Abstract
Alcohol use is highly prevalent in modern society and ramifications of alcohol abuse pose a large public health concern. Previous work investigating the effects of alcohol exposure on the brain have implicated microglia, the resident immune cells of the central nervous system (CNS), as critical participants in the brain’s response to chronic and developmental ethanol (EtOH) exposure. As rapid sensors of their environment, microglia also have the capacity to rapidly respond to alcohol administration and to contribute to acute effects of alcohol on the brain, however their acute responses have not been assessed. Here for the first time, we have examined the acute response of microglia to alcohol intoxication in vivo utilizing two-photon microscopy to assess the dynamics of these motile cells in both visual cortex and the cerebellum of mice. We found that microglia respond rapidly to EtOH exposure with fast changes in morphology, motility, parenchyma surveillance, and injury response. However, regional differences between the responses of cerebellar and cortical microglial populations indicate that subtle differences in microglial physiology may alter their vulnerability to acute alcohol intoxication. Our findings suggest that the longer-term effects of repeated EtOH exposure on microglia, may result from repeat acute alterations in microglial physiology by single exposure to alcohol which rapidly alter behavior in specific microglial populations.
Keywords: mouse, two-photon microscopy, neuroimmunology, glia, alcohol
Graphical Abstract
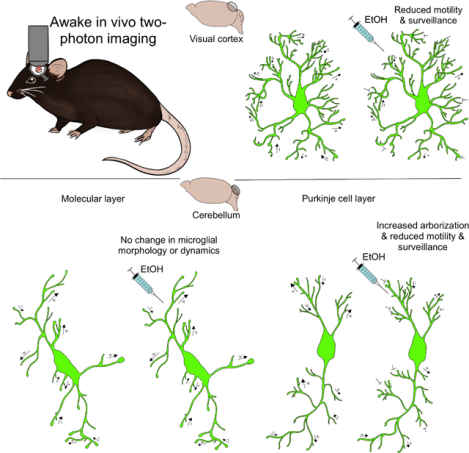
Microglia are the immune sentinels of the central nervous system, with their highly motile cell processes they survey the parenchyma and interact with neural elements. Alcohol abuse causes many deleterious effects within the brain and some of these occur through signaling to microglia. Using in vivo two-photon imaging in the visual cortex and cerebellum we found that ethanol intoxication causes heterogenous changes in microglial morphology, motility, and surveillance dependent on brain region.
Introduction
Alcohol is one of the most widely used recreational drugs and globally there are millions of individuals suffering from disorders involving alcohol abuse. The high prevalence of alcohol use and abuse globally poses a strong public health need to understand the ramifications of alcohol exposure on the central nervous system (CNS). Acute ethanol (EtOH) exposure has heterogeneous effects on neuronal activity across the striatum, hippocampus, cerebellum, amygdala, substantia nigra, and ventral tegmental area (Abrahao et al., 2017). EtOH drives diverse changes in both GABAergic and glutamatergic signaling in neurons leading to changes in both long-term potentiation and depression across the CNS (Abrahao et al., 2017). Gene expression analysis has shown that neurovascular blood flow, astrocyte reactivity, and neuronal glutamatergic signaling and ion channel transport are all altered by drinking alcohol to the point of intoxication (Mulligan et al., 2011). For example, astrocytic glutamate uptake through excitatory amino acid transporters is increased by acute EtOH exposure (Wu et al., 2010). Chronic binge drinking of EtOH causes long-term changes in neuronal networks (Abrahao et al., 2017), reactive astrogliosis (Hayes et al., 2013), decreased glymphatic function (Lundgaard et al., 2018), and inflammation that includes immune activation of microglia, the brain’s immune cells (Kettenmann et al., 2011; Wong et al., 2017).
While neuronal and astrocytic responses to acute EtOH exposure have been studied and likely serve as a substrate for more profound changes after chronic alcohol exposure, many questions remain concerning how microglia respond to acute alcohol presentation in the adult brain. Microglia are the resident innate immune cells of the CNS with numerous roles in CNS health and disease (Kettenmann et al., 2011). In the healthy and intact CNS the microglial process arbor displays a high degree of motility and efficient rapid surveillance of the CNS parenchyma (Davalos et al., 2005; Nimmerjahn et al., 2005). As the innate immune cells of the CNS, microglia are the first rapid responders to pathological insults such as injury or infection. In the case of an acute injury microglia rapidly respond by extending their processes to the site of injury (Davalos et al., 2005; Nimmerjahn et al., 2005). During pathological insults microglia have a diverse range of responses tailored to CNS insults including: retraction of primary processes, assuming an ameboid morphology, cell proliferation, phagocytosis of pathogens and debris, and release of pro- or anti-inflammatory cytokines (Kettenmann et al., 2011). In the absence of pathological perturbations microglia are essential participants in synaptic plasticity processes (Parkhurst et al., 2013; Schafer et al., 2013; Sipe et al., 2016; Stowell et al., 2019) and the developmental wiring of the CNS (Bialas & Stevens, 2013; Pagani et al., 2015; Paolicelli et al., 2011; Schafer et al., 2012; Squarzoni et al., 2014; Zhan et al., 2014). Thus, microglial roles are diverse and impact both normal brain function and responses to pathological events.
Microglia have been a key point of interest in understanding the neuroimmune activation and damage caused by EtOH due to their exquisite sensitivity to acute CNS perturbations (Coleman & Crews, 2018; Wong et al., 2017). Previous research has suggested that microglia may be very sensitive to alcohol treatment, as alcohol can robustly alter neuroimmune signaling in patients and animal models of EtOH exposure (Ahlers et al., 2015; Fernandez-Lizarbe et al., 2009; He & Crews, 2008; Qin et al., 2008; Qin & Crews, 2012b). Post mortem histological studies of humans who suffered from alcoholism show marked immunological activation in the CNS, reflected by increased Iba-1 immunoreactivity and proinflammatory cytokines (He & Crews, 2008). Work with mouse models has demonstrated that microglial Toll-like Receptor 4 (TLR4) may be responsible for the microglial inflammatory responses to EtOH with TLR4 receptor ablation reducing EtOH-induced microglial reactivity (Fernandez-Lizarbe et al., 2009). Interestingly, in the periphery, EtOH can have an acute immunosuppressive effect on macrophages, a cell type closely related to microglia, through suppression of TLR driven cytokine production (Pruett et al., 2003; Pruett et al., 2004). While, both microglia and macrophages show shifts in cytokine production of Tumor necrosis factor α, and interleukin 6 after EtOH exposure, the directionality of these shifts differ between the two populations (Doremus-Fitzwater et al., 2014). Additionally, EtOH can have priming effects on microglia, as EtOH exposure causes microglia to have enhanced proinflammatory marker and cytokine expression upon subsequent endotoxin challenge (Qin et al., 2008).
Previous work has examined how acute and repeated exposure influences the time course of cytokine expression and microglial activation, however it is unclear whether and how EtOH can impact more subtle aspects of microglial behavior that drive their physiological and pathological functions, such as their morphological dynamics in vivo (Bell-Temin et al., 2013; Kane et al., 2014; McClain et al., 2011). Here, utilizing chronic cranial window preparations in CX3CR1-GFP mice where microglia and monocytes are selectively labeled with green fluorescent protein (GFP)(Jung et al., 2000), we were able to evaluate the impact of acute EtOH exposure on microglial morphology, motility, and focal tissue injury response in both visual cortex (V1) and cerebellum. We sought to compare these areas because emerging evidence suggests that microglia within distinct regions of the CNS differ in how they respond to EtOH exposure (Kane et al., 2014; Riikonen et al., 2002). Additionally, we have recently identified cerebellar microglia as a unique population with distinct dynamic properties which could differentially respond to EtOH challenge (Stowell et al., 2018). We focused on the first hour immediately following administration to capture microglial behavior during the onset of EtOH entry into the brain. We found that EtOH did not uniformly impact microglial physiology, rather microglial populations in the cortex and cerebellum showed a different degree of responses to EtOH exposure suggesting that, while EtOH rapidly affects microglial dynamics, regional diversity drives sensitivity to EtOH exposure.
Methods
Animals
All experimental protocols strictly adhered to the policies of the University of Rochester Committee on Animal Resources and National Institutes of Health Guidelines. Adult mice (P60–90) were bred on a C57BL/6 background in house. All the imaging experiments were done on CX3CR1-GFP (JAX: 005582) heterozygous mice (Jung et al., 2000), which allow for the specific visualization of microglia in the CNS. Mice were genotyped utilizing the JAX protocol (JAX: 00582). Both male and female mice were used in the experiments and no significant sex-specific differences were observed.
Blood EtOH Concentration
A small cohort (n=2M/4F), of animals was dosed with EtOH (3.0g/kg, i.p.) and sacrificed 15 minutes later by decapitation. Whole trunk blood was collected and frozen immediately at −80°C. Blood EtOH concentration was later analyzed by gas chromatography (Hewlett Packard 5890 series II, Wilmington, DE, USA) as described in (Ramirez et al., 2011). to confirm the efficacy of our acute binge model of EtOH administration.
Cranial Window Surgery
Animals were anesthetized with a fentanyl cocktail delivered i.p. (fentanyl 0.05mg/kg, midazolam 5.0mg/kg, and dexmedatomadin 0.5mg/kg) for the cranial window implantation. Lubricant ointment was applied to the eyes and body temperature was maintained at 37°C for the duration of the procedure. Aseptic technique was observed during all surgeries: tools were autoclaved by steam sterilization. A stereotaxic frame was used to head-fix the mice for the surgical procedure. Alternating washes of betadine and 70% EtOH were applied to the scalp. The skull was exposed through a scalp incision and all connective tissues were cleared. A 3mm biopsy core (Integra, Upper Saddle River, NJ, USA) was then used to create a circular score on the skull over either V1 or cerebellum. A 0.5 mm drill bit (FST, Foster City, CA, USA) was then used to drill the 3mm craniotomy. A 5-mm coverslip attached to a 3-mm coverslip (Warner Instruments, Hamden, CT, USA) by UV glue (Norland Optical Adhesive, Norland Inc, Cranbury, NJ, USA) was then fit into the craniotomy and secured utilizing C&B metabond dental cement (Parkell Inc, Edgewood, NY, USA). A custom headplate produced by emachine shop (www.Emachineshop.com; designs courtesy of the Mriganka Sur Lab, MIT) was then secured onto the skull while the remaining exposed skull was coated with C&B metabond dental cement. Animals were administered slow release Buprenorphine (5mg/kg) for analgesia. Animals recovered from surgery for a minimum of 2 weeks prior to imaging sessions.
Two-Photon Microscopy
A custom two-photon laser-scanning microscope was used for in vivo imaging (Ti: Sapphire, Mai-Tai, Spectra Physics; modified Fluoview confocal scan head, 20X water-immersion objective, 0.95 numerical aperture, Olympus). Excitation for fluorescent imaging was achieved with 100fs laser pulses (80 MHz) at 920nm with a power of ~40mW measured at the sample. For all imaging a 580/180 filter was used. Prior to imaging, mice were first accustomed to handling and underwent 3 days of habituation (day 1: 30min, day 2: 45min, day 3: 60min) to being head-fixed on a stationary platform for awake imaging. Imaging was conducted at 4–5x digital zoom and 1μm z-step, with time-lapse imaging at 5-min intervals for 1 hour per imaging session. Experimental design allowed within subject analysis. To avoid confounds such as photobleaching from repeated imaging of the same area, we selected distinct areas within the imaging window for each session. Each animal had 4 total imaging sessions: awake motility, awake +EtOH motility, awake laser ablation, and awake +EtOH laser ablation (Fig. 1A). The two imaging sessions with EtOH exposure were separated by a minimum of 1 week. For EtOH exposure sessions, mice were administered 3.0g/kg EtOH i.p. (20% w/v EtOH in 0.9% saline) approximately 5 minutes prior to the start of imaging allowing adequate time for EtOH to reach its peak concentration in the CNS (Jamal et al., 2016; Quoilin et al., 2012). All image analysis was run offline using ImageJ and Matlab.
Figure 1|. EtOH impairs microglial dynamics in V1.

(A) Example timeline of surgical and imaging procedures (B) Gas chromatography results depicting blood ethanol concentrations 15 minutes post 3g/kg EtOH i.p. (C) Two-photon images of representative microglia in the V1 in the same awake mouse with and without EtOH treatment. (D) Sholl profile of visual cortical microglia in awake mice with and without EtOH treatment. (E) Maximum intersection (#) of Sholl profiles; Paired t-test, n.s. (F) Area under the curve (AUC) of the Sholl profiles; Paired t-test, n.s. (G) Overlay of two images taken 5 minutes apart representing microglial motility analysis (green represents retraction, magenta represents extension while white represents pixels that remained stable between the two timepoints). (H) EtOH exposure reduces microglial motility in V1; Paired t-test p<0.01. (I) Image of microglia coverage of the CNS parenchyma at t=0min and total surveillance over the 1hr imaging session (all 12 images taken during this time are overlaid). (H) EtOH decreases microglial surveillance of the ML parenchyma; Paired t-test, n.s. Scale bars = 20μm.
Microglial Morphology
Individual microglia were selected from our t=10min z-stacks for morphological analysis: 2 microglia per layer of cerebellum and 2–3 for V1. The selected microglia were projected in Z to capture their full arbor and the arbor was manually traced. This tracing was then subjected to Sholl Analysis using the ImageJ plugin (developed by the Anirvan Ghosh Laboratory). This analysis places concentric circles radiating out from the soma in 2-μm steps out to the end of the arbor. The number of intersections at each step from the soma was used to generate Sholl profile curves. For each animal the average of all the measured microglia was used as the final value for that animal. To quantify differences in the Sholl profile, we calculated the area under the curve (AUC) of the profile and the maximum number of intersections. The AUC was calculated using the trapz trapezoidal integration function in Matlab 2018.
Microglial Motility and Surveillance
Microglial motility analysis was done using ImageJ and Matlab as previously described (Sipe et al., 2016). Briefly, Z-stacks were collected in layer II/III of V1 and cerebellum every 5 min for 1hr producing 12 time points. Z stacks were 95–115μm in depth and for analysis in V1 40 μm of the stacks was z-projected. In cerebellum the molecular layer (ML) and the Purkinje cell layers (PCL) were projected separately, and these stacks were each 50 μm in depth. Lateral motion artifact was corrected in ImageJ (Stackreg plugin, ImageJ). A custom Matlab algorithm (Sipe et al., 2016) was used to threshold the images and compare pixels across individual time points to generate a motility index. The motility index reflects any loss (retraction) or gain (extension) of pixels between each pair of consecutive time points. The motility index is then defined as the total number of lost and gained pixels divided by the number of stable pixels. The surveillance ratio was calculated by collapsing the 12 time points in ImageJ with the max-projection function. This max-projection was then binarized to calculate the total pixels occupied by microglia. The surveillance ratio was calculated as the ratio of microglia occupied pixels out of the total pixels in the field of view. The surveillance ratio quantifies the proportion of the imaging field of view that microglial processes occupy within a 1 hour imaging session.
Laser Ablation
Focal tissue injury was generated using a point scan for 8s at 780nm with ~75mW laser power at the sample. The microglial injury response was measured as previously described (Davalos et al., 2005; Nimmerjahn et al., 2005; Stowell et al., 2018). Z-stacks of 50–60μm were collected every 5 minutes for an hour post-laser ablation injury. All analysis was done in ImageJ. For each laser ablation 5 individual microglial processes were tracked as they converged on the core of the injury (manual track plugin). The average velocity of those processes was calculated for each injury and the response curves plotted. From these curves we analyzed both peak velocity and the full-width half max of the response curves.
Statistics
Statistical tests were run with Prism V statistical analysis software (GraphPad). Paired t-tests were used to assess changes in microglia from awake to awake +EtOH. The ns for the experiments reflect the individual animals. Reported values are the mean ± s.e.m. For all analyses, α=0.05.
Results
EtOH impairs microglial dynamics in V1
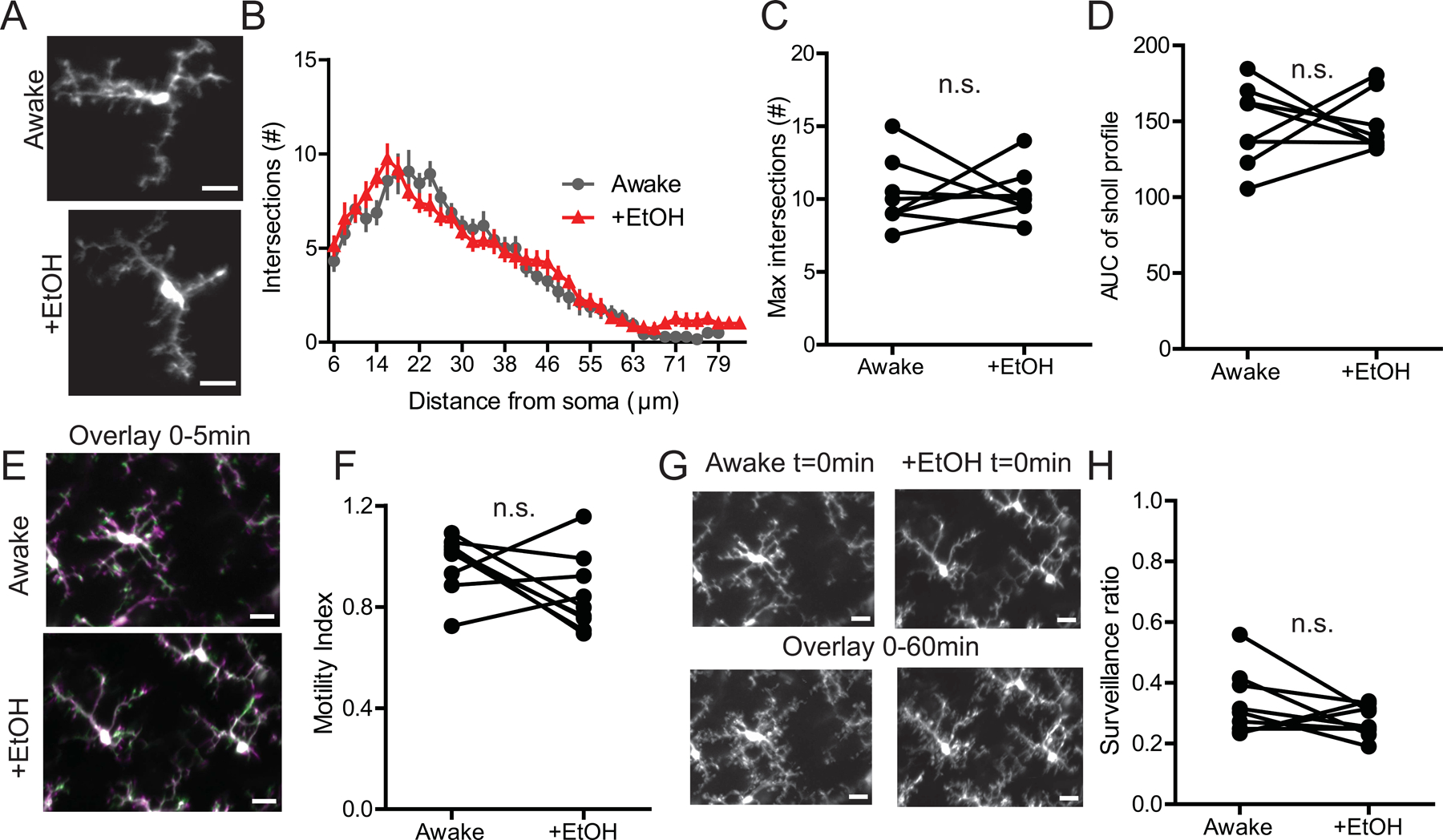
Microglial function in the physiological and pathological conditions is driven by dynamic microglial processes that contact other brain cells, monitor for disturbances and navigate to sites of injury. To assay how acute EtOH exposure impacts these critical microglial behaviors, we assayed baseline microglial morphology and motility in V1 in head restrained, awake CX3CR1-GFP mice, in which microglia are fluorescently labeled (Jung et al., 2000) using in vivo two-photon microscopy. We compared microglial morphology and dynamics within the same animals imaged awake with or without acute alcohol administration by i.p. injection (3g/kg; Fig. 1A)(Quoilin et al., 2012), which resulted in blood EtOH concentrations of ~400mg/dL 15 minutes after injection (as assayed in a separate cohort of mice; Fig. 1B). We found that in V1 microglial morphology remained unaltered by EtOH exposure. Using Sholl analysis to assay the complexity of the microglial arbor, we found no qualitative differences in the Sholl profile. The area under the curve of the profile or the maximum number of process intersections was not statistically different with and without EtOH administration (Fig.1 C–F; n=3F/4M; Max intersections, Paired t-test, t(6)=1.235, p=0.2630; AUC, Paired t-test, t(6)=0.6226, p=0.5565). Despite the unchanged microglial arbor, we did find a robust reduction in microglial process motility (Videos 1 & 2; Fig.1 G, H; n=3F/4M; Paired t-test, t(6)=5.259, p=0.0019), and surveillance of the parenchyma over the 1 hour imaging session (Fig.1 I,J; n=3F/4M; Paired t-test, t(6)=4.808, p=0.0030). Thus, in V1 we see that acute EtOH exposure does not alter microglial arborization, but it does perturb microglial process dynamics resulting in decreased microglial monitoring of the CNS microenvironment.
EtOH has different effects on microglia in different layers of the cerebellum
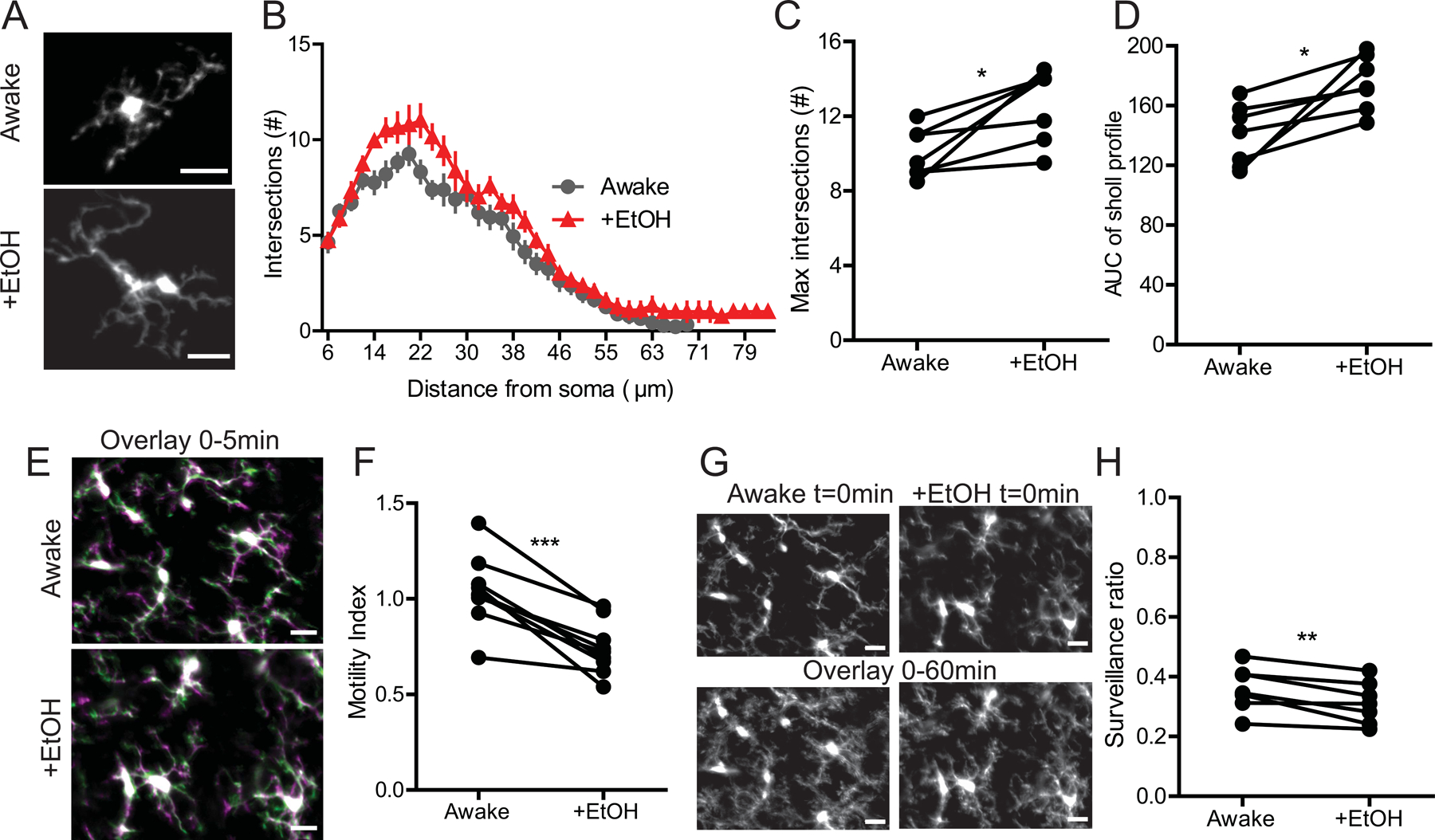
Cerebellar microglia have distinct morphologies and dynamic behavior from their cortical counterparts, hence we wondered whether the EtOH would have different effects on this microglial population (Stowell et al., 2018). Previous work in our lab found baseline differences in microglial dynamics between the ML and the PCL (Stowell et al., 2018), as a result we analyzed microglia in these regions separately in the present study. In the ML, we found that, similarly to V1, acute EtOH exposure did not reduce microglial arborization as measured by the AUC of the Sholl profile and the maximum number of process intersections (Fig.2 A–D; n=4M/4F; Max intersections, Paired t-test, t(7)=0.02784, p=0.9786; AUC, Paired t-test, t(7)=0.0002849, p=0.9998). However, unlike in V1, EtOH did not affect microglial process motility (Fig.2 E, F; n=4M/5F; Paired t-test, t(8)=1.809, p=0.1081) or microglial surveillance of the parenchyma (Fig.2 G, H; n=4M/5F; Paired t-test, t(8)=1.480, p=0.1771) in the ML.
Figure 2|. EtOH does not alter microglial dynamics in the ML of the cerebellum.
(A) Image of microglia in the ML of the same awake mouse with and without EtOH treatment. (B) Sholl profile of ML microglia in awake mice with and without EtOH treatment. (C) Maximum intersection (#) of Sholl profiles; Paired t-test, n.s. (D) Area under the curve (AUC) of the Sholl profiles; Paired t-test, n.s. (E) Overlay of two images taken 5 minutes apart representing microglial motility analysis (green represents retraction, magenta represents extension while white represents pixels that remained stable between the two time points). (F) EtOH exposure does not alter microglial motility in the ML; Paired t-test, n.s. (G) Image of microglia coverage of the CNS parenchyma at t=0min and total surveillance over the 1hr imaging session (all 12 images taken during this time are overlaid). (H) EtOH does not affect microglial surveillance of the ML parenchyma; Paired t-test, n.s. Scale bars = 20μm.
In the PCL we found that EtOH drove two distinct changes in microglial physiology. First, we observed a hyper-ramification of the microglial arbor, as measured by Sholl analysis which we quantified with the area under the curve of the profile and the maximum number of process intersections (Fig.3 A–D; n=4M/3F; Intersections, Paired t-test, t(6)=3.479, p=0.0132; AUC, Paired t-test, t(6)=3.411, p=0.0143). Despite this increase in process complexity, we saw decreased motility of the microglial processes (Videos 3 & 4; Fig.3 E, F; n=4M/3F; Paired t-test, t(8)=6.485, p=0.0002), and a significant loss of microglial surveillance (Fig.3 G,H; n=3M/4F; Paired t-test, t(6)=3.794, p=0.0090), as in V1.
Figure 3|. EtOH alters microglial dynamics in the PCL of the cerebellum.
(A) Representative images of microglia in the PCL in the same awake mouse with and without EtOH treatment. (B) EtOH increases ramification of microglia in the PCL as seen by Sholl analysis. (C) Maximum intersection (#) of Sholl profiles; Paired t-test, p<0.05. (D) Area under the curve (AUC) of the Sholl profiles; Paired t-test, p<0.05. (E) Overlay of two images taken 5 minutes apart representing microglial motility analysis (green represents retraction, magenta represents extension while white represents pixels that remained stable between the two time points) (F) EtOH exposure reduces microglial motility in the PCL; Paired t-test p<0.001. (G)) Image of microglia coverage of the CNS parenchyma at t=0min and total surveillance over the 1hr imaging session (all 12 images taken during this time are overlaid). (H) EtOH decreases microglial surveillance of the PCL parenchyma; Paired t-test, p<0.01. Scale bars = 20μm.
EtOH alters the microglial tissue injury response in the ML but not in V1
To determine whether EtOH also impacts microglial ability to respond to brain injury, we assayed the microglial response to a focal laser ablation injury in the brain of the same awake mice with and without EtOH administration. This analysis was carried out in the same mice a week after motility measurements were made (Fig. 1A). Because injury is harder to elicit deeper in the brain, we limited our analysis of the cerebellum to the more accessible ML. In both V1 and ML of the cerebellum, we observed a robust recruitment of microglial processes to the injury core over the course of 1 hour (Fig.4 A, E). The velocity of the processes responding to the injury peaked between 5–10min post ablation with most processes reaching the core of the injury around 35–40min post ablation (Fig.4 B, F). We observed no significant difference in the response in V1 with EtOH exposure (Videos 5 & 6; Fig.4 C, D; n=3M/4F; FWHM Paired t-test, t(6)=0.4743, p=0.6521; Peak velocity Paired t-test, t(6)=0.07978, p=0.9390). In the ML of the cerebellum, the response appeared to be decreased after EtOH exposure, with a larger velocity of processes sustained for a longer time in awake mice (Videos 7 & 8; Fig.4 G, H; n=3M/3F; FWHM Paired t-test, t(5)=2.614, p=0.0474). However the peak velocity did not differ with EtOH exposure (Fig.4 H Peak velocity Paired t-test; n=3M/3F, t(5)=1.559, p=0.1798). This suggests that EtOH does not impair the ability of microglial processes to respond to focal injury but may subtly affect the timescale of the response in the cerebellum. With the within subjects design of our experiments it may also be that prior ablations impacted microglial responses to the focal tissue injury during the EtOH challenge.
Figure 4|. EtOH impacts focal tissue injury response in the cerebellum but not in the V1.
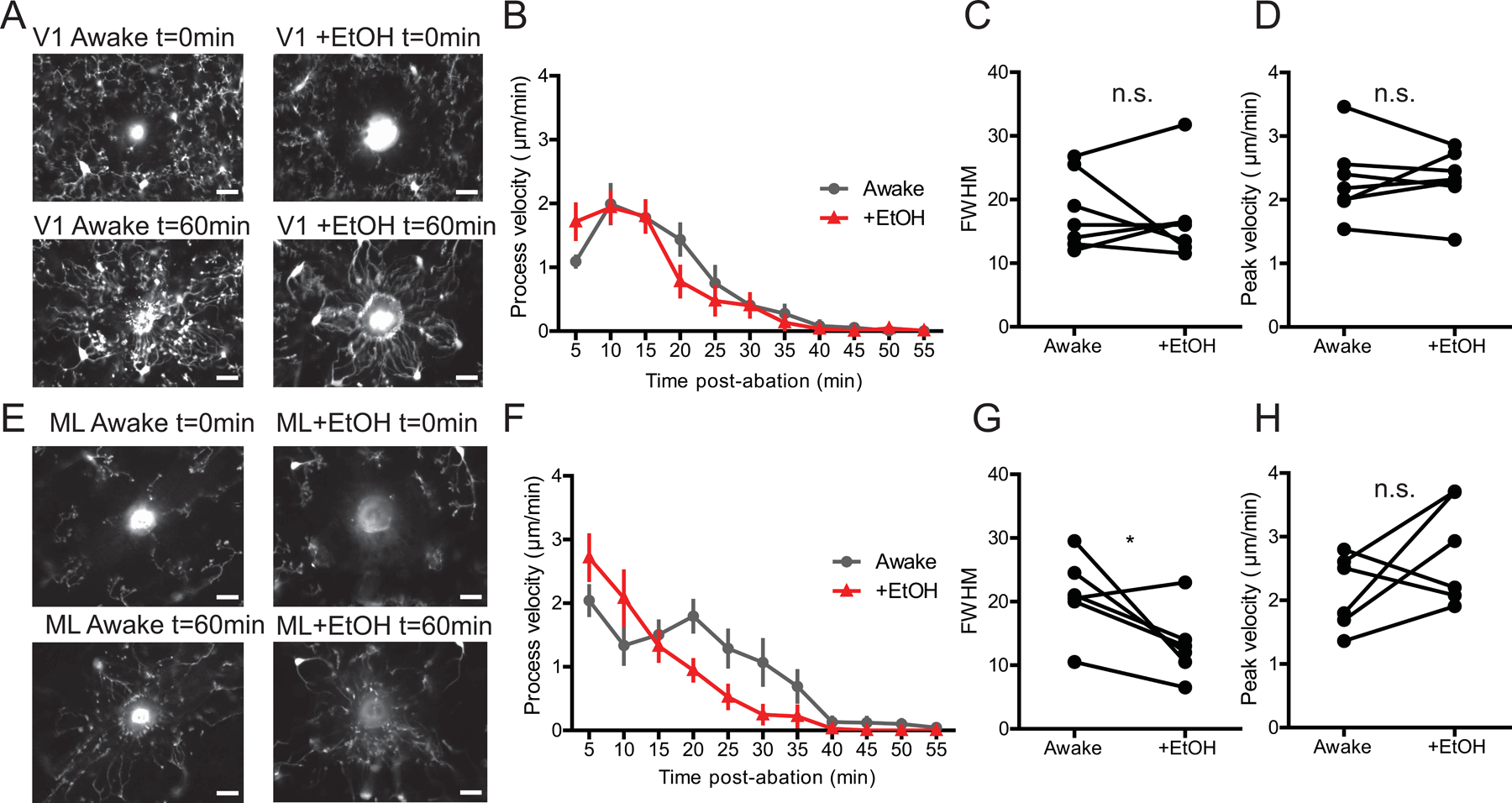
(A) Representative images of microglia in the V1 at t=0min of a laser ablation injury and at t=60min in awake mice with and without EtOH treatment. (B) Average velocity of V1 microglial processes responding to laser ablation over the course of an hour. (C) EtOH does not alter the timing of the V1 response when comparing Full-width half-max of the velocity curves; Paired t-test, n.s. (D) EtOH does not alter the peak velocity of responding microglial processes; Paired t-test, n.s. (E) Representative images of ML microglia at t=0min of a laser ablation injury and at t=60min in awake mice with and without EtOH exposure. (F) Average velocity of ML microglial processes responding to laser ablation over the course of an hour. (G) EtOH changes the Full-width half-max of the velocity curves; Paired t-test, p<0.05. (H) EtOH does not change the peak velocity of responding ML microglial processes; Paired t-test, n.s. Scale bars = 20μm.
Discussion
In the present study we observe acute rapid changes in microglial physiology in both the V1 and cerebellum in response to EtOH administration. The most pronounced effect of EtOH on microglia was the reduction in microglial motility, which occurred rapidly in both the V1 and PCL of the cerebellum leading to a reduction in microglial surveillance. Even acute reductions in microglial surveillance, an important facet of microglial interaction with other CNS elements such as neuronal cell bodies and dendritic spines, could have ramifications for structural plasticity and neural homeostasis (Miyamoto et al., 2016; Stowell et al., 2018; Stowell et al., 2019; Tremblay et al., 2010). It was surprising to find such robust and rapid changes in microglial dynamics from a single acute intoxicating dose of EtOH, and this finding could have widespread implications for understanding even moderate consumption of alcohol throughout the lifespan. In particular, if microglia respond similarly to EtOH in developing and adolescent animals, perturbing microglial surveillance and interactions with neurons could alter developmental wiring of the CNS in areas where microglia are known to participate in pruning and circuit establishment (Hoshiko et al., 2012; Schafer et al., 2012; Squarzoni et al., 2014). The present study was conducted in adult mice, but future work could begin to evaluate if EtOH has the similar effects on microglia in the developing brain.
Interestingly, we found that EtOH elicited morphological changes in microglia only in the PCL, with a significant increase in process ramification. This finding is in line with previous analysis of gene expression has found that the cerebellum can be more responsive than the cortex to acute ethanol exposure (Kane et al., 2014). In classical pathological inflammatory activation microglia tend to assume an ameboid morphology (Kettenmann et al., 2011). We did not observe this type of morphological change in any of the areas we studied, suggesting that acute EtOH exposure does not generate a stereotypical inflammatory response in microglia in the early stages of EtOH intoxication, which may only affect microglial homeostatic functions dependent on their process dynamics. Our findings agree with the prior work showing that microglia tend to have nuanced cytokine and gene expression responses to acute EtOH exposure rather than classical activation (Bell-Temin et al., 2013; Doremus-Fitzwater et al., 2014; Kane et al., 2014). Within our imaging timeframe, we also did not observe robust changes in focal tissue injury responses, which was unexpected as much of the EtOH literature suggests that EtOH generates immunoreactivity, and inflammation (Coleman & Crews, 2018). However, these proinflammatory effects and immunological perturbations have been observed after repeated or developmental administration which differs from our acute study in adult mice. This suggests that the larger changes in neuroimmune function may develop on a slower timescale from the effects of repeated exposure or the deleterious compounds present during withdrawal after EtOH exposure (Doremus-Fitzwater et al., 2014; Qin & Crews, 2012a; Walter & Crews, 2017).
Interestingly, the effects of EtOH on microglia differ by both brain region and layer within the cerebellum. We anticipated that microglia in the cortex and cerebellum may respond differently to the EtOH challenge as previous work from our lab found baseline differences in microglial physiology between these areas (Stowell et al., 2018). Transcriptomic analyses of these regions also show differences in expression of both metabolic and immune surveillance transcripts (Ayata et al., 2018; Grabert et al., 2016; Tay et al., 2017). Additionally, previous work has shown heterogenous responses within cerebellar layers to repeated EtOH exposure (Riikonen et al., 2002). However, it was surprising that in the ML we observed no significant changes in baseline morphology, motility, or surveillance while in the PCL we had extensive changes in all these facets of microglial physiology with EtOH exposure. The robust effects of EtOH on microglia in the PCL may indicate a specific vulnerability in this layer to perturbations from EtOH. Indeed, exposure to EtOH during the brain growth spurt has been shown to result in selective loss of Purkinje neurons, leading to motor deficits later in life (Idrus & Napper, 2012; Topper et al., 2015). Microglia in the PCL produce Interleukin 1β and Tumor necrosis factor α in response to developmental EtOH exposure which causes Purkinje cell apoptosis, but in the cortex, microglial activation by developmental EtOH exposure is largely driven by neuronal apoptosis (Ahlers et al., 2015) (see also Wong et al., 2018 where no effects of developmental EtOH exposure on microglia were observed). This suggests that PCs and cerebellar microglia may interact to potentiate EtOH’s effects in the PCL. While these studies were carried out in the context of developmental EtOH-driven neural apoptosis which is delayed from EtOH exposure, it is possible that microglia rapidly respond to changes in neural activity or to the direct presence of EtOH on acute timescales, and at later timepoints react to secondary effects of EtOH such as neural cell death or the presence of toxic byproducts from EtOH metabolism.
Several limitations need to be considered when evaluating these results. Our study included both male and female mice and while we saw similar trends in both sexes, future work with larger cohorts could elucidate if some of our variability represents significant effects of sex which is known to alter microglial genetic profiles (Guneykaya et al., 2018). Also, our study was designed to allow for repeated awake imaging to study microglial dynamics in vivo with acute EtOH exposure and avoid inter-animal variability. While this approach is relatively non-invasive, allowing microglia to be studied in their native milieu rather than in reduced preparations that can alter microglia function and cause inflammation, it is possible that our within subjects design produced mice with a sensitized microglial state due to the cranial window and repeat dosing procedures. While this was unavoidable with our experimental design future work can seek to further evaluate microglial acute dynamic responses with other preparations.
Here we have demonstrated for the first time that even a single moderately high intoxicating dose of EtOH leads to heterogeneous rapid changes in baseline microglial dynamics in vivo. We observed differential microglial responses based on CNS region, supporting recent work which has emphasized fundamental differences in microglial transcriptomics across brain areas (Grabert et al., 2016; Tay et al., 2017). Our results also agree with recent transcriptomic and proteomic analyses of regional heterogeneity in microglial responses to acute binge EtOH (Bell-Temin et al., 2013; Kane et al., 2014). Future studies are needed to evaluate how EtOH can produce such rapid modulation of microglial behavior, and what signals may differ between cortex and the cerebellum to produce selective microglial vulnerability to EtOH exposure.
Supplementary Material
Video 1 Time lapse movie showing the motility of V1 microglia in an awake mouse taken over 1 hour at 5-minute intervals (40μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 2 Time lapse movie showing the motility of V1 microglia in an awake mouse with EtOH treatment taken over 1 hour at 5-minute intervals (40μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 3 Time lapse movie showing the motility of PCL microglia in an awake mouse taken over 1 hour at 5-minute intervals (50μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 4 Time lapse movie showing the motility of PCL microglia in an awake mouse with EtOH treatment taken over 1 hour at 5-minute intervals (50μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 5 Time-lapse movie of V1 microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 6 Time-lapse movie of V1 microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse with EtOH treatment (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 7 Time-lapse movie of ML microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 8 Time-lapse movie of ML microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse with EtOH treatment (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Acknowledgements
We would like to thank Kim Papastrat and Dr. Linda Spear of the Spear lab, SUNY Binghamton, for assistance with the completion of gas chromatography analysis of blood EtOH concentrations. This work was supported by the Nation Institutes of Health (NIH) grants R01 AA02711 (A.K.M), R21 NS099973 (A.K.M), F31 NS105249 (R.D.S), T32 NS007489 (R.D.S), National Science Foundation grant NSF 1557971 (A.K.M).
Abbreviations:
- AUC
Area under the curve
- CNS
Central Nervous System
- EtOH
Ethanol
- FWHM
Full-width half-max
- GFP
Green fluorescent protein
- ML
Molecular Layer
- V1
Primary visual cortex
- PC
Purkinje Cell
- PCL
Purkinje Cell Layer
- TLR4
Toll-like Receptor 4
Footnotes
Competing Interests
The authors declare no competing financial interests.
Data Availability
The primary data are maintained in a data repository at the University of Rochester. They can be made available by request to the corresponding author Dr. Ania Majewska.
Works Cited
- Abrahao KP, Salinas AG & Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron, 96, 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ & Dailey ME (2015) Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia, 63, 1694–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh YE, Ebert A, Pimenova AA, Ramirez BR, Chan AT, Sullivan JM, Purushothaman I, Scarpa JR, Goate AM, Busslinger M, Shen L, Losic B & Schaefer A (2018) Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci, 21, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Temin H, Zhang P, Chaput D, King MA, You M, Liu B & Stevens SM Jr. (2013) Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. J Proteome Res, 12, 2067–2077. [DOI] [PubMed] [Google Scholar]
- Bialas AR & Stevens B (2013) TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci, 16, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Coleman LG Jr. & Crews FT (2018) Innate Immune Signaling and Alcohol Use Disorders. Handb Exp Pharmacol, 248, 369–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML & Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci, 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME & Deak T (2014) Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res, 38, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M & Guerri C (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol, 183, 4733–4744. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM & McColl BW (2016) Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci, 19, 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Comert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H & Wolf SA (2018) Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep, 24, 2773–2783 e2776. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Deeny MA, Shaner CA & Nixon K (2013) Determining the threshold for alcohol-induced brain damage: new evidence with gliosis markers. Alcohol Clin Exp Res, 37, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J & Crews FT (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol, 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N & Audinat E (2012) Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci, 32, 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrus NM & Napper RM (2012) Acute and long-term Purkinje cell loss following a single ethanol binge during the early third trimester equivalent in the rat. Alcohol Clin Exp Res, 36, 1365–1373. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Tanaka N, Ito A, Takakura A, Kumihashi M & Kinoshita H (2016) Ethanol and Acetaldehyde After Intraperitoneal Administration to Aldh2-Knockout Mice-Reflection in Blood and Brain Levels. Neurochem Res, 41, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A & Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol, 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS & Drew PD (2014) Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res, 38, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M & Verkhratsky A (2011) Physiology of microglia. Physiol Rev, 91, 461–553. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R & Nedergaard M (2018) Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep, 8, 2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM & Nixon K (2011) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun, 25 Suppl 1, S120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y & Nabekura J (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun, 7, 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Harris RA & Ponomarev I (2011) Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res, 35, 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F & Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Pagani F, Paolicelli RC, Murana E, Cortese B, Di Angelantonio S, Zurolo E, Guiducci E, Ferreira TA, Garofalo S, Catalano M, D’Alessandro G, Porzia A, Peruzzi G, Mainiero F, Limatola C, Gross CT & Ragozzino D (2015) Defective microglial development in the hippocampus of Cx3cr1 deficient mice. Front Cell Neurosci, 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D & Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science, 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR & Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R & Zheng Q (2003) Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci, 72, 1825–1839. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K & Schwab C (2004) Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol, 33, 147–155. [DOI] [PubMed] [Google Scholar]
- Qin L & Crews FT (2012a) Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation, 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L & Crews FT (2012b) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation, 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS & Crews FT (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E & Quertemont E (2012) Chronic ethanol exposure during adolescence alters the behavioral responsiveness to ethanol in adult mice. Behav Brain Res, 229, 1–9. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Varlinskaya EI & Spear LP (2011) Effect of the selective NMDA NR2B antagonist, ifenprodil, on acute tolerance to ethanol-induced motor impairment in adolescent and adult rats. Alcohol Clin Exp Res, 35, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K & Hervonen A (2002) Intermittent ethanol exposure increases the number of cerebellar microglia. Alcohol Alcohol, 37, 421–426. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA & Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK & Stevens B (2013) The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia, 61, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE & Majewska AK (2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun, 7, 10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F & Garel S (2014) Microglia modulate wiring of the embryonic forebrain. Cell Rep, 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Stowell RD, Sipe GO, Dawes RP, Batchelor HN, Lordy KA, Whitelaw BS, Stoessel MB, Bidlack JM, Brown E, Sur M & Majewska AK (2019) Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell RD, Wong EL, Batchelor HN, Mendes MS, Lamantia CE, Whitelaw BS & Majewska AK (2018) Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev Neurobiol, 78, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar Datta, M., Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O & Prinz M (2017) A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci, 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Topper LA, Baculis BC & Valenzuela CF (2015) Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation, 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL & Majewska AK (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol, 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ & Crews FT (2017) Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J Neuroinflammation, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EL, Stowell RD & Majewska AK (2017) What the Spectrum of Microglial Functions Can Teach us About Fetal Alcohol Spectrum Disorder. Front Synaptic Neurosci, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lee MR, Choi S, Kim T & Choi DS (2010) ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res, 34, 1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D & Gross CT (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci, 17, 400–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 Time lapse movie showing the motility of V1 microglia in an awake mouse taken over 1 hour at 5-minute intervals (40μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 2 Time lapse movie showing the motility of V1 microglia in an awake mouse with EtOH treatment taken over 1 hour at 5-minute intervals (40μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 3 Time lapse movie showing the motility of PCL microglia in an awake mouse taken over 1 hour at 5-minute intervals (50μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 4 Time lapse movie showing the motility of PCL microglia in an awake mouse with EtOH treatment taken over 1 hour at 5-minute intervals (50μm z-stacks were compressed in each time point). Scale bar = 20μm
Video 5 Time-lapse movie of V1 microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 6 Time-lapse movie of V1 microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse with EtOH treatment (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 7 Time-lapse movie of ML microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse (10μm z-stacks were compressed at each time point). Scale bar = 20μm
Video 8 Time-lapse movie of ML microglia taken over 1 hour at 5 minute intervals after focal laser injury in an awake mouse with EtOH treatment (10μm z-stacks were compressed at each time point). Scale bar = 20μm


