The combination of extracorporeal membrane oxygenation with lung‐protective ventilation strategies could be a potential treatment option for intractable pneumothorax with coronavirus disease 2019 (COVID‐19) to avoid unnecessary surgical procedures and aerosol generation.
Keywords: Acute respiratory distress syndrome, aerosols, COVID‐19, extracorporeal membrane oxygenation, pneumothorax
Abstract
Background
Some patients with coronavirus disease 2019 (COVID‐19) develop pneumothorax. Tube thoracotomy and bulla resection could generate aerosols and cause virus transmission; the optimal treatment strategy remains unclear.
Case Presentation
A 57‐year‐old male was transferred as a severe COVID‐19 pneumonia case. On the 16th day after admission, the patient’s respiratory condition deteriorated, and the chest X‐ray revealed the presence of severe right‐sided pneumothorax. A chest drain was immediately inserted; however, a significant air leak continued, and severe ventilator settings were required. Thus, veno‐venous extracorporeal membrane oxygenation (VV‐ECMO) treatment was initiated to allow the lungs to rest. After 10 days of lung‐protective ventilation, the patient was weaned from ECMO and the chest drain was removed on the following day with no major comorbidities.
Conclusion
The combination of ECMO with lung rest strategy could be a treatment option for intractable pneumothorax with COVID‐19 to avoid unnecessary surgical procedures and aerosol generation.
Introduction
Some cases of pneumonia of unknown origin were reported from China in December 2019. Later, the cause was identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The diseases caused by SARS‐CoV‐2 were defined as coronavirus disease 2019 (COVID‐19); unfortunately, the pandemic is continuing. Chen et al. 1 reported that 1% of patients with COVID‐19 developed pneumothorax. The optimal treatment strategy for pneumothorax with COVID‐19 remains unclear. This study reports a case of a patient with COVID‐19 pneumonia who developed pneumothorax and underwent veno‐venous extracorporeal membrane oxygenation (VV‐ECMO) treatment.
Case report
A 57‐year‐old male with a 7‐day history of fever and dyspnea was admitted to a local hospital and intubated for respiratory failure. COVID‐19 was confirmed by a positive polymerase chain reaction (PCR) test result obtained via a nasopharyngeal swab. Thus, he was transferred as a severe COVID‐19 pneumonia case in need of intensive care. He had a medical history of hypertension, dyslipidemia, and asthma. His height was 173 cm and his weight was 71.6 kg.
On arrival, the physical examination revealed body temperature of 38°C, blood pressure of 112/47 mmHg, pulse of 106 beats/min, and respiratory rate of 14 breaths/min. He was deeply sedated (Glasgow Coma Scale score: E1VtM4) and suffered severe respiratory failure; arterial blood gas analysis showed pH 7.283, partial pressure of carbon dioxide (PaCO2) 45 mmHg, partial pressure of oxygen (PaO2) 64 mmHg with invasive mechanical ventilation [pressure control ventilation with positive end‐expiratory pressure (PEEP) of 12 cmH2O, peak inspiratory pressure (PIP) of 24 cmH2O, the fraction of inspired O2 (FiO2) of 0.75, inspiratory time of 1.6 s, frequency of 14/min, and the delivered tidal volume was about 470 mL]. The computed tomography scan revealed a bilateral consolidation with lower lung predominance; bulla or emphysema was not observed (Fig. 1). Favipiravir, hydroxychloroquine, and tocilizumab were administered for the COVID‐19 treatment. As mechanical ventilation, in accordance with the acute respiratory distress syndrome (ARDS) strategy, did not show sufficient improvement, prone position ventilation was also initiated. His oxygenation improved slightly but not enough; PaO2 was 78.4 mmHg with FiO2 of 0.6.
Fig. 1.
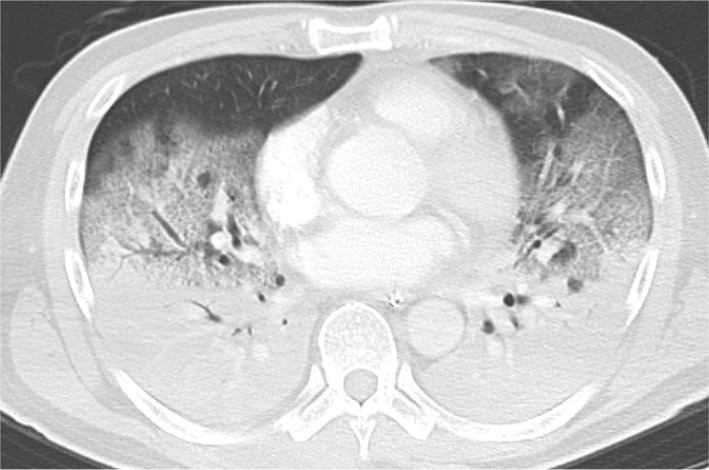
Chest computed tomography scan showed a bilateral consolidation with lower lung predominance. Bulla or emphysema was not observed.
On the 15th day after admission, the chest X‐ray suggested a giant bulla in the right lung (Fig. 2). One day later, his respiratory condition deteriorated and the chest X‐ray revealed the presence of severe right‐sided pneumothorax (Fig. 3). A 24‐Fr chest drain was immediately inserted, covering the patient’s right chest with a plastic sheet and attaching an in‐line filter to the suction tubing to minimize aerosolization. However, a significant air leak continued, and severe ventilator settings were still required (pressure control ventilation with PEEP of 12 cmH2O, PIP of 28 cmH2O, FiO2 of 0.6, inspiratory time of 0.9 s, frequency of 20/min, and the delivered tidal volume was about 320 mL) to compensate for the respiratory acidosis (pH 7.266, PaCO2 62.2 mmHg, PaO2 73.3 mmHg). The Murray score was 3. In addition, the pneumothorax was considered to be intractable and the continuous air leak increased the risk of medical staffs’ exposure. Thus, VV‐ECMO treatment (MERA centrifugal blood pump system HCS‐CFP; Senko Medical Instrument, Tokyo, Japan) was promptly initiated for lung protection at 5 h after performing the tube thoracotomy. The right internal jugular vein was cannulated with a 19‐Fr heparin‐coated cannula for blood return, and the right femoral vein was cannulated with a 24‐Fr heparin‐coated cannula for blood access. The blood flow was 4.5 L/min and sweep gas was 5.0 L/min. Heparin was given to maintain an activated partial thromboplastin time of about 50 s.
Fig. 2.
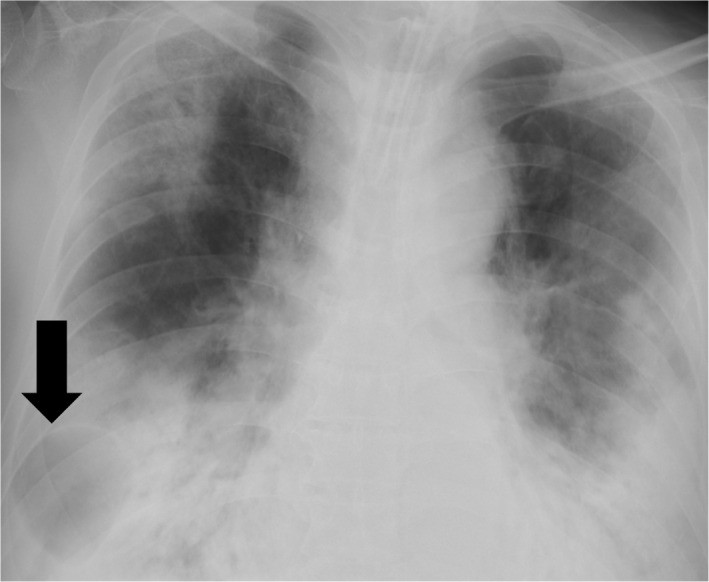
The chest X‐ray suggested a giant bulla in the right lung (arrow).
Fig. 3.
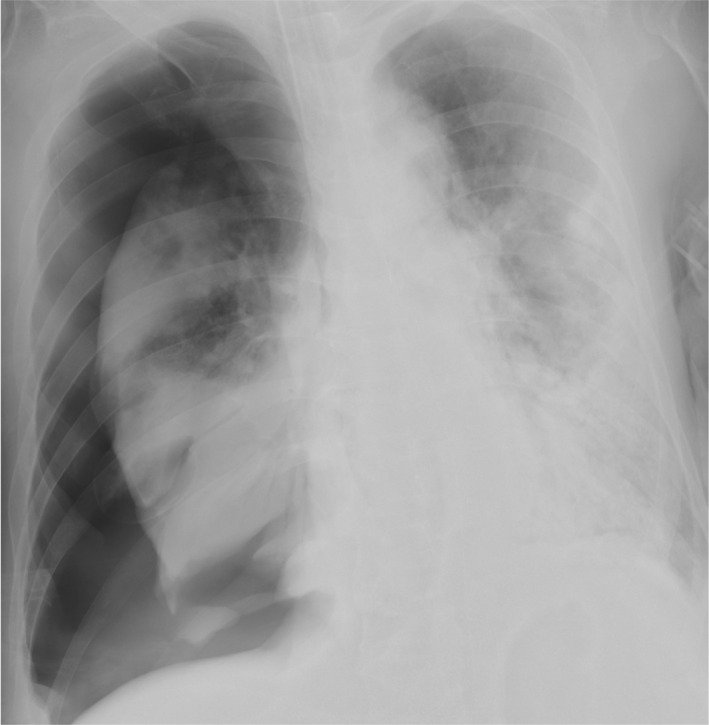
Chest X‐ray revealed the presence of severe right‐sided pneumothorax.
The PEEP was initially set to 0 cmH2O and the PIP was set to 5 cmH2O. After the air leak diminished, we gradually increased the pressure. On the 3rd day after starting ECMO, the PEEP was set to 5 cmH2O and the PIP was set to 10 cmH2O. On the 7th day, the PEEP was set to 8 cmH2O and the PIP was set to 12 cmH2O. On the 10th day, during the sweep gas off test, the arterial blood gas analysis showed pH 7.383, PaCO2 49.4 mmHg, PaO2 81.7 mmHg with PEEP of 10 cmH2O, PIP of 22 cmH2O, FiO2 of 0.4, inspiratory time of 1.1 s, frequency of 18/min, and the delivered tidal volume was about 480 mL. Thus, the patient was weaned from the ECMO and the chest drain was removed on the following day with no major comorbidities.
We performed the PCR test every other week. The PCR test result was negative on the 31st day after admission. The patient underwent tracheostomy on the 34th day. The ventilator management was completed on the 52nd day and he was transferred for rehabilitation on the 61st day.
Discussion
Severe COVID‐19 pneumonia can progress to ARDS. 1 ARDS could result in a low lung compliance and requires severe ventilator settings, eventually leading to ventilator‐induced injury and pneumothorax. 2 Furthermore, some reports advocated that alveolar damage owing COVID‐19 pneumonia could be associated with pneumothorax. The pathological features of patients with severe COVID‐19 pneumonia showed bilateral diffuse alveolar damage with cellular fibromyxoid exudates, desquamation of pneumocytes, and pulmonary edema with hyaline membrane formation. Pulmonary cystic lesions may form in response to cellular fibromyxoid exudates, which form a valve in the bronchus. 3 , 4 This case also presented with ARDS that required prolonged high‐pressure ventilation despite the low tidal volume strategy, which might have contributed to pneumothorax.
Treatment for pneumothorax with COVID‐19 could be more challenging than usual. Tube thoracotomy, the general treatment for pneumothorax, is a potentially aerosol‐generating procedure. A continuous air leak could spread aerosols to the surroundings. Thus, the guide gives some recommendations to minimize exposure risk to staff, such as using a drainage system with viral filters and fully covering patients' chest with drape when insertting or removing chest tube. 5 Moreover, the resection of bulla, which would normally be the first choice of the treatment for intractable pneumothorax, could generate aerosols. 6 In addition, transportation to the operating room increased a risk of exposure, especially in cases with an air leak in the tube drainage system. 7 Aiolfi et al. 8 reported two cases that underwent bulla resection in intractable pneumothorax with COVID‐19 pneumonia in the intensive care unit without transportation. However, this would be a difficult approach for many institutions, and cannot be generalized. Moreover, as in the current case, surgical procedure would not be practical owing to the severe respiratory condition.
The efficacy of ECMO for pneumothorax has been already reported. 9 , 10 ECMO could be a treatment option for pneumothorax with severe ventilator settings, as ECMO would allow the lungs to rest, reduce lung inflation, and avoid overdistension of the lungs. 9 He et al. 10 reported a successful case of ECMO for pneumothorax with severe respiratory infection to treat the air leak with protective ventilation, subsequently avoiding additional surgical procedures. Thus, for the pneumothorax with severe respiratory infection such as the COVID‐19 pneumonia, which has high infectivity and requires severe ventilator settings, early initiation of ECMO for lung protection might be a suitable treatment to avoid unnecessary surgical procedures and aerosol generation. Moreover, the effect of lung rest following initiation of ECMO could contribute to the treatment of ARDS. Recent reports indicated the possibility of ECMO treatment for ARDS accompanying COVID‐19. 11 By contrast, despite no obvious scientific evidence, some experts insisted that plasma leakage through the deteriorated membrane of ECMO might have infectivity; thus, medical workers need to manage ECMO circuit carefully enough. 12
Conclusion
The combination of ECMO with lung rest strategies could be a potential treatment option for intractable pneumothorax with COVID‐19 to avoid unnecessary surgical procedures and aerosol generation.
Disclosure
Approval of the research protocol: N/A. Informed consent about this case report was obtained from the patient.
Informed Consent: Informed consent for publication of the clinical details and images was obtained from the patient.
Registry and the Registration No. of the study: N/A.
Animal studies: N/A.
Conflict of interest: Authors declare no Conflict of interests for this article.
Funding information
No funding information provided.
References
- 1. Chen N, Zhou M, Dong X et al Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slutsky AS, Ranieri VM. Ventilator‐induced lung injury. N. Engl. J.. Med. 2013; 369: 2126–36. [DOI] [PubMed] [Google Scholar]
- 3. Liu K, Zeng Y, Xie P et al COVID‐19 with cystic features on computed tomography. Medicine (Baltimore). 2020; 99: e20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhe Xu, Shi L, Wang Y et al Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet. Respir. Med. 2020; 8: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pieracci FM, Burlew CC, Spain D et al Tube thoracostomy during the COVID‐19 pandemic: Guidance and recommendations from the AAST Acute Care Surgery and Critical Care Committees. Trauma Surg. Acute Care Open. 2020; 5: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rakovich G, Urbanowicz R, Issa R, Wang HT. Minimizing the risk of aerosol contamination during elective lung resection surgery. Ann. Surg. 2020; 272: e125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandey AS, Ringer AJ, Rai AT et al Minimizing SARS‐CoV‐2 exposure when performing surgical interventions during the covid‐19 pandemic. J. Neurointerv. Surg. 2020; 12: 643–7. [DOI] [PubMed] [Google Scholar]
- 8. Aiolfi A, Biraghi T, Montisci A et al Management of persistent pneumothorax with thoracoscopy and blebs resection in Covid‐19 patients. Ann. Thorac. Surg. 2020; 110: e413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rozencwajg S, Guihot A, Franchineau G et al Ultra‐protective ventilation reduces biotrauma in patients on venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit. Care Med. 2019; 47: 1505–12. [DOI] [PubMed] [Google Scholar]
- 10. He H, Wang H, Li X et al Successful rescue combination of extracorporeal membrane oxygenation, high‐frequency oscillatory ventilation and prone positioning for the management of severe methicillin‐resistant Staphylococcus aureus pneumonia complicated by pneumothorax: A case report . BMC Pulm. Med. 2017; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramanathan K, Antognini D, Combes A et al Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet. Respir. Med. 2020; 8: 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu K. An assessment of aerosolization via membranous oxygenator and coagulopathy in COVID‐19. [homepage on the internet].; [update March 2002]. Available from: https://ecmoedblog.files.wordpress.com/2020/03/elso‐webinar‐slides‐keibun‐liu.pdf


