Abstract
High-quality health care not only includes timely access to effective new therapies but timely abandonment of therapies when they are found to be ineffective or unsafe. Little is known about changes in use of medications after they are shown to be ineffective or unsafe. In this study, we examine changes in use of two medications: fenofibrate, which was found to be ineffective when used with statins among patients with Type 2 diabetes (ACCORD lipid trial); and dronedarone, which was found to be unsafe in patients with permanent atrial fibrillation (PALLAS trial). We examine the patient and provider characteristics associated with a decline in use of these medications.
Using Medicare fee-for-service claims from 2008–2013, we identified two cohorts: patients with Type 2 diabetes using statins (7 million patient-quarters), and patients with permanent atrial fibrillation (83 thousand patient-quarters). We used interrupted time-series regression models to identify the patient-and provider-level characteristics associated with changes in medication use after new evidence emerged for each case. After new evidence of ineffectiveness emerged, fenofibrate use declined by 0.01 percentage points per quarter (95% CI −0.02 to −0.01) from a baseline of 6.9 percent of all diabetes patients receiving fenofibrate; dronedarone use declined by 0.13 percentage points per quarter (95% CI −0.15 to −0.10) from a baseline of 3.8 percent of permanent atrial fibrillation patients receiving dronedarone. For dronedarone, use decline more quickly among patients dually-enrolled for Medicare and Medicaid compared to Medicare-only patients (p<0.001), among patients seen by male providers compared to female providers (p=0.01), and among patients seen by cardiologists compared to primary care providers (p<0.001).
Keywords: de-adoption, physician behavior, disparities
INTRODUCTION
High-quality health care not only includes timely access to effective new therapies but timely abandonment of therapies when they are found to be ineffective or unsafe. Little is known, however, about how quickly use of ineffective or unsafe therapies declines. Patterns of reduction in use of ineffective or unsafe therapies may not mirror patterns of adoption of effective therapies, since populations that receive low levels of effective care may not be the same ones that receive high levels of ineffective or unsafe care (Van Bodegom-Vos, Davidoff, and Marang-van de Mheen 2016; Davidoff 2015; Ubel and Asch 2015). Reversals of medical evidence are common, so it is imperative to understand whether reversals lead to disparities in ongoing use of unsafe or ineffective care, and the types of providers likely to respond to new evidence (Prasad et al. 2013).
Racial, socioeconomic and rural/urban disparities in use of effective health care treatments are well known, including differences in how quickly new therapies are adopted (Agency for Healthcare Research and Quality 2016; Groeneveld, Laufer, and Garber 2005b; Skinner et al. 2003; Weinstein et al. 2006). However, little evidence exists on whether disparities exist in how quickly use of unsafe or ineffective medical practices are reduced (Howard and Shen 2014; Kozhimannil et al. 2017; Mohan et al. 2014; Niven, Rubenfeld, et al. 2015; Qato et al. 2016). Several studies showed that in the Medicaid youth population, antidepressant use declined more among whites relative to blacks and Latinos in response to a Food and Drug Administration (FDA) black box warning on increased suicidal ideation (Carson et al. 2017; Cook et al. 2019; DePetris and Cook 2013).
There is mixed evidence about the role of provider characteristics in adoption and use of treatments, and even less is known about the provider characteristics associated with reductions in use of treatments (Karaca-Mandic, Town, and Wilcock 2016). Several studies pointed to provider age, specialty, and patient mix with varied findings (Bekelis et al. 2017; van Bodegom-Vos, Davidoff, and Marang-van de Mheen 2016; Cook et al. 2019; Howard, David, and Hockenberry 2017; Howard and Hockenberry 2019; Wallaert et al. 2016).
Using Medicare claims, we analyzed changes in use of two medications, fenofibrate and dronedarone, that were found ineffective and unsafe (respectively) after initially being approved by the FDA. We chose these two case studies for several reasons. First, the medications were found to be ineffective or unsafe in well-powered, randomized, double-blind, placebo controlled landmark trials. Second, the medications were relevant for diseases with important public health impact and costs, and for which disparities in outcomes and treatment are well-documented. Third, we required the relevant study populations and medications to be identifiable from claims data.
Fenofibrate, approved in 2001 to lower cholesterol, was found in the 2010 ACCORD lipid trial to be ineffective in reducing major adverse cardiac events when used in combination with statins among patients with Type 2 diabetes (Ginsburg, Elam, and Lovato 2010). Dronedarone, an antiarrhythmic drug approved in 2009 as an alternative to amiodarone, was found in the 2011 PALLAS trial to be unsafe for patients with permanent atrial fibrillation (permanent AF) due to increased risk of heart failure, stroke, and death from cardiovascular events (Connolly et al. 2011). We examined changes in use of these medications by patient race/ethnicity, socioeconomic status (SES), and geographic region; and by provider sex, provider age, and provider specialty.
METHODS
Study Population
We used administrative claims data from a 20% random sample of Medicare fee-for-service (FFS) beneficiaries age 65 and older with at least 12 consecutive months of enrollment in Medicare Parts A, B, and D (Centers for Medicare and Medicaid Services 2014).
For the fenofibrate case study, we constructed a cohort of patients with diabetes based on two or more outpatient claims or one or more inpatient claim for Type 2 Diabetes (DM2) over the previous two years relative to each index quarter from 2008 through 2013 (Centers for Medicare and Medicaid Services 2014). We identified additional DM2 patients by flagging the use of medications commonly used to treat DM2, following the Health Effectiveness Data and Information Set (HEDIS) diabetes algorithm (Centers for Medicare and Medicaid Services 2014). We further restricted the cohort to those using statins in a given quarter, identified using pharmacy claims in the Part D files. The resulting analytic dataset was at the patient-quarter level. The cohort sample was dynamic, meaning patients could move in and out of the cohort over the course of the study period based on whether or not they met the DM2 and statin-use inclusion criteria in each quarter.
For the dronedarone case study, we similarly constructed a dynamic cohort of patients with permanent AF. We used the CMS Chronic Conditions Warehouse (CCW) to identify patients with AF between 2009 and 2013. The cohort was restricted to patients with permanent AF based on the presence of more than 20 AF claims (the 90th percentile of the distribution of AF claims in the past year across all AF beneficiaries during the study period) and no evidence of attempts to restore sinus rhythm in the past year. As a sensitivity analysis, we varied this claims threshold and found no changes to our findings. Detailed definitions for each cohort are provided in Supplemental Digital Content tables A1 and A2.
Primary Outcomes
The primary outcome for the diabetes cohort was a dichotomous indicator for concurrent statin and fenofibrate therapy in a given patient-quarter (the unit of analysis). The indicator was equal to one if the patient had a supply of a fibrate (fenofibrate, fenofibric acid, or gemfibrozil) during that quarter and zero otherwise. For the cohort of patients with permanent AF, dronedarone use was defined similarly in each patient-quarter.
Patient Characteristics
We analyzed differences in reduction in use according to three patient characteristics: race and ethnicity, SES, and rurality. For race and ethnicity, we created indicators for race/ethnicity groups (non-Hispanic White, non-Hispanic Black, Hispanic, Asian, and other non-Hispanic/non-White) using the Research Triangle Institute (RTI) race codes in the Medicare beneficiary file (Eicheldinger and Bonito 2008). For SES, we used AHRQ’s composite SES index for Medicare beneficiaries. This index aggregates seven census block measures related to SES, including measures of employment, income, wealth, education, and crowding (Agency for Healthcare Research and Quality n.d.). We created a low-SES indicator for patients living in zip codes in the bottom quartile of this index. As a second proxy for SES, we used an indicator for dual enrollment in Medicare and Medicaid. For rurality, we created an indicator for whether the patient lived in a non-metropolitan county (i.e, rural county), according to rural-urban continuum codes in the Area Health Resource File (United States Department of Agriculture Economic Research Service 2018).
As additional covariates, patient age, sex, and comorbidities were taken from Medicare claims. We used comorbidities to construct a Charlson comorbidity index score for each patient (Stagg 2006).
Provider characteristics
We attributed each patient-year to a provider responsible for the patient’s care using a validated attribution algorithm based on the plurality of condition-specific Evaluation and Management visits in office settings, supplemented by medication refills (Higuera and Carlin 2017; Mehrotra et al. 2010; Pham et al. 2007). Details of the attribution algorithm are described in Supplemental Digital Content section A3.
To analyze changes in reduction use by provider characteristics, we obtained data on the attributed provider’s sex, age, and specialty from a comprehensive database assembled by Doximity that collects provider data from well-validated data sources such as the National Provider Identifier Registry and state medical boards. This database has been previously described and validated (Jena et al. 2015; Jena, Olenski, and Blumenthal 2016; Tsugawa et al. 2018). We created two dichotomous variables: an indicator for female providers and an indicator for providers age 65 or older. We identified the provider specialties representing at least five percent of patient-quarters in each cohort, and based on this created a categorical variable for specialty type: primary care (internal medicine, family practice, pediatrics, or nurse practitioner), cardiology, endocrinology (for the diabetes cohort only), and all other specialty types.
Statistical Analysis
The ACCORD lipid trial was published on April 29, 2010 and the PALLAS trial for permanent AF was published on November 14, 2011. The unit of analysis was patient-calendar quarter. We estimated changes in the level and trend in the use of both medications after evidence introduction using standard interrupted time series analysis (Kontopantelis et al. 2015; Penfold and Zhang 2013; Wagner et al. 2002). We then interacted both the level and the trend changes with a dichotomous variable for the patient or provider characteristic of interest to assess differences in changes by group. In sensitivity analyses, we excluded data from the quarters in which the trials were published and one quarter afterwards to avoid possible anticipatory effects or effects due to uncertainty in evidence introduction (Supplemental Digital Content Table A5.1). All models adjusted for the other patient and provider characteristics; standard errors were clustered at the patient level. Supplemental Digital Content section A4 provides details of the statistical specification.
To assess whether the changes in level and trend in use of medications were affected by changes in patient population after the trial publication, we also estimated the same models on a subset of patients with qualifying diagnoses before trial evidence introduction (prevalent cohort).
All analyses were performed with SAS software, Version 9.4 (Copyright © 2002–2012 SAS Institute Inc.) and Stata 14.2 (StataCorp, College Station, TX). The study was deemed exempt from review by the University of Minnesota Institutional Review Board because the data were de-identified.
RESULTS
Patient Cohort Characteristics
Among patients in the diabetes cohort, 7.1 million patient-quarters were analyzed during 2008–2013 from 808,449 unique patients. Overall, 74.5% were non-Hispanic White; 9.7% were non-Hispanic Black; 9.3% were Hispanic; 5.0% were Asian; and 1.5% were other non-Hispanic, non-White; 21.8% lived in a non-metropolitan county; and 29.8% lived in a socioeconomically disadvantaged zip code. Roughly 82.7% of patient-quarters were attributed to providers under the age of 65; 77.9% of attributed providers were male; 79.3% were primary care providers, 5.0% were cardiologists; and 7.0% were endocrinologists (Table I). The permanent AF cohort included 830 thousand patient-quarters during 2009–2013 from 114,535 unique patients. Of these patients, 91.4% were non-Hispanic White; 3.4% were non-Hispanic Black; 3.3% were Hispanic; 1.3% were Asian; and 0.6% were other non-Hispanic, non-White; 19.9% lived in a non-metropolitan county; and 19.9% lived in a socioeconomically disadvantaged zip code. Roughly 84.0% of patient-quarters were attributed to providers under the age of 65; 85.5% of attributed providers were male; 47.9% were primary care providers; and 47.2% were cardiologists (Table II).
Table I:
Type 2 Diabetes (DM2) Cohort Descriptive Statistics, 2008–2013 (N=7,081,113)
| Number bene-quarters (in millions) | Percent | |
|---|---|---|
| PATIENT CHARACTERISTICS | ||
| Age Group | ||
| 65–74 | 3.37 | 47.6 |
| 75–84 | 2.73 | 38.6 |
| 85+ | 0.97 | 13.8 |
| Sex | ||
| Male | 2.90 | 41.0 |
| Female | 4.18 | 59.0 |
| Non-white race/ethnicity | 1.81 | 25.5 |
| Black | 0.69 | 9.7 |
| Asian | 0.35 | 5.0 |
| Hispanic | 0.66 | 9.3 |
| Other non-Hispanic, non-white | 0.11 | 1.5 |
| Lives in socioeconomically disadvantaged zip code | 2.11 | 29.8 |
| Dually Eligible for Medicare & Medicaid | 2.46 | 34.8 |
| Lives in non-metropolitan county | 1.54 | 21.8 |
| Prescribed statins only | 6.60 | 93.2 |
| Prescribed statins & fibrates concurrently | 0.48 | 6.8 |
| Mean (Standard Deviation) | ||
| Charlson comorbidity score | 1.64 (2.00) | |
| PROVIDER CHARACTERISTICS | ||
| Provider age group | ||
| Under 65 | 5.85 | 82.7 |
| 65 or older | 1.23 | 17.3 |
| Provider sex | ||
| Male | 5.52 | 77.9 |
| Female | 1.56 | 22.1 |
| Provider Specialty | ||
| Primary Care | 5.62 | 79.3 |
| Cardiology | 0.35 | 5.0 |
| Endocrinology | 0.50 | 7.0 |
| All other specialties | 0.62 | 8.7 |
| FIBRATE USE | ||
| All years | 0.48 | 6.8 |
| 2008 | 0.04 | 6.4 |
| 2009 | 0.07 | 6.5 |
| 2010 | 0.08 | 6.9 |
| 2011 | 0.09 | 6.9 |
| 2012 | 0.10 | 6.8 |
| 2013 | 0.10 | 6.8 |
Table II:
Permanent Atrial Fibrillation Cohort Descriptive Statistics, 2009–2013 (N=830,392)
| Number bene-quarters (in millions) | Percent | |
|---|---|---|
| PATIENT CHARACTERISTICS | ||
| Age group | ||
| 65–74 | 0.19 | 23.0 |
| 75–84 | 0.37 | 44.9 |
| 85+ | 0.27 | 32.1 |
| Sex | ||
| Male | 0.32 | 38.1 |
| Female | 0.51 | 61.9 |
| Non-white race/ethnicity | 0.07 | 8.6 |
| Black | 0.03 | 3.4 |
| Asian | 0.01 | 1.3 |
| Hispanic | 0.03 | 3.3 |
| Other non-Hispanic, non-white | <0.01 | 0.6 |
| Lives in socioeconomically disadvantaged zip code | 0.16 | 19.9 |
| Dually Eligible for Medicare & Medicaid | 0.21 | 25.0 |
| Lives in non-metropolitan county | 0.17 | 19.9 |
| Mean (Standard Deviation) | ||
| Charlson comorbidity score | 1.97 (2.19) | |
| PROVIDER CHARACTERISTICS | ||
| Provider age group | ||
| Under 65 | 0.70 | 84.0 |
| 65 or older | 0.13 | 16.0 |
| Provider sex | ||
| Male | 0.71 | 85.5 |
| Female | 0.12 | 14.5 |
| Provider Specialty | ||
| Primary Care | 0.40 | 47.9 |
| Cardiology | 0.39 | 47.2 |
| All other specialties | 0.41 | 4.9 |
| DRONEDARONE USE | ||
| All years | 0.021 | 2.5 |
| 2009 | 0.000 | 0.0 |
| 2010 | 0.005 | 3.0 |
| 2011 | 0.006 | 3.8 |
| 2012 | 0.005 | 2.9 |
| 2013 | 0.005 | 2.5 |
Changes in Use over Time
Concurrent fenofibrate and statin therapy
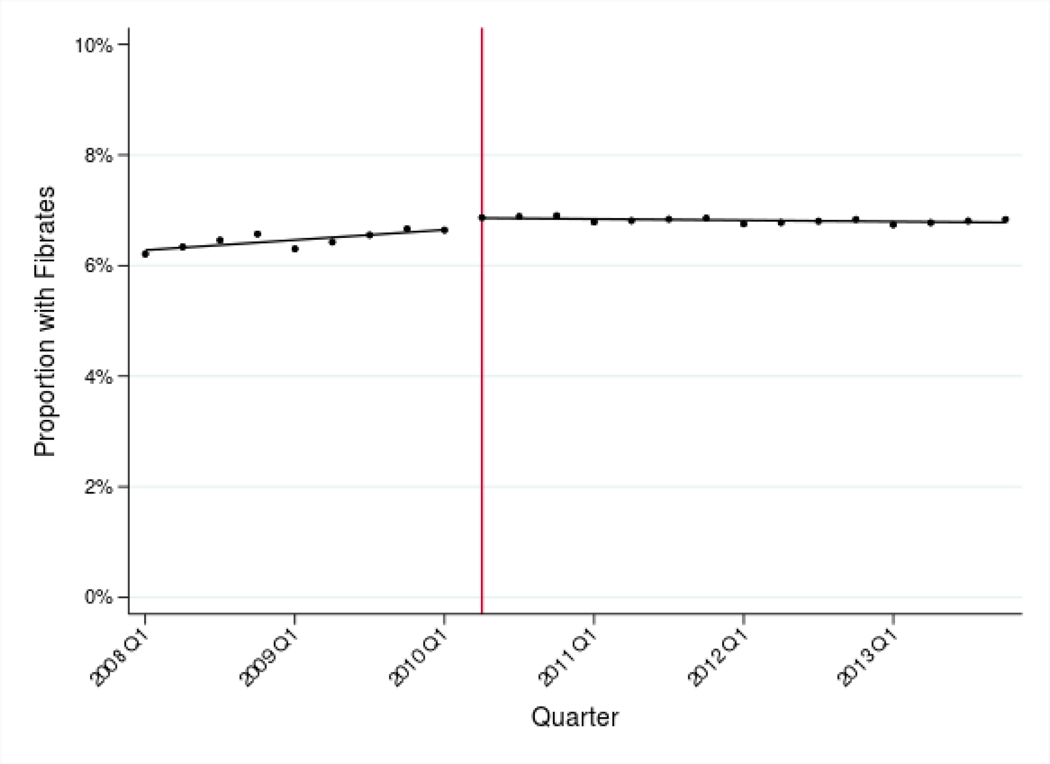
In the nine quarters before the ACCORD trial showed fenofibrate-statin combination therapy to be ineffective, the proportion of statin users prescribed fenofibrates steadily increased, from 6.4% of patients to 6.9% (Table I). Following the 2010 publication, adjusted use of concurrent statin and fenofibrate therapy decreased by 0.01 percentage points per quarter [95% CI −0.02 to −0.01] from Q2 2010 through Q4 2013. (Table III, Panel A and Figure I, Panel A).
Table III:
Trends in adjusted rates of fenofibrate and dronedarone use before and after evidence introduction, by patient and provider characteristics
| Panel A: Concurrent fenofibrate and statin therapy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | After-Before (Change in Trends) | After-Before (Change in Level) | |||||
| All patients in the cohort | 0.09 (0.07, 0.11) | −0.01 (−0.02, −0.01) | −0.10 (−0.12, −0.08) | 0.10 (0.03, 0.18) | ||||
|
Patient Characteristics | ||||||||
| Non-Hispanic White | 0.09 (0.07, 0.11) | −0.01 (−0.02, −0.00) | −0.10 (−0.13, −0.08) | 0.06 (−0.03, 0.16) | ||||
| Non-Hispanic Black | 0.06 (0.03, 0.08) | −0.01 (−0.02, −0.01) | −0.06 (−0.10, −0.03) | 0.03 (−0.11, 0.17) | ||||
| Diff. P-value | 0.054 | 0.38 | 0.05 | 0.69 | ||||
| Non-Hispanic White | 0.09 (0.07, 0.11) | −0.01 (−0.02, −0.00) | −0.10 (−0.13, −0.08) | 0.06 (−0.03, 0.16) | ||||
| Asian | 0.17 (0.10, 0.24) | −0.01 (−0.04, 0.02) | −0.18 (−0.26, −0.10) | 0.04 (−0.30, 0.38) | ||||
| Diff. P-value | 0.05 | 0.89 | 0.10 | 0.92 | ||||
| Non-Hispanic White | 0.09 (0.07, 0.11) | −0.01 (−0.02, −0.00) | −0.10 (−0.13, −0.08) | 0.10 (0.02, 0.17) | ||||
| Other non-Hispanic, non-white | 0.17 (0.04, 0.31) | −0.07 (−0.12, −0.01) | −0.24 (−0.39, −0.08) | −0.13 (−0.77, 0.52) | ||||
| Diff. P-value | 0.20 | 0.10 | 0.08 | 0.50 | ||||
| Non-Hispanic White | 0.09 (0.07, 0.11) | −0.01 (−0.02, −0.00) | −0.10 (−0.13, −0.08) | 0.06 (−0.03, 0.15) | ||||
| Hispanic | 0.11 (0.06, 0.16) | −0.01 (−0.04, 0.01) | −0.12 (−0.19, −0.06) | 0.31, (0.05, 0.57) | ||||
| Diff. P-value | 0.50 | 0.90 | 0.60 | 0.07 | ||||
| High socioeconomic status | 0.09 (0.07, 0.11) | −0.02 (−0.03, −0.01) | −0.11 (−0.14, −0.09) | 0.09 (−0.00, 0.18) | ||||
| Low socioeconomic status | 0.08 (0.05, 0.11) | 0.00 (−0.01, 0.02) | −0.08 (−0.11, −0.04) | 0.13 (−0.01, 0.27) | ||||
| Diff. P-value | 0.38 | 0.01 | 0.09 | 0.65 | ||||
| Non-Dual eligible patients | 0.08 (0.06, 0.10) | −0.01 (−0.01, 0.01) | −0.08 (−0.11, −0.06) | 0.09 (−0.01, 0.19) | ||||
| Dual eligible patients | 0.09 (0.06, 0.12) | −0.02 (−0.03, −0.01) | −0.11 (−0.15, −0.08) | 0.13 (0.00, 0.26) | ||||
| Diff. P-value | 0.43 | 0.04 | 0.14 | 0.61 | ||||
| Metropolitan | 0.09 (0.07, 0.11) | −0.02 (−0.03, −0.01) | −0.11 (−0.13, −0.08) | 0.10 (0.02, 0.19) | ||||
| Non-Metropolitan | 0.09 (0.05, 0.13) | −0.01 (−0.02, 0.01) | −0.09 (−0.14, −0.05) | 0.10 (−0.01, 0.27) | ||||
| Diff. P-value | 0.93 | 0.31 | 0.64 | 1.00 | ||||
|
Provider Characteristics | ||||||||
| Male provider | 0.09 (0.07, 0.11) | −0.01 (−0.02, 0.00) | −0.10 (−0.12, −0.08) | 0.09 (−0.00, 0.18) | ||||
| Female provider | 0.09 (0.05, 0.13) | −0.04 (−0.05, −0.02) | −0.12 (−0.17, −0.08) | 0.16 (−0.17, 0.33) | ||||
| Diff. P-value | 0.94 | 0.01 | 0.29 | 0.48 | ||||
| Provider under 65 | 0.10 (0.08, 0.12) | −0.01 (−0.02, −0.01) | −0.12 (−0.14, −0.09) | 0.07 (−0.02, 0.16) | ||||
| Provider 65 or over | 0.04 (0.01, 0.08) | −0.02 (−0.03, 0.00) | −0.06 (−0.10, −0.01) | 0.22 (0.05, 0.39) | ||||
| Diff. P-value | 0.003 | 0.89 | 0.02 | 0.15 | ||||
| Cardiologist | 0.00 (−0.11, 0.11) | −0.03 (−0.07, 0.01) | −0.03 (−0.15, 0.09) | 0.10 (−0.31, 0.51) | ||||
| Primary care provider | 0.10 (0.08, 0.12) | −0.02 (−0.03, −0.01) | −0.12 (−0.14, −0.09) | 0.10 (0.02, 0.19) | ||||
| Diff. P-value | 0.08 | 0.49 | 0.16 | 0.98 | ||||
| Endocrinologist | 0.23 (0.13, 0.32) | −0.06 (−0.11, −0.01) | −0.29 (−0.40, −0.18) | 0.21 (0.85, 2.65) | ||||
| Primary care provider | 0.10 (0.08, 0.12) | −0.02 (−0.03, −0.01) | −0.12 (−0.14, −0.09) | 0.10 (0.02, 0.19) | ||||
| Diff. P-value | 0.01 | 0.06 | 0.00 | 0.65 | ||||
| Other specialty types | 0.05 (−0.01, 0.10) | 0.02 (−0.01, 0.05) | −0.03 (−0.09, 0.04) | 0.02 (−0.26, 0.30) | ||||
| Primary care provider | 0.10 (0.08, 0.12) | −0.02 (−0.03, −0.01) | −0.12 (−0.14, −0.09) | 0.10 (0.02, 0.19) | ||||
| Diff. P-value | 0.09 | 0.01 | 0.02 | 0.60 | ||||
| Panel B: Dronedarone | ||||||||
| Before | After | After-Before (Change in Trends) | After-Before (Change in Level) | |||||
| All patients in the cohort | 0.47 (0.45, 0.49) | −0.13 (−0.15, −0.10) | −0.59 (−0.64, −0.55) | −1.64 (−1.80, −1.48) | ||||
|
Patient Characteristics | ||||||||
| Non-Hispanic White | 0.47 (0.45, 0.49) | −0.12 (−0.15, −0.09) | −0.59 (−0.63, −0.55) | −1.64 (−1.81, −1.48) | ||||
| Non-Hispanic Black | 0.43 (0.32, 0.54) | −0.20 (−0.33, −0.06) | −0.63 (−0.84, −0.42) | −1.33 (−2.14, −0.52) | ||||
| Diff. P-value | 0.51 | 0.27 | 0.72 | 0.46 | ||||
| Non-Hispanic White | 0.47 (0.45, 0.49) | −0.12 (−0.15, −0.09) | −0.59 (−0.63, −0.55) | −1.64 (−1.81, −1.48) | ||||
| Asian | 0.63 (0.43, 0.83) | −0.28 (−0.51, −0.06) | −0.91 (−1.29, −0.54) | −2.68 (−4.23, −1.14) | ||||
| Diff. P-value | 0.13 | 0.15 | 0.09 | 0.19 | ||||
| Non-Hispanic White | 0.47 (0.45, 0.49) | −0.12 (−0.15, −0.10) | −0.59 (−0.63, −0.55) | −1.64 (−1.79, −1.48) | ||||
| Other non-Hispanic, non-white | 0.55 (0.28, 0.83) | −0.25 (−0.54, 0.05) | −0.80 (−1.28, −0.33) | −2.24 (−4.43, −0.05) | ||||
| Diff. P-value | 0.55 | 0.41 | 0.39 | 0.59 | ||||
| Non-Hispanic White | 0.47 (0.45, 0.49) | −0.12 (−0.15, −0.09) | −0.59 (−0.63, −0.55) | −1.64 (−1.81, −1.48) | ||||
| Hispanic | 0.42 (0.31, 0.53) | −0.13 (−0.28, 0.01) | −0.55 (−0.77, −0.33) | −1.33 (−2.18, −0.48) | ||||
| Diff. P-value | 0.35 | 0.86 | 0.73 | 0.48 | ||||
| High socioeconomic status | 0.47 (0.45, 0.50) | −0.13 (−0.16, −0.10) | −0.60 (−0.65, −0.55) | −1.64 (−1.82, −1.47) | ||||
| Low socioeconomic status | 0.46 (0.42, 0.51) | −0.11 (−0.17, −0.05) | −0.58 (−0.67, −0.49) | −1.63 (−1.99, −1.26) | ||||
| Diff. P-value | 0.76 | 0.71 | 0.69 | 0.94 | ||||
| Non-Dual eligible patients | 0.51 (0.48, 0.54) | −0.15 (−0.18, −0.11) | −0.66 (−0.71, −0.61) | −1.69 (−1.88, −1.50) | ||||
| Dual-eligible patients | 0.36 (0.32, 0.39) | −0.08 (−0.12, −0.03) | −0.43 (−0.50, −0.36) | −1.49 (−1.77, −1.22) | ||||
| Diff. P-value | P<0.001 | 0.02 | P<0.001 | 0.25 | ||||
| Metropolitan | 0.47 (0.45, 0.50) | −0.12 (−0.16, −0.09) | −0.60 (−0.64, −0.55) | −1.62 (−0.12, −1.44) | ||||
| Non-Metropolitan | 0.46 (0.41, 0.50) | −0.13 (−0.18, −0.07) | −0.58 (−0.67, −0.49) | −1.73 (−2.07, −1.38) | ||||
| Diff. P-value | 0.49 | 0.98 | 0.74 | 0.59 | ||||
|
Provider Characteristics | ||||||||
| Male provider | 0.49 (0.46, 0.51) | −0.13 (−0.16, −0.10) | −0.62 (−0.66, −0.57) | −1.70 (−1.87, −1.52) | ||||
| Female provider | 0.38 (0.33, 0.43) | −0.10 (−0.17, −0.03) | −0.48 (−0.58, −0.38) | −1.31 (−1.69, −0.92) | ||||
| Diff. P-value | P<0.001 | 0.42 | 0.01 | 0.07 | ||||
| Provider under 65 | 0.49 (0.46, 0.51) | −0.13 (−0.16, −0.10) | −0.61 (−0.66, −0.57) | −1.73 (−1.91, −1.54) | ||||
| Provider 65 or over | 0.40 (0.36, 0.45) | −0.13 (−0.18, −0.06) | −0.53 (−0.62, −0.44) | −1.29 (−1.64, −0.95) | ||||
| Diff. P-value | 0.001 | 0.97 | 0.10 | 0.03 | ||||
| Cardiologist | 0.70 (0.66, 0.73) | −0.19 (−0.24, −0.15) | −0.89 (−0.96, −0.82) | −2.47 (−2.77, −2.18) | ||||
| Primary care provider | 0.28 (0.25, 0.30) | −0.06 (−0.09, −0.03) | −0.34 (−0.38, −0.29) | −0.94 (−1.12, −0.76) | ||||
| Diff. P-value | P<0.001 | P<0.001 | P<0.001 | P<0.001 | ||||
| Other specialty types | 0.26 (0.19, 0.33) | −0.09 (−0.18, −0.01) | −0.35 (−0.48, −0.21) | −0.83 (−1.44, −0.22) | ||||
| Primary care provider | 0.28 (0.25, 0.30) | −0.06 (−0.09, −0.03) | −0.34 (−0.38, −0.29) | −0.94 (−1.12, −0.76) | ||||
| Diff. P-value | 0.68 | 0.61 | 0.89 | 0.73 | ||||
Notes: Estimates represent linear time trends in the proportion of patient-quarters in the diabetes/permanent AF cohorts using fenofibrates/dronedarone. 95% confidence intervals reported in parentheses. Models adjusted for patient characteristics (race/ethnicity, SES, rurality, age, sex, and Charlson comorbidity index) and provider characteristics (provider age, sex, and specialty). All models were estimated using multivariate linear regression. The “Diff. P-value” rows provide p-values for a test that the time trends or level differences are different for the two patient subgroups. For Panel A, “Before” refers to Q1 2008-Q1 2009, prior to evidence from the ACCORD trial on April 29, 2010 declaring fenofibrate-statin combination therapy to be ineffective, and “After” refers to Q2 2010-Q4 2013. For Panel B, “Before” refers to Q1 2009-Q3 2011, prior to evidence from the PALLAS trial on November 14, 2011 declaring dronedarone to be unsafe, and “After” refers to Q4 2012-Q4 2013.
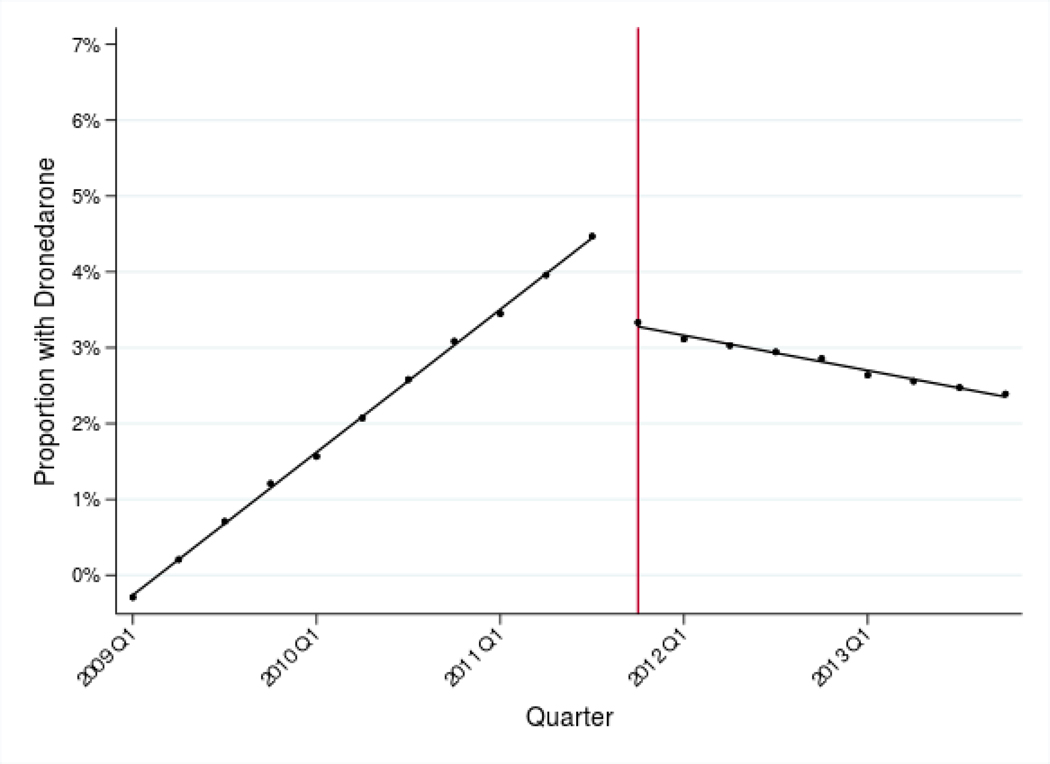
Figure I: Adjusted use of fenofibrates and dronedarone before and after new evidence.
Panel A: Adjusted use of fenofibrates, 2008–2013
Panel B: Adjusted use of dronedarone, 2009–2013
Notes: Estimates represent fitted values from multivariate regression models that adjust for patient race/ethnicity, SES, rurality, age, sex, and Charlson comorbidity index, and for provider age, sex, and specialty. The vertical lines indicate when the de-adoption evidence was introduced (April 2010 for fenofibrates and November 2011 for dronedarone).
Dronedarone
From dronedarone’s initial FDA approval, its use increased among permanent AF patients and reached a peak of 3.8% of patients in 2011 (Table II). Following the PALLAS trial published in November of 2011, adjusted dronedarone use decreased by 0.13 percentage points per quarter (95% CI −0.15 to −0.10) from Q4 2011 through Q4 2013 (Table III, Panel B and Figure I, Panel B).
Changes in Trends of Use by Race/Ethnicity, Socioeconomic Status and Rurality
Concurrent fenofibrate and statin therapy (Table III, Panel A)
Before the ACCORD lipid trial, fenofibrate gradually increased for all patient sub-groups (Non-Hispanic White/Non-White, High/Low socio-economic status, Metropolitan/Non-Metropolitan). Following trial publication, use declined modestly in each sub-group, with a statistically significant change in trend following the new evidence (Non-Hispanic White: −0.10 percentage points [95% CI −0.13 to −0.08]; Non-Hispanic Black: −0.06 percentage points [95% CI −0.10 to −0.03]; Hispanic: −0.12 [95% CI −0.19 to −0.06]; Asian: −0.18 [95% CI −0.26 to −0.10]; other non-Hispanic, non-White: −0.24 [95% CI −0.39 to −0.08]; High-SES: −0.11 percentage points [95% CI −0.14 to −0.09] and Low-SES: −0.08 percentage points [95% CI −0.11 to −0.08]; Dual-enrolled: −0.11 [95% CI −0.15 to −0.08]; non-dual-enrolled: −0.08 [95% CI −0.11 to −0.06]; Metropolitan: −0.11 percentage points per quarter [95% CI −0.13 to −0.08]; Non-Metropolitan: −0.11 percentage points per quarter [95% CI −0.14 to −0.05]). However, the change in trends did not differ significantly between the sub-groups (non-Hispanic Black/non-Hispanic White: P=0.05; Hispanic/non-Hispanic White: P=0.60; Asian/non-Hispanic White: P=0.10; Other non-Hispanic, non-White/non-Hispanic White: P=0.08; socioeconomic status: P=0.09; dual eligibility: P=0.14; rurality: P=0.64).
Dronedarone (Table III, Panel B)
Use of dronedarone increased steadily before the PALLAS trial, and declined modestly after trial publication in each sub-group. The change in trends following trial publication was statistically significant within each group (Non-Hispanic White: −0.59 percentage points [95% CI −0.63 to −0.55]; Non-Hispanic Black: −0.63 percentage points [95% CI −0.84 to −0.42]; Hispanic: −0.55 [95% CI −0.77 to −0.33]; Asian: −0.87 [95% CI −1.27 to −0.47]; other non-Hispanic, non-White: −0.69 [95% CI −1.21 to −0.18]; High-SES: −0.55 percentage points [95% CI −0.59 to − 0.50]; Low-SES: −0.53 percentage points [95% CI −0.63 to −0.44]; Dual-enrolled: −0.43 [95% CI −0.50 to −0.36]; non-dual-enrolled: −0.66 [95% CI −0.71 to −0.6]; Metropolitan: −0.60 percentage points [95% CI −0.64 to −0.55]; Non-Metropolitan: −0.58 percentage points [95% CI − 0.67 to −0.49]). The change in trends was significantly larger for patients dually-enrolled in Medicare and Medicaid compared to non-dually enrolled patients (P<0.001). The change in trends was not different between any other two groups (P=0.72; P=0.73; P=0.09; P=0.39; P=0.69, P=0.74 for non-Hispanic Black, Hispanic, Asian, other non-Hispanic non-White [each relative to non-Hispanic White], socioeconomic status, and rurality, respectively).
Additional analyses conducted on a subset of our study cohort using the drug before the trial was published (prevalent cohort) revealed similar results to those discussed above across all specifications (Supplemental Digital Content Table A5.2).
Changes in Trends of Use by Provider Characteristics
Concurrent fenofibrate and statin therapy (Table III, Panel A)
Before the ACCORD lipid trial, fenofibrate use increased very gradually, or remained constant, for patients attributed to each provider characteristic category (sex, age, and specialty). Following the trial’s publication, use declined modestly, with a statistically significant change in trend after the publication of new evidence among all provider categories except cardiologists, for whom there was no significant change in the trend (Male: −0.10 percentage points [95% CI − 0.12 to −0.08] and Female: −0.12 percentage points [95% CI −0.17 to −0.08]; Under 65: −0.12 percentage points [95% CI −0.14 to −0.09] and 65+: −0.06 percentage points [95% CI −0.10 to − 0.01]; Cardiologists: −0.03 percentage points [95% CI −0.15 to 0.09]; PCPs: −0.12 percentage points [95% CI −0.14 to −0.09]; Endocrinologists: −0.b percentage points [95% CI −0.40 to −0.18]; Other Specialists: −0.03 [95% CI −0.09 to −0.04]). There were no significant differences in the changes in trends by provider sex (P=0.29). Providers under 65 had a larger change in trend relative to providers age 65+, but they also had a steeper positive trend before the evidence reversal. Endocrinologists had a larger change in trend relative to PCPs (P=0.00), but PCPs had a larger change in trend relative to all other specialty types (P=0.02). There were no significant differences between PCPs and cardiologists (P=0.16).
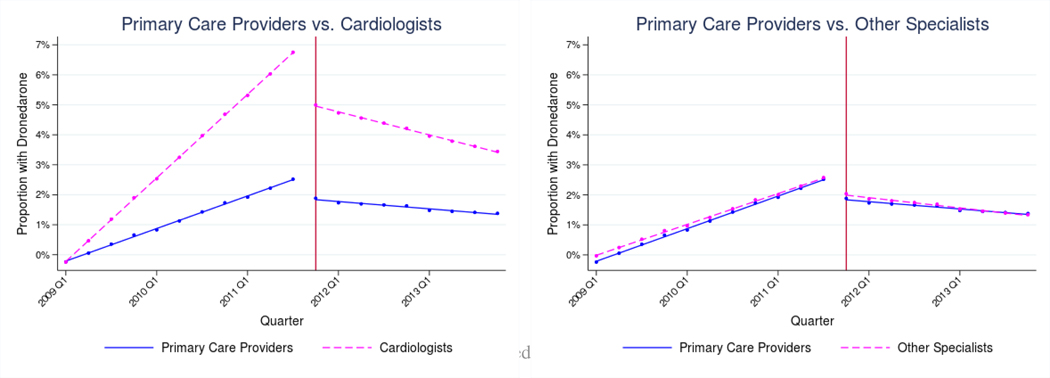
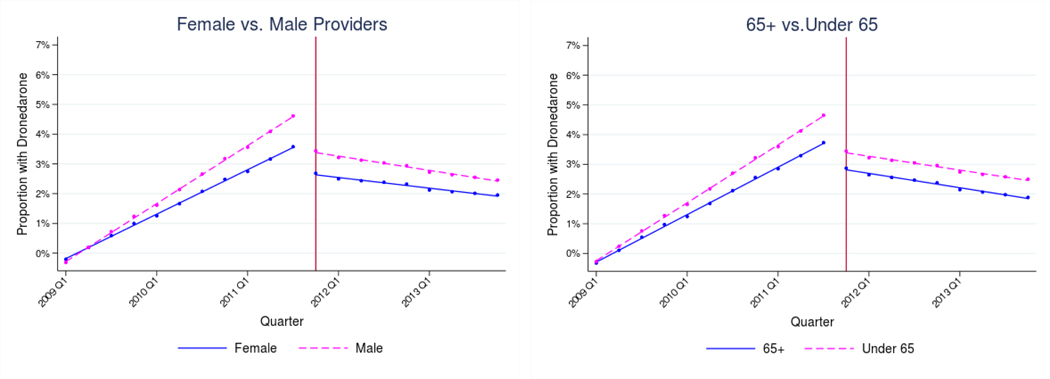
Dronedarone (Table III, Panel B and Figure II)
Figure II: Adjusted use of Dronedarone by provider characteristics, 2009–2013.
Notes: Estimates represent fitted values from multivariate regression models that adjust for patient race/ethnicity, SES, rurality, age, sex, and Charlson comorbidity index, and for provider age, sex, and specialty. The vertical lines indicate when the de-adoption evidence was introduced (November 2011).
Among all providers, use of dronedarone increased steadily prior to the PALLAS trial and declined after its publication. The change in trends following trial publication was statistically significant within each provider category (Male: −0.62 percentage points [95% CI − 0.66 to −0.57] and Female: −0.48 percentage points [95% CI −0.58 to −0.38]; Under 65: −.61 percentage points [95% CI −0.66 to −0.57] and 65+: −0.53 percentage points [95% CI −0.62 to − 0.44]; Cardiologists: −0.89 percentage points per quarter [95% CI −0.96 to −0.82]; PCPs: −0.34 percentage points per quarter [95% CI −0.38 to −0.29]; Other Specialists: −0.35 [95% CI −0.48 to −0.21]). The change in trends was larger among male providers relative to female (P=0.01). There were no significant differences in the changes in trends by provider age group (P=0.10), but providers under age 65 did have a larger immediate level-shift at the time of the new evidence (Under 65: −1.73 percentage points [95% CI −1.91 to −1.54] and 65+: −1.29 percentage points [95% CI −1.64 to −0.95]; P=0.03). The change in trends was larger among cardiologists relative to PCPs (P<0.001), and cardiologists also had a larger immediate level-shift (Cardiologists: −2.47 percentage points per quarter [95% CI −2.77 to −2.18]; PCPs: − 0.94 percentage points per quarter [95% CI −1.12 to −0.76]; P<0.001). There was no significant difference between among non-cardiology specialists and PCPs (P=0.89) (Figure II).
DISCUSSION
The change in use of treatments in response to evidence about ineffectiveness or safety has received little attention in the clinical literature, and this is one of the first studies to analyze the patient and provider characteristics associated with changes in use (Bekelis et al. 2017). In a national random sample of Medicare FFS beneficiaries, we found that the use of two medications that were ultimately found to be ineffective or unsafe – fenofibrate and dronedarone – decreased after evidence emerged about their ineffectiveness or lack of safety. Reduction in use of fenofibrate was overall very modest. The most notable differences by patient and provider characteristics were found in the reduction of dronedarone, according to patients’ dual enrollment status, provider sex, and provider specialty. Specifically, dronedarone was de-adopted faster among patients not dually-enrolled in Medicare and Medicaid compared to patients dually-enrolled, among patients seen by male providers compared to female, and among patients treated by cardiologists compared to PCPs.
Our findings suggest that reductions in use may vary significantly depending on the type of medication, and whether new evidence found the medication to be ineffective versus unsafe. We found overall very modest reduction in use of fenofibrates (found to be ineffective), but more significant reductions in use of dronedarone (found to be unsafe). We did not find evidence of disparities according to patient race or ethnicity, inconsistent with prior case studies in de-adoption (Carson et al. 2017; Cook et al. 2019; DePetris and Cook 2013). However, we did find that patients dually-enrolled for Medicare and Medicaid experienced slower reductions in use of the dronedarone, suggesting there may be socioeconomic disparities – consistent with prior work (Qato et al. 2016). Our most notable finding was that reduction in use of dronedarone was faster among cardiologists, the specialty most commonly treating patients with permanent AF. This suggests cardiologists had more information about the new evidence – perhaps disseminated to them through a specialty association – and/or were more likely to act on new information compared to PCPs. Cardiologists also had the highest adoption rates before the Black Box warning, consistent with them being more responsive to new information.
Identifying the characteristics driving reduction in use is critical for several reasons. First, understanding how information regarding the effectiveness and/or safety of existing practices affects clinical practice contributes to a better understanding of how the health care system can address safety and outcomes disparities. Second, identifying patient and provider characteristics associated with faster or slower response to clinical evidence may inform policy and organizational strategies to promote timely and equitable reduction in use (Niven, Mrklas, et al. 2015). Third, disparities in care may relate to where patients receive care; a growing literature finds care for minority and lower socioeconomic status patients may be clustered among providers and facilities of lower quality and weaker credentials (Anderson et al. 2014; Bach et al. 2004; Barnato et al. 2005; Girotti et al. 2014; Groeneveld, Laufer, and Garber 2005a; Khera et al. 2015; Reschovsky and O’Malley 2008; Rhoads et al. 2015; Rothenberg et al. 2004; Tsai, Orav, and Joynt 2014). Documenting differences in response to evidence on ineffectiveness and safety could improve our understanding of whether racial and ethnic minorities and lower socioeconomic status patients are clustered among a group of lower quality providers and facilities, and how this may contribute to disparities.
Our study had several limitations. First, this was a retrospective, observational study using administrative data; our findings reflect associations, not causal relationships. Second, patient populations were identified using diagnoses codes and medication use in administrative claims, which may not match a gold-standard population definition. Third, our analysis was limited to Medicare FFS beneficiaries – an important population with a high burden of morbidity and mortality – but the findings may not be the same in other groups. Finally, other factors – such as medication costs, patient preferences, and providers’ clinical assessments of the treatment effectiveness for patients – likely influence use of medications, and we are unable to adjust for these factors.
Future work should consider other medications and other patient populations, including Medicare Advantage beneficiaries and commercially insured patients. Further, while this study focuses on differences based on patient and provider characteristics, future research should investigate other potential mechanisms that may affect changes in use of medications in response to evidence. Other potential mechanisms include the role of specialty organizations, pharmaceutical sales representatives, and continuing medical education in disseminating information to providers; health care delivery organization factors; and market-level factors. In summary, after evidence emerged about medications being ineffective or harmful, we found use of the ineffective medication declined very modestly among Medicare patients, and we found no evidence of racial or ethnic, socioeconomic, or rural/urban disparities in reductions in use. Use of the unsafe medication declined more substantially, but there was some evidence of differences in de-adoption according to patient socioeconomic status, provider sex, and provider specialty. These results suggest de-adoption may look different on a case-by-case basis, and more work is needed to promote timely reduction in use of ineffective and unsafe medications for all patients.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported a pilot grant by the National Institute on Aging of the National Institutes of Health under Award Number P01AG005842. We also acknowledge support from the National Heart, Lung, and Blood Institute (R56 HL130496) and Agency for Healthcare Research and Quality (R01 HS025164). Laura Barrie Smith and Alexander Everhart and also acknowledge support from the AHRQ doctoral training program at the University of Minnesota (T32 HS000036). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by AHRQ’s Comparative Health System Performance Initiative under Grant # 1U19HS024075, which studies how health care delivery systems promote evidence-based practices and patient-centered outcomes research in delivering care.
Disclosures: In the past 36 months, Dr. Shah has received research support through Mayo Clinic from the Food and Drug Administration to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Centers of Medicare and Medicaid Innovation under the Transforming Clinical Practice Initiative (TCPI), from the Agency for Healthcare Research and Quality (R01HS025402; R03HS025517), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HL131535), National Science Foundation, and from the Patient Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). Support to Dr. Jena was provided by the Office of the Director, National Institutes of Health (1DP5OD017897). Dr. Jena reports receiving consulting fees unrelated to this work from Pfizer, Hill Rom Services, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Biogen, Precision Health Economics, and Analysis Group. Dr. Ross has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from Medtronic, Inc. and the Food and Drug Administration (FDA) to develop methods for postmarket surveillance of medical devices (U01FD004585), from the Food and Drug Administration to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Blue Cross Blue Shield Association to better understand medical technology evaluation, from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting (HHSM-500–2013-13018I), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale. Dr. Herrin has recieved additional support for unrelated research from the Centers for Medicare and Medicaid Services and Mayo Clinic. Mr. Everhart and Mr. Higuera are paid research fellows at Medtronic for unrelated projects. Dr. Jeffery is supported by two grants that funded this study (NIH/R56 HL130496 and Agency for Healthcare Research and Quality/R01 HS025164); by an American Cancer Society funded study on biosimilar drug uptake (131611-RSGI-17–154-01-CPHPS); by the CERSI (U01FD 05938) for a project addressing unsafe prescribing of opioids subject to REMS; and by NHLBI for a project on step-down of asthma biologics (R21HL 140287). She has additional unrelated funding from NCATS (UL1TR 02377) and NIDA (UG3DA047003). Dr. Karaca-Mandic serves as the Principal Investigator to grants that funded this study (NIA/P01AG005842; NIH/R56 HL130496 and Agency for Healthcare Research and Quality/R01 HS025164). She is also the Principal Investigator to an American Cancer Society funded study on biosimilar drug uptake (131611-RSGI-17–154-01-CPHPS). In the past 36 months, she reports receiving consulting fees unrelated to this work from Tactile Medical and Precision Health Economics.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Laura Barrie Smith, Division of Health Policy and Management, University of Minnesota School of Public Health.
Nihar R. Desai, Cardiovascular Medicine, Yale School of Medicine; Center for Outcomes Research and Evaluation, Yale School of Medicine.
Bryan Dowd, Division of Health Policy and Management, University of Minnesota School of Public Health.
Alexander Everhart, Division of Health Policy and Management, University of Minnesota School of Public Health.
Jeph Herrin, Department of Health Sciences Research, Mayo Clinic.
Lucas Higuera, Division of Health Policy and Management, University of Minnesota School of Public Health.
Molly Moore Jeffery, Department of Health Sciences Research, Mayo Clinic; Emergency Medicine Research, Mayo Clinic.
Anupam B. Jena, Department of Health Care Policy, Harvard Medical School; Department of Medicine, Massachusetts General Hospital; National Bureau of Economic Research, Cambridge, MA.
Joseph S. Ross, General Internal Medicine, Yale School of Medicine; Center for Outcomes Research and Evaluation, Yale School of Medicine; Health Policy and Management, Yale School of Public Health.
Nilay D. Shah, Department of Health Sciences Research, Mayo Clinic.
Pinar Karaca-Mandic, National Bureau of Economic Research, Cambridge, MA; Carlson School of Management, University of Minnesota.
References
- Agency for Healthcare Research and Quality. 2016. 2016 National Healthcare Quality and Disparities Report. Content Last Reviewed June 2018. Rockville, MD. [Google Scholar]
- Agency for Healthcare Research and Quality. “Creation of New Race-Ethnicity Codes and SES Indicators for Medicare Beneficiaries -Chapter 3: Creating and Validating an Index of Socioeconomic Status. Publication # 08–0029-EF.” https://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators3.html#sect3.3 (June 29, 2018).
- Anderson Ryan E., Ayanian John Z., Zaslavsky Alan M., and Michael McWilliams J.. 2014. “Quality of Care and Racial Disparities in Medicare Among Potential ACOs.” Journal of General Internal Medicine 29(9): 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Peter B. et al. 2004. “Primary Care Physicians Who Treat Blacks and Whites.” New England Journal of Medicine 351(6): 575–84. [DOI] [PubMed] [Google Scholar]
- Barnato Amber E et al. 2005. “Hospital-Level Racial Disparities in Acute Myocardial Infarction Treatment and Outcomes.” Medical care 43(4): 308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekelis Kimon, Skinner Jonathan, Gottlieb Daniel, and Goodney Philip. 2017. “De-Adoption and Exnovation in the Use of Carotid Revascularization: Retrospective Cohort Study.” BMJ (Clinical research ed.) 359: j4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegom-Vos Leti, Davidoff Frank, and Marang-van de Mheen Perla J. 2016. “IImplementation and De-Implementation: Two Sides of the Same Coin?” BMJ quality & safety. [DOI] [PubMed] [Google Scholar]
- Van Bodegom-Vos Leti, Davidoff Frank, and Marang-van de Mheen Perla J. 2016. “Implementation and De-Implementation: Two Sides of the Same Coin?” BMJ quality & safety 0: 1–7. [DOI] [PubMed] [Google Scholar]
- Carson Nicholas J., Progovac Ana M., Wang Ye, and Cook Benjamin L.. 2017. “A Decline in Depression Treatment Following FDA Antidepressant Warnings Largely Explains Racial/Ethnic Disparities in Prescription Fills.” Depression and Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. 2014. “Quality Rating System Measure Technical Specifications.” : 1–181. [Google Scholar]
- Connolly Stuart J et al. 2011. “Dronedarone in High-Risk Permanent Atrial Fibrillation for the PALLAS Investigators*.” N Engl J Med 365: 2268–76. [DOI] [PubMed] [Google Scholar]
- Cook Benjamin L. et al. 2019. “Assessing Provider and Racial/Ethnic Variation in Response to the FDA Antidepressant Box Warning.” Health Services Research 54: 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff Frank. 2015. “On the Undiffusion of Established Practices.” JAMA Internal Medicine 175(5): 809. [DOI] [PubMed] [Google Scholar]
- DePetris Andrea Elizabeth, and Cook Benjamin L 2013. “Differences in Diffusion of FDA Antidepressant Risk Warnings across Racial-Ethnic Groups.” Psychiatric services (Washington, D.C.) 64(5): 466–71, 471.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicheldinger Celia, and Bonito Arthur. 2008. “More Accurate Racial and Ethnic Codes for Medicare Administrative Data.” Health Care Financing Review 29(3): 27–42. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg Henry N., Elam Marshall B., and Lovato Laura C.. 2010. “Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus.” New England Journal of Medicine 362(17): 1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti Micah E., Shih Terry, Sha’Shonda Revels, and Dimick Justin B.. 2014. “Racial Disparities in Readmissions and Site of Care for Major Surgery.” Journal of the American College of Surgeons 218(3): 423–30. [DOI] [PubMed] [Google Scholar]
- Groeneveld Peter W, Laufer Sara B, and Garber Alan M. 2005a. 43 Care Technology Diffusion, Hospital Variation, and Racial Disparities among Elderly Medicare. [DOI] [PubMed] [Google Scholar]
- Groeneveld Peter W, Laufer Sara B, and Garber Alan M. 2005b. “Technology Diffusion, Hospital Variation, and Racial Disparities among Elderly Medicare Beneficiaries 1989–2000.” Medical Care 43(4): 320–29. [DOI] [PubMed] [Google Scholar]
- Higuera Lucas, and Carlin Caroline. 2017. “A Comparison of Retrospective Attribution Rules.” The American Journal of Managed Care 23(6): e180–85. [PubMed] [Google Scholar]
- Howard David H., David Guy, and Hockenberry Jason. 2017. “Selective Hearing: Physician-Ownership and Physicians’ Response to New Evidence.” Journal of Economics & Management Strategy 26(1): 152–68. [Google Scholar]
- Howard David H., and Hockenberry Jason. 2019. “Physician Age and the Abandonment of Episiotomy.” Health Services Research 54(3): 650–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard David H., and Shen Yu-Chu. 2014. “Trends in PCI Volume after Negative Results from the COURAGE Trial.” Health Services Research 49(1): 153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena Anupam B. et al. 2015. “Sex Differences in Academic Rank in US Medical Schools in 2014.” JAMA 314(11): 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena Anupam B., Olenski Andrew R., and Blumenthal Daniel M.. 2016. “Sex Differences in Physician Salary in US Public Medical Schools.” JAMA Internal Medicine 176(9): 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca-Mandic Pinar, Town Robert J., and Wilcock Andrew. 2016. “The Effect of Physician and Hospital Market Structure on Medical Technology Diffusion.” Health Services Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera Rohan, Mary Vaughan-Sarrazin Gary E. Rosenthal, and Girotra Saket. 2015. “Racial Disparities in Outcomes After Cardiac Surgery: The Role of Hospital Quality.” Current Cardiology Reports 17(5): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis Evangelos et al. 2015. “Regression Based Quasi-Experimental Approach When Randomisation Is Not an Option: Interrupted Time Series Analysis.” BMJ (Clinical research ed.) 350: h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil Katy B. et al. 2017. “Uptake and Utilization of Practice Guidelines in Hospitals in the United States: The Case of Routine Episiotomy.” The Joint Commission Journal on Quality and Patient Safety 43(1): 41–48. [DOI] [PubMed] [Google Scholar]
- Mehrotra Ateev, Adams John L., Thomas J. William, and McGlynn Elizabeth A.. 2010. “The Effect of Different Attribution Rules on Individual Physician Cost Profiles.” Annals of Internal Medicine 152(10): 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan AV et al. 2014. “Changes in Geographic Variation in the Use of Percutaneous Coronary Intervention for Stable Ischemic Heart Disease After Publication of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial.” Circulation: Cardiovascular Quality and Outcomes 7(1): 125–30. [DOI] [PubMed] [Google Scholar]
- Niven Daniel J., Mrklas Kelly J., et al. 2015. “Towards Understanding the De-Adoption of Low-Value Clinical Practices: A Scoping Review.” BMC Medicine 13(1): 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven Daniel J., Rubenfeld Gordon D., Kramer Andrew A., and Stelfox Henry T.. 2015. “Effect of Published Scientific Evidence on Glycemic Control in Adult Intensive Care Units.” JAMA Internal Medicine 175(5): 801. [DOI] [PubMed] [Google Scholar]
- Penfold Robert B., and Zhang Fang. 2013. “Use of Interrupted Time Series Analysis in Evaluating Health Care Quality Improvements-ClinicalKey.” Academic Pediatrics 13(6): S38–44. [DOI] [PubMed] [Google Scholar]
- Pham Hoangmai H. et al. 2007. “Care Patterns in Medicare and Their Implications for Pay for Performance.” New England Journal of Medicine 356(11): 1130–39. [DOI] [PubMed] [Google Scholar]
- Prasad Vinay et al. 2013. “A Decade of Reversal: An Analysis of 146 Contradicted Medical Practices.” Mayo Clinic Proceedings 88(8): 790–98. [DOI] [PubMed] [Google Scholar]
- Qato Danya M., Trivedi Amal N., Mor Vincent, and Dore David D.. 2016. “Disparities in Discontinuing Rosiglitazone Following the 2007 FDA Safety Alert.” Medical Care 54(4): 406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschovsky James D., and O’Malley Ann S.. 2008. “Do Primary Care Physicians Treating Minority Patients Report Problems Delivering High-Quality Care?” Health Affairs 27(3): w222–31. [DOI] [PubMed] [Google Scholar]
- Rhoads Kim F, Patel Manali I, Ma Yifei, and Schmidt Laura A. 2015. “How Do Integrated Health Care Systems Address Racial and Ethnic Disparities in Colon Cancer?” Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33(8):854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg Barbara M, Thomas Pearson, Jack Zwanziger, and Dana Mukamel. 2004. “Explaining Disparities in Access to High-Quality Cardiac Surgeons.” The Annals of Thoracic Surgery 78(1): 18–24. [DOI] [PubMed] [Google Scholar]
- Skinner Jonathan, Weinstein James N., Sporer Scott M., and Wennberg John E.. 2003. “Racial, Ethnic, and Geographic Disparities in Rates of Knee Arthroplasty among Medicare Patients.” New England Journal of Medicine 349(14): 1350–59. [DOI] [PubMed] [Google Scholar]
- Stagg Vicki. 2006. “CHARLSON: Stata Module to Calculate Charlson Index of Comorbidity.” Statistical Software Components S456719, Boston College Department of Economics. https://ideas.repec.org/c/boc/bocode/s456719.html. [Google Scholar]
- Tsai Thomas C E John Orav, and Joynt Karen E. 2014. “Disparities in Surgical 30-Day Readmission Rates for Medicare Beneficiaries by Race and Site of Care.” Annals of surgery 259(6): 1086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa Yusuke et al. 2018. “Association between Physician US News & World Report Medical School Ranking and Patient Outcomes and Costs of Care: Observational Study.” BMJ 362: 3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubel Peter A, and Asch David A. 2015. “Creating Value in Health by Understanding and Overcoming Resistance to De-Innovation.” Health affairs (Project Hope) 34(2): 239–44. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture Economic Research Service. 2018. “Rural-Urban Continuum Codes -Data.Gov” https://catalog.data.gov/dataset/rural-urban-continuum-codes.
- Wagner AK, Soumerai SB, Zhang F, and Ross-Degnan D.. 2002. “Segmented Regression Analysis of Interrupted Time Series Studies in Medication Use Research.” Journal of Clinical Pharmacy and Therapeutics 27(4): 299–309. [DOI] [PubMed] [Google Scholar]
- Wallaert Jessica B et al. 2016. “Physician Specialty and Variation in Carotid Revascularization Technique Selected for Medicare Patients.” Journal of vascular surgery 63(1): 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein James N. et al. 2006. “United States’ Trends and Regional Variations in Lumbar Spine Surgery: 1992–2003.” Spine 31(23): 2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.