Abstract
Background
The impact of asthma on coronavirus disease 2019 (COVID-19) remains largely unknown.
Objective
To investigate the asthma prevalence among patients with COVID-19 and compare outcomes between patients with and without asthma.
Methods
In this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, bioRxiv, and medRxiv for studies reporting asthma prevalence in general patients with COVID-19 or comparing outcomes between patients with and without asthma, and excluded duplicate publications, reviews, editorials, comments, single case reports, or small case series (<10 cases). We determined the pooled estimates of effect using random-effect model.
Results
On the basis of 131 studies (410,382 patients), we found great variability in the prevalence of comorbid asthma among patients with COVID-19 in different countries or regions ranging from 1.1% to 16.9%. No significant difference in asthma prevalence was found between hospitalized and nonhospitalized (risk ratio [RR], 1.15; 95% CI, 0.92-1.43), severe and nonsevere (RR, 1.21; 95% CI, 0.92-1.57), intensive care unit and non–intensive care unit (RR, 1.19; 95% CI, 0.92-1.54), dead and survived (RR, 0.90; 95% CI, 0.73-1.11), intubated/mechanically ventilated and nonintubated/mechanically ventilated (RR, 0.91; 95% CI, 0.71-1.17) patients with COVID-19. Patients with asthma have a lower risk of death compared with patients without asthma (RR, 0.65; 95% CI, 0.43-0.98). Asthma is not associated with a higher risk of intubation or mechanical ventilation (RR, 1.03; 95% CI, 0.72-1.46).
Conclusions
There is great variability in asthma prevalence among patients with COVID-19 in different countries or regions. Asthma is not associated with higher COVID-19 severity or worse prognosis, and patients with asthma are found to have a lower risk of death compared with patients without asthma.
Key words: COVID-19, Asthma, Prevalence, Severity, Prognosis
Abbreviations used: COVID-19, Coronavirus disease 2019; ICU, Intensive care unit; RR, Risk ratio; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
What is already known about this topic? Asthma was proposed to potentially confer increased risk for coronavirus disease 2019 (COVID-19) due to deficiency in antiviral response and tendency of virus-induced exacerbation. However, data from several countries showed a low prevalence of asthma among patients with COVID-19, and attributed this to a potential TH2-mediated protection. The impact of asthma on COVID-19 remains largely unknown.
What does this article add to our knowledge? There is great variability in asthma prevalence among patients with COVID-19 in different countries and regions. Asthma is not associated with higher COVID-19 severity or worse prognosis, and patients with asthma are found to have a lower risk of death compared with patients without asthma.
How does this study impact current management guidelines? Asthma was not found to be associated with more severe COVID-19 phenotypes. Patients with asthma should continue their treatment regiments based on the current guidelines. Treatment according to COVID-19 severity would be reasonable for patients with preexisting asthma.
Introduction
The pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide since December 2019, posing a great challenge to global public health. As of October 1, 2020, there have been more than 33,000,000 confirmed cases and more than 1,000,000 deaths reported to the World Health Organization.1
Identifying fragile populations with higher susceptibility and worse prognosis is important in the fight against COVID-19. Currently, a series of risk factors have been identified to be associated with COVID-19 severity and mortality, such as older age, male sex, comorbidities, and metabolic abnormalities.2, 3, 4 Patients with asthma have deficient antiviral immune responses and a tendency of exacerbation elicited by common respiratory viruses.5 , 6 Thus, asthma was proposed to potentially confer increased risk for COVID-19 in the early stage of the pandemic.7 , 8 This was supported by several studies from the United States reporting that comorbidity rates of asthma in patients with COVID-19 were higher than those in the local population.9 , 10 However, data from China11 , 12 and Italy13 showed a low prevalence of asthma among patients with COVID-19, and attributed this to a potential TH2-mediated protection from COVID-19 in patients with asthma.11 In addition, it remains unclear whether patients with asthma are subject to a more severe disease course and worse prognosis compared with patients without asthma. Considering the large population base of patients with asthma, it is urgent to clarify the risk of SARS-CoV-2 infection and severity in asthma, which is a focus of concern during this potentially protracted COVID-19 pandemic. A better understanding of the relationship between COVID-19 and asthma may also provide new insights into the pathophysiology and clinical aspects of COVID-19.
Therefore, we did a systematic review and meta-analysis of studies to report the prevalence of comorbid asthma among patients with COVID-19 and further performed subgroup analyses based on the geographical region and disease severity. We also compared clinical outcomes between patients with and without asthma.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The protocol was registered in PROSPERO (CRD42020202253). We searched PubMed, Embase, Web of Science, bioRxiv, and medRxiv databases on July 21, 2020 (updated on August 18, 2020), for articles published since January 1, 2020. Given the overwhelming number of articles on COVID-19, the search was restricted to certain study types. The detailed search strategy is presented in this article's Online Repository at www.jaci-inpractice.org. The records were managed with EndNote (version X9.0) to exclude duplicates. The remaining were further reviewed to determine whether to include in further analyses.
Studies were included if they met the following criteria: (1) allowing for calculation of asthma prevalence among patients with COVID-19 from the original data; or (2) reported severity and mortality (hospitalized vs nonhospitalized, severe vs nonsevere, intensive care unit [ICU] vs non-ICU, death vs nondeath, intubation/mechanical ventilation vs not requiring intubation/mechanical ventilation) of patients with COVID-19 with and without asthma. The definition of COVID-19 severity may vary among studies, and we selected the articles that explicitly reported the number of patients with asthma among “severe” and “nonsevere” patients with COVID-19. The following studies were excluded: (1) duplicate publications; (2) studies in non-English languages; (3) reviews, editorials, comments, guidelines, consensus, and small case series (<10 cases)14; and (4) studies focusing on special population such as children and pregnant women. The institution and time period of each study were recorded to avoid double counting. For example, if one institution publishes multiple studies during overlapping time periods, or participates in both single-center and multicenter studies, only the study with the largest number of patients was retained. Two reviewers (S.L. and Y.C.) independently conducted the literature search and screening and resolved discrepancy by consensus. The quality of included studies was rated by the National Institutes of Health Quality Assessment Tool.
Data analysis
Two investigators (S.L. and Y.C.) independently extracted the data from included studies and disagreements were resolved by consensus. The following information (if available) was extracted and recorded: (1) first author, (2) year of publication, (3) study title, (4) study design, (5) country and city, (6) specific populations (eg, children, adult, and women), (7) number of confirmed patients with COVID-19, (7) number of patients complicated with asthma, (9) number of patients with different clinical severity and mortality (as described above), and (10) demographic characteristics: including mean or median age and sex ratio.
The statistical analyses were performed with the package “meta” on R (version 4.0.0).15 The “metaprop” command was used for pooled estimate for the prevalence of asthma. The “metabin” command was used to calculated the risk ratio (RR) of comorbid asthma in patients with COVID-19 with different severity or mortality, and the RR of outcomes between patients with COVID-19 with and without asthma. Considering the interstudy heterogeneity, a random-effects model was adopted to estimate the pooled effect for a more conservative estimate of the 95% CI. τ2 was estimated using the DerSimonian-Laird method. The Hartung-Knapp-Sidik-Jonkman method was used to produce a more robust estimate of var (θF).16 The I 2 statistic and Cochran Q test were used to assess statistical heterogeneity. Contour-enhanced funnel-plot and Egger test were used to investigate the publication bias. The package “rworldmap” was used to visualize the difference in asthma comorbidity rate.17
Results
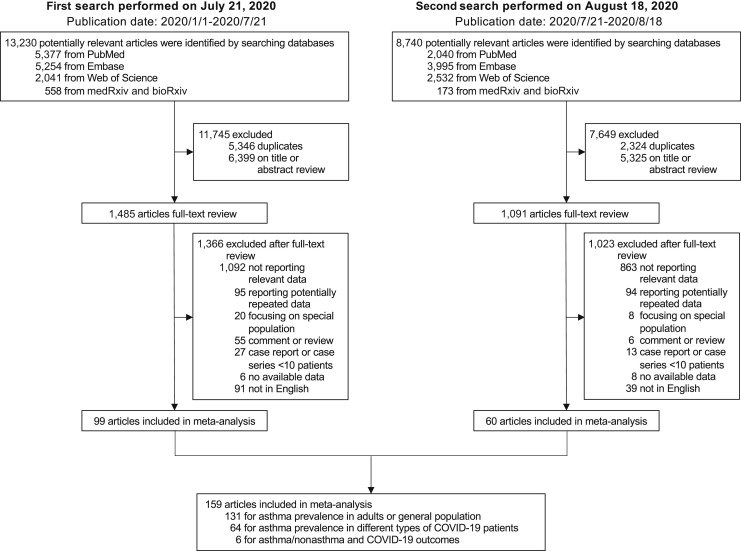
On the first search, 13,230 potentially relevant articles were identified. A total of 5346 records were recognized as duplicates, and 6399 articles were excluded after review of titles or abstracts. Among the 1485 articles undergoing full-text review, 99 articles were included in further meta-analysis after excluding articles not reporting relevant data or potentially repeated data, review articles, editorials or comments, case report or small-scale case series (<10 patients), and non-English or inaccessible articles. On the second search, the same protocol was followed and another 60 articles were identified. As shown in Figure 1 , a total of 159 articles were included in later analyses for various purposes, including 105 observational cohort or cross-sectional studies, 42 case series, 9 case-control studies, and 3 controlled intervention studies. The quality of included studies was rated as good (n = 108) or fair (n = 51) according to the National Institutes of Health Quality Assessment Tool (see Table E1 in this article's Online Repository at www.jaci-inpractice.org). The data sets used in each analysis are listed in Table E2 in this article's Online Repository at www.jaci-inpractice.org.
Figure 1.
Study selection. PubMed, Embase, Web of Science, bioRxiv, and medRxiv databases were searched on July 21, 2020, for articles published since January 1, 2020. Another search was performed on August 18, 2020, to include eligible articles that were published between the 2 searches.
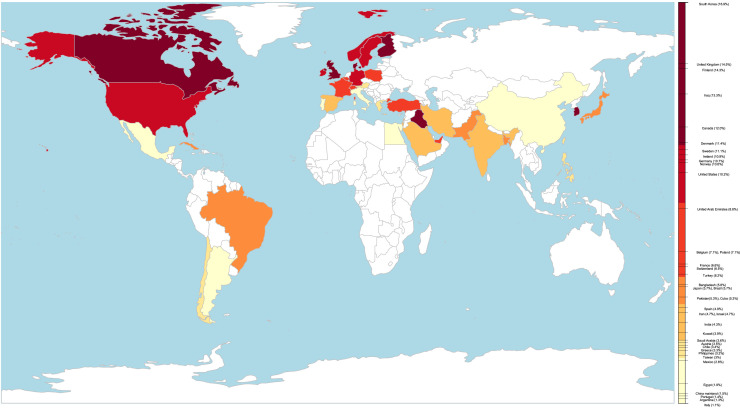
Among the 159 included studies, 131 studies from 39 countries (410,382 patients) reported asthma prevalence in adult or all-age-group patients with COVID-19. The regional asthma comorbidity rates were estimated as follows: East Asia and the Pacific, 2.2% (95% CI, 1.2-3.8%; I 2 = 98.0%; n = 25,534); Europe, 6.4% (95% CI, 3.8-9.6%; I 2 = 99.2%; n = 58,620); Latin America and the Caribbean, 3.5% (95% CI, 1.6-6.0%; I 2 = 97.2%; n = 219,879); Middle East and North Africa, 4.9% (95% CI, 4.0%-6.0%; I 2 = 53.2%; n = 8503); North America, 10.2% (95% CI, 8.8%-11.6%; I 2 = 97.5%; n = 97,312) (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). The prevalence of comorbid asthma in patients with COVID-19 in each country or region ranged from 1.1% to 16.9%, varying by as much as 15-fold. Table I presents the pooled asthma comorbidity rate in patients with COVID-19 by country or region. The difference in asthma comorbidity rate is visualized as a colored world map in Figure 2 .
Table I.
The prevalence of asthma in patients with COVID-19 in different countries or regions
| Country/region | Asthma prevalence in patients with COVID-19 |
Country/region | Asthma prevalence in patients with COVID-19 |
||||
|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients | Calculated prevalence | No. of studies | No. of patients | Calculated prevalence | ||
| Egypt | 2 | 242 | 1.9% | United Kingdom | 1 | 17,535 | 14.5% |
| China mainland | 18 | 7,948 | 1.5% | Argentina | 1 | 78 | 1.3% |
| Taiwan | 1 | 100 | 3% | Brazil | 1 | 8,637 | 5.7% |
| Japan | 4 | 126 | 5.7% | Chile | 1 | 29 | 3.4% |
| South Korea | 1 | 9,148 | 16.9% | Cuba | 1 | 19 | 5.3% |
| Austria | 1 | 259 | 3.5% | Mexico | 1 | 211,003 | 2.8% |
| Belgium | 1 | 28 | 7.1% | Iran | 6 | 429 | 4.7% |
| Denmark | 1 | 175 | 11.4% | Iraq | 1 | 15 | 13.3% |
| Finland | 1 | 28 | 14.3% | Israel | 1 | 4,151 | 4.7% |
| France | 5 | 4,918 | 6.6% | Kuwait | 1 | 1,096 | 3.9% |
| Germany | 3 | 88 | 10.7% | Saudi Arabia | 1 | 1,519 | 3.6% |
| Greece | 1 | 90 | 3.3% | United Arab Emirates | 1 | 34 | 8.8% |
| Ireland | 2 | 239 | 10.9% | Turkey | 2 | 1,017 | 6.2% |
| Italy | 2 | 6,314 | 1.1% | Canada | 1 | 117 | 12.0% |
| Norway | 1 | 66 | 10.6% | United States | 48 | 97,195 | 10.2% |
| Poland | 1 | 28 | 7.1% | Bangladesh | 1 | 103 | 5.8% |
| Portugal | 1 | 20,293 | 1.4% | India | 1 | 280 | 4.3% |
| Spain | 7 | 8,204 | 4.9% | Pakistan | 1 | 30 | 5.3% |
| Sweden | 1 | 27 | 11.1% | Philippines | 1 | 8,212 | 3.2% |
| Switzerland | 3 | 328 | 6.5% | ||||
For each country/region with 2 or more studies reporting the prevalence of asthma in patients with COVID-19, a meta-analysis was performed to estimate the pooled prevalence. For countries or regions with only 1 available study reporting asthma prevalence in patients with COVID-19, asthma prevalence was calculated as (total patients with COVID-19 with asthma)/(total patients with COVID-19).
Figure 2.
World map of asthma comorbidity rates in patients with COVID-19. The difference in asthma comorbidity rate is visualized as a colored world map, in which a darkening color represents higher prevalence. The asthma comorbidity rate was calculated as follows: for countries with 2 or more studies reporting the prevalence of asthma in patients with COVID-19, a meta-analysis was performed to estimate the pooled prevalence; for countries with only 1 study on asthma prevalence in patients with COVID-19, asthma prevalence was calculated as (total patients with COVID-19 with asthma)/(total patients with COVID-19).
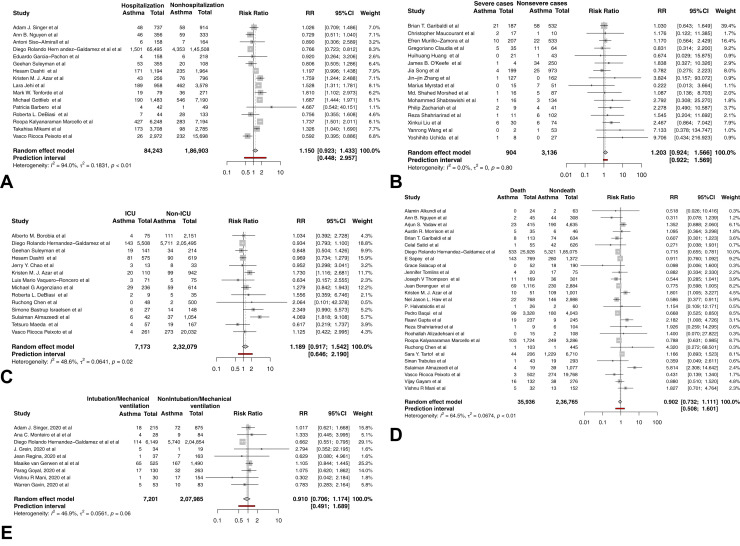
To explore whether asthma affects the disease course of COVID-19, we first analyzed the difference in asthma comorbidity rates between patients with different severity and mortality. No significant difference in asthma prevalence was found between hospitalized and nonhospitalized patients with COVID-19 (RR, 1.15; 95% CI, 0.92-1.43; I 2 = 94.0%; P = .19; Figure 3 , A), severe and nonsevere patients (RR, 1.21; 95% CI, 0.92-1.57; I 2 = 0.0%; P = .16; Figure 3, B), ICU and non-ICU patients (RR, 1.19; 95% CI, 0.92-1.54; P = .17; I 2 = 48.6%; Figure 3, C), patients who did and did not die (RR, 0.90; 95% CI, 0.73-1.11; P = 0.31; I 2 = 64.5%; Figure 3, D), and patients requiring and not requiring intubation/mechanical ventilation (RR, 0.91; 95% CI, 0.71-1.17; P = .42; I 2 = 46.9%; Figure 3, E). These findings suggest that asthma was not more prevalent among patients with COVID-19 with more severe disease courses. No significant publication bias was detected by contour-enhanced funnel-plot and Egger test.
Figure 3.
Forest plots of the prevalence of comorbid asthma in patients with COVID-19 with different severity. (A) Forest plot of the prevalence of comorbid asthma in hospitalized and nonhospitalized patients with COVID-19. (B) Forest plot of the prevalence of comorbid asthma in severe and nonsevere patients with COVID-19. (C) Forest plot of the prevalence of comorbid asthma in patients with COVID-19 requiring and not requiring ICU admission. (D) Forest plot of the prevalence of comorbid asthma in dead and survived patients with COVID-19. (E) Forest plot of the prevalence of comorbid asthma in patients with COVID-19 requiring and not requiring intubation/mechanical ventilation.
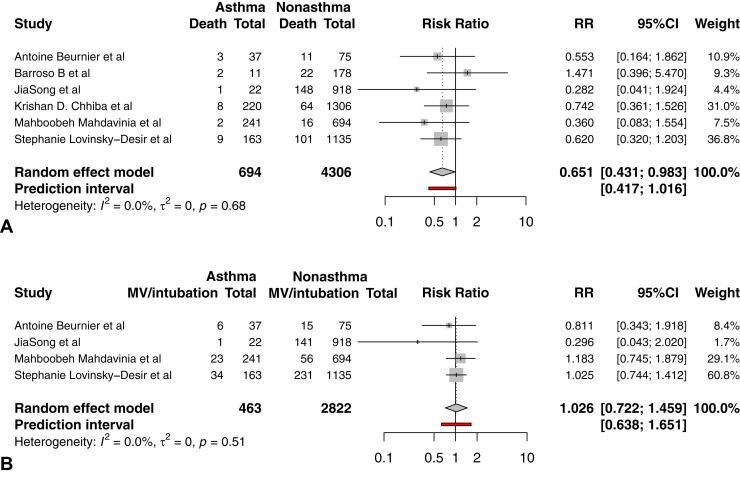
In addition, a comparison of clinical outcomes between patients with and without asthma could provide better information on the relationship between comorbid asthma and COVID-19 mortality. By combining 6 studies reporting the outcome of death among patients with COVID-19 with and without asthma, we found that the death risk was even lower in patients with COVID-19 with asthma (RR, 0.65; 95% CI, 0.43-0.98; P = .04; I 2 = 0.0%; Figure 4 , A) than in patients with COVID-19 without asthma. We also found that patients with asthma were not associated with a significantly higher risk of intubation or mechanical ventilation (RR, 1.03; 95% CI, 0.72-1.46; P = .83; I 2 = 0.0%; Figure 4, B) based on 4 studies reporting relevant outcomes. No significant publication bias was detected by contour-enhanced funnel-plot and Egger test. Table II lists the reported COVID-19 outcomes in patients with and without asthma. No significant difference in asthma prevalence was reported in terms of length of hospitalization, ICU admission, and severity rate.
Figure 4.
COVID-19 outcomes in patients with and without asthma. (A) Forest plot of death risk in patients with COVID-19 with and without asthma. (B) Forest plot of intubation or mechanical ventilation need in patients with COVID-19 with and without asthma. MV, Mechanical ventilation.
Table II.
COVID-19 outcomes in patients with and without asthma
| Outcome | Study | City | Asthma group | Nonasthma group | P value∗ |
|---|---|---|---|---|---|
| Hospitalization | Mahdavinia et al18 | Chicago, USA | 73 of 241 | 224 of 694 | .65 |
| Chhiba et al19 | Chicago, USA | 115 of 220 | 738 of 1306 | .242 | |
| Length of hospital stay (d) | Lovinsky-Desir et al20 | New York City, USA | Median: 6, IQR: 9 | Median: 5, IQR: 9 | .25 |
| Mahdavinia et al18 | Chicago, USA | Mean: 8.36, SD: 6.02 | Mean: 7.81, SD: 5.19 | .25 | |
| Barroso et al21 | Madrid, Spain | Mean: 9.72, SD: 8.14 | Mean: 10.9, SD: 9.67 | NS | |
| Acute respiratory distress syndrome | Mahdavinia et al18 | Chicago, USA | 22 of 241 | 65 of 694 | .92 |
| Song et al22 | Wuhan, China | 1 of 22 | 128 of 918 | .35 | |
| Severe cases | Song et al22 | Wuhan, China | 1 of 22 | 229 of 918 | .02 |
| ICU admission | Beurnier et al23 | Paris, France | 11 of 37 | 33 of 75 | .21 |
| Barroso et al21 | Madrid, Spain | 2 of 11 | 30 of 178 | >.99 | |
| Mechanical ventilation/ intubation | Lovinsky-Desir et al20 | New York City, USA | 34 of 163 | 231 of 1135 | .92 |
| Mahdavinia et al18 | Chicago, USA | 23 of 241 | 56 of 694 | .35 | |
| Beurnier et al23 | Paris, France | 6 of 37 | 15 of 75 | .82 | |
| Song et al22 | Wuhan, China | 1 of 22 | 141 of 918 | .23 | |
| Death | Lovinsky-Desir et al20 | New York City, USA | 9 of 163 | 101 of 1135 | .18 |
| Mahdavinia et al18 | Chicago, USA | 2 of 241 | 16 of 694 | .22 | |
| Beurnier et al23 | Paris, France | 3 of 37 | 11 of 75 | .38 | |
| Song et al22 | Wuhan, China | 1 of 22 | 148 of 918 | .23 | |
| Chhiba et al19 | Chicago, USA | 8 of 220 | 64 of 1306 | .413 | |
| Barroso et al21 | Madrid, Spain | 2 of 11 | 22 of 178 | .63 |
IQR, Interquartile range; NS, not significant.
P values provided in the article are shown in regular type, while P values calculated with Fisher exact tests or χ2 tests are shown in italic type.
Discussion
Currently, meta-analysis focusing on the relationship between COVID-19 and asthma is lacking. To our knowledge, this meta-analysis is the first to produce a pooled estimate of asthma comorbidity rate and related outcomes based on a large COVID-19 population (more than 400,000 patients). To identify the most comprehensive original studies, we performed systematic literature search in both databases and preprint websites, and updated the search during the analysis. Our results revealed great variability in the prevalence of comorbid asthma among patients with COVID-19 in different countries or regions. We also found that the prevalence of asthma was not higher in patients with COVID-19 who need more intensive treatment (hospitalization, ICU admission, intubation or mechanical ventilation), with higher clinical severity, or have worse outcome (death). When compared with those without asthma, a lower risk of death was found in patients with COVID-19 with asthma. No significant difference was reported in terms of other clinical outcomes between these 2 groups of patients. Altogether, our results suggest that asthma is not associated with more severe COVID-19 phenotypes.
From the perspective of pathophysiology, it is reasonable to anticipate asthma as a risk factor for higher susceptibility to COVID-19. First, patients with asthma are known to have deficient antiviral immune response, which may predispose them to elevated susceptibility to viral infection.6 Interferon responses, which play a central role in antiviral immune response, are deficient in patients with asthma due to impaired production24, 25, 26, 27 and damping effect of IgE cross-linking.27 Second, patients with asthma have a tendency for exacerbation elicited by common respiratory viruses, and are associated with severe adverse outcomes.28 Based on these theoretical associations, current Centers for Disease Control guidelines warned that patients with moderate to severe asthma may be at increased risk for more severe disease course with COVID-19.29 However, this theoretical association was not validated by real-world observations. The results based on currently available data showed high variable asthma comorbidity rates, and most of the original studies failed to distinguish physician-diagnosed from self-reported asthma, making it hard to determine whether asthma plays a predisposing or protective role in COVID-19 susceptibility.
Despite the concern that patients with asthma might suffer more severe COVID-19, the current analyses did not indicate that asthma aggravates the COVID-19 course. We first reported that asthma was not more prevalent among patients with COVID-19 with more severe disease courses. Then, by comparing the clinical outcomes between patients with and without asthma, we further found that asthma did not increase the risk of intubation or mechanical ventilation, and might even be associated with lowered death risk. The above findings are consistent in suggesting that asthma is not associated with elevated death risk of COVID-19, which is against the previous public concern. The theoretical mechanisms may include the role of type 2 immune response and other physiological changes associated with the asthmatic state, asthma medications, and behavioral aspects. As stated in our previous review article,30 certain aspects of type 2 immune response have the potential to provide protection against COVID-19 to some extent. Some type 2 cytokines (IL-4, IL-5, IL-13, etc) have inhibitory effects on the production of proinflammatory cytokines (IL-6, TNF-α, etc), the overactivation of which is proposed to be a potential pathological mechanism for COVID-19 progression. Recruitment and activation of effector cells, including mast cells, eosinophils, and basophils, may also modulate the immune response against SARS-CoV-2. Local eosinophilia, which is characteristic of asthma, may also act on viral clearance by releasing ribonucleases, although the association between eosinophil and COVID-19 course is inconclusive.31 In addition, mucus overproduced during the type 2 immune response may serve as a physical barrier against viral invasion.32 Respiratory allergy and controlled allergen exposures are associated with reduced expression of angiotensin-converting enzyme 2, which is the entry receptor for SARS-CoV-2 associated with in vitro susceptibility.33 Another consideration is the role of asthma medication, including inhaled corticosteroids, allergen immunotherapy, and biological agents (anti-IgE mAb, anti–IL-5 mAb), which might also be beneficial through alleviating inflammation or enhancing antiviral defense. In addition, warned of the increased risk since the beginning of the pandemic, patients with asthma may better observe recommendations such as social distancing, personal protection, and hygiene rules, which are also supposed to have potential protective effects. However, the question whether asthma is associated with lowered death risk of COVID-19 should be answered with great caution in case of giving misleading information. Our results provided potential clues; however, considering the relatively small number of available studies (n = 6), further evidence is still needed to confirm the observed association.
There is also evidence that patients with severe asthma did not tend to develop into more severe types of COVID-19. In a large cohort study based on the Severe Asthma Network in Italy, an infrequent rate of confirmed or highly-suspected COVID-19 (26 of 1504 [1.73%]) is reported, which coincides with the underreported asthma cases among patients with COVID-19.34 In the abovementioned cohort, the COVID-19–related mortality rate was 7.7%, which was lower than that of the general population in Italy, indicating that severe asthma does not increase the risk of SARS-CoV-2 infection and disease severity. In another follow-up study in Spain, 3 of 80 (3.8%) patients with severe asthma had confirmed COVID-1928 but no patient developed cytokine storm or acute respiratory distress syndrome.35 In addition, no increase in exacerbation of severe asthma due to COVID-19 was observed during the study period.35
Efforts have been made to explore the potential influence of comorbid asthma on the COVID-19 course. Wang et al36 performed a meta-analysis based on 4 studies from 744 patients with asthma and 8151 patients without asthma and found that presence of asthma had no significant effect on mortality (odds ratio, 0.96; 95% CI, 0.70-1.30; I 2 = 0%; P = .79). No strong conclusions regarding asthma and other clinical outcomes could be made due to limited data. Wang et al37 performed a meta-analysis based on 14 publications representing data from 17,694 participants and found that severe COVID-19 was not associated with an increased risk of asthma compared with nonsevere COVID-19 while asthma was not associated with increased risk of mortality in patients with COVID-19. In another study exploring population risk factors for COVID-19 case mortality rate, Hashim et al38 reported that the COVID-19 case mortality rates were higher in countries where the population had risk factors such as Alzheimer disease, lung cancer, asthma, and chronic obstructive pulmonary disease, but case mortality rate was found to be only weakly correlated with asthma (r = 0.28).
The above articles have provided valuable information on the relationship between asthma and COVID-19. However, our article also provides additional information regarding this issue. First, to explore the association between asthma comorbidity and COVID-19 severity, we performed several subgroup analyses rather than only compare whether severe COVID-19 was associated with increased risk of asthma. Our results revealed that the risk of asthma was not increased in patients requiring hospitalization compared with those not requiring hospitalization, as well as in patients needing ICU admission versus no ICU admission, in patients having severe diseases versus nonsevere diseases, in patients with outcomes of death versus survive, and in patients requiring intubation/mechanical ventilation versus not requiring these measures. Therefore, our study provided more detailed evidence to support the previous conclusion. Second, by incorporating more available studies, we found that patients with asthma had lower death risk than patients without asthma, which was never proposed by previous studies. Third, our analyses are based on a larger population. For analysis on the association of COVID-19 severity and asthma risk, we used data from 64 articles of 308,775 participants. For analysis on the asthma comorbidity and COVID-19 clinical outcomes, we used data from 6 articles representing 694 patients with asthma and 4306 patients without asthma. Therefore, although new articles are emerging in this quickly-moving field, our study could still provide new perspectives for readers.
This study has several potential limitations. First, because available COVID-19 patients' data do not represent the proportion of population in each country, a pooled asthma prevalence based on meta-analysis is not directly comparable to the global asthma prevalence. In this sense, it is almost impossible to know the exact asthma comorbidity rate among patients with COVID-19, and to know the difference between asthma prevalence among patients with COVID-19 and the general population. Therefore, we only calculated country-specific and regional prevalence of comorbid asthma. Second, strict exclusion criteria were adopted to avoid double counting, resulting in exclusion of many studies from the final analysis. However, the asthma comorbidity rate might be overestimated, because we included only those studies that explicitly report asthma prevalence and possibly missed some studies that failed to list asthma as a comorbidity. Third, most of the original studies failed to provide detailed information on asthma, including clinical phenotypes, severity, medication regimen, and controlling states, making it hard to draw further conclusion on the influence of asthma on COVID-19 course and prognosis. This hinders further exploration into the pathophysiological interactions between asthma and COVID-19. Fourth, we excluded non-English articles during the full-text review stage of study selection, which might introduce a potential bias to conclusion. However, because these non-English articles account for only a relatively small part of candidate articles, and it has been found that exclusion of non-English publications from systematic reviews on clinical interventions confer a minimal effect on overall conclusions,39 the current analyses in this article still provide comprehensive and credible information.
There are still many unanswered questions on asthma management during the pandemic. Do patients with asthma need higher levels of personal protection? Would patients with well-controlled asthma be protected from SARS-CoV-2 infection and severe disease course? Would asthma treatment provide benefit to combat SARS-CoV-2? More detailed original studies are needed to provide reliable evidence. Currently, considering the ambiguous role of comorbid asthma in COVID-19 susceptibility, and that uncontrolled asthma is a risk factor for greater virus-induced exacerbation severity, we still recommend continuing asthma control and ensure personal protection in clinical practice. Because no association was found between comorbid asthma and more severe COVID-19 phenotypes, treatment according to COVID-19 severity might be reasonable for these patients with preexisting asthma.
Conclusions
Our results do not suggest that asthma is associated with more severe COVID-19 phenotypes. On the basis of data of more 400,000 patients with COVID-19 worldwide, we found great variability in the prevalence of comorbid asthma among patients with COVID-19 in different countries or regions. Asthma was not overrepresented in patients with COVID-19 who need more intensive treatment, with higher clinical severity, or have worse outcome. In addition, patients with asthma showed a tendency of lower death risk compared with patients without asthma, and no increase in need for intubation/mechanical ventilation. Therefore, we recommended continuing asthma control based on the current guidelines and giving treatment according to COVID-19 severity for patients with preexisting asthma.
Acknowledgment
We thank Dr Kun Zhao for his support and advice on the manuscript.
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int Available from:
- 2.Liu D., Cui P., Zeng S., Wang S., Feng X., Xu S. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25:100471. doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales-van Horn S.R., Farrar J.D. Interferon at the crossroads of allergy and viral infections. J Leukoc Biol. 2015;98:185–194. doi: 10.1189/jlb.3RU0315-099R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention People with certain medical conditions. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available from:
- 8.National Health Service Who’s at higher risk from coronavirus. 2020. https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/ Available from:
- 9.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 12.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IntHout J., Ioannidis J.P., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.South A. rworldmap: a new R package for mapping global data. R J. 2011;3:35–43. [Google Scholar]
- 18.Mahdavinia M., Foster K.J., Jauregui E., Moore D., Adnan D., Andy-Nweye A.B. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020;8:2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovinsky-Desir S., Deshpande D.R., De A., Murray L., Stingone J.A., Chan A. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146:1027–1034.e4. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barroso B., Valverde-Monge M., Cañas J.A., Rodrigo-Muñoz J.M., Gonzalez-Cano B., Villalobos-Violan V. Presenting prevalence, characteristics and outcome of asthmatic patients with T2 diseases in hospitalized subjects with COVID-19 in Madrid, Spain. J Investig Allergol Clin Immunol. 2020;30:382–384. doi: 10.18176/jiaci.0627. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Zeng M, Wang H, Qin C, Hou HY, Sun ZY, et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19 [published online ahead of print July 27, 2020]. Allergy. https://doi.org/10.1111/all.14517. [DOI] [PubMed]
- 23.Beurnier A., Jutant E.M., Jevnikar M., Boucly A., Pichon J., Preda M. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56:2001875. doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cakebread J.A., Xu Y., Grainge C., Kehagia V., Howarth P.H., Holgate S.T. Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154.e9. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., Message S.D., Mallia P., Kebadze T., Contoli M., Ward C.K. Bronchial mucosal IFN-alpha/beta and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2019;143:114–125.e4. doi: 10.1016/j.jaci.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 27.Gill M.A., Bajwa G., George T.A., Dong C.C., Dougherty, Jiang N. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennington E. Asthma increases risk of severity of COVID-19. [published online ahead of print May 5, 2020]. Cleve Clin J Med. [DOI] [PubMed]
- 30.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59:78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanin M., Baviskar P., Webster R., Webby R. The interaction between respiratory pathogens and mucus. Cell Host Microbe. 2016;19:159–168. doi: 10.1016/j.chom.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma and expression of the SARS-CoV-2 receptor, ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heffler E., Detoraki A., Contoli M., Papi A., Paoletti G., Malipiero G. COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. [published online ahead of print August 1, 2020]. Allergy. [DOI] [PMC free article] [PubMed]
- 35.Haroun-Díaz E., Vázquez de la Torre M., Ruano F.J., Somoza Álvarez M.L., Alzate D.P., González P.L. Severe asthma during the COVID-19 pandemic: clinical observations. J Allergy Clin Immunol Pract. 2020;8:2787–2789. doi: 10.1016/j.jaip.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Chen J., Chen W., Liu L., Dong M., Ji J. Does asthma increase the mortality of patients with COVID-19? A systematic review and meta-analysis. Int Arch Allergy Immunol. 2020:1–7. doi: 10.1159/000510953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Ao G., Qi X., Xie B. The association between COVID-19 and asthma: a systematic review and meta-analysis. Clin Exp Allergy. 2020;50:1274–1277. doi: 10.1111/cea.13733. [DOI] [PubMed] [Google Scholar]
- 38.Hashim M.J., Alsuwaidi A.R., Khan G. Population risk factors for COVID-19 mortality in 93 countries. J Epidemiol Glob Health. 2020;10:204–208. doi: 10.2991/jegh.k.200721.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nussbaumer-Streit B., Klerings I., Dobrescu A.I., Persad E., Stevens A., Garritty C. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54. doi: 10.1016/j.jclinepi.2019.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.