transcription factor, CT antigens such as XAGE-1 could be used as potential targets for vaccines or TCR-transduced T cells. The advantage of this family of antigens consists of their tumor-restricted expression, their broad off-the-shelf applicability, and their immunogenicity. Another advantage of CT antigens is that many of these proteins have been shown to play a central role in inducing and maintaining the malignant phenotype and the fact that their expression can be further enhanced by treating the patient with demethylating agents.
The identification of promising tumor antigens is not the only requirement for the development of effective immunotherapies for ES, but it is equally important to address the immunosuppressive microenvironment of the tumor. The lack of inflammatory signals within the tumor, for example, can severely inhibit homing of tumor-reactive T cells to the target tissue. As outlined previously, type 1-associated proinflammatory chemokines (such as CXCL9, CXCL10, and CCL5) seem to be able to recruit effector T cells to the ES tumor tissue,60 and one could envision several ways of overexpressing these inflammatory signals in the tumor microenvironment to improve T cell homing and targeting of the cancer cells.
Absence of HLA class I molecules from the tumor tissue is another obstacle, and in order for vaccine-based approaches, adoptively transferred TILs, or TCR-transduced T cells to be effective, one would have to find ways to significantly upregulate the local expression of HLA molecules, for example, by delivering interferon into the tumor tissue. Conversely, immunosuppressive molecules overexpressed in the tumor microenvironment, such as PD-L1 and HLA-G, could be blocked using checkpoint inhibitors in combination with a given antitumor immunotherapy.
Finally, immunosuppressive cells, including Tregs, MDSCs, and F2 fibrocytes, accumulate in the microenvironment of ES and have been shown to inhibit local antitumor immune responses. There are several ways to eliminate these cells from the tumor tissue. For example, trabectedin, a chemotherapy used as a standard treatment for sarcoma, has been shown to reduce the number of intratumoral, immunosuppressive MDSCs and M2-like macrophages.121 In a mouse model, treatment of sarcoma-bearing mice with ATRA largely eradicated monocytic MDSCs and diminished the suppressive capacity of granulocytic MDSCs. As a consequence, combined CAR T cell plus ATRA treatment significantly improved antitumor efficacy against sarcoma xenografts.49 These systemic treatments should be investigated as potential combination partners in order to improve the efficacy of different immunotherapeutic approaches in ES.
Footnotes
Contributors: DA conceptualized and designed the manuscript. EM, FI, SD, TL, and DA wrote the paper. MO made the figures.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Riggi N, Cironi L, Provero P, et al. . Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res 2005;65:11459–68. 10.1158/0008-5472.CAN-05-1696 [DOI] [PubMed] [Google Scholar]
- 2.Riggi N, Suva M-L, Suva D, et al. . EWS-FLI-1 Expression Triggers a Ewing’s Sarcoma Initiation Program in Primary Human Mesenchymal Stem Cells. Cancer Res 2008;68:2176–85. 10.1158/0008-5472.CAN-07-1761 [DOI] [PubMed] [Google Scholar]
- 3.Desai SS, Jambhekar NA. Pathology of Ewing's sarcoma/PNET: current opinion and emerging concepts. Indian J Orthop 2010;44:363–8. 10.4103/0019-5413.69304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallen ME, Hornick JL. The 2020 who classification: what's new in soft tissue tumor pathology? Am J Surg Pathol 2020. 10.1097/PAS.0000000000001552. [Epub ahead of print: 12 Aug 2020]. [DOI] [PubMed] [Google Scholar]
- 5.Stahl D, Gentles AJ, Thiele R, et al. . Prognostic profiling of the immune cell microenvironment in Ewing's sarcoma family of tumors. Oncoimmunology 2019;8:e1674113. 10.1080/2162402X.2019.1674113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renzi S, Anderson ND, Light N, et al. . Ewing‐like sarcoma: an emerging family of round cell sarcomas. J Cell Physiol 2019;234:7999–8007. 10.1002/jcp.27558 [DOI] [PubMed] [Google Scholar]
- 7.Thangaretnam KP, Gopisetty G, Ramanathan P, et al. . A polypeptide from the junction region sequence of EWS-FLI1 inhibits Ewing’s sarcoma cells, interacts with the EWS-FLI1 and partner proteins. Sci Rep 2017;7:7172 10.1038/s41598-017-07482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delattre O, Zucman J, Plougastel B, et al. . Gene fusion with an Ets DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992;359:162–5. 10.1038/359162a0 [DOI] [PubMed] [Google Scholar]
- 9.Sorensen P, Triche TJ. Gene fusions encoding chimaeric transcription factors in solid tumours. Semin Cancer Biol 1996;7:3–14. 10.1006/scbi.1996.0002 [DOI] [PubMed] [Google Scholar]
- 10.Sorensen PHB, Lessnick SL, Lopez-Terrada D, et al. . A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS–family transcription factor, ERG. Nat Genet 1994;6:146–51. 10.1038/ng0294-146 [DOI] [PubMed] [Google Scholar]
- 11.Urano F, Umezawa A, Hong W, et al. . A Novel Chimera Gene betweenEWSandE1A-F, Encoding the Adenovirus E1A Enhancer-Binding Protein, in Extraosseous Ewing’s Sarcoma. Biochem Biophys Res Commun 1996;219:608–12. 10.1006/bbrc.1996.0281 [DOI] [PubMed] [Google Scholar]
- 12.Peter M, Couturier J, Pacquement H, et al. . A new member of the Ets family fused to EWS in Ewing tumors. Oncogene 1997;14:1159–64. 10.1038/sj.onc.1200933 [DOI] [PubMed] [Google Scholar]
- 13.Le Loarer F, Pissaloux D, Coindre JM, et al. . Update on families of round cell sarcomas other than classical Ewing sarcomas. Surg Pathol Clin 2017;10:587–620. 10.1016/j.path.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 14.Antonescu C. Round cell sarcomas beyond Ewing: emerging entities. Histopathology 2014;64:26–37. 10.1111/his.12281 [DOI] [PubMed] [Google Scholar]
- 15.Graham C, Chilton-MacNeill S, Zielenska M, et al. . The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol 2012;43:180–9. 10.1016/j.humpath.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 16.Haidar A, Arekapudi S, DeMattia F, et al. . High-grade undifferentiated small round cell sarcoma with t(4;19)(q35;q13.1) CIC-DUX4 fusion: emerging entities of soft tissue tumors with unique histopathologic features--a case report and literature review. Am J Case Rep 2015;16:87–94. 10.12659/AJCR.892551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Italiano A, Sung YS, Zhang L, et al. . High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer 2012;51:207–18. 10.1002/gcc.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbiah V, Anderson P, Lazar AJ, et al. . Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol 2009;10:126–40. 10.1007/s11864-009-0104-6 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Iwakuma T, Harimaya K, et al. . EWS-Fli1 antisense oligodeoxynucleotide inhibits proliferation of human Ewing’s sarcoma and primitive neuroectodermal tumor cells. J. Clin. Invest. 1997;99:239–47. 10.1172/JCI119152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu-Lieskovan S, Heidel JD, Bartlett DW, et al. . Sequence-Specific Knockdown of EWS-FLI1 by Targeted, Nonviral Delivery of Small Interfering RNA Inhibits Tumor Growth in a Murine Model of Metastatic Ewing’s Sarcoma. Cancer Res 2005;65:8984–92. 10.1158/0008-5472.CAN-05-0565 [DOI] [PubMed] [Google Scholar]
- 21.Toretsky JA, Kalebic T, Blakesley V, et al. . The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J. Biol. Chem. 1997;272:30822–7. 10.1074/jbc.272.49.30822 [DOI] [PubMed] [Google Scholar]
- 22.Casey DL, Lin T-Y, Cheung N-KV. Exploiting signaling pathways and immune targets beyond the standard of care for Ewing sarcoma. Front Oncol 2019;9:537 10.3389/fonc.2019.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer 2005;5:468–79. 10.1038/nrc1631 [DOI] [PubMed] [Google Scholar]
- 24.Maksimenko A, Malvy C. Oncogene-targeted antisense oligonucleotides for the treatment of Ewing sarcoma. Expert Opin Ther Targets 2005;9:825–30. 10.1517/14728222.9.4.825 [DOI] [PubMed] [Google Scholar]
- 25.Mateo-Lozano S, Gokhale PC, Soldatenkov VA, et al. . Combined Transcriptional and Translational Targeting of EWS/FLI-1 in Ewing’s Sarcoma. Clinical Cancer Research 2006;12:6781–90. 10.1158/1078-0432.CCR-06-0609 [DOI] [PubMed] [Google Scholar]
- 26.Kovar H, Ban J, Pospisilova S. Potentials for RNAi in sarcoma research and therapy: Ewing’s sarcoma as a model. Semin Cancer Biol 2003;13:275–81. 10.1016/S1044-579X(03)00041-5 [DOI] [PubMed] [Google Scholar]
- 27.Minas TZ, Han J, Javaheri T, et al. . YK-4-279 effectively antagonizes EWS-Fli1 induced leukemia in a transgenic mouse model. Oncotarget 2015;6:37678–94. 10.18632/oncotarget.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toretsky JA, Erkizan V, Levenson A, et al. . Oncoprotein EWS-Fli1 activity is enhanced by RNA helicase a. Cancer Res 2006;66:5574–81. 10.1158/0008-5472.CAN-05-3293 [DOI] [PubMed] [Google Scholar]
- 29.Erkizan HV, Kong Y, Merchant M, et al. . A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med 2009;15:750–6. 10.1038/nm.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankar S, Bell R, Stephens B, et al. . Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene 2013;32:5089–100. 10.1038/onc.2012.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner JC, Feng FY, Han S, et al. . PARP-1 Inhibition as a Targeted Strategy to Treat Ewing’s Sarcoma. Cancer Res 2012;72:1608–13. 10.1158/0008-5472.CAN-11-3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choy E, Butrynski JE, Harmon DC, et al. . Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer 2014;14:813. 10.1186/1471-2407-14-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engert F, Schneider C, Weiβ LM, Weiss LM, et al. . Parp inhibitors sensitize Ewing sarcoma cells to temozolomide-induced apoptosis via the mitochondrial pathway. Mol Cancer Ther 2015;14:2818–30. 10.1158/1535-7163.MCT-15-0587 [DOI] [PubMed] [Google Scholar]
- 34.Sakimura R, Tanaka K, Nakatani F, et al. . Antitumor effects of histone deacetylase inhibitor on Ewing's family tumors. Int J Cancer 2005;116:784–92. 10.1002/ijc.21069 [DOI] [PubMed] [Google Scholar]
- 35.Sonnemann J, Dreyer L, Hartwig M, et al. . Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. J Cancer Res Clin Oncol 2007;133:847–58. 10.1007/s00432-007-0227-8 [DOI] [PubMed] [Google Scholar]
- 36.Jaboin J, Wild J, Hamidi H, et al. . MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res 2002;62:6108–15. [PubMed] [Google Scholar]
- 37.Ma Y, Baltezor M, Rajewski L, et al. . Targeted inhibition of histone deacetylase leads to suppression of Ewing sarcoma tumor growth through an unappreciated EWS-FLI1/HDAC3/HSP90 signaling axis. J Mol Med 2019;97:957–72. 10.1007/s00109-019-01782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza BK, da Costa Lopez PL, Menegotto PR, et al. . Targeting histone deacetylase activity to arrest cell growth and promote neural differentiation in Ewing sarcoma. Mol Neurobiol 2018;55:7242–58. 10.1007/s12035-018-0874-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauchamp EM, Ringer L, Bulut G, et al. . Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest 2011;121:148–60. 10.1172/JCI42874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Hu H-M, Zielinska-Kwiatkowska A, et al. . FOXO1 is a direct target of EWS-Fli1 oncogenic fusion protein in Ewing’s sarcoma cells. Biochem Biophys Res Commun 2010;402:129–34. 10.1016/j.bbrc.2010.09.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauchamp E, Bulut G, Abaan O, et al. . Gli1 is a direct transcriptional target of EWS-Fli1 oncoprotein. J Biol Chem 2009;284:9074–82. 10.1074/jbc.M806233200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwerner JP, Joo J, Warner KL, et al. . The EWS/FLI1 oncogenic transcription factor deregulates Gli1. Oncogene 2008;27:3282–91. 10.1038/sj.onc.1210991 [DOI] [PubMed] [Google Scholar]
- 43.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 45.Altvater B, Kailayangiri S, Theimann N, et al. . Common Ewing sarcoma-associated antigens fail to induce natural T cell responses in both patients and healthy individuals. Cancer Immunol Immunother 2014;63:1047–60. 10.1007/s00262-014-1574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Nagaraj S. Myeloid-Derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253–68. 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006;16:53–65. 10.1016/j.semcancer.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 49.Long AH, Highfill SL, Cui Y, et al. . Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunology Research 2016;4:869–80. 10.1158/2326-6066.CIR-15-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Maric I, DiPrima MJ, et al. . Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 2013;122:1105–13. 10.1182/blood-2012-08-449413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spurny C, Kailayangiri S, Altvater B, et al. . T cell infiltration into Ewing sarcomas is associated with local expression of immune-inhibitory HLA-G. Oncotarget 2018;9:6536–49. 10.18632/oncotarget.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gooden MJM, de Bock GH, Leffers N, et al. . The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machado I, López-Guerrero JA, Scotlandi K, et al. . Immunohistochemical analysis and prognostic significance of PD-L1, PD-1, and CD8+ tumor-infiltrating lymphocytes in Ewing’s sarcoma family of tumors (ESFT). Virchows Arch 2018;472:815–24. 10.1007/s00428-018-2316-2 [DOI] [PubMed] [Google Scholar]
- 54.van Erp AEM, Versleijen-Jonkers YMH, Hillebrandt-Roeffen MHS, et al. . Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8+ lymphocytes in primary sarcomas is subtype dependent. Oncotarget 2017;8:71371–84. 10.18632/oncotarget.19071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majzner RG, Simon JS, Grosso JF, et al. . Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer 2017;123:3807–15. 10.1002/cncr.30724 [DOI] [PubMed] [Google Scholar]
- 56.Shamamian P, Mancini M, Kawakami Y, et al. . Recognition of neuroectodermal tumors by melanoma-specific cytotoxic T lymphocytes: evidence for antigen sharing by tumors derived from the neural crest. Cancer Immunol Immunother 1994;39:73–83. 10.1007/BF01525312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tait BD. Hla class I expression on human cancer cells. Implications for effective immunotherapy. Hum Immunol 2000;61:158–65. 10.1016/s0198-8859(99)00150-0 [DOI] [PubMed] [Google Scholar]
- 58.Berghuis D, de Hooge ASK, Santos SJ, et al. . Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol 2009;218:222–31. 10.1002/path.2537 [DOI] [PubMed] [Google Scholar]
- 59.Yabe H, Tsukahara T, Kawaguchi S, et al. . Prognostic significance of HLA class I expression in Ewing’s sarcoma family of tumors. J Surg Oncol 2011;103:380–5. 10.1002/jso.21829 [DOI] [PubMed] [Google Scholar]
- 60.Berghuis D, Santos SJ, Baelde HJ, et al. . Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8 + T-lymphocyte infiltration and affect tumour progression. J Pathol 2011;223:347–57. 10.1002/path.2819 [DOI] [PubMed] [Google Scholar]
- 61.Tawbi HA, Burgess M, Bolejack V, et al. . Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18:1493–501. 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chalmers ZR, Connelly CF, Fabrizio D, et al. . Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang T-C, Carter RA, Li Y, et al. . The neoepitope landscape in pediatric cancers. Genome Med 2017;9:78 10.1186/s13073-017-0468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spurny C, Kailayangiri S, Jamitzky S, et al. . Programmed cell death ligand 1 (PD-L1) expression is not a predominant feature in Ewing sarcomas. Pediatr Blood Cancer 2018;65:e26719 10.1002/pbc.26719 [DOI] [PubMed] [Google Scholar]
- 65.Dyson KA, Stover BD, Grippin A, et al. . Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol 2019;12:78 10.1186/s13045-019-0756-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanindratarn P, Dean DC, Nelson SD, et al. . Advances in immune checkpoint inhibitors for bone sarcoma therapy. J Bone Oncol 2019;15:100221. 10.1016/j.jbo.2019.100221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004;4:540–50. 10.1038/nrc1388 [DOI] [PubMed] [Google Scholar]
- 68.Lo JC, Chin RK, Lee Y, et al. . Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest 2003;112:1495–505. 10.1172/JCI19188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma S, Yang S-C, Hillinger S, et al. . SLC/CCL21-mediated anti-tumor responses require IFNgamma, MIG/CXCL9 and IP-10/CXCL10. Mol Cancer 2003;2:22 10.1186/1476-4598-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Y, Sharma S, John M. Ccl21 cancer immunotherapy. Cancers 2014;6:1098–110. 10.3390/cancers6021098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong CY, Lee H-J, Kim H-J, et al. . The lymphoid chemokine CCL21 enhances the cytotoxic T lymphocyte-inducing functions of dendritic cells. Scand J Immunol 2014;79:173–80. 10.1111/sji.12145 [DOI] [PubMed] [Google Scholar]
- 72.Lee JM, Lee M-H, Garon E, et al. . Phase I Trial of Intratumoral Injection of CCL21 Gene–Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8 + T-cell Infiltration. Clin Cancer Res 2017;23:4556–68. 10.1158/1078-0432.CCR-16-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sand LGL, Buckle T, van Leeuwen FWB, et al. . Fluorescent CXCR4 targeting peptide as alternative for antibody staining in Ewing sarcoma. BMC Cancer 2017;17:383. 10.1186/s12885-017-3352-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah W, Yan X, Jing L, et al. . A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4+Foxp3+ regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol 2011;8:59–66. 10.1038/cmi.2010.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.García-Martínez E, Gil GL, Benito AC, et al. . Tumor-Infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res 2014;16:488 10.1186/s13058-014-0488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burger JA, Kipps TJ. Cxcr4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006;107:1761–7. 10.1182/blood-2005-08-3182 [DOI] [PubMed] [Google Scholar]
- 77.Marchesi F, Monti P, Leone BE, et al. . Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res 2004;64:8420–7. 10.1158/0008-5472.CAN-04-1343 [DOI] [PubMed] [Google Scholar]
- 78.Müller A, Homey B, Soto H, et al. . Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6. 10.1038/35065016 [DOI] [PubMed] [Google Scholar]
- 79.Orimo A, Gupta PB, Sgroi DC, et al. . Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335–48. 10.1016/j.cell.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 80.Bennani-Baiti IM, Cooper A, Lawlor ER, et al. . Intercohort gene expression co-analysis reveals chemokine receptors as prognostic indicators in Ewing's sarcoma. Clin Cancer Res 2010;16:3769–78. 10.1158/1078-0432.CCR-10-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reddy K, Zhou Z, Jia S-F, et al. . Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing's sarcoma tumor growth in the absence of vascular endothelial growth factor. Int J Cancer 2008;123:831–7. 10.1002/ijc.23582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berghuis D, Schilham MW, Santos SJ, et al. . The CXCR4-CXCL12 axis in Ewing sarcoma: promotion of tumor growth rather than metastatic disease. Clin Sarcoma Res 2012;2:24 10.1186/2045-3329-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hotte SJ, Hirte HW, Moretto P, et al. . 405 poster final results of a phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers. Eur J Cancer 2008;6:127 10.1016/S1359-6349(08)72339-5 [DOI] [Google Scholar]
- 84.Duda DG, Kozin SV, Kirkpatrick ND, et al. . CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res 2011;17:2074–80. 10.1158/1078-0432.CCR-10-2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krook MA, Nicholls LA, Scannell CA, et al. . Stress-Induced CXCR4 promotes migration and invasion of Ewing sarcoma. Mol Cancer Res 2014;12:953–64. 10.1158/1541-7786.MCR-13-0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berning P, Schaefer C, Clemens D, et al. . The CXCR4 antagonist plerixafor (AMD3100) promotes proliferation of Ewing sarcoma cell lines in vitro and activates receptor tyrosine kinase signaling. Cell Commun Signal 2018;16:21. 10.1186/s12964-018-0233-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pardali E, van der Schaft DWJ, Wiercinska E, et al. . Critical role of endoglin in tumor cell plasticity of Ewing sarcoma and melanoma. Oncogene 2011;30:334–45. 10.1038/onc.2010.418 [DOI] [PubMed] [Google Scholar]
- 88.van der Schaft DWJ, Hillen F, Pauwels P, et al. . Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res 2005;65:11520–8. 10.1158/0008-5472.CAN-05-2468 [DOI] [PubMed] [Google Scholar]
- 89.Borowski A, van Valen F, Ulbrecht M, et al. . Monomorphic HLA class I-(non-A, non-B) expression on Ewing's tumor cell lines, modulation by TNF-alpha and IFN-gamma. Immunobiology 1999;200:1–20. 10.1016/S0171-2985(99)80029-1 [DOI] [PubMed] [Google Scholar]
- 90.Carpentieri DF, Nichols K, Chou PM, et al. . The expression of WT1 in the differentiation of rhabdomyosarcoma from other pediatric small round blue cell tumors. Mod Pathol 2002;15:1080–6. 10.1097/01.MP.0000028646.03760.6B [DOI] [PubMed] [Google Scholar]
- 91.McCarty G, Awad O, Loeb DM. Wt1 protein directly regulates expression of vascular endothelial growth factor and is a mediator of tumor response to hypoxia. J Biol Chem 2011;286:43634–43. 10.1074/jbc.M111.310128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katuri V, Gerber S, Qiu X, et al. . Wt1 regulates angiogenesis in Ewing sarcoma. Oncotarget 2014;5:2436–49. 10.18632/oncotarget.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallegos ZR, Taus P, Gibbs ZA, et al. . EWSR1-FLI1 activation of the cancer/testis antigen FATE1 promotes Ewing sarcoma survival. Mol Cell Biol 2019;39 10.1128/MCB.00138-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brinkmann U, Vasmatzis G, Lee B, et al. . Novel genes in the page and GAGE family of tumor antigens found by homology walking in the dbEST database. Cancer Res 1999;59:1445–8. [PubMed] [Google Scholar]
- 95.Liu XF, Helman LJ, Yeung C, et al. . XAGE-1, a new gene that is frequently expressed in Ewing's sarcoma. Cancer Res 2000;60:4752–5. [PubMed] [Google Scholar]
- 96.Zendman AJW, Van Kraats AA, Weidle UH, et al. . The XAGE family of cancer/testis-associated genes: alignment and expression profile in normal tissues, melanoma lesions and Ewing's sarcoma. Int J Cancer 2002;99:361–9. 10.1002/ijc.10371 [DOI] [PubMed] [Google Scholar]
- 97.Jacobs JFM, Brasseur F, Hulsbergen-van de Kaa CA, et al. . Cancer-Germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer 2007;120:67–74. 10.1002/ijc.22118 [DOI] [PubMed] [Google Scholar]
- 98.Mahlendorf DE, Staege MS. Characterization of Ewing sarcoma associated cancer/testis antigens. Cancer Biol Ther 2013;14:254–61. 10.4161/cbt.23298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodeberg DA, Nuss RA, Elsawa SF, et al. . Generation of tumoricidal Pax3 peptide antigen specific cytotoxic T lymphocytes. Int J Cancer 2006;119:126–32. 10.1002/ijc.21817 [DOI] [PubMed] [Google Scholar]
- 100.Meyer-Wentrup F, Richter G, Burdach S. Identification of an immunogenic EWS-FLI1-derived HLA-DR-restricted T helper cell epitope. Pediatr Hematol Oncol 2005;22:297–308. 10.1080/08880010590935194 [DOI] [PubMed] [Google Scholar]
- 101.Peng W, Huang X, Yang D. EWS/FLI-l peptide-pulsed dendritic cells induces the antitumor immunity in a murine Ewing's sarcoma cell model. Int Immunopharmacol 2014;21:336–41. 10.1016/j.intimp.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 102.Dagher R, Long LM, Read EJ, et al. . Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Med Pediatr Oncol 2002;38:158–64. 10.1002/mpo.1303 [DOI] [PubMed] [Google Scholar]
- 103.Mackall CL, Rhee EH, Read EJ, et al. . A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res 2008;14:4850–8. 10.1158/1078-0432.CCR-07-4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geiger JD, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001;61:8513–9. [PubMed] [Google Scholar]
- 105.Suminoe A, Matsuzaki A, Hattori H, et al. . Immunotherapy with autologous dendritic cells and tumor antigens for children with refractory malignant solid tumors. Pediatr Transplant 2009;13:746–53. 10.1111/j.1399-3046.2008.01066.x [DOI] [PubMed] [Google Scholar]
- 106.Merchant MS, Bernstein D, Amoako M, et al. . Adjuvant immunotherapy to improve outcome in high-risk pediatric sarcomas. Clinical Cancer Research 2016;22:3182–91. 10.1158/1078-0432.CCR-15-2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghisoli M, Barve M, Schneider R, et al. . Pilot Trial of FANG Immunotherapy in Ewing’s Sarcoma. Molecular Therapy 2015;23:1103–9. 10.1038/mt.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghisoli M, Barve M, Mennel R, et al. . Three-year Follow up of GMCSF/bi-shRNA furin DNA-transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing’s Sarcoma. Molecular Therapy 2016;24:1478–83. 10.1038/mt.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miser JS, Goldsby RE, Chen Z, et al. . Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—a report from the Children’s Oncology Group. Pediatr Blood Cancer 2007;49:894–900. 10.1002/pbc.21233 [DOI] [PubMed] [Google Scholar]
- 110.Kaur B, Antonio Chiocca E, P. Cripe T. Oncolytic HSV-1 virotherapy: clinical experience and opportunities for progress. Curr Pharm Biotechnol 2012;13:1842–51. 10.2174/138920112800958814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cripe TP, Ngo MC, Geller JI, et al. . Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther 2015;23:602–8. 10.1038/mt.2014.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todo T, Martuza RL, Rabkin SD, et al. . Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A 2001;98:6396–401. 10.1073/pnas.101136398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Young JS, Kim JW, Ahmed AU, et al. . Therapeutic cell carriers: a potential road to cure glioma. Expert Rev Neurother 2014;14:651–60. 10.1586/14737175.2014.917964 [DOI] [PubMed] [Google Scholar]
- 114.Shulak L, Beljanski V, Chiang C, et al. . Histone Deacetylase Inhibitors Potentiate Vesicular Stomatitis Virus Oncolysis in Prostate Cancer Cells by Modulating NF- B-Dependent Autophagy. J Virol 2014;88:2927–40. 10.1128/JVI.03406-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parato KA, Senger D, Forsyth PAJ, et al. . Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005;5:965–76. 10.1038/nrc1750 [DOI] [PubMed] [Google Scholar]
- 116.Stojdl DF, Lichty BD, tenOever BR, et al. . Vsv strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003;4:263–75. 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- 117.Breitbach CJ, Paterson JM, Lemay CG, et al. . Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Molecular Therapy 2007;15:1686–93. 10.1038/sj.mt.6300215 [DOI] [PubMed] [Google Scholar]
- 118.Spurrell E, Gangeswaran R, Wang P, et al. . Stat1 interaction with E3-14.7K in monocytes affects the efficacy of oncolytic adenovirus. J Virol 2014;88:2291–300. 10.1128/JVI.02829-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Streby KA, Geller JI, Currier MA, et al. . Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin Cancer Res 2017;23:3566–74. 10.1158/1078-0432.CCR-16-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Streby KA, Currier MA, Triplet M, et al. . First-In-Human intravenous Seprehvir in young cancer patients: a phase 1 clinical trial. Molecular Therapy 2019;27:1930–8. 10.1016/j.ymthe.2019.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Denton NL, Chen C-Y, Hutzen B, et al. . Myelolytic treatments enhance oncolytic herpes virotherapy in models of Ewing sarcoma by modulating the immune microenvironment. Mol Ther Oncolytics 2018;11:62–74. 10.1016/j.omto.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denton N, Chen C-Y, Scott T, et al. . Tumor-Associated macrophages in oncolytic virotherapy: friend or foe? Biomedicines 2016;4:13 10.3390/biomedicines4030013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng YH, Deng YY, Lai W, et al. . Effect of bone marrow mesenchymal stem cells on the polarization of macrophages. Mol Med Rep 2018;17:4449–59. 10.3892/mmr.2018.8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen C-H, Chen W-Y, Lin S-F, et al. . Epithelial-Mesenchymal transition enhances response to oncolytic herpesviral therapy through nectin-1. Hum Gene Ther 2014;25:539–51. 10.1089/hum.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meisen WH, Wohleb ES, Jaime-Ramirez AC, et al. . The impact of macrophage- and Microglia-Secreted TNFα on oncolytic HSV-1 therapy in the glioblastoma tumor microenvironment. Clin Cancer Res 2015;21:3274–85. 10.1158/1078-0432.CCR-14-3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakai T, Imura Y, Tamiya H, et al. . Trabectedin is a promising antitumor agent potentially inducing melanocytic differentiation for clear cell sarcoma. Cancer Med 2017;6:2121–30. 10.1002/cam4.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herzog J, von Klot-Heydenfeldt F, Jabar S, et al. . Trabectedin followed by irinotecan can stabilize disease in advanced Translocation-Positive sarcomas with acceptable toxicity. Sarcoma 2016;2016:1–6. 10.1155/2016/7461783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grohar PJ, Segars LE, Yeung C, et al. . Dual targeting of EWS-Fli1 activity and the associated DNA damage response with trabectedin and SN38 synergistically inhibits Ewing sarcoma cell growth. Clin Cancer Res 2014;20:1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hernando-Cubero J, Sanz-Moncasi P, Hernández-García A, et al. . Metastatic extraskeletal Ewing’s sarcoma treated with trabectedin: A case report. Oncol Lett 2016;12:2936–41. 10.3892/ol.2016.4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Germano G, Frapolli R, Belgiovine C, et al. . Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249–62. 10.1016/j.ccr.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 131.Borgoni S, Iannello A, Cutrupi S, et al. . Depletion of tumor-associated macrophages switches the epigenetic profile of pancreatic cancer infiltrating T cells and restores their anti-tumor phenotype. Oncoimmunology 2018;7:e1393596 10.1080/2162402X.2017.1393596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Igarashi K, Murakami T, Kawaguchi K, et al. . A patient-derived orthotopic xenograft (PDOX) mouse model of a cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: implications for precision oncology. Oncotarget 2017;8:62111–9. 10.18632/oncotarget.19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, Merchant MS, Chua KS, et al. . Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther 2003;2:579–86. 10.4161/cbt.2.5.545 [DOI] [PubMed] [Google Scholar]
- 134.Kirschner A, Thiede M, Grünewald TGP, et al. . Pappalysin-1 T cell receptor transgenic allo-restricted T cells kill Ewing sarcoma in vitro and in vivo. Oncoimmunology 2017;6:e1273301. 10.1080/2162402X.2016.1273301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thiel U, Pirson S, Müller-Spahn C, et al. . Specific recognition and inhibition of Ewing tumour growth by antigen-specific allo-restricted cytotoxic T cells. Br J Cancer 2011;104:948–56. 10.1038/bjc.2011.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blaeschke F, Thiel U, Kirschner A, et al. . Human HLA-A*02:01/CHM1+ allo-restricted T cell receptor transgenic CD8+ T Cells specifically inhibit Ewing sarcoma growth in vitro and in vivo. Oncotarget 2016;7:43267–80. 10.18632/oncotarget.9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schirmer D, Grünewald TGP, Klar R, et al. . Transgenic antigen-specific, HLA-A*02:01-allo-restricted cytotoxic T cells recognize tumor-associated target antigen STEAP1 with high specificity. Oncoimmunology 2016;5:e1175795 10.1080/2162402X.2016.1175795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thiel U, Schober SJ, Einspieler I, et al. . Ewing sarcoma partial regression without GVHD by chondromodulin-I/HLA-A*02:01-specific allorestricted T cell receptor transgenic T cells. Oncoimmunology 2017;6:e1312239 10.1080/2162402X.2017.1312239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Town J, Pais H, Harrison S, et al. . Exploring the surfaceome of Ewing sarcoma identifies a new and unique therapeutic target. Proc Natl Acad Sci U S A 2016;113:3603–8. 10.1073/pnas.1521251113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yee D, Favoni RE, Lebovic GS, et al. . Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest 1990;86:1806–14. 10.1172/JCI114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. . Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004;5:221–30. 10.1016/S1535-6108(04)00050-9 [DOI] [PubMed] [Google Scholar]
- 142.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–69. 10.1038/nrc3215 [DOI] [PubMed] [Google Scholar]
- 143.van Valen F, Winkelmann W, Jürgens H. Type I and type II insulin-like growth factor receptors and their function in human Ewing’s sarcoma cells. J Cancer Res Clin Oncol 1992;118:269–75. 10.1007/BF01208615 [DOI] [PubMed] [Google Scholar]
- 144.Scotlandi K, Benini S, Sarti M, et al. . Insulin-Like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res 1996;56:4570–4. [PubMed] [Google Scholar]
- 145.Hofbauer S, Hamilton G, Theyer G, et al. . Insulin-Like growth factor-I-dependent growth and in vitro chemosensitivity of Ewing's sarcoma and peripheral primitive neuroectodermal tumour cell lines. Eur J Cancer 1993;29A:241–5. 10.1016/0959-8049(93)90183-g [DOI] [PubMed] [Google Scholar]
- 146.Hamilton G, Mallinger R, Hofbauer S, et al. . The monoclonal HBA-71 antibody modulates proliferation of thymocytes and Ewing's sarcoma cells by interfering with the action of insulin-like growth factor I. Thymus 1991;18:33–41. [PubMed] [Google Scholar]
- 147.Scotlandi K, Benini S, Nanni P, et al. . Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing's sarcoma in athymic mice. Cancer Res 1998;58:4127–31. [PubMed] [Google Scholar]
- 148.Juergens H, Daw NC, Geoerger B, et al. . Preliminary efficacy of the Anti-Insulin–Like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. JCO 2011;29:4534–40. 10.1200/JCO.2010.33.0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malempati S, Weigel B, Ingle AM, et al. . Phase I/II Trial and Pharmacokinetic Study of Cixutumumab in Pediatric Patients With Refractory Solid Tumors and Ewing Sarcoma: A Report From the Children’s Oncology Group. JCO 2012;30:256–62. 10.1200/JCO.2011.37.4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pappo AS, Patel SR, Crowley J, et al. . R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II sarcoma alliance for research through collaboration study. J Clin Oncol 2011;29:4541–7. 10.1200/JCO.2010.34.0000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rizk VT, Walko CM, Brohl AS. Precision medicine approaches for the management of Ewing sarcoma: current perspectives. Pharmgenomics Pers Med 2019;12:9–14. 10.2147/PGPM.S170612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fukuda T, Chen L, Endo T, et al. . Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify Ror1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A 2008;105:3047–52. 10.1073/pnas.0712148105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dave H, Anver MR, Butcher DO, et al. . Restricted cell surface expression of receptor tyrosine kinase Ror1 in pediatric B-lineage acute lymphoblastic leukemia suggests targetability with therapeutic monoclonal antibodies. PLoS One 2012;7:e52655 10.1371/journal.pone.0052655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cui B, Zhang S, Chen L, et al. . Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res 2013;73:3649–60. 10.1158/0008-5472.CAN-12-3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hudecek M, Schmitt TM, Baskar S, et al. . The B-cell tumor–associated antigen Ror1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 2010;116:4532–41. 10.1182/blood-2010-05-283309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rottman J, et al. ROR1-Directed chimeric antigen receptor T cell recognition of self-antigen is associated with acute toxicity T cell dysfunction, and poor tumor control. Blood 2017;130. [Google Scholar]
- 157.Balakrishnan A, Goodpaster T, Randolph-Habecker J, et al. . Analysis of Ror1 protein expression in human cancer and normal tissues. Clin Cancer Res 2017;23:3061–71. 10.1158/1078-0432.CCR-16-2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berger C, Sommermeyer D, Hudecek M, et al. . Safety of targeting Ror1 in primates with chimeric antigen Receptor–Modified T cells. Cancer Immunol Res 2015;3:206–16. 10.1158/2326-6066.CIR-14-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Choi MY, Widhopf GF, Wu CCN, et al. . Pre-Clinical specificity and safety of UC-961, a first-in-class monoclonal antibody targeting Ror1. Clin Lymphoma Myeloma Leuk 2015;15 Suppl:S167–9. 10.1016/j.clml.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gohil S, Paredes-Moscosso S, Harrasser M, et al. . Preclinical development of novel humanised Ror1 targeting chimeric antigen receptor T cells and bispecific T-cell engagers. The Lancet 2017;389:S40 10.1016/S0140-6736(17)30436-1 [DOI] [Google Scholar]
- 161.Gohil SH, Paredes-Moscosso SR, Harrasser M, et al. . An Ror1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. Oncoimmunology 2017;6:e1326437 10.1080/2162402X.2017.1326437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Townsend MH, Shrestha G, Robison RA, et al. . The expansion of targetable biomarkers for CAR T cell therapy. J Exp Clin Cancer Res 2018;37:163 10.1186/s13046-018-0817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kontny HU, Hämmerle K, Klein R, et al. . Sensitivity of Ewing’s sarcoma to TRAIL-induced apoptosis. Cell Death Differ 2001;8:506–14. 10.1038/sj.cdd.4400836 [DOI] [PubMed] [Google Scholar]
- 164.Wakelee HA, Patnaik A, Sikic BI, et al. . Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol 2010;21:376–81. 10.1093/annonc/mdp292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cretney E, Takeda K, Yagita H, et al. . Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 2002;168:1356–61. 10.4049/jimmunol.168.3.1356 [DOI] [PubMed] [Google Scholar]
- 166.Staniek J, Lorenzetti R, Heller B, et al. . Trail-R1 and TRAIL-R2 mediate TRAIL-Dependent apoptosis in activated primary human B lymphocytes. Front Immunol 2019;10:951 10.3389/fimmu.2019.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merchant MS, Geller JI, Baird K, et al. . Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol 2012;30:4141–7. 10.1200/JCO.2012.44.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pasello M, Manara MC, Scotlandi K. Cd99 at the crossroads of physiology and pathology. J Cell Commun Signal 2018;12:55–68. 10.1007/s12079-017-0445-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guan H, Zhou Z, Wang H, et al. . A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing's sarcoma growth in a xenograft mouse model. Clin Cancer Res 2005;11:2662–9. 10.1158/1078-0432.CCR-04-1206 [DOI] [PubMed] [Google Scholar]
- 170.Ackermann M, Morse BA, Delventhal V, et al. . Anti-VEGFR2 and anti-IGF-1R-Adnectins inhibit Ewing’s sarcoma A673-xenograft growth and normalize tumor vascular architecture. Angiogenesis 2012;15:685–95. 10.1007/s10456-012-9294-9 [DOI] [PubMed] [Google Scholar]
- 171.Zhou Z, Bolontrade MF, Reddy K, et al. . Suppression of Ewing's sarcoma tumor growth, tumor vessel formation, and vasculogenesis following anti vascular endothelial growth factor receptor-2 therapy. Clin Cancer Res 2007;13:4867–73. 10.1158/1078-0432.CCR-07-0133 [DOI] [PubMed] [Google Scholar]
- 172.Pollack SM, Ingham M, Spraker MB, et al. . Emerging targeted and immune-based therapies in sarcoma. JCO 2018;36:125–35. 10.1200/JCO.2017.75.1610 [DOI] [PubMed] [Google Scholar]
- 173.Christian S, Winkler R, Helfrich I, et al. . Endosialin (TEM1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol 2008;172:486–94. 10.2353/ajpath.2008.070623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rouleau C, Curiel M, Weber W, et al. . Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res 2008;14:7223–36. 10.1158/1078-0432.CCR-08-0499 [DOI] [PubMed] [Google Scholar]
- 175.Rouleau C, Smale R, Fu Y-S, et al. . Endosialin is expressed in high grade and advanced sarcomas: evidence from clinical specimens and preclinical modeling. Int J Oncol 2011;39:73–89. 10.3892/ijo.2011.1020 [DOI] [PubMed] [Google Scholar]
- 176.Rouleau C, Smale R, Sancho J, et al. . Endosialin: a novel malignant cell therapeutic target for neuroblastoma. Int J Oncol 2011;39:841–51. 10.3892/ijo.2011.1091 [DOI] [PubMed] [Google Scholar]
- 177.Tomkowicz B, Rybinski K, Sebeck D, et al. . Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther 2010;9:908–15. 10.4161/cbt.9.11.11731 [DOI] [PubMed] [Google Scholar]
- 178.Tomkowicz B, Rybinski K, Foley B, et al. . Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A 2007;104:17965–70. 10.1073/pnas.0705647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Norris RE, Fox E, Reid JM, et al. . Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: a report from the children's Oncology Group phase 1 pilot Consortium (ADVL1213). Pediatr Blood Cancer 2018;65:e26944. 10.1002/pbc.26944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dobrenkov K, Ostrovnaya I, Gu J, et al. . Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer 2016;63:1780–5. 10.1002/pbc.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu AL, Gilman AL, Ozkaynak MF, et al. . Anti-Gd2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–34. 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheung N-KV, Cheung IY, Kushner BH, et al. . Murine Anti-GD2 Monoclonal Antibody 3F8 Combined With Granulocyte-Macrophage Colony-Stimulating Factor and 13- Cis -Retinoic Acid in High-Risk Patients With Stage 4 Neuroblastoma in First Remission. JCO 2012;30:3264–70. 10.1200/JCO.2011.41.3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lawrence MS, Stojanov P, Mermel CH, et al. . Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505:495–501. 10.1038/nature12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lawrence MS, Stojanov P, Polak P, et al. . Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–8. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jamal-Hanjani M, Quezada SA, Larkin J, et al. . Translational implications of tumor heterogeneity. Clinical Cancer Research 2015;21:1258–66. 10.1158/1078-0432.CCR-14-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kandoth C, McLellan MD, Vandin F, et al. . Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333–9. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. . Signatures of mutational processes in human cancer. Nature 2013;500:415–21. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Remšík J, Fedr R, Navrátil J, et al. . Plasticity and intratumoural heterogeneity of cell surface antigen expression in breast cancer. Br J Cancer 2018;118:813–9. 10.1038/bjc.2017.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marusyk A, Almendro V, Polyak K. Intra-Tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012;12:323–34. 10.1038/nrc3261 [DOI] [PubMed] [Google Scholar]
- 190.Malladi S, Macalinao DG, Jin X, et al. . Metastatic latency and immune evasion through autocrine inhibition of Wnt. Cell 2016;165:45–60. 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol 2019;2019:1–12. 10.1155/2019/4508794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lipman NS, Jackson LR, Trudel LJ, et al. . Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR Journal 2005;46:258–68. 10.1093/ilar.46.3.258 [DOI] [PubMed] [Google Scholar]
- 193.Bühnemann C, Li S, Yu H, et al. . Quantification of the heterogeneity of prognostic cellular biomarkers in Ewing sarcoma using automated image and random survival forest analysis. PLoS One 2014;9:e107105 10.1371/journal.pone.0107105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bosma SE, van Driel PBAA, Hogendoorn PCW, et al. . Introducing fluorescence guided surgery into orthopedic oncology: a systematic review of candidate protein targets for Ewing sarcoma. J Surg Oncol 2018;118:906–14. 10.1002/jso.25224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Davila ML, Riviere I, Wang X, et al. . Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:p. 224ra25 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hirayama AV, Gauthier J, Hay KA, et al. . High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019;134:636–40. 10.1182/blood.2019000905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet 2015;385:517–28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang X, Park H, Greene J, et al. . IGF1R- and ROR1-Specific CAR T cells as a potential therapy for high risk sarcomas. PLoS One 2015;10:e0133152 10.1371/journal.pone.0133152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kailayangiri S, Altvater B, Spurny C, et al. . Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2017;6:e1250050 10.1080/2162402X.2016.1250050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ahmed N, Brawley VS, Hegde M, et al. . Human epidermal growth factor receptor 2 (HER2) –Specific chimeric antigen Receptor–Modified T cells for the immunotherapy of HER2-positive sarcoma. JCO 2015;33:1688–96. 10.1200/JCO.2014.58.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Evans CH, Liu F, Porter RM, et al. . EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clin Cancer Res 2012;18:5341–51. 10.1158/1078-0432.CCR-12-1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Celli R, Cai G. Ewing Sarcoma/Primitive neuroectodermal tumor of the kidney: a rare and lethal entity. Arch Pathol Lab Med 2016;140:281–5. 10.5858/arpa.2014-0367-RS [DOI] [PubMed] [Google Scholar]
- 203.Kailayangiri S, Altvater B, Meltzer J, et al. . The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer 2012;106:1123–33. 10.1038/bjc.2012.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Modak S, Kramer K, Gultekin SH, et al. . Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001;61:4048–54. [PubMed] [Google Scholar]
- 205.Grunewald TGP, Ranft A, Esposito I, et al. . High STEAP1 expression is associated with improved outcome of Ewing’s sarcoma patients. Ann Oncol 2012;23:2185–90. 10.1093/annonc/mdr605 [DOI] [PubMed] [Google Scholar]
- 206.Zhao H, Guo L, Zhao H, et al. . Cxcr4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget 2015;6:5022–40. 10.18632/oncotarget.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Körner M, Reubi JC. Npy receptors in human cancer: a review of current knowledge. Peptides 2007;28:419–25. 10.1016/j.peptides.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 208.Körner M, Waser B, Reubi JC. High expression of neuropeptide Y1 receptors in Ewing sarcoma tumors. Clin Cancer Res 2008;14:5043–9. 10.1158/1078-0432.CCR-07-4551 [DOI] [PubMed] [Google Scholar]
- 209.Kara IO, Gonlusen G, Sahin B, et al. . A general aspect on soft-tissue sarcoma and c-kit expression in primitive neuroectodermal tumor and Ewing's sarcoma. is there any role in disease process? Saudi Med J 2005;26:1190–6. [PubMed] [Google Scholar]
- 210.Baldi A, De Falco M, De Luca L, et al. . Characterization of tissue specific expression of Notch-1 in human tissues. Biol Cell 2004;96:303–11. 10.1111/j.1768-322X.2004.tb01418.x [DOI] [PubMed] [Google Scholar]
- 211.Bennani-Baiti IM, Aryee DN, Ban J, et al. . Notch signalling is off and is uncoupled from Hes1 expression in Ewing's sarcoma. J Pathol 2011;225:353–63. 10.1002/path.2966 [DOI] [PubMed] [Google Scholar]