Abstract
The rodent dorsal hippocampus is essential for episodic memory consolidation, a process heavily modulated by dopamine D1-like receptor (D1/5R) activation. It was previously thought that the ventral tegmental area provided the only supply of dopamine release to dorsal hippocampus, but several recent studies have established the locus coeruleus (LC) as the major source for CA1. Here we show that selective blockade of the norepinephrine transporter (NET) prevents dopamine-dependent, late long-term synaptic potentiation (LTP) in dorsal CA1, a neural correlate of memory formation that relies on LC-mediated activation of D1/5Rs. Since dopamine activation of D1/5Rs by vesicular release is expected to be enhanced by NET antagonism, our data identify NET reversal as a plausible mechanism for LC-mediated DA release. We also show that genetic deletion of LC NMDA receptors (NMDARs) blocks D1R-mediated LTP, suggesting the requirement of both a functional NET and presynaptic NMDARs for this release. As LC activity is highly correlated with attentional processes and memory, these experiments provide insight into how selective attention influences memory formation at the synaptic and circuit levels.
Keywords: norepinephrine transporter, dopamine, LTP, long-term potentiation, locus coeruleus, dorsal hippocampus, synaptic plasticity, NMDA receptors
Introduction
Adrenergic signaling in the mammalian brain is largely controlled by a network of remarkably divergent axon projections arising from locus coeruleus (LC) neurons [1, 2]. These LC axons were once thought to exclusively release norepinephrine (NE)[3], but recent chemical evidence reveals that their specific activation can also increase extracellular dopamine (DA)[4–7]. In accordance with this, LC stimulation is sufficient to modulate DA-dependent changes in learning and synaptic physiology within the rodent dorsal hippocampus [5, 8–10]. Dopamine D1-like receptors are abundantly expressed in this region, where they play a vital role in promoting many forms of long-term synaptic potentiation (LTP), especially in area CA1 [11]. Surprisingly, within CA1, projections arising from canonical DA-releasing nuclei such as the ventral tegmental area (VTA) are extremely sparse compared to those of the LC [8, 12], indicating that DA receptor activation in this subregion is mainly due to LC activity. However, despite the data supporting the LC as the major source of dorsal hippocampal DA, the mechanism underlying its release has never been investigated.
The most intuitive hypothesis for this mechanism is vesicular release. In LC terminals, DA is synthesized in the cytosol, and is then transported into synaptic vesicles via VMAT2 where it is converted into NE by the enzyme dopamine β-hydroxylase [13]. Work in adrenal chromaffin cells has estimated that the rate of this conversion is slower than the rate DA transport into vesicles [14]. In LC neurons, this could mean that DA does not get fully converted into NE during times of high vesicle turnover due to increased firing, causing DA to be released from the same vesicles as NE.
A novel alternative hypothesis for LC-DA’s release mechanism is by reverse transport through the norepinephrine transporter (NET). Under normal conditions, the NET is responsible for the reuptake of both NE and DA after they are released [15, 16]. In contrast, the presence of amphetamines causes the NET to efflux catecholamines [17], and DA released in this way can potentiate synaptic strength in dorsal CA1 [18]. Furthermore, the closely related dopamine transporter (DAT) can reverse its flux under more physiological conditions than amphetamine application (for a review, see [19]). These conditions include a rise in intracellular [Na+] and [Ca2+] following action potential firing [20, 21], activation of NMDA receptors [22, 23], and phosphorylation by CAMKII or PKC [24]. Because the amino acid sequences of DAT and NET are almost 80% homologous [25], we propose that the NET can also efflux cytosolic DA from LC axons under similar physiological conditions.
In support of a more detailed model for LC-mediated DA release, an existing theory posits that high-frequency glutamate activity may also play a role [26]. The authors postulate that elevated pyramidal cell firing in response to environmental stimuli can result in glutamate spillover [27], leading to activation of presynaptic NMDA receptors on LC terminals and enhanced NE release. This theory is corroborated by studies from isolated dorsal hippocampal synaptosomes reporting increases in both extracellular NE and DA after NMDA receptor agonist application [28, 29]. These findings, taken together, suggest the possibility of a role for both the NET and presynaptic NMDARs in the release of DA from LC terminals.
Below we investigate these hypotheses in the dorsal hippocampus, where DAT expression is not detectable [18, 30]. If DA is primarily released from LC synaptic vesicles, then blockade of its reuptake with a NET antagonist may be expected to both increase local concentration and time spent near the release sites, thus enhancing DA-dependent synaptic potentiation. If NET reversal is a major contributor to LC DA release, then blocking the NET should decrease DA-dependent LTP. Finally, if presynaptic NMDA receptors contribute to this DA release, their genetic deletion should also attenuate DA-dependent potentiation.
Results
The norepinephrine transporter (NET) contributes to dopamine-dependent potentiation in the dorsal hippocampus
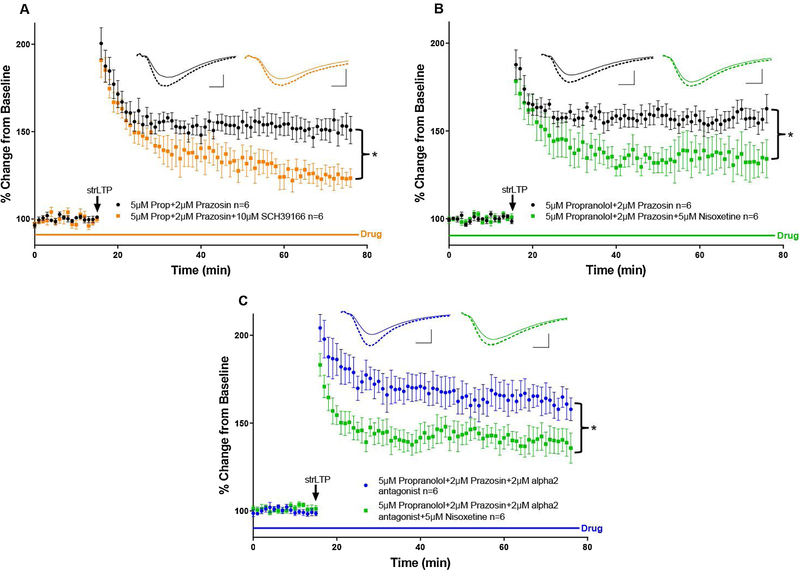
To test whether LC-DA dependent potentiation is mediated by DA originating from LC vesicles or the NET, we developed a strong theta-burst LTP protocol (strLTP) by stimulating CA3 axons and recording the slope of field excitatory postsynaptic potentials (fEPSPs) from stratum radiatum dendrites of CA1 (Fig.1A–D). This protocol was based on previous methods used to generate catecholamine-dependent potentiation in hippocampus [31, 32]. Importantly, our strLTP was not blocked by co-application of β-adrenergic (propranolol) and α1-adrenergic (prazosin) receptor antagonists (Fig. 1D, comparison between strLTP with no-drug and strLTP with drug from Fig. 2A). However, following the addition of the D1-like receptor antagonist SCH 23390, a robust attenuation of LTP occurred over the last 30 minutes of recording (Fig. 2A, orange traces), indicating that strLTP is modulated by DA receptors, but not adrenergic receptors.
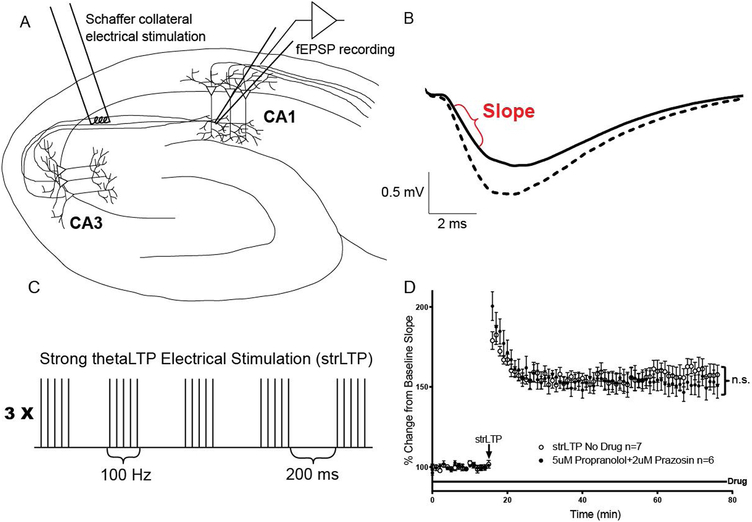
Figure 1. Establishment of long-term potentiation (LTP) protocol not blocked by adrenergic receptor antagonists.
A, Diagram of a hippocampal slice with electrodes in place. The stimulating electrode (left) is placed in contact with Schaffer collateral axons from the CA3 region about 400 μm from the recording electrode. The recording electrode (right) measures the extracellular field excitatory postsynaptic potential (fEPSP) in stratum radiatum dendrites of CA1. B, Example fEPSP from CA1 before LTP (solid line) and 60 minutes after administration of the strLTP stimulation (dashed line). Data is taken as the initial slope of the voltage trace as shown in red. Scale bars represent the 0.5 millivolt amplitude and 2.0 millisecond duration in all of the following figures. C, Strong (strLTP) Schaffer collateral thetaLTP stimulation protocol (see methods for more details). D, strLTP (open circles, n=7) is not blocked by the addition of prazosin and propranolol to the bath (closed circles, n=6), F(1, 17) = 0.06472, p=0.8022, ‘n.s.’ stands for ‘not significant’. All data points are represented as mean +/− SEM. Tests for significance were done using a two-way repeated measures ANOVA over the last 30 minutes of strLTP.
Figure 2. The norepinephrine transporter (NET) contributes to dopamine-dependent long-term potentiation.
A, The previously established strong LTP protocol (strLTP, black arrow) was not blocked by application of antagonists for β- and α1-adrenergic receptors, propranolol and prazosin, respectively (black circles, n=6, see Fig. 1D). However, application of SCH 23390, a dopamine D1-like receptor antagonist, along with the β- and α1 blockers was enough to significantly reduce LTP (orange circles, n=6), F(1, 10) = 9.265, p=0.0124. B, Similar to A, but the D1/5 receptor antagonist was replaced with the NET blocker nisoxetine (green squares, n=6), which was sufficient to attenuate the dopamine-dependent LTP (black circles, n=6), F(1, 10) = 5.028, p=0.0488. C, Even with all adrenergic receptors blocked (blue triangles, n=6) the application of nisoxetine was still able to significantly reduce LTP (green triangles, n=6), F(1, 10) = 5.521, p=0.0407. All data points are represented as mean +/− SEM. Tests for significance were done using a two-way repeated measures ANOVA over the last 30 minutes of strLTP. Asterisks represent p-values <0.05.
Next, we administered the same strLTP stimulation, but substituted nisoxetine, a NET blocker, for SCH 23390. We observed that treatment with nisoxetine produced a reduction in LTP of similar amplitude to the complete blockade of D1/5 receptors by SCH 23390 (Fig. 2B, green traces), suggesting that DA signaling in the dorsal hippocampus requires NET activity. A constitutive deletion of the NET from LC neurons also greatly reduced strLTP amplitude after 1 hour (Supplementary Fig. 1) when a different strLTP protocol (no adrenergic receptor antagonists, and a one second 100hz tetanus that also induces new protein dependent strLTP [11]) was employed. It should be noted that these mice will have altered catecholamine homeostasis, including greatly reduced stored NE.
Blocking α2-adrenergic receptors does not reduce the effect of NET antagonism
Because blocking the NET will flood synapses with NE, one possible confound is over-activation of inhibitory α2-adrenergic autoreceptors, leading to a decrease in overall LC excitability and subsequently less neurotransmitter release [33]. This may cause a reduction in LTP based on an indirect decrease in total NE and/or DA levels. To control for this, we repeated the aforementioned experiments with the inclusion of RS 79948, an α2-receptor antagonist, in the bath with propranolol and prazosin (Fig. 2C, blue traces). In line with our prior results, the further addition of nisoxetine was still able to diminish the magnitude of strLTP over the last 30 minutes (Fig. 2C, green traces), reinforcing the finding that NET contributes to DA signaling in dorsal hippocampus.
NMDA receptor knock-out from catecholamine neurons reduces the magnitude of dopamine-dependent LTP
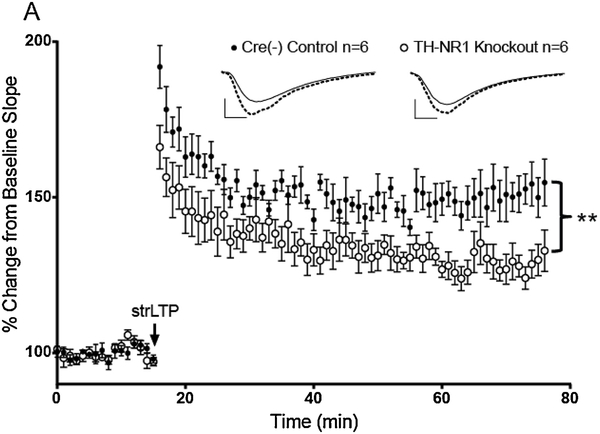
Activation of glutamate receptors, in particular NMDARs, is capable of locally enhancing catecholamine release in hippocampus [28, 29]. Expanding on the mechanism of DA release from the NET, we asked if presynaptic NMDARs on LC terminals were involved in our DA-dependent LTP. To approach this question, the NR1 subunits of NMDARs were selectively deleted from catecholamine neurons by crossing a mouse expressing Cre recombinase under the control of the tyrosine hydroxylase (TH) promoter with a floxed NMDAR-NR1 subunit mouse. Cre-negative, floxed control mice showed normal strLTP (Fig. 3, filled circles), whereas the NR1 knockouts exhibited decreased LTP magnitude throughout the full hour after LTP induction (Fig. 3, open circles). We did not notice any difference in baseline activity between these groups (data not shown).
Figure 3. Knocking out NMDA receptors from catecholamine neurons reduces the magnitude of dopamine-dependent LTP in dorsal hippocampus.
A, The same strong LTP protocol used previously (strLTP, black arrow) was administered in slices from Cre(−) control mice (black circles, n=6) and NMDA-NR1 subunit knockout mice (open circles, n=6). All data points are represented as mean +/− SEM. Tests for significance were done using a two-way repeated measures ANOVA over the last 30 minutes of strLTP. Double asterisk represents a significant difference <0.01, F(1, 10) = 13.24, p=0.0046.
Discussion
Taken together, our results allude to the LC orchestrating the co-release of NE and DA in the dorsal hippocampus using two separate mechanisms. The first is the widely accepted vesicular release of NE [34], and the second is a putative reverse transport of DA from the NET, consistent with the blockade rather than enhancement of DA-dependent LTP that results from NET antagonism. A reason for separate release mechanisms is still unclear, but one plausible explanation is that it would provide a means for independent modulation of attentional selectivity and memory consolidation [35], especially considering the LC’s involvement in both cognitive processes at the behavioral level [36].
A well-known theory of how the LC controls selective attention is by modulating the gain of glutamatergic circuits [37]. This gain control is most effective during times of relatively high LC firing and NE release, a phenomenon observed when animals experience something salient in their environment [8, 38]. In CA1, these high levels of NE simultaneously activate lower affinity β-adrenergic receptors on postsynaptic glutamatergic pyramidal cells, and higher affinity α1-adrenergic receptors located mostly on CA1 interneurons involved in lateral inhibition. Both receptor types increase excitability in their respective cell types [39]. Thus, the end result of salience-directed increases in LC activity is a feed-forward augmentation of the most active glutamatergic ensembles and a suppression of the less active ones.
In conjunction with the LC’s putative role in hippocampal gain control, NET-dependent DA release can provide a form of coincidence detection at CA1 synapses. We predict this DA would potentiate only the most active glutamatergic circuits that were selected by the preceding gain modulation of glutamatergic attentional resources by NE [37]. Given that DA seems to be involved in the synaptic tagging process [40], this could lead to tagging of specific salience-relevant synapses recruited for memory consolidation. For this reason, having DA and NE release separated in time might enable more efficient signal processing and storage of new information, since DA released out of the NET would not necessarily interfere with the formation of neural representations initially shaped by vesicular NE release and gain modulation [38]. In the hippocampus, this system presumably optimizes circuit organization to reduce the overlap between stored memory traces.
One possibility for the initiation of NET-dependent DA release is by a switch to higher intracellular [Na+] during barrages of LC action potentials. This scenario seems likely, since monoamine transporters are known to move neurotransmitters using the energy stored in Na+ gradients [41] and DA is readily available in the LC cytosol. In favor of this idea, it is known that the NET can reuptake DA about as well as NE in the mouse hippocampus [16], hinting that the reverse mechanism is possible under the correct conditions.
Our data also suggest that presynaptic LC-NMDARs are somehow involved in dorsal hippocampal LC-DA signaling, since their genetic deletion abolishes the DA-dependent facilitation of late LTP. This effect makes sense within the framework of sustained attention being a driving force for memory formation [35]. For instance, strong glutamatergic signaling in response to salient stimuli can cause glutamate to overflow from the synapse and bind to NMDARs on LC terminals [26]. At the same time, salience-evoked, enhanced LC action potential firing will depolarize terminals, removing the Mg2+ block and allowing Ca2+ influx through NMDARs. This could lead to DA release via activation of downstream Ca2+ effector proteins known to cluster with NMDA receptors. Two of these effectors are CAMKII or PKC, kinases capable of interacting with [42] and phosphorylating [43] catecholamine transporters. The modifications may shift the conformational equilibrium of these transporters to a state more permissive to efflux than reuptake [19].
In closing, our findings support the idea that the NET and presynaptic LC-NMDARs contribute to DA signaling in the CA1 region of dorsal hippocampus where they help regulate late LTP-dependent memory storage. One drawback of our methods is that LC fibers were not selectively stimulated. Instead, catecholamine release was elicited by electrical stimulation of all fibers within the range of the stimulating electrode, which could include any other neuromodulatory inputs into CA1 that might interact with the effects of NE and DA (e.g. acetylcholine or serotonin).
We have not ruled out the possibility that the conditional NMDAR gene (Grin1) deletion alters the expression of enzymes necessary for the synthesis of DA and NE (tyrosine hydroxylase and dopamine β-hydroxylase, respectively), independently of any loss of NMDAR’s at the terminals. Importantly, our use of TH-Cre mice in the knockout experiments should not pose a problem with interpretation of our results, as TH-Cre mice on an inbred C57BL/6 background have been extensively shown to not exhibit any deficits in the DA system. This includes expression of the TH enzyme and dopamine synaptic homeostasis [44]. For the pharmacology experiments, it is possible that the effects we see due to NET blockade result from the elimination of an excitatory current that has been observed to occur through the NET during NE reuptake [45, 46]. Even if this were the case, it means that the NET is still involved in DA signaling to a large enough extent to modulate DA-dependent synaptic potentiation, which is a novel finding.
Future studies should employ specific optogenetic activation of the LC to study this question with greater precision. It may also be necessary to utilize the recently developed genetically encoded fluorescent catecholamine sensors [47–49] to probe the dynamics of LC DA and NE co-release in greater detail. In conclusion, although our evidence is indirect, it presents a vital first step towards showing a crucial role for both NET function and NMDAR activation in the complex interplay between glutamate activity and catecholamine release, not only within the hippocampus, but in potentially all LC terminal fields throughout the central nervous system.
Methods
Animal approval and mouse lines
All animal procedures performed were approved by the animal care and use committee (IACUC) at the University of Texas Southwestern Medical Center and comply with federal regulations set forth by the National Institutes of Health.
Tyrosine hydroxylase-Cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME) (B6.Cg-Tg (TH-Cre)1Tmd/J; #008601). Floxed NMDA-NR1 subunit mice were also obtained from The Jackson Laboratory (Bar Harbor, ME) (B6.129S4-Grin1tm2Stl/J; #005246). Norepinephrine transporter knockout mice were a generous gift from Dr. Marc G. Caron, and creation of these mice can be found in Wang, Xu [50].
Ex vivo slice preparation
Coronal slices (300 μm thick) containing dorsal hippocampus were made from male, wild type, C57BL/6J mice (6–12 weeks old) in low-light conditions to prevent photooxidation of catecholamines. Animals were anesthetized under 1.5–2% isoflurane, after which brains were removed and blocked following rapid decapitation. Slices were prepared using a Leica VT1000S vibratome (Wetzlar, Germany) in ice-cold NMDG ringer solution containing (in mM): 5 NaCl, 90 NMDG (N-Methyl-d-Glucosamine), 37.5 Na-Pyruvate, 12.5 Na-Lactate, 5 Na-Ascorbate, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 Glucose, 10 MgSO4.7H20, 0.5 CaCl2.2H20. The pH was set between 7.3 and 7.4 using 12 N HCl, the osmolarity was adjusted as needed to ~315 mOsm using glucose, and the solution was continuously bubbled with 95% O2 and 5% CO2 gas during slicing. Slices were then transferred and maintained for up to 6 hours, while protected from light, at 30 °C in artificial cerebrospinal fluid containing (aCSF; in mM): 120 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, and 11 dextrose continuously bubbled with 95% O2 and 5% CO2 gas.
Field recordings
After at least 1 hour of recovery in aCSF, slices were transferred to a submersion recording chamber and perfused with aCSF at a rate of 2–3 ml/min at 31–32 °C. Extracellular voltage recordings from the stratum radiatum field of dorsal CA1 were acquired using a borosilicate glass electrode (1–2 MΩ, Sutter Instrument (Novato, CA)) filled with normal aCSF. A bipolar stimulating electrode (FHC, Inc. (Bowdoin, ME)) was also placed in the stratum radiatum of CA1 within ~400 μm of the recording electrode (see Figure 1A), and stimulus strength was controlled with a stimulus isolator unit (World Precision Instruments, Sarasota, FL). Stimulus strength was set to produce a baseline excitatory field postsynaptic potential (fEPSP) slope (Figure 1B) that was ~50% of the slope measured following the first appearance of a population spike. This method led to a typical baseline stimulation current of 20–30 μA, while stimulus duration was set to 0.2 ms. Schaffer collateral stimulation was given once every 30 seconds and the average of every two consecutive stimuli was taken. For the NET blockade experiments, the entire experiment was run in the presence of various antagonists (indicated in figures). A 15 minute baseline was obtained, followed by a strong theta-burst tetanus containing 15 bursts (given at 5 Hz), with each burst containing 5 spikes at 100 Hz (75 total spikes). Baseline stimulation then resumed as described above for 60 minutes. For Supplementary Figure 1, a different LTP stimulation protocol was used that consisted of a 1 second long train of 100 Hz (100 total spikes), without any antagonist application. All experiments were performed in low-light conditions to avoid photooxidation of catecholamines. Data was acquired using a Multiclamp 700B amplifier and pCLAMP 10 software (Molecular Devices, San Jose, CA). The signal was low-pass filtered online at 2 kHz using the Multiclamp 700B Commander software, and then digitized at 20 kHz using a Digidata 1440A (Molecular Devices, San Jose, CA).
Drugs
Where indicated, the following drugs (dissolved in water unless specified) were used: prazosin hydrochloride (α1-adrenergic antagonist; 2 μM, dissolved in DMSO), propranolol hydrochloride (β-adrenergic inhibitor; 5 μM), SCH 23390 hydrochloride (D1-like receptor antagonist; 10 μM), nisoxetine hydrochloride (norepinephrine transporter blocker; 5μM), RS 79948 hydrochloride (α2-adrenergic antagonist; 5 μM). All drugs were purchased from Tocris Bioscience (Minneapolis, MN).
Statistical analysis
In all cases, ‘n’ represents the number of different animals per group. For the pharmacology experiments, at least two slices were taken per animal, but slices from the same animal were never included in the same group. All electrophysiological data points are represented as the mean ± SEM. Statistical significance was tested using two-way repeated measures ANOVAs with time as an independent variable while looking for a main effect between groups. All ANOVAs were run over the last 30 minutes of recording after LTP stimulation, with the assumption that early-LTP was over based on unpublished observations form our lab using the same LTP stimulus protocol. All analyses were performed using GraphPad Prism 7 software (San Diego, CA).
Supplementary Material
Acknowledgements
We thank Dr. Marc G. Caron for his generous gift of NET knockout mice. We would also like to thank To Thai for his technical assistance.
This work was supported by NIH grants 5R01MH080297 awarded to Robert W. Greene, and NIDA-T32-DA7290 Basic Science Training Program in Drug Abuse awarded to Alex Sonneborn.
Footnotes
Additional information
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kebschull JM, et al. , High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron, 2016. 91(5): p. 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarz LA, et al. , Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature, 2015. 524(7563): p. 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge CW and Waterhouse BD, The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews, 2003. 42(1): p. 33–84. [DOI] [PubMed] [Google Scholar]
- 4.Beas BS, et al. , The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci, 2018. 21(7): p. 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempadoo KA, et al. , Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A, 2016. 113(51): p. 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devoto P, et al. , Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J Neurochem, 2005. 92(2): p. 368–74. [DOI] [PubMed] [Google Scholar]
- 7.Zerbi V, et al. , Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron, 2019. 103(4): p. 702–718 e5. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi T, et al. , Locus coeruleus and dopaminergic consolidation of everyday memory. Nature, 2016. 537(7620): p. 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagatsuma A, et al. , Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc Natl Acad Sci U S A, 2018. 115(2): p. E310–E316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemon N and Manahan-Vaughan D, Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex, 2012. 22(9): p. 2131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisman J, Grace AA, and Duzel E, A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci, 2011. 34(10): p. 536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura S, et al. , Noradrenalin and dopamine receptors both control cAMP-PKA signaling throughout the cerebral cortex. Front Cell Neurosci, 2014. 8: p. 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devoto P and Flore G, On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol, 2006. 4(2): p. 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn NG and Klinman JP, Nature of rate-limiting steps in a compartmentalized enzyme system. Quantitation of dopamine transport and hydroxylation rates in resealed chromaffin granule ghosts. J Biol Chem, 1989. 264(21): p. 12259–65. [PubMed] [Google Scholar]
- 15.Moron JA, et al. , Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci, 2002. 22(2): p. 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borgkvist A, et al. , Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol, 2012. 15(4): p. 531–40. [DOI] [PubMed] [Google Scholar]
- 17.Robertson SD, Matthies HJ, and Galli A, A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol, 2009. 39(2): p. 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CC and Greene RW, CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci, 2012. 32(18): p. 6072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leviel V, Dopamine release mediated by the dopamine transporter, facts and consequences. J Neurochem, 2011. 118(4): p. 475–89. [DOI] [PubMed] [Google Scholar]
- 20.Khoshbouei H, et al. , Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem, 2003. 278(14): p. 12070–7. [DOI] [PubMed] [Google Scholar]
- 21.Gnegy ME, et al. , Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol, 2004. 66(1): p. 137–43. [DOI] [PubMed] [Google Scholar]
- 22.Olivier V, Guibert B, and Leviel V, Direct in vivo comparison of two mechanisms releasing dopamine in the rat striatum. Brain Res, 1995. 695(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Ihalainen JA, Riekkinen P Jr., and Feenstra MG, Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett, 1999. 277(2): p. 71–4. [DOI] [PubMed] [Google Scholar]
- 24.Feenstra MG, et al. , Dopamine and noradrenaline release in the prefrontal cortex of rats during classical aversive and appetitive conditioning to a contextual stimulus: interference by novelty effects. Neurosci Lett, 1999. 272(3): p. 179–82. [DOI] [PubMed] [Google Scholar]
- 25.Andersen J, et al. , Binding site residues control inhibitor selectivity in the human norepinephrine transporter but not in the human dopamine transporter. Sci Rep, 2015. 5: p. 15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mather M, et al. , Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci, 2016. 39: p. e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okubo Y, et al. , Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci U S A, 2010. 107(14): p. 6526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaki S, et al. , Regulation of NMDA-induced [3H]dopamine release from rat hippocampal slices through sigma-1 binding sites. Neurochem Int, 1998. 33(1): p. 29–34. [DOI] [PubMed] [Google Scholar]
- 29.Malva JO, Carvalho AP, and Carvalho CM, Modulation of dopamine and noradrenaline release and of intracellular Ca2+ concentration by presynaptic glutamate receptors in hippocampus. Br J Pharmacol, 1994. 113(4): p. 1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon OB, et al. , Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A, 2008. 105(40): p. 15587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen PV and Kandel ER, Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem, 1997. 4(2): p. 230–43. [DOI] [PubMed] [Google Scholar]
- 32.Larson J and Munkacsy E, Theta-burst LTP. Brain Res, 2015. 1621: p. 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abercrombie ED, Keller RW Jr., and Zigmond MJ, Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience, 1988. 27(3): p. 897–904. [DOI] [PubMed] [Google Scholar]
- 34.Chiti Z and Teschemacher AG, Exocytosis of norepinephrine at axon varicosities and neuronal cell bodies in the rat brain. FASEB J, 2007. 21(10): p. 2540–50. [DOI] [PubMed] [Google Scholar]
- 35.Chun MM and Turk-Browne NB, Interactions between attention and memory. Curr Opin Neurobiol, 2007. 17(2): p. 177–84. [DOI] [PubMed] [Google Scholar]
- 36.Sara SJ, The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 2009. 10(3): p. 211–23. [DOI] [PubMed] [Google Scholar]
- 37.Aston-Jones G and Cohen JD, An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci, 2005. 28: p. 403–50. [DOI] [PubMed] [Google Scholar]
- 38.Devilbiss DM, Consequences of tuning network function by tonic and phasic locus coeruleus output and stress: Regulating detection and discrimination of peripheral stimuli. Brain Res, 2019. 1709: p. 16–27. [DOI] [PubMed] [Google Scholar]
- 39.O’Dell TJ, et al. , Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal, 2010. 22(5): p. 728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramar EA, et al. , A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci, 2004. 24(22): p. 5151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesner RP, Behavioral analysis of the contribution of the hippocampus and parietal cortex to the processing of information: interactions and dissociations. Hippocampus, 2000. 10(4): p. 483–90. [DOI] [PubMed] [Google Scholar]
- 42.Fog JU, et al. , Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron, 2006. 51(4): p. 417–29. [DOI] [PubMed] [Google Scholar]
- 43.Darracq L, et al. , Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci, 1998. 18(7): p. 2729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Runegaard AH, et al. , Preserved dopaminergic homeostasis and dopamine-related behaviour in hemizygous TH-Cre mice. Eur J Neurosci, 2017. 45(1): p. 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galli A, et al. , Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol, 1995. 198(Pt 10): p. 2197–212. [DOI] [PubMed] [Google Scholar]
- 46.Galli A, Blakely RD, and DeFelice LJ, Norepinephrine transporters have channel modes of conduction. Proc Natl Acad Sci U S A, 1996. 93(16): p. 8671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng J, et al. , A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron, 2019. 102(4): p. 745–761 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patriarchi T, et al. , Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science, 2018. 360(6396). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun F, et al. , A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell, 2018. 174(2): p. 481–496 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang YM, et al. , Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry, 1999. 46(9): p. 1124–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.