Abstract
Cancer immunotherapy aims to leverage the immune system to suppress the growth of tumors and to inhibit metastasis. The recent promising clinical outcomes associated with cancer immunotherapy have prompted research and development efforts towards enhancing the efficacy of immune checkpoint blockade, cancer vaccines, cytokine therapy, and adoptive T cell therapy. Advancements in biomaterials, nanomedicine, and micro-/nano-technology have facilitated the development of enhanced local delivery systems for cancer immunotherapy, which can enhance treatment efficacy while minimizing toxicity. Furthermore, locally administered cancer therapies that combine immunotherapy with chemotherapy, radiotherapy, or phototherapy have the potential to achieve synergistic antitumor effects. Herein, the latest studies on local delivery systems for cancer immunotherapy are surveyed, with an emphasis on the therapeutic benefits associated with the design of biomaterials and nanomedicines.
Summary and Outlook
Recent advances in the fields of molecular pharmaceuticals, biomaterials, and micro-/nano-technology have inspired enhanced local delivery systems for cancer immunotherapy, which can enhance efficacy and minimize the risk of adverse effects caused by systemic toxicity. Local administration using intratumoral injection, peritumoral injection, sprayable gel, or transdermal microneedle array can be effective to overcome potential systemic transport limitations and to enhance the retention time of therapeutics at the diseased site. Local administration also partially addresses the toxicity issue, as locally administered immunotherapeutics often have lower minimum effective doses than those of systemically administered immunotherapeutics. Sustained release formulations, which utilize materials such as hydrogels and micro-/nano-particles, can further address toxicity issues by establishing control over the release kinetics of the encapsulated therapeutic agents and can sometimes directly serve as adjuvants that help increase activation of the immune system. Importantly, compared to the local administration of small molecule chemotherapeutic drugs, local treatment with immunotherapeutics could also have efficacy toward metastasized sites. Locating and injecting internal tumors could be technically challenging, but these challenges could be addressed by imaging-guided injection and minimally invasive surgical techniques. Examples presented in this review show that locally administered immunotherapeutics can induce systemic antitumor responses specific to the tumor antigens at the injection site, and thus can be effective in inhibiting tumor recurrence and metastasis potential. However, detailed in vivo studies of pharmacokinetics and pharmacodynamics should be evaluated, associated with the distant diseased sites.
A detailed understanding of the intercellular interactions and signaling molecules in the tumor microenvironment can be leveraged to design combination therapies for synergistic antitumor effects. Therefore, continued research aimed at elucidating the immunological mechanisms that play a role in the establishment and development of tumors is crucial for further progress in the field of cancer immunotherapy. Moreover, for accelerating clinical translation of anticancer immunotherapeutic delivery systems, the rational design of formulations and devices in the initial development phase is crucial, especially regarding issues of biocompatibility of materials, feasibility of large-scale manufacturing, and quality control. The selection of formulations and delivery routes should be tightly linked to the clinical needs. For example, hydrogel- based systems are well-suited to deliver multiple types of therapeutics, such as immune checkpoint antibodies and chemo- therapy small molecules. By altering the chemical composition, properties such as the drug release rate, biocompatibility, degradation triggers, and physical properties of the hydrogel can be tuned to the desired specifications. Nano-/micro- formulations can also be incorporated to adjust these properties. Moreover, the location of the tumor and the disease degree should be carefully considered when selecting the delivery systems. For example, in the case of unresectable melanoma, a transdermal patch might be highly suitable. If only a partial surgical resection is possible, an injectable hydrogel may be suitable to replace the volume removed. If a tumor can be fully resected, a sprayable gel may be preferred to treat the post-surgical site with sufficient area covered. By fine-tuning the selection of therapeutics, formulation, delivery methods, and potential combination with other treatment modalities such as radiotherapy, clinical efficacy and safety can be enhanced. As personalized medicine is emerging as an overarching theme in healthcare, the choice of the most suitable therapeutic agent(s), formulation(s), and delivery method(s) should be tailored for each individual patient.
Graphical/Visual Abstract and Caption
Caption: Recent advances in local delivery systems for cancer immunotherapy show promise for enhancing therapeutic efficacy while minimizing toxicity.
Introduction
Cancer treatment strategies include surgical resection (Pavri, Clune, Ariyan, & Narayan, 2016), chemotherapy (Diaby, Tawk, Sanogo, Xiao, & Montero, 2015), radiotherapy (Allen, Her, & Jaffray, 2017), hormone therapy (Forster, Stoffel, & Gasser, 2002), stem cell therapy (T. Wang et al., 2015), and immunotherapy (Velcheti & Schalper, 2016). Representative cancer immunotherapy approaches include immune checkpoint blockade, cytokine therapy, cancer vaccines, and adoptive T cell therapy (Kakimi, Karasaki, Matsushita, & Sugie, 2017; Lohmueller & Finn, 2017; Y. Yang, 2015; Yousefi, Yuan, Keshavarz-Fathi, Murphy, & Rezaei, 2017). The immune system can normally play a key role in eliminating cancer cells. Specifically, the immune system is able to identify, target, and eliminate cancer cells (C. Wang, Ye, Hu, Bellotti, & Gu, 2017). In the cancer-immunity cycle, released tumor-associated antigens are internalized by antigen-presenting cells (APCs) and then presented to naïve T cells, which become cytotoxic T lymphocytes (CTLs) that can recognize and kill cancer cells (Shi & Lammers, 2019). In order to avoid elimination by the immune system, cancer cells can create an immunosuppressive tumor microenvironment that hinders the ability of immune cells to eliminate cancer cells - in effect “pushing the brakes” (Jeanbart & Swartz, 2015). Therefore, the goal of immunotherapy is either to limit the ability of cancer cells to inhibit the immune response and/or to increase activation of immune cells to promote their ability to target cancer cells.
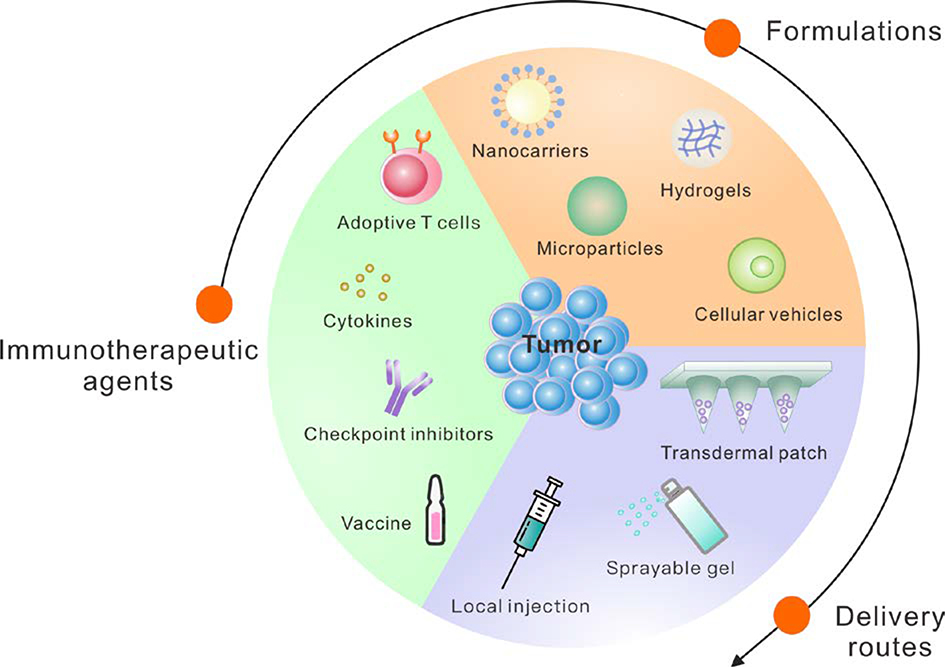
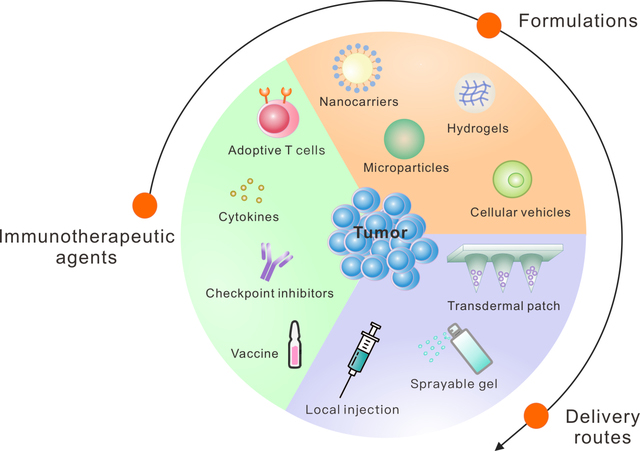
Although the advances in the development of cancer immunotherapy are promising, considerable limitations and risks still need to be addressed. Limitations of immune checkpoint blockade include systemic toxicity and relatively low clinical objective response rates (A. Ribas & Wolchok, 2018). Many studies are attempting to elucidate the biological factors that determine the efficacy or lack thereof of immunotherapeutics. For example, it is known that the efficacy of immune checkpoint blockade could be affected by factors related to the gut microbiome (Gopalakrishnan et al., 2018; Sivan et al., 2015; Vétizou et al., 2015), signal transducer and activator of transcription 1 (STAT1) signaling, Toll-like receptor 3 (TLR3) signaling, interleukin-10 (IL-10) signaling, and by the number of infiltrating-activated natural killer (NK) cells (Zemek et al., 2019). Limitations of adoptive T cell therapy include the risk of cytokine release syndrome, a severe reaction to therapy that is potentially life-threatening, and limited efficacy towards solid tumors when administered systemically, as a result of issues relating to trafficking and expansion within tumors (Beatty & O’Hara, 2016; Gill, Maus, & Porter, 2016). For these reasons, delivery methods that enhance therapeutic efficacy while mitigating the risk of systemic toxicity and adverse side effects are needed (A. S. Cheung & Mooney, 2015; Hotaling, Tang, Irvine, & Babensee, 2015; Koshy & Mooney, 2016; Marabelle, Kohrt, Caux, & Levy, 2014; Riley, June, Langer, & Mitchell, 2019; Weber & Mule, 2015). Advances in nano-/micro-technologies have the potential to address some of these limitations by enhancing the local delivery of immunotherapeutic agents and by modulating the local tumor microenvironment (Fan & Moon, 2015; Irvine, Hanson, Rakhra, & Tokatlian, 2015; Ji, Zhao, Ding, & Nie, 2013; Shao et al., 2015; Sun et al., 2019). Furthermore, nanomedicine-based immunotherapies have demonstrated the potential to inhibit cancer metastasis in preclinical cancer models (P. Zhang, Zhai, Cai, Zhao, & Li, 2019). Among them, the local delivery systems have potential to enhance the efficacy and safety of immunotherapy by facilitating sustained delivery of immunotherapeutics directly to the disease site, while minimizing the side effects that are often associated with systemic administration (Qian Chen, Wang, Chen, Hu, & Gu, 2018; Gu & Mooney, 2016; Huitong Ruan et al., 2019; Wen, Chen, et al., 2019; Wolf et al., 2019). Therefore, in cases when solid tumors cannot be fully resected due to technical or other reasons, local immunotherapy drug delivery systems could be preferable compared to systemic administration. Furthermore, drug delivery-assisted cancer immunotherapy can be enhanced when combined with other cancer therapeutics, including chemotherapy, radiotherapy, and phototherapy (Qian Chen, Chen, & Liu, 2019; D. G. Leach, Young, & Hartgerink, 2019; H. Wang & Mooney, 2018). In this review, we will discuss advances in enhancing the efficacy of cancer immunotherapeutics through the rational design of local drug delivery systems (Figure 1).
Figure 1: Local Delivery Strategies for Cancer Immunotherapy.
Recent advances in local delivery systems for cancer immunotherapy show promise for enhancing therapeutic efficacy while minimizing toxicity.
1. Local Delivery of Immune Checkpoint Inhibitors
Immune checkpoints include intercellular inhibitory pathways and immunosuppressive enzymes, such as indoleamine-2,3-dioxygenase (IDO) (Pardoll, 2012; Ricciuti et al., 2019). Because cancer cells can exploit immune checkpoints to avoid immune system-mediated elimination, immune checkpoint inhibition can be an effective anticancer strategy. Food and Drug Administration (FDA) approved immune checkpoint inhibitors work by targeting cytotoxic T lymphocyte-associated protein 4 (CTLA-4 or CTLA4) or the interaction between programmed cell death 1 (PD-1) receptors and the associated programmed cell death ligand 1 (PD-L1) (R. Park, Winnicki, Liu, & Chu, 2019; A. Ribas & Wolchok, 2018). CTLA-4 is a cell surface protein expressed by T cells that inhibits the T cell response against cancer cells by outcompeting for signaling molecules (CD80 and CD86) necessary for effector T cell activation and by facilitating T cell inhibitory signaling (Bengsch, Knoblock, Liu, McAllister, & Beatty, 2017; A. Ribas & Wolchok, 2018). The interaction between CTLA-4 and CD80/CD86 results in a diminished ability of T cells to kill cancer cells, which permits continued tumor growth. Ipilimumab is an FDA-approved antibody therapy designed to bind to CTLA-4 in order to counteract its inhibitory effects (A. Ribas & Wolchok, 2018). Another inhibitory effect is due to the secretion of Interferon-γ (IFNγ) by T cells in response to cancer cells, which triggers the expression of PD-L1 in cancer cells (Alsaab et al., 2017). When the PD-1 receptors on T cells interact with PD-L1, the antitumor response of the T cells is diminished, allowing continued tumor growth (Alsaab et al., 2017). Several FDA-approved antagonistic antibodies, which inhibit binding of PD-1 to PD-L1 by blocking either PD-1 (Pembrolizumab, Nivolumab) or PD-L1 (Atezolizumab, Avelumab, Durvalumab) are currently in the market (A. Ribas & Wolchok, 2018; Zou, Wolchok, & Chen, 2016). CD47 immune checkpoint blockade is also another promising strategy that has been under recent investigation in clinical trials (Uger & Johnson, 2020). CD47, a cell surface protein often over-expressed on the surface of cancer cells, binds to SIRPα receptors on macrophages, which inhibits their phagocytosis capability and allows cancer cells to avoid phagocytic attack (Jaiswal et al., 2009; Soto-Pantoja et al., 2014; Vonderheide, 2015). The local delivery of immune checkpoint inhibiting antibodies for solid tumors has been reported to enhance therapeutic efficacy and reduce systemic toxicities when compared to systemic administration (Aznar et al., 2017; Huynh et al., 2019; Marabelle, Kohrt, & Levy, 2013; Simmons et al., 2008; C. Wang, Ye, & Gu, 2017). Furthermore, advances in the fields of biomaterials and nanomedicine have facilitated enhanced therapeutic efficacy and delivery of immune checkpoint blockade therapy (Francis & Thomas, 2017; Lamichhane et al., 2019; Marabelle, Tselikas, de Baere, & Houot, 2017; Zhao, Li, Wei, & Luo, 2018). In this section, we will summarize several examples of local delivery systems for immune checkpoint inhibiting antibodies and IDO inhibitors. Additional methods to locally deliver immune checkpoint inhibitors in combination with other cancer therapeutics will be discussed in the combination therapy section.
In one example, Lei et al. reported that functionalized mesoporous silica (FMS) nanoporous supports loaded with anti-CTLA-4 antibodies (aCTLA-4) locally administered at the tumor site could enhance therapeutic efficacy (Lei et al., 2010). The authors had previously reported a method for protein loading and immobilization in FMS (Lei et al., 2006). Unfunctionalized mesoporous silica was modified with -COOH and -NH2 functional groups to generate FMS and to facilitate electrostatic, hydrophilic, and hydrogen bonding interactions with the loaded protein. The authors demonstrated that controlled release of the antibodies could be tuned by altering the pore size of the FMS and by changing the type and concentration of functional groups present in the FMS. The authors investigated how significant toxicities associated with systemically administered aCTLA-4, such as enteritis and endocrine deficiencies, could be avoided by means of local delivery (J. C. Yang et al., 2007). They found that direct injection of aCTLA-4 loaded FMS into a murine melanoma cancer model effectively inhibited tumor growth. Furthermore, FMS particles exhibited negligible toxicity and could facilitate sustained local release of the antibodies with less systemic exposure compared to directly injected antibodies.
In another work, Fransen et al. conducted a study to investigate the effects of local and systemic delivery on the efficacy of CTLA-4 blockade in murine cancer models (Fransen, van der Sluis, Ossendorp, Arens, & Melief, 2013). The authors reported that an 8-fold lower dose administered locally was as effective as a systemic high dose, and that mice given the low dose treatment systemically had similar survivorship outcomes to untreated mice. The authors also found that local aCTLA-4 treatment of tumors could decrease the tumor volume of distant tumors. The authors proposed that this phenomenon is due to the effect of aCTLA-4 on tumor-specific CD8+ T cells. The authors also compared the effect of a local, low dose aCTLA-4 in a slow release formulation of Montanide ISA-51, a water in oil emulsion that is also known as incomplete Freund’s adjuvant (IFA), to a systemic high dose of aCTLA-4, and found that serum levels of aCTLA-4 were 1,000-fold higher in the systemic high dose condition. By decreasing systemic exposure to aCTLA-4, controlled release formulations could help reduce systemic toxicity. In a related study, Sandin et al. investigated the effects of local vs. systemic delivery of aCTLA-4 for the treatment of a murine model of pancreatic adenocarcinoma cancer (Sandin, Eriksson, et al., 2014). The authors found that an approximately 6-fold higher systemic dose was necessary to achieve a comparable outcome to the local dose.
Rahimian et al. engineered aCTLA-4 loaded poly(lactic-co-hydroxymethyl-glycolic acid) (pLHMGA) polymer-based microparticles and demonstrated their efficacy in treating murine models of cancer (Leemhuis et al., 2006; Rahimian et al., 2015). By varying the polymer concentration, the investigators could control the microparticle size, loading efficiency, duration of release, and burst release properties. Increasing polymer concentration resulted in decreased burst release, and all formulations exhibited a sustained release kinetic profile due to polymer degradation. The authors compared the effects of locally administered aCTLA-4 loaded microparticles with locally administered aCTLA-4 in an IFA formulation and found that serum antibody levels were 5 to 10 times lower using microparticles than with the IFA formulation. Furthermore, the authors observed no remainder of the microparticle formulation at the injection site during postmortem examination, whereas the IFA formulation was still observable.
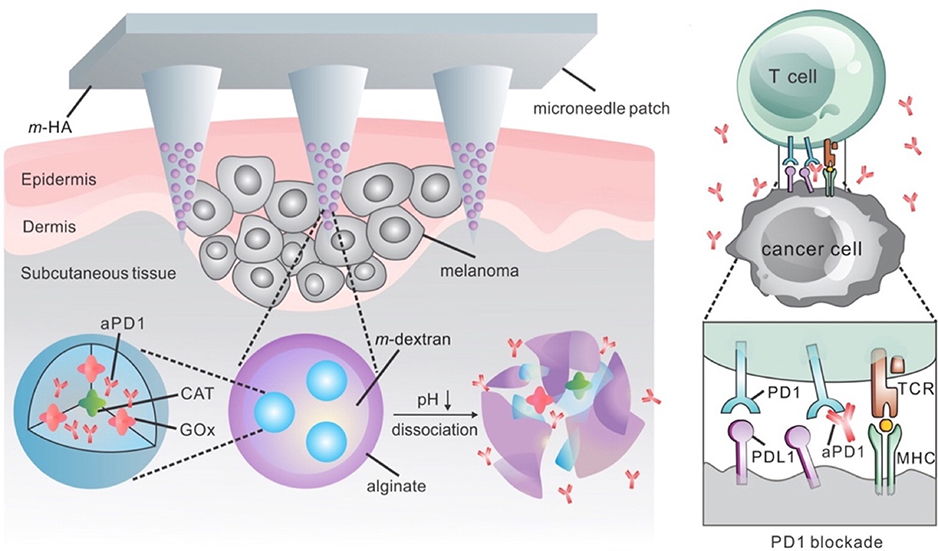
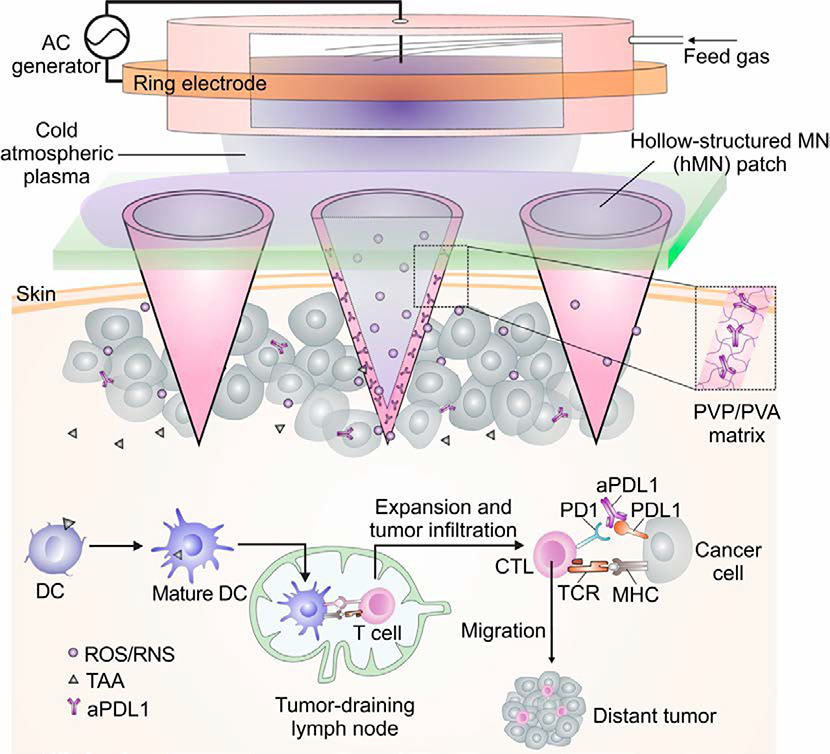
Microneedle array patches have been engineered to enhance local immune checkpoint inhibition therapy. For the first time, Wang et al. demonstrated the delivery of anti-PD-1 antibodies (aPD-1) to treat melanoma using a transdermal microneedle patch (Figure 2) (C. Wang, Ye, Hochu, Sadeghifar, & Gu, 2016). Nanoparticles were prepared using a biocompatible pH sensitive dextran matrix, polyelectrolyte-based surfactant, a glucose oxidase (GOx)-catalase enzymatic system, and aPD-1. The surfaces of the nanoparticles were coated with alginate to achieve negatively charged outer surfaces. The nanoparticles were embedded and concentrated at the tips of a hyaluronic acid (HA)-based microneedles. This system released aPD-1 in a glucose and pH-dependent manner, due to the catalytic effect of the GOx enzyme and the acid degradable polymeric component. By changing the amount of GOx enzyme present, the release kinetics of the aPD-1 could be adjusted to facilitate controlled release. The authors then tested a similar microneedle formulation to load both aCTLA-4 and aPD-1, which outperformed aCTLA-4 alone and aPD-1 alone, validating that simultaneous delivery of aCTLA-4 and aPD-1 immune checkpoint inhibitors could increase treatment efficacy. Ye et al. reported a method to deliver aPD-1 and 1-methyl-ᴅʟ-tryptophan (1-MT), an IDO inhibitor, via a transdermal microneedle patch to treat murine models of melanoma (Y. Ye et al., 2016). IDO is an enzyme that has been shown to limit T effector cell function while promoting the proliferation and differentiation of T regulatory cells, which together make the tumor microenvironment more immunosuppressive (Munn, 2012). Therefore, by inhibiting this enzyme with 1-MT, the authors sought to further enhance the therapeutic effects of the microneedle-based immune checkpoint inhibitor system previously designed. The authors utilized a 1-MT-conjugated HA microneedle formulation to encapsulate the anti-PD-1 loaded nanoparticles. Accumulation of 1-MT in the tumor site was 3-fold greater in the microneedle formulation with 1-MT than that of free 1-MT, and furthermore, tumor uptake of aPD-1 increased in the 1-MT containing microneedle. The mice treated with the microneedle formulation containing 1-MT had significantly higher survivorship than the mice treated with the microneedle formulation without 1-MT. Recently, Chen et. al. developed a transdermal hollow-structured microneedle array patch to facilitate cold atmospheric plasma-mediated aPD-L1 therapy for melanoma treatment (Figure 3) (G. Chen et al., 2020). The microneedle patch facilitated the entry of cold atmospheric plasma into the tumor, causing the release of tumor-associated antigens. The authors found that such release of tumor-associated antigens and PD-L1 blockade inhibited tumor growth in both treated and distant (untreated) tumors, indicating that a systemic anti-tumor immune response had been achieved.
Figure 2: Microneedle Patch Assisted Delivery of PD-1 Blockade.
The schematic showing the delivery of PD-1 blockade using nanoparticles released from a microneedle patch for melanoma treatment. Adapted with permission from (C. Wang et al., 2016). Copyright 2016, American Chemical Society.
Figure 3: Microneedle Patch Assisted Cold Atmospheric Plasma-mediated Immune Checkpoint Blockade.
The schematic for the microneedle patch enabled cold atmospheric plasma-mediated immune checkpoint blockade. Adapted from (G. Chen et al., 2020). Copyright 2020, National Academy of Sciences.
Ultrasound-guided injections can be used to deliver a variety of therapies to internal unresectable tumors in a minimally invasive manner. Van Hooren et al. conducted a study to investigate the effect of local vs. systemic delivery of CTLA-4 and PD-1 checkpoint inhibitors to treat murine models of bladder cancer (van Hooren et al., 2017). The authors first performed a titration experiment wherein mice bearing subcutaneous murine bladder 49 tumors (MB49) were injected with either 3, 10, or 30 μg aCTLA-4 either peritumorally or intravenously. The authors found that only the 30 μg dose was effective, and that both routes of administration exhibited similar therapeutic efficacy. However, intravenous injections produced significantly higher serum levels of aCTLA-4 and IL-6 than local injections at four hours post injection. Lower serum aCTLA-4 levels decrease the risk of adverse events due to the toxicity effects of CTLA-4 therapy. The authors then investigated the effect of an ultrasound-guided intratumoral injection of aCTLA-4 on an orthotopically growing MB49-GFP-Luc bladder tumor and found a more than ten-fold reduction in serum aCTLA-4 levels in comparison to systemic administration.
Ishihara et al. reported a method to locally deliver aCTLA-4 and aPD-L1 via conjugation to high affinity extracellular matrix binding peptides to treat murine tumor models of melanoma and breast cancer (Ishihara et al., 2017). Sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1- carboxylate (sulfo-SMCC) was used to conjugate PlGF-2123–144 peptides, which have high affinity for cellular extracellular matrices (ECM), to either aPD-L1 or aCTLA-4. The results show that each antibody could be conjugated to about six PlGF-2123–144 peptides without altering its target recognition capability. Both PlGF-2123–144-anti-CTLA-4 and PlGF-2123–144-anti-PD-L1 were found to bind to the ECM proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and type I, II, III, and IV collagen. The purpose of the modification was to increase the retention of the antibodies at the tumor site, enhance their therapeutic effects, and minimize their toxic side effects by increasing T cell activation and by decreasing systemic exposure. Peritumorally injected PlGF-2123–144–antibodies were retained at the tumor site for a longer time than freely administered antibodies. Reduced systemic toxicity in comparison to unmodified antibodies given via peritumoral and intra-peritoneal routes of administration was also achieved with lower serum concentrations of antibodies, tumor necrosis factor–α (TNFα), IFNγ, the liver damage marker, and alanine aminotransferase (ALT) in mice. The combination of PlGF-2123–144–anti-CTLA-4 and PlGF-2123–144–anti-PD-L1 resulted in T cell activation and increased the number of tumor-infiltrating CD8+ T cells relative to free antibodies.
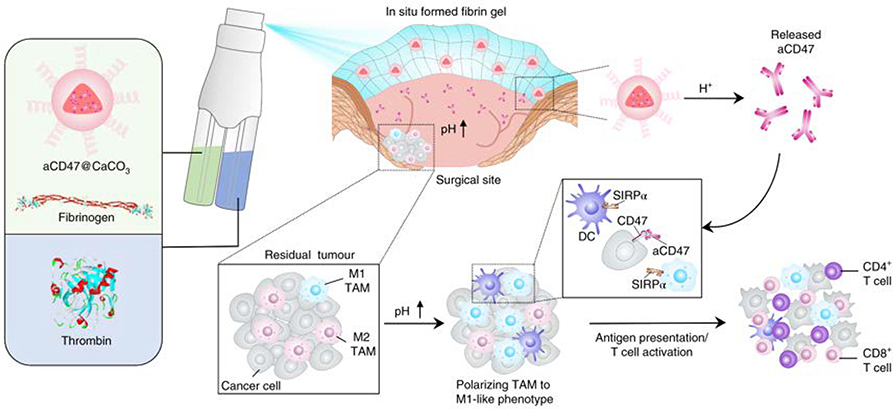
Since the tumor microenvironment is often acidic, delivery systems that are pH-dependent are desirable for bioresponsive delivery of local immune checkpoint blockade. In one example, Chen et al. designed a sprayable in situ formed pH responsive fibrin gel to deliver anti-CD47 antibodies (aCD47) to the post-surgical tumor site (Figure 4) (Qian Chen, Ci, & Gu, 2019; Qian Chen, Chao Wang, et al., 2019). The aCD47 induced tumor associated macrophages to phagocytize cancer cells by blocking the inhibitory ‘don’t eat me’ signaling between cancer cells and macrophages. The dispenser for this therapy contains two compartments; one compartment contains thrombin, and the other compartment contains fibrinogen and aCD47 loaded CaCO3 nanoparticles. When the two liquids mix at the tumor site, the reaction between thrombin and fibrinogen results in the formation of a biodegradable fibrin gel. The nanoparticles degrade in the presence of the relatively acidic tumor microenvironment and release aCD47.
Figure 4: In Situ Sprayed Bioresponsive Immunotherapeutic Gel.
The schematic of the in situ formed fibrin gel containing aCD47-loaded nanoparticles. Adapted with permission from (Qian Chen, Chao Wang, et al., 2019). Copyright 2018, Springer Nature.
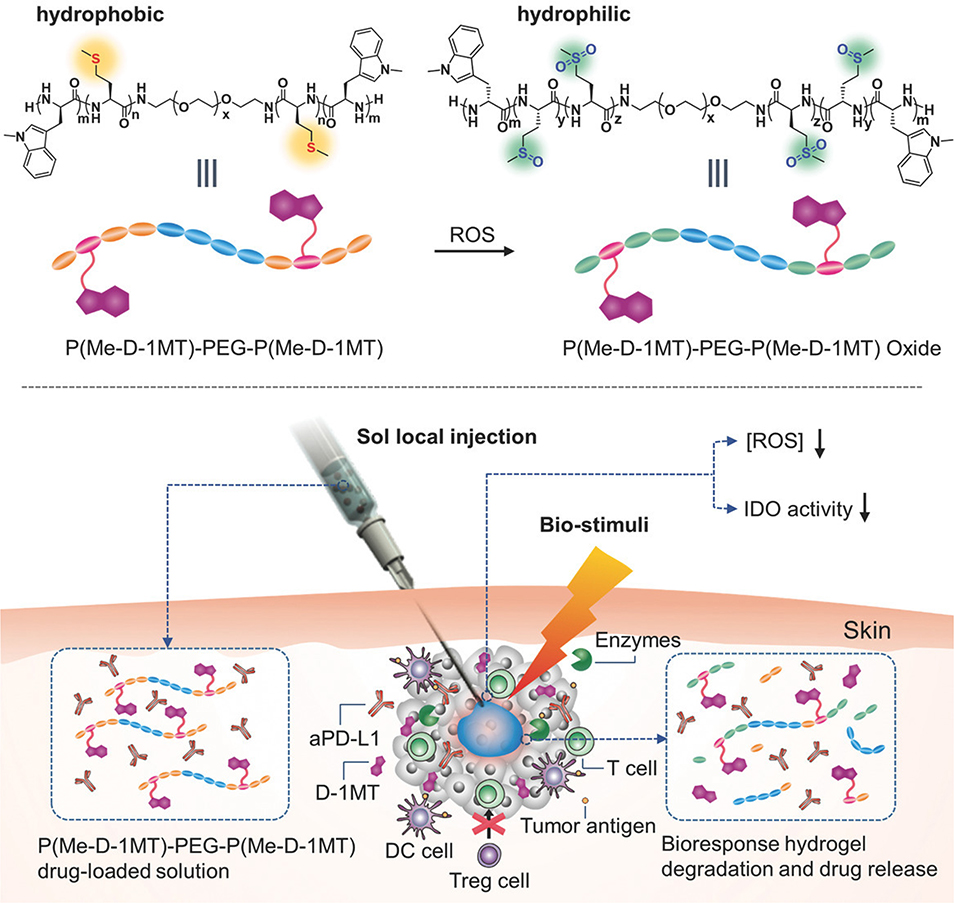
In addition to being relatively acidic, the tumor microenvironment often contains reactive oxygen species (ROS), so ROS-dependent delivery systems are also rational choices for local immune checkpoint blockade. In one example, Yu et al. engineered an injectable ROS-responsive polypeptide-based hydrogel for co-delivery of anti-PD-L1 antibodies (aPD-L1) and dextro-1-methyl tryptophan (D-1MT) to the tumor microenvironment (Figure 5) (Yu et al., 2018). The authors were able to achieve synergistic antitumor effects in tumor-bearing mice using this combination therapy. In another example, Chen et al. designed ROS-sensitive protein complexes for controlled local release of aCD47 and aPD-1 to the tumor site (Qian Chen, Guojun Chen, et al., 2019). Due to the considerable presence of ROS in the tumor microenvironment, the authors hypothesized that the presence of ROS would be a suitable trigger for controlled release of the antibodies. The antibody complexes were prepared by cross-linking aPD-1 and aCD47 using an ROS-sensitive cross-linker: bis-N-hydroxy succinimide (NHS) modified 2,2′-[propane-2,2-diylbis(thio)]diacetic acid (NHS-IE-NHS) (Qian Chen, Guojun Chen, et al., 2019). In addition to serving as a trigger for controlled release, the ability of the ROS-sensitive cross-linker-based particles to scavenge the tumor microenvironment for ROS resulted in a significantly reduced population of immunosuppressive cells, including M2-type macrophages and regulatory T cells.
Figure 5: Bioresponsive Hydrogel for PD-L1 Blockade and IDO Inhibitor Delivery.
The schematic showing the local delivery of PD-L1 blockade therapy and the IDO inhibitor, D-1MT, using a bioresponsive hydrogel. Adapted with permission from (Yu et al., 2018). Copyright 2018, John Wiley & Sons, Inc.
2. Local Delivery of Immunostimulatory Agents
In contrast to immune checkpoint blockade, which inhibits the ability of cancer cells to inactivate T cells, immunostimulatory agents aim to directly activate immune cells; if immune checkpoint blockade limits the ability of cancer cells to “push the brakes,” then immunostimulatory agents can be thought of as “pushing the accelerator.” This can be achieved by several strategies, including targeting co-stimulatory immune cell receptors, delivering cytokines to the tumor microenvironment, or administering immunostimulatory adjuvants.
2.1. Targeting Co-Stimulatory Immune Cell Receptors
Co-stimulatory immune cell receptors, including CD40, OX40 (CD134), and CD137, are potential therapeutic targets for immunotherapy. The binding of agonistic antibodies to these receptors results in downstream signaling as if the natural ligand was bound. This mechanism is the opposite of that used in immune checkpoint blockade, in which antagonistic antibodies that inhibit downstream signaling are used. For example, agonistic antibodies and adenoviral vectors carrying CD40L complementary DNA have been developed to target CD40 (Beatty, Li, & Long, 2017; Lindqvist, Sandin, Fransson, & Loskog, 2009). CD40 is a costimulatory cell surface receptor belonging to the Tumor Necrosis Factor (TNF) superfamily and is often present on APCs, and its ligation to CD40L on T cells causes APC activation and promotes a T cell mediated antitumor response (Vonderheide & Glennie, 2013). Therefore, agonistic CD40 antibodies (aCD40) can promote APC activation and subsequent antitumor immune responses. OX40 is a costimulatory cell surface receptor belonging to the TNF superfamily that can be present on T cells, and its ligation to OX40L on APCs results in enhanced antitumor T cell activity (Aspeslagh et al., 2016). CD137 is a costimulatory cell surface receptor belonging to the TNF superfamily that can be present on T cells and NK cells, and its ligation to 137L on APCs results in enhanced antitumor CTL responses (Pardoll, 2012; Yonezawa, Dutt, Chester, Kim, & Kohrt, 2015).
Sandin et al. conducted a study to compare the local and systemic delivery of aCD40 in MB49 tumor-bearing mice (Sandin, Orlova, et al., 2014). By radiolabeling the aCD40 and measuring serum radiation, the authors determined that local delivery resulted in lower serum antibody levels compared to systemic delivery. The authors reported that local low-dose aCD40 therapy increased survival and decreased toxicity compared to systemic high-dose aCD40 therapy. Furthermore, the locally delivered antibodies could result in tumor regression of both the primary tumor and distant tumors in murine cancer models. To decrease toxicity associated with aCD40 therapy, Fransen et al. developed a delivery system that implemented the slow-release formulation, Montanide ISA-51, to stimulate local tumor antigen-presenting dendritic cells with aCD40 (Fransen, Sluijter, Morreau, Arens, & Melief, 2011). The authors found that a low local dose of aCD40 in Montanide ISA-51 resulted in decreased serum levels of the liver damage markers, ALT and AST, compared to the high systemic dose treatment group.
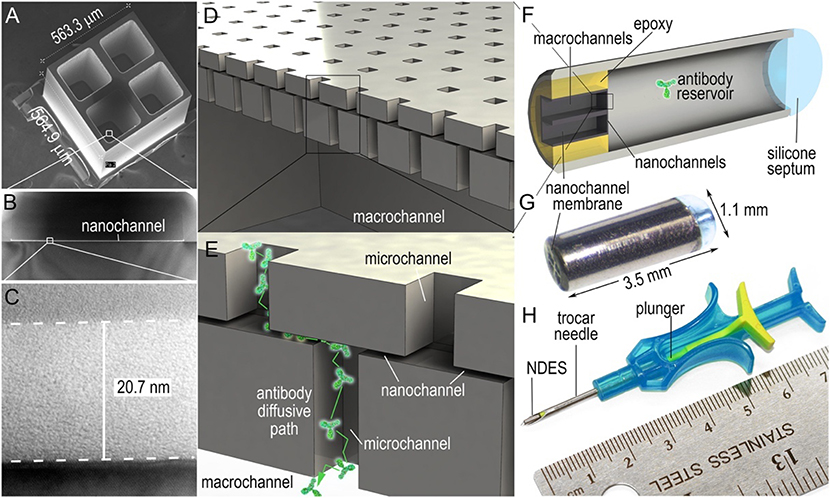
To enhance the therapeutic efficacy of local delivery of aCD40 therapy and reduce its toxicity, Ishihara et al. utilized super-affinity peptides derived from placenta growth factor-2 (PIGF-2123–144) (Ishihara et al., 2018). The authors found that PIGF-2123–144 peptides conjugated to aCD40 improved its stability in the tumor microenvironment and decreased systemic toxicity. Another local delivery strategy for CD40 therapy was reported by Rahimian et al., who engineered aCD40 loaded poly (lactic-co-hydroxymethyl-glycolic acid) (pLHMGA) polymer-based microparticles and tested them in murine cancer models (Rahimian et al., 2015). Compared to the IFA formulation, serum aCD40 levels after delivery of aCD40-loaded microparticles were lower, and therefore the risk of adverse side effects due to systemic exposure was mitigated. In another work, Chua et al. engineered a nanofluidic drug-eluting seed (NDES) to enhance the therapeutic efficacy of locally delivered aCD40 and agonistic OX40 antibodies (aOX40), and validated the system in murine models of triple negative breast cancer (Figure 6) (Chua et al., 2018). The authors found that the high interstitial pressure usually associated with intratumoral injection could be avoided by gradually releasing the CD40 and OX40 therapeutic antibodies intratumorally over time using the NDES (Chua et al., 2018). The authors found that the NDES therapy allowed for enhanced controlled release of the antibodies, which resulted in both a local and systemic antitumor immune response. In other works, amphiphilic poly (γ-glutamic acid) nanoparticles and luminescent porous silicon nanoparticles were developed to facilitate local delivery of aCD40 (Broos et al., 2012; Gu et al., 2012).
Figure 6: Nanofluidic Drug-Eluting Seed.
The panel describing the properties of the nanofluidic drug-eluting seed. Adapted with permission from (Chua et al., 2018). Copyright 2018, Elsevier B.V.
2.2. Cytokines
IL-2, a pro-inflammatory cytokine, is reported to be the first effective immunotherapy for treating human cancer (Rosenberg, 2014). Other cytokines under investigation for use as immunotherapeutics include Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-10, IL-12, IL-15, transforming growth factor- β (TGF-β) and TNF-α (Berraondo et al., 2019). Due to the potential lethal toxicity associated with the systemic administration of certain cytokines, it is essential to design drug systems that mitigate the risk of systemic cytokine toxicity while enhancing their antitumor efficacy.
Momin et al. engineered a local delivery system for the immune cytokines IL-2 and IL-12 by fusing them with lumican, a collagen binding protein (Momin et al., 2019). The authors observed that this modification enhanced retention of the cytokines in the tumor microenvironment while decreasing their systemic exposure. Sabel et al. encapsulated IL-12 and TNFα in poly-lactic acid microspheres for intratumoral immunotherapy, and reported that this combination resulted in a systemic and synergistic antitumor response, which was mediated by NK cells and cytotoxic T cells (Sabel et al., 2007). Hydrogels have also been designed to locally deliver cytokines for cancer immunotherapy (C. G. Park et al., 2018). In one example, Zaharoff et al. reported that intratumoral injection of IL-12 in chitosan solution resulted in systemic tumor specific immunity in tumor-bearing mice (Zaharoff, Hance, Rogers, Schlom, & Greiner, 2010). Chitosan was used to facilitate the local and sustained delivery of IL-12 in the tumor microenvironment and resulted in a longer retention time of IL-12 in the tumor region than locally administered free IL-12. The chitosan/IL-12 therapy partially or completely protected the mice from tumor rechallenge at non-injected sites. In another work, Kwong et al. engineered a local delivery system for the IL-2 fusion protein (IL-2Fc) and anti-CD137 by attaching them to the surface of PEGylated liposomes (Kwong, Gai, Elkhader, Wittrup, & Irvine, 2013). Intratumoral injection of anti-CD137-liposomes and IL-2Fc-liposomes in tumor-bearing mice treated most of the primary tumors and also resulted in systemic tumor immunity in a CD8+ T cell dependent manner. Liposome therapy decreased systemic toxicity to negligible levels and slowed the growth of both primary and distant tumors.
2.3. Immunostimulatory Adjuvants
Immunostimulatory adjuvants are a class of immunotherapeutics that are often used in cancer vaccine formulations but can also be used as a monotherapy. Oligodeoxynucleotides containing unmethylated CpG-rich regions (CpG) are favored adjuvants for cancer immunotherapy vaccines due to their ability to activate cells of the innate immune system by binding to TLR9 receptors (Furumoto, Soares, Engleman, & Merad, 2004; Kawarada et al., 2001). For example, intratumoral injection of CpG and adenovirus type 5 encoding a tumor antigen was shown to have therapeutic benefit in murine cancer models (Geary, Lemke, Lubaroff, & Salem, 2011). In one example, Zhang et al. engineered a CpG delivery system using 3-aminopropyltriethoxysilane (APTES)-modified Fe3O4 magnetic (FeNP) nanoparticles (X. Zhang et al., 2018). APTES modification increased CpG loading capacity by creating more CpG binding sites on the FeNPs. The therapeutic effect of this formulation for inhibiting tumor growth in a murine cancer model was superior to that of free CpG. Due to their superparamagnetic properties, FeNP nanoparticles could potentially be concentrated at the tumor site by applying an external magnetic field (Majidi et al., 2016). In another work, Zhu et al. engineered DNA-inorganic hybrid nanovaccines (hNVs) for delivery of CpG analogs (Zhu et al., 2016). hNVs were fabricated via self-assembly of concatemer CpG analogs and magnesium pyrophosphate (Mg2PPi), and subsequently injected intratumorally into murine melanoma models. The hNVs were found to dissociate faster in acidic conditions, such as in internalized endosomes, facilitating the release of CpG from the hNVs. In comparison to free CpG, the hNV delivery vehicles carrying CpG significantly inhibited tumor growth, prolonged the retention time of CpG in the tumor microenvironment, and mitigated adverse systemic side effects. Another method to increase the retention time of CpG in the tumor microenvironment was reported by Liu et al., who engineered lipid-conjugated CpG for insertion into the membranes of tumor cells (Liu, Kwong, & Irvine, 2011).
In addition to CpG oligonucleotides, cyclic dinucleotides (CDNs) are also another effective immunostimulatory adjuvant. In one work, Wilson et al. engineered poly(beta-amino ester) nanoparticles to deliver CDNs, which activate the stimulator of interferon genes (STING) in APCs, upon intratumoral injection (Wilson et al., 2018). CDNs can also be delivered locally using hydrogels, as was demonstrated by Leach et. al., who engineered a “STINGel” - a MultiDomain Peptide hydrogel loaded with CDNs (David G. Leach et al., 2018). The authors demonstrated that this MultiDomain Peptide-based hydrogel drug delivery system significantly enhanced the efficacy of CDN immunotherapy in vivo by decreasing the release rate of CDNs by 8-fold, in comparison to a collagen-based hydrogel. Such extended release corresponded to a higher local concentration of CDNs and increased survival rate in preclinical experiments.
Recently, tumor-associated macrophages (TAMs), which are capable of supporting tumor cell proliferation and metastasis, have been under intensive investigation as potential therapeutic targets (Komohara, Fujiwara, Ohnishi, & Takeya, 2016; Muraoka et al., 2019; Nie et al., 2017; B. Z. Qian & Pollard, 2010). Depending on their phenotypic polarization, TAMs can either support or suppress tumor growth; M2 polarized macrophages often support tumor growth, metastasis, and angiogenesis, whereas M1 polarized macrophages are tumor destructive (W. Hu et al., 2016; Rodell et al., 2018). An increased number of M2 polarized macrophages within the tumor microenvironment is associated with poor clinical prognosis and limited treatment efficacy (Zheng et al., 2017). Therefore, one strategy for immunotherapy is to increase the number of M1 TAMs while decreasing the number of M2 TAMs within the tumor microenvironment (Mills, Lenz, & Harris, 2016). For example, Jaynes et. al. reported that the RP-182 peptide could convert the polarization of TAMs from M2 to M1 upon binding to CD206 receptors, which are expressed on M2 TAMs (Jaynes et al., 2020).
Another strategy for macrophage-mediated immunotherapy involves using the toll-like receptor 7/8 agonist small molecule, R848, to increase macrophage M1 polarization and production of pro-inflammatory immune cytokines (Manome et al., 2018; Rodell et al., 2018). Park et al. demonstrated that extended peritumoral release of R848 from a hydrogel-based scaffold could significantly enhance treatment efficacy compared to local administration of R848 in solution (C. G. Park et al., 2018). Furthermore, the authors demonstrated that perioperative implantation of a hydrogel loaded with R848 could inhibit local and systemic tumor recurrence and metastasis, and could eliminate existing metastases. In a related work, Tsai et. al. designed a drug delivery system for Imiquimod (R837), a TLR7 agonist that has similar effects on macrophage polarization as R848, by first generating Imiquimod liposomes using 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine, and then loading them into a thermosensitive pluronic F127 hydrogel (Tsai et al., 2019). At cold temperatures, the liposomes and hydrogel are in a soluble form, while at body temperature, the mixture converts into a gel, facilitating the ease of administration.
3. Local Delivery of Cancer Immunotherapy Vaccines
Cancer immunotherapy vaccines aim to induce the host immune system to attack cancer cells by decreasing immune tolerance to tumor antigens, which can be achieved by co-delivery of tumor antigens and immunostimulatory adjuvants (Ali, Emerich, Dranoff, & Mooney, 2009). Many intratumorally administered therapeutic cancer immunotherapy vaccines aim to induce a CTL response against tumor cells by introducing strongly immunogenic epitope(s) of the specific cancer antigen(s) (Berzofsky et al., 2018). Cancer vaccines can contain several types of immunogenic components, including peptides, DNA, whole cell lysates, recombinant viruses, or modified immune cells (Thomas & Prendergast, 2016). Patient-specific neoantigens, which can arise as a result of tumor-specific mutations, can be utilized to formulate patient-specific cancer immunotherapy vaccines (Schumacher & Schreiber, 2015). To date, the FDA has approved Sipuleucel-T (Kantoff et al., 2010), talimogene laherparepvec (Gatti-Mays, Redman, Collins, & Bilusic, 2017; Johnson, Puzanov, & Kelley, 2015), and Bacillus Calmettle-Guerin (Gatti-Mays et al., 2017) for the treatment of prostate cancer, melanoma, and bladder cancer, respectively.
In situ cancer immunotherapy vaccines contain immunostimulatory components that activate host immune cells in the tumor microenvironment and utilize locally present tumor antigens, eliminating the need to pre-identify and deliver tumor antigens (Sheen & Fiering, 2019). Oncolytic viruses can be used to produce tumor-associated antigens for in situ vaccination, directly lysing tumor cells, and activating of cells of the innate immune system (P. K. Bommareddy & Kaufman, 2018; P. K. Bommareddy, Shettigar, & Kaufman, 2018; Kaufman & Bommareddy, 2019; Lichty, Breitbach, Stojdl, & Bell, 2014). The underlying immunological mechanisms by which a selected oncolytic virus functions as an immune adjuvant have been investigated (M. C. Brown et al., 2017). Oncolytic viruses have been used in clinic to treat various cancers, such as neuroblastoma, hepatocellular carcinoma, Ewing sarcoma, and retinoblastoma (Cripe et al., 2015; B. H. Park et al., 2008; Pascual-Pasto et al., 2019).
The efficacy of cancer vaccines can be enhanced by increasing the availability of tumor-associated antigens to antigen-presenting cells. An et al. described a method to utilize the cytotoxic effects of intratumorally injected cationic silica nanoparticles to induce necrotic tumor cell death, and by doing so, the release of tumor-associated antigens could activate antigen-presenting cells and induce antitumor immunity (An et al., 2018). To further enhance the activation of antigen-presenting cells, the authors complexed the cationic silica nanoparticles (CSiNPs) with CDN adjuvants, which significantly increased retention time in the tumor. The authors found that c-di-GMP/CSiNPs caused increased CD8+ T cell activation and infiltration into the tumor microenvironment. The sustained local release of a pre-identified tumor antigen could also be an effective way to design a vaccine, as was demonstrated by Umeki et al., who engineered a CpG DNA-containing hydrogel encapsulating the modified model antigen, cationized ovalbumin (Umeki et al., 2015). The electrostatic interactions created between the positively charged antigen and negatively charged hydrogel significantly prolonged the release of the antigen from the CpG DNA hydrogel and produced more potent antitumor effects compared to that of unmodified ovalbumin released from the hydrogel.
Several intratumorally injected cancer immunotherapy vaccine formulations make use of plant virus-based nanoparticles, including cowpea mosaic virus, tobacco mosaic virus, potato virus X, and papaya mosaic virus-based nanoparticles (Lebel et al., 2016; Lee et al., 2017; Lizotte et al., 2016; Murray, Wang, Fiering, & Steinmetz, 2018). In one example, Lizotte et al. engineered self-assembling, empty cowpea mosaic virus (eCPMV) nanoparticles and found that intratumoral injection of eCPMV nanoparticles resulted in cancer cell necrosis in the tumor microenvironment and systemic antitumor immunity in tumor-bearing mice (Lizotte et al., 2016). In another work, Murray et al. reported that nanoparticles from tobacco mosaic virus were less potent than CPMV-derived nanoparticles for the treatment of a murine model of dermal melanoma due to differences in immune activation (Murray et al., 2018). Plant virus-based nanoparticles can be standalone therapies or can be used as drug delivery vehicles to carry other therapeutics.
4. Local Delivery of Adoptive T Cell Therapy
Cell-based therapies are emerging as a novel therapeutic strategy to potentially enhance treatment efficacy for many diseases, such as cancer and diabetes (Z. Chen, Hu, & Gu, 2018; Fischbach, Bluestone, & Lim, 2013; Wen, Wang, et al., 2019; X. Xu et al., 2019). In the case of engineered T cells, tumor antigen recognition specificity can be achieved by chimeric antigen receptor (CAR) T cell therapy and T cell receptor (TCR) therapy (Klebanoff, Rosenberg, & Restifo, 2016). In CAR T cell therapy, peripheral blood mononuclear cells are harvested from the patient’s blood and are stimulated to become T cells, after which, DNA encoding a CAR engineered to recognize a certain antigen is incorporated into the genome of each cell (Gill et al., 2016). The CAR allows the T cell to recognize and kill cells which have those antigens (Gill et al., 2016; Zhen, Carrillo, & Kitchen, 2017). The modified CAR T cells are cultured to increase the total number of cells and are infused back into the patient, where they initiate an antitumor immune response (Gill et al., 2016; Zhen et al., 2017). Currently, the FDA has approved two CAR T cell therapies to treat blood cancers: Axicabtagene ciloleucel and tisagenlecleucel, which aim to treat refractory diffuse large B-cell lymphoma and B-cell precursor acute lymphoblastic leukemia, respectively (Leahy, Elgarten, Grupp, Maude, & Teachey, 2018; Roberts, Better, Bot, Roberts, & Ribas, 2018). In addition, several CAR T cell therapies for diseases, including multiple myeloma, human immunodeficiency virus (HIV), and neuroblastoma are under investigation (Ghosh et al., 2018; Gill et al., 2016; Zhen et al., 2017). Systemically administered CAR T cells are often of limited therapeutic efficacy for the treatment of solid tumors due to inefficient delivery to the tumor site(s) (Beatty & O’Hara, 2016). It has been demonstrated in both preclinical cancer models and clinical trials that local delivery of CAR T cells is preferable to systemic delivery for treating solid tumors of several types cancer, including head and neck squamous cell carcinoma and carcinoembryonic antigen peritoneal tumors (Katz et al., 2016; Papa, van Schalkwyk, & Maher, 2015; van Schalkwyk et al., 2013). Several examples of locally delivered CAR T cells are discussed here to highlight the potential advantages of their local delivery as opposed to their systemic delivery for the treatment of solid tumors.
Adusumilli et al. reported that intrapleurally administered CAR T cells were superior to intravenously administered CAR T cells for the treatment of pleural tumors as well as metastatic sites (Adusumilli et al., 2014). The authors engineered mesothelin (a tumor cell surface marker)-targeted CD28 co-stimulated (M28z) CAR T cells and evaluated their efficacy in murine orthotopic models of malignant pleural mesothelioma (MPM). In contrast to the intravenously administered T cells, which produced only a marginal antitumor effect compared to the control CAR T cells, intrapleurally administered CAR T cells produced a stronger antitumor response. Even when administered at a 30-fold lower dose than the intravenous dose, the intrapleural dose produced an enhanced antitumor effect and resulted in systemic tumor immunity at extrathoracic tumor sites. Moreover, a similar amount of intravenously administered CAR T cells accumulated at the tumor site did not generate a comparable antitumor effect.
Katz et al. reported that intraperitoneal infusion of CAR T cells resulted in enhanced protection over systemically infused CAR T cells in murine models of peritoneal carcinomatosis (Katz et al., 2016). The authors found that intraperitoneal injection of CAR T cells resulted in a 37-fold reduction in tumor burden, whereas intravenously injected mice only showed a 3-fold reduction. Priceman et al. reported that local intracranial administration of HER2 targeted CAR T cells resulted in significant antitumor effects in orthotopic xenograft murine models of brain tumors resulting from breast cancer metastasis (Priceman et al., 2018). The authors evaluated three delivery routes: intravenous (systemic), intraventricular (regional), and intratumoral (local). Equivalent antitumor efficacy for both regional and local delivery of CAR T cells was observed, although regional delivery sometimes resulted in a delayed response. Systemic delivery of a ten-fold greater dose than that of regional delivery resulted in only marginal tumor regression. Nellan et al. reported that regional and intravenous administration of CAR T cells targeting HER2 resulted in tumor regression in murine models of medulloblastoma, although a 5-fold higher dose of intravenously administered CAR T cells was required to produce a comparable effect to intratumorally injected CAR T cells in murine models (Nellan et al., 2018). Murad et al. generated CAR T cells against tumor-associated glycoprotein 72 with a 4–1BB intracellular co-stimulatory signaling domain (TAG72-BBζ) and tested their efficacy in murine models of peritoneal ovarian tumors using a regional, intraperitoneal injection (Murad et al., 2018). The authors found that mice showed rapid antitumor responses after an intraperitoneal injection of TAG72-BBζ CAR T cells, while an intravenous injection of the same therapy showed only a marginal antitumor response.
The beneficial effects of local delivery of CAR T cells have been investigated in clinical trials; for example, Van Schalkwyk et al. described a clinical trial (NCT01818323) to evaluate the efficacy of intratumoral injection of CAR T cells targeting ErbB in patients with head and neck squamous cell carcinoma (HNSCC) in an effort to minimize toxicity (van Schalkwyk et al., 2013). In another work, Brown et al. reported that intracranial infusion of CAR T cells targeting IL13Rα2 in a patient with glioblastoma (NCT02208362) resulted in regression of all observable intracranial and spinal tumors with no major toxic effects (C. E. Brown et al., 2016).
To aid in the local delivery of engineered T cells, biopolymer implants have been engineered to facilitate sustained local delivery of adoptive T cells directly to solid tumors (Smith et al., 2017; Stephan et al., 2015). Because a large number of T cells must be generated ex vivo in a short period of time prior to therapy, techniques that can increase the speed and efficiency of this process are highly desirable. Currently, the commercially available superparamagnetic Dynabeads coated with anti-CD3 antibodies (aCD3) and anti-CD28 antibodies (aCD28) are considered the standard for ex vivo T cell isolation, activation and expansion (Neurauter et al., 2007). Biomaterials can facilitate the ex vivo expansion and activation of T cells prior to their administration (Lin et al., 2018; Schluck, Hammink, Figdor, Verdoes, & Weiden, 2019). Specifically, hydrogel-based scaffolds have been investigated for use in local cell delivery as well as in the ex vivo expansion and activation of T cells (Monette, Ceccaldi, Assaad, Lerouge, & Lapointe, 2016; Weiden et al., 2018). In one example, Tsao et al. engineered a thermosensitive poly(ethylene glycol)-g-chitosan hydrogel to serve as a local T cell delivery depot for the treatment of glioblastoma (Tsao et al., 2014). The authors demonstrated that the T cells released from the hydrogel could kill the glioblastoma model cells, U-87 MG, in vitro. In another work, Guasch engineered polyethylene glycol hydrogels cross-linked with integrin-activating fibronectin-derived peptides, decorated with gold nanoparticles functionalized with aCD3, and co-stimulated with aCD28 for enhanced ex vivo T cell activation and proliferation (Guasch, Muth, Diemer, Riahinezhad, & Spatz, 2017). Therefore, the authors demonstrated that by providing extracellular matrix interaction cues to T cells, T cell activation and expansion could be enhanced.
In addition to hydrogels, polydimethylsiloxane (PDMS) microbeads coated with aCD3 and aCD28 have also been engineered to enhance T cell ex vivo expansion, and were reported to increase T cell population faster than Dynabeads (Lambert et al., 2017). To increase ex vivo T cell growth rate, Fadel et al. bound carbon nanotube-polymer composites carrying antigens and biotinylated T cell stimuli to PLGA nanoparticles carrying IL-2 and magnetite, in order to create artificial APC cues to T cells (Fadel et al., 2014). The authors validated the resulting therapeutic T cells in animal tumor models and reported that a 3 order of magnitude higher concentration of IL-2 was necessary to produce an approximately similar number of T cells without the carbon nanotube-based therapy. In a related work, Cheung et al. designed a scaffold to mimic antigen-presenting cells to increase T cell ex vivo expansion rate using fluid lipid bilayers supported by mesoporous silica micro-rods carrying aCD3, aCD28, and IL-2 (Alexander S. Cheung, Zhang, Koshy, & Mooney, 2018). Collectively, these examples demonstrate how recent advances in the fields of biomaterials and nanomedicine can enhance both the local delivery and ex vivo preparation of adoptive T cell immunotherapies.
5. Local Delivery of Combination Therapy
Local immunotherapy can be enhanced through combination with chemotherapy, phototherapy, radiotherapy, or another type of immunotherapy (Qian Chen, Muchao Chen, et al., 2019). Several local combination therapies have demonstrated increased efficacy and minimal toxicity in the clinic. For example, Chesney et al. reported that intralesional injection of Talimogene laherparepvec and systemic aCTLA-4 led to significantly higher objective response rates than that of aCTLA-4 alone in patients with advanced, unresectable melanoma (Chesney et al., 2018). Some combinations result in synergistic antitumor effects, while others result in enhanced but no synergistic effects (Q. Hu, Sun, Wang, & Gu, 2016). To lower the additional costs associated with combination therapies (Almutairi et al., 2019), it is desirable to achieve synergistic rather than additive effects since synergistic combinations produce greater results than the sum of both agents. Therefore, it is important to understand the mechanisms by which different therapies function in order to achieve the most potent synergistic antitumor effect (Nam et al., 2019; Smyth, Ngiow, Ribas, & Teng, 2016). For example, it has been reported that cancer vaccine formulation dictates synergy or lack thereof with CTLA-4 and PD-L1 checkpoint blockade therapy (Hailemichael et al., 2018). Furthermore, the timing of different therapeutics administered in combination immunotherapy could be critical for achieving the most potent antitumor effects. For example, Messenheimer et al. reported that OX40 therapy followed by PD-1 therapy resulted in significantly enhanced therapeutic effects compared to PD-1 therapy followed by OX40 therapy (Messenheimer et al., 2017). A thorough understanding of interferon and innate immune signaling pathways as well as cancer immunology is crucial for the rational design of personalized combination cancer immunotherapy treatment strategies (Minn & Wherry, 2016; Moynihan et al., 2016; Huitong Ruan et al., 2019).
5.1. Combination Immunotherapy
Numerous combinations of immunotherapeutics have been reported to result in enhanced antitumor efficacy, including PD-1 blockade and CTLA-4 blockade (Curran, Montalvo, Yagita, & Allison, 2010), PD-1 blockade and IL-21 therapy (Pan et al., 2013), PD-L1 blockade and IL-2 therapy (West et al., 2013), aCD40 and a toll-like receptor-4 agonist (Van De Voort, Felder, Yang, Sondel, & Rakhmilevich, 2013), CTLA-4 or PD-1 blockade and CpG (Mangsbo et al., 2010), PD-1 blockade and aOX40 (Z. Guo et al., 2014), CTLA-4 or PD-1 blockade and a tumor-selective oncolytic vaccinia virus encoding IL-12 and IL-7 (Nakao et al., 2020), and also, a mixture of mRNAs encoding IL-23, IL-36γ, and OX40L (Hewitt et al., 2019). In one study, Sagiv-Barfi et al. investigated the mechanisms by which a combination therapy consisting of an intratumoral injection of aOX40 and CpG results in a synergistic and systemic antitumor host immune response for treating multiple types of cancer (Sagiv-Barfi et al., 2018). It was reported that CpG causes myeloid-derived cells to secrete pro-inflammatory cytokines, including IL-12, IFNγ, and TNFα, which causes CD4+ T cells in the tumor microenvironment to upregulate OX40. The aOX40 in this formulation then binds OX40, which activates tumor-infiltrating effector T cells (Teffs) while inhibiting regulatory T cell (Treg) activity, resulting in a therapeutic antitumor immune response. Tregs have been reported to induce tumor-infiltrating CTL dysfunction (Bauer et al., 2014). The authors found that the systemic antitumor immune response was specific to the tumor antigens present at the locally injected site; therefore, this strategy has the potential to reduce the chance of tumor recurrence at metastatic sites. The authors were able to increase antitumor efficacy while minimizing systemic toxicity by using a lower local dose of CpG and aOX40 than if given systemically. Other combination therapies incorporating CpG have shown promising results; Kwong et al. engineered PEGylated liposomes with surface-conjugated CpG and aCD40 for intratumoral injection, and found reduced toxicities associated with systemic exposure to CpG and aCD40 (Kwong, Liu, & Irvine, 2011). In another work, Wang et al. designed DNA nanococoons to locally release aPD-1 and CpG in the presence of inflammatory conditions, and reported that this bioresponsive formulation resulted in synergistic therapeutic effects (C. Wang, Sun, Wright, Wang, & Gu, 2016).
Several oncolytic virotherapies have been reported to induce enhanced or synergistic antitumor effects when combined with checkpoint inhibitors in both experimental cancer models and in clinical trials (Bourgeois-Daigneault et al., 2018; C. Y. Chen, Hutzen, Wedekind, & Cripe, 2018; LaRocca & Warner, 2018; Antoni Ribas et al., 2017; Saha, Martuza, & Rabkin, 2018; Zamarin et al., 2014). Intratumoral injection of oncolytic viruses in combination with immune checkpoint blockade can increase objective clinical response rates, especially in patients with immune checkpoint blockade resistant tumors (Shekarian et al., 2019). In one example, Bartee et al. designed a locally injected oncolytic virus, which induced host antitumor responses and caused infected cells to produce a soluble form of PD-1 (Bartee, Dunlap, & Bartee, 2017). The authors found that local treatment with this virus combined with systemic depletion of CD4 resulted in enhanced antitumor effects in melanoma-bearing tumor models. Oncolytic viruses have also been utilized to generate therapeutic immune cytokines at the tumor site; Wang et al. designed a tumor-targeted oncolytic virus encoding a modified version of IL-12 to limit systemic exposure to IL-12, and verified its antitumor efficacy in a subcutaneous animal model of pancreatic cancer (P. Wang et al., 2017). Plant virus nanoparticles, which are potentially safer than mammalian viruses due to their lack of infectious properties in mammals (C. Wang, Beiss, & Steinmetz, 2019), have also been under investigation for local combination immunotherapy. For instance, Wang et al. demonstrated that in situ treatment with cowpea mosaic virus nanoparticles and CD47 blockade achieved the most significant reduction in tumor volume in 4T1 breast tumor model bearing mice (C. Wang & Steinmetz, 2019).
Hydrogels can facilitate the local delivery of multiple therapeutics at the tumor site and can be engineered to degrade and release their contents under certain desired conditions (Bae & Kurisawa, 2016; Elias et al., 2015; Ishii, Kaneko, & Nagasaki, 2016; Sharifzadeh & Hosseinkhani, 2017; Thambi, Li, & Lee, 2017; Van Tomme & Hennink, 2007). For example, Lemdani et al. designed a thermosensitive mucoadhesive hydrogel for the sustained local delivery of two immunomodulators: Heat-killed Mycobacterium tuberculosis (HKMT) and GM-CSF (Lemdani et al., 2019). The bioadhesive properties of this gel enhanced its efficacy by prolonging its in vivo half-life. In another work, Hori et al. designed an alginate-based hydrogel for peritumoral delivery of therapeutic dendritic cells and IL-15 superagonist (IL-15SA), and found that peritumoral injections resulted in an approximately 40-fold higher concentration of IL-15SA at the tumor site in comparison to systemically injected IL-15SA (Hori, Stern, Hynes, & Irvine, 2009). In another example, Song et al. engineered an injectable PEG-b-poly(L-alanine) hydrogel for sustained local co-delivery of tumor cell lysate, GM-CSF, aCTLA-4, and aPD-1 (H. Song et al., 2019). This therapy resulted in an enhanced tumor specific CTL response, while decreasing systemic exposure to the antibodies. Oh et al. engineered a gelatin-based hydrogel for local delivery of dendritic cells and oncolytic adenoviruses expressing IL-12 and GM-CSF, which enhanced retention of the therapeutic agents at the tumor site (Oh et al., 2017). Nanoscale liposomal polymeric gels with hydrogel cores to deliver IL-2 and a TGF- β inhibitor have also been engineered (J. Park et al., 2012).
5.2. Immunochemotherapy
Immunochemotherapy (or equivalently, chemoimmunotherapy) aims to enhance the efficacy of cancer treatment by combining a chemotherapeutic agent to directly inhibit tumor growth while also potentiating the patient’s immune system to attack the tumor using an immunotherapeutic agent (Y. L. Chen, Chang, & Cheng, 2017). In addition to causing cell cycle arrest and subsequent cell apoptosis in both cancerous and non-malignant cells, antineoplastic chemotherapeutic drugs may also indirectly serve as immunotherapeutic agents by activating dendritic cells upon release of “danger” signals, such as HMGB1 from dying tumor cells, or by directly enhancing the antigen-presenting function of dendritic cells (Shurin, Tourkova, Kaneno, & Shurin, 2009; Tanaka, Matsushima, Mizumoto, & Takashima, 2009). Combination oncolytic virotherapy and chemotherapy has made tremendous progress in preclinical cancer models and has advanced to clinical trials (Praveen K. Bommareddy, Aspromonte, Zloza, Rabkin, & Kaufman, 2018; Simpson, Relph, Harrington, Melcher, & Pandha, 2016). In one example, Yang et al. reported that a combination therapy consisting of an engineered adenovirus expressing IL-24 and temozolomide induced an antitumor effect in tumor-bearing mice (M. Yang et al., 2018). Several nanoparticle-based local immunochemotherapy delivery strategies have been engineered to enhance treatment efficacy. For instance, Lee et al. found that intratumoral injection of potato virus X (PVX)-based nanoparticles and doxorubicin resulted in an enhanced antitumor response, although PVX nanoparticles carrying doxorubicin produced inferior antitumor effects (Lee et al., 2017). Doxorubicin is a chemotherapeutic drug that interferes with the cellular machinery necessary for DNA replication (Rivankar, 2014). In another example, Lu et al. engineered self-assembling indoximod prodrug nanovesicles for local co-administration with the chemotherapeutic drug, oxaliplatin, and validated this immunogenic cell death combination therapy using syngeneic murine models of pancreatic ductal adenocarcinoma (J. Lu et al., 2017). Locally injected formulations, which can potentially enhance the efficacy of immunochemotherapy treatment, include hydrogel-based formulations, porous silica-based formulations, nanogel-based formulations, and engineered nanoparticles.
By facilitating a sustained local release of therapeutic agent(s), hydrogels can enhance the efficacy of immunochemotherapy while minimizing the risk of systemic toxicity (Fakhari & Anand Subramony, 2015; J. Li & Mooney, 2016; Oliva, Conde, Wang, & Artzi, 2017; Singh & Peppas, 2014). In one example, Li et al. reported the local co-delivery of celecoxib and aPD-1 from an alginate hydrogel could synergistically enhance antitumor immunity (Y. Li et al., 2016). The angiogenesis inhibition effect of celecoxib limits the ability of the tumor to obtain the necessary nutrients and eliminate waste, and thereby inhibits tumor growth. The authors found that relatively slow, local, sustained release of both celecoxib and aPD-1 from the alginate hydrogel resulted in enhanced antitumor efficacy in comparison to celecoxib and aPD-1 administered in PBS. In addition to limiting tumor vascularization, anti-angiogenesis therapy has been found to reprogram the tumor microenvironment from an immunosuppressive state to an immune-supportive state (Yi et al., 2019). In another work, Jiang et al. designed a thermosensitive poly(D,L-lactide-co-glycolide)-poly(ethylene glycol)-poly(D,L-lactide-co-glycolide) (PLGA-PEG-PLGA) injectable hydrogel-based drug delivery system containing multifunctional dendritic nanoparticles to locally deliver doxorubicin and L-Arginine to the tumor site (Jiang et al., 2018). L-Arginine serves as the substrate for inducible nitric oxide synthase enzymes present in M1 macrophages in the tumor microenvironment, which then kills tumor cells by producing nitric oxide (NO) (Jiang et al., 2018). A hydrogel formulation that makes use of the abundant ROS present in the tumor microenvironment was reported by Wang et al., who engineered an in situ formed ROS-responsive hydrogel-based scaffold to locally deliver gemcitabine and aPD-L1 to the tumor site upon reactive oxygen species-dependent hydrogel degradation (C. Wang et al., 2018). Gemcitabine is a chemotherapeutic agent that inhibits cellular processes necessary for DNA synthesis, and has been reported to reduce the number of myeloid suppressor cells in tumor-bearing animals (Plunkett et al., 1995; Suzuki, Kapoor, Jassar, Kaiser, & Albelda, 2005). In another work, Ruan et al. engineered a pH and reactive oxygen species-dependent hydrogel-based delivery system for local co-delivery of aPD-1 and zebularine, which is a hypomethylating agent that is reported to enhance tumor-associated antigen expression (H. Ruan et al., 2019). A chitosan hydrogel-based delivery system for the local delivery of doxorubicin and vaccinia virus vaccine, expressing a protein that enhances antigenicity to the tumor site, has been reported to result in synergistic antitumor effects (Han et al., 2008). Additionally, hydrogel-based delivery systems for immunochemotherapy have been engineered to locally deliver GM-CSF and doxorubicin, cisplatin, or cyclophosphamide (Seo, Han, Noh, Kim, & Son, 2009), doxorubicin, IL-2 and IFNγ (Lv, He, Quan, Yu, & Chen, 2018), IL-15 and cisplatin (X. Wu et al., 2017), as well as doxorubicin and melittin (Jin et al., 2018).
Several porous silica delivery systems have been reported to facilitate enhanced local immunochemotherapy treatment. For example, Qian et al. investigated the use of intratumorally injected mesoporous silica-zinc oxide micro-rosettes (MS-Zn) containing doxorubicin and polyinosinic-polycytidylic acid sodium salt (PIC) for enhanced immunochemotherapy (G. Qian et al., 2019). PIC is a TLR3 ligand which causes the release of pro-inflammatory cytokines from dendritic cells, which could help counteract the immunosuppressive tumor microenvironment (Han et al., 2016). In addition, the MS and Zn are both immune potentiators that further counteract the immunosuppressive tumor microenvironment (X. Wang et al., 2016). The authors found that this formulation resulted in both local tumor regression as well as systemic antitumor response. Furthermore, the authors engineered the MS-Zn micro-rosettes to exhibit increased release rate of the loaded drugs at lower pH values. Since the tumor microenvironment is slightly acidic, it is desirable for drug delivery vehicles to exhibit increased release rates in more acidic environments. Mice treated with the MS-Zn-DOX-PIC were found to have a greater CD4+ and CD8+ T cell populations in their splenocytes than mice treated with free DOX-PIC, demonstrating that the MS-Zn delivery vehicle played a crucial role in promoting systemic antitumor immunity.
Nanogels, which are gels with diameters usually less than 100 nm, can be useful drug delivery vehicles for local or systemic immunochemotherapy due to their ability to hold large therapeutic biomolecules and potential to serve as inherent immunostimulatory agents (Purwada et al., 2016; Song et al., 2017; Tahara & Akiyoshi, 2015). In one example, Wu et al. engineered nanogel-incorporated chemical and physical hybrid hydrogels for extended peritumoral release of IL-2, IFNγ, and doxorubicin (Xilong Wu, He, Wu, Chen, & Cheng, 2015). By facilitating local and sustained release of IL-2, IFNγ, and doxorubicin, the nanogel-integrated hydrogel carrying system demonstrated a significant synergistic effect in experimental A549 xenograft tumors compared to protein therapy or chemotherapy alone. A syringeable immunomodulatory multidomain nanogel (iGel), formed by positively charged nanoliposomes and negatively charged multi-nanodomain vesicles carrying gemcitabine and R837, was engineered by Song et al. for local immunochemotherapy treatment (C. Song et al., 2019). By extending the release time to over one week, the iGel significantly reduced systemic toxicity, inhibited tumor metastasis, and achieved systemic antitumor immunity in murine cancer models. In another work, Phuengkham et al. engineered a 3D hyaluronic acid and collagen-based porous scaffold for postoperative peritumoral implantation, which consists of gemcitabine, a whole tumor lysate vaccine, and nanogels loaded with the TLR3 agonist, polyinosinic:polycytidylic acid (Phuengkham, Song, Um, & Lim, 2018). The authors found that postsurgical implantation of this nanogel-containing scaffold inhibited tumor recurrence and tumor metastasis, while inducing a systemic antitumor immune response.
5.3. Photoimmunotherapy
Photothermal therapy (PTT) and photodynamic therapy (PDT) are two types of phototherapy under investigation for use in combination immunotherapy (Sanchez-Barcelo & Mediavilla, 2014). In photothermal therapy, photosensitizer molecules absorb light and convert it into heat to ablate tumor cells and induce the release of tumor antigens (Liang, Xu, Song, & Liu, 2016). Photothermal immunotherapy can enhance the therapeutic efficacy of immune checkpoint blockade, therapeutic cancer immunotherapy vaccines, and CAR T cell therapy (Qian Chen, Quanyin Hu, et al., 2019; T. Wang et al., 2018; X. Ye et al., 2019). However, due to immune-related adverse events observed in patients receiving in situ photothermal immunotherapy, it is desirable to engineer local delivery strategies that can mitigate these side effects while also increasing therapeutic efficacy (X. Li et al., 2010).
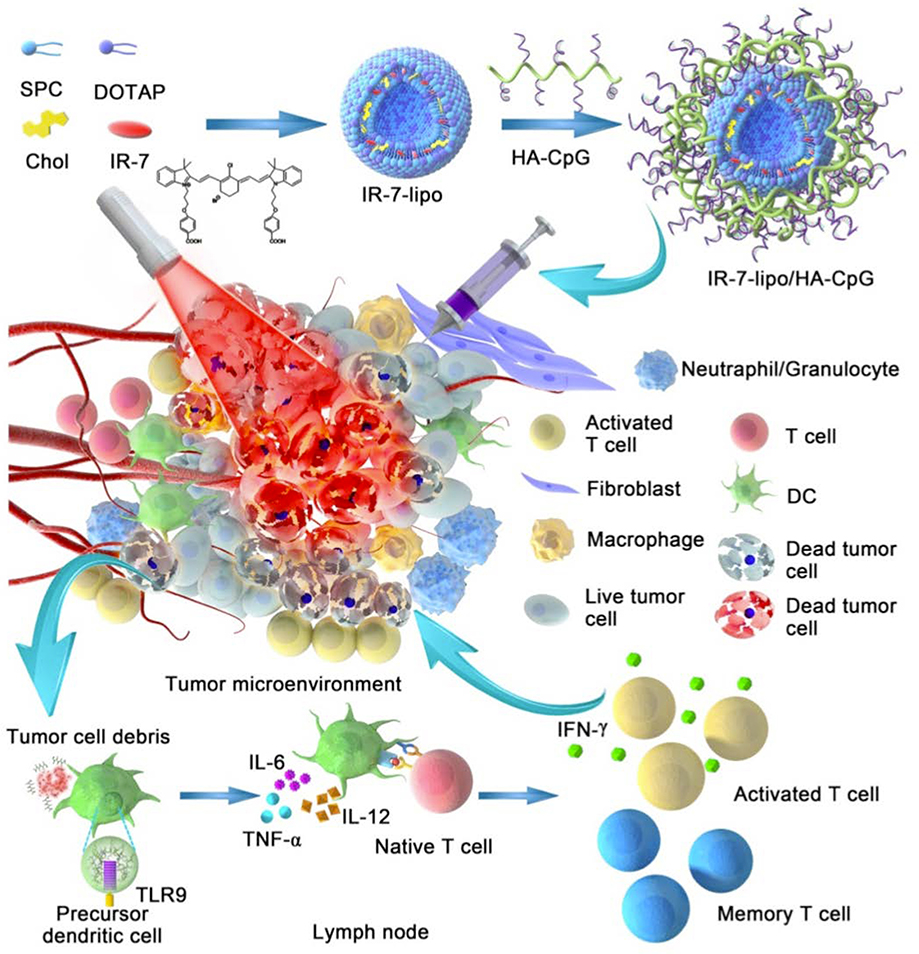
Chen et al. demonstrated that the release of tumor antigens during local photothermal immunotherapy could induce tumor immunity following intratumoral injection of poly(lactic-co-glycolic) acid (PLGA) nanoparticles carrying a photothermal agent, indocyanine green, and the toll-like receptor-7 agonist, R837, followed by irradiation with a 808 nm laser (Q. Chen et al., 2016). R837 serves as an adjuvant that primes host immune cells to recognize the released tumor antigens and to subsequently induce an antitumor response. Similarly, Xu et al. engineered upconversion nanoparticles carrying clorin e6, a photothermal agent, and R837 for intratumoral injection (J. Xu et al., 2017). In another work, Cano-Mejia et al. designed a photothermal immunotherapy using intratumorally injected pH-sensitive and immunostimulatory Prussian blue nanoparticles with local 808 nm laser irradiation, and found that this therapy could rapidly decrease tumor burden in the murine model of neuroblastoma (Cano-Mejia et al., 2017). These nanoparticles remain stable and reach higher temperatures in the relatively acidic tumor microenvironment (pH 5–6) but degrade at systemic pH (7.4). Therefore, this response increases cancer cell ablation at the tumor site while minimizing systemic activity. Several local photothermal immunotherapy strategies utilize CpG in order to induce immunostimulatory activity of antigen-presenting cells in the tumor microenvironment. For example, Guo et al. engineered intratumorally injectable chitosan-coated hollow copper sulfide nanoparticles containing CpG for photothermal immunotherapy (L. Guo et al., 2014). Similarly, CpG-containing hydrogel nanoparticles have been engineered (Dong et al., 2019; Yata et al., 2017). In one work, Tao et al. engineered CpG-loaded polyethylene glycol and polyethylenimine dual-polymer-functionalized cationic graphene oxide (GO-PEG-PEI-CpG) for enhanced intratumoral local photothermal immunotherapy (Tao, Ju, Ren, & Qu, 2014). The authors found that GO-PEG-PEI-CpG therapy could induce the local release of cytokines, including TNF-α and IL-6. Li et al. engineered IR-7-loaded liposomes coated with hyaluronic acid-CpG for local photothermal immunotherapy, and reported that this combination therapy could induce tumor cell necrosis and subsequent release of tumor-associated antigens, and could activate antigen-presenting cells (Figure 7) (L. Li et al., 2018).
Figure 7: Local Photothermal Immunotherapy.
The schematic showing the delivery of IR-7-loaded liposomes coated with hyaluronic acid-CpG for local photothermal immunotherapy. Adapted from (L. Li et al., 2018) under a Creative Commons Attribution (CC BY-NC) license.
Single-walled carbon nanotubes are also useful for photothermal therapy due to their ability to convert optical energy into thermal energy upon irradiation. A photothermal immunotherapy approach using intratumorally injected PEGylated single-walled carbon nanotubes exposed to 808 nm laser irradiation in combination with systemic CTLA-4 blockade therapy resulted in significantly reduced lung metastases and inhibited the development of secondary tumors (C. Wang et al., 2014). Another method to enhance the efficacy of single-walled carbon nanotubes for photothermal immunotherapy was reported by Zhou et al., who engineered glycated chitosan modified single-walled carbon nanotubes that could enhance tumor immunogenicity (Zhou et al., 2012). In a related example, Li et al. studied the effects of combined intratumorally injected glycated chitosan modified single-walled carbon nanotubes and intraperitoneally injected CTLA-4 immune checkpoint blockade, and found that this combination resulted in systemic antitumor immunity in murine models of metastatic breast cancer (Y. Li et al., 2019).
Whereas PTT uses heat to locally ablate the tumor, PDT combines photosensitizers, light, and oxygen to generate ROS to induce immunogenic cell death in situ. In one example, Lu et al. engineered chlorin-based nanoscale metal−organic framework, TBC-Hf, to locally deliver a small molecule IDO inhibitor and locally generate ROS upon light irradiation (K. Lu et al., 2016). ROS caused the release of tumor associated antigens, which were subsequently presented to T cells, and the authors reported that this combination therapy achieved systemic antitumor immunity in murine cancer models. Another photodynamic-immunotherapy approach was reported by Meng et al., who engineered a photosensitizer-modified catalase poly(ethylene glycol) double acrylate (PEGDA)-based light triggered in situ gelation system for local delivery of R837-loaded PLGA nanoparticles, and in situ generation of ROS (Meng et al., 2019). Combination therapies utilizing both photothermal therapy and photodynamic therapy have also been reported (Yan et al., 2019). In one recent example, Wang et al. fabricated antigen-capturing nanoplatforms for simultaneous photothermal and photodynamic immunotherapy via the self-assembly of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG-mal) and indocyanine green onto upconversion nanoparticles, which were further modified with the photosensitizer, rose bengal (UCNP/ICG/RB-mal) (M. Wang et al., 2019). The authors reported that upon intratumoral injection and subsequent NIR laser irradiation, UCNP/ICG/RB-mal therapy could eliminate the treated tumor while significantly decreasing the growth of untreated distant tumors.
5.4. Radiotherapy-enhanced Immunotherapy
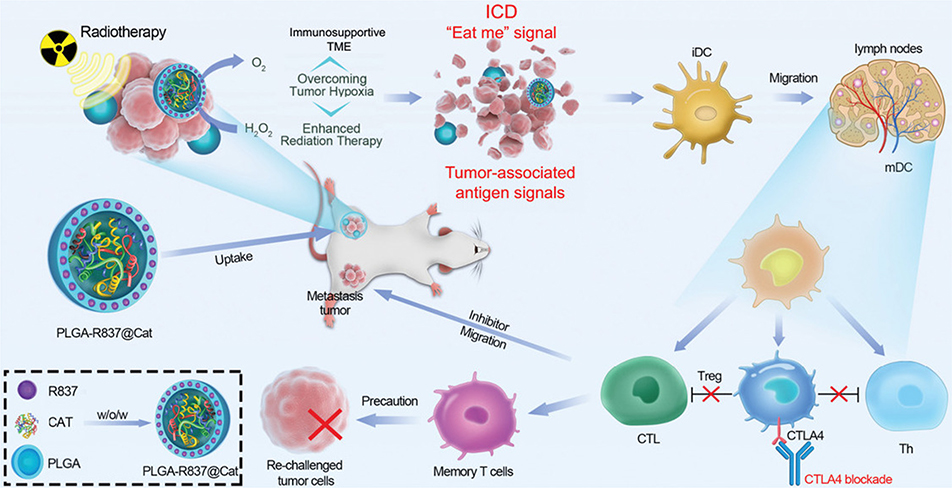
Several preclinical studies and clinical trials have investigated the potential therapeutic benefits of combining immunotherapy with radiotherapy (Formenti & Demaria, 2013; Levy, Massard, Soria, & Deutsch, 2016; Mujoo et al., 2018; Reynders, Illidge, Siva, Chang, & De Ruysscher, 2015; Tsui, Mihalcioiu, & Cury, 2018), as well as the underlying biological mechanisms that can explain these benefits (Apetoh et al., 2007; Barker, Paget, Khan, & Harrington, 2015). The mechanisms by which radiotherapy can induce antitumor immune responses were reviewed by Spiotto et al., who reported that radiotherapy increases inflammation in tumors, facilitates dendritic cell maturation following the release of tumor-associated antigens, and increases T cell priming and infiltration into tumors (Spiotto, Fu, & Weichselbaum, 2016). Furthermore, radiation therapy enhances immunotherapy in part by increasing the diversity of T cell receptors in the tumor microenvironment and by causing release of tumor antigens, which play a crucial role in the establishment of tumor immunity (Mujoo et al., 2018; Twyman-Saint Victor et al., 2015). Radiotherapy-induced cell death can cause the release of endogenous immune adjuvants, which alert the immune system to danger (Kono & Rock, 2008). Local radiotherapy combined with immune checkpoint blockade can jump-start a systemic response of tumors previously unresponsive to immune checkpoint blockade alone (Demaria, Coleman, & Formenti, 2016; Kalbasi, June, Haas, & Vapiwala, 2013). In one preclinical study, radiation and PD-L1 therapy resulted in a synergistic antitumor immunity through a CTL–mediated mechanism (Deng et al., 2014). However, due to the toxicities and adverse effects observed in patients receiving combination radiotherapy with immune checkpoint blockade therapy, further work is necessary to mitigate such risks (Hwang, Pike, Royce, Mahal, & Loeffler, 2018). Radiotherapy is able to directly induce cell death at the specific location where it is administered, but usually fails to result in regression of cancer cells at untreated sites, which is known as the abscopal effect (Z. I. Hu, McArthur, & Ho, 2017). Several reports of locally delivered immunotherapeutics in combination with radiation therapy have been reported to achieve abscopal effects. For instance, Yasmin-Karim et al. reported that intratumoral injection of aCD40 in combination with stereotactic body radiation resulted in synergistic regression of untreated tumors in murine models of pancreatic cancer, and achieved an abscopal effect (Yasmin-Karim et al., 2018).
Biomaterials and nanomedicines have been utilized to enhance the efficacy and safety of local immunotherapy administered in combination with radiotherapy. Nanoscale metal-organic frameworks have recently been utilized in local radiotherapy-enhanced immunotherapy, owing to their biocompatibility and ability to serve as radioenhancers by absorbing X-ray energy. In one example, intratumoral injection of nanoscale metal-organic frameworks loaded with IDO inhibitors in combination with low-dose X-ray radiation treatment resulted in an abscopal antitumor effect in murine models of breast and colorectal cancer (K. D. Lu et al., 2018). Nanoscale metal-organic frameworks have also been engineered to enhance combination immune checkpoint blockade radiotherapy. Ni et al. reported that two porous, Hf-based nanoscale metal-organic frameworks could enhance the efficacy of radiotherapy upon intratumoral injection, and could trigger tumor regression in untreated tumors when combined with PD-L1 checkpoint blockade therapy, thereby achieving an abscopal effect (Ni et al., 2018). In another work, Min et al. fabricated antigen-capturing nanoparticles to enhance the delivery of tumor specific antigens released during radiotherapy to APCs in order to generate an abscopal effect (Min et al., 2017). In another example, Chen et al. designed PLGA nanoparticles containing catalase enzymes and the TLR7 agonist, R837, and found that intratumoral injection of these nanoparticles along with systemic administration of aCTLA-4 achieved an abscopal effect in murine models of cancer undergoing radiation therapy (Figure 8) (Q. Chen et al., 2019). The authors found that the ability of the catalase enzyme to decrease hypoxia in the tumor microenvironment enhanced the therapeutic efficacy of radiotherapy.
Figure 8: Nanoparticle Assisted Radiotherapy.
The schematic showing the mechanism for PLGA-R837@CAT nanoparticle-assisted radiotherapy. Adapted with permission from (Q. Chen et al., 2019). Copyright 2019, John Wiley & Sons, Inc.
Plant-derived virus-based nanoparticles have also been reported to enhance radiation therapy. Patel et al. reported that intratumoral injection of cowpea mosaic virus in combination with radiation therapy in animal models of serous ovarian cancer resulted in enhanced therapeutic efficacy in comparison to radiation therapy alone (Patel, Czapar, Fiering, Oleinick, & Steinmetz, 2018). In another work, Hoopes et al. reported that intratumoral injection of plant-based virus-like nanoparticles in two canine oral melanoma patients undergoing hypofractionated radiation effectively treated the local tumor and showed no relapse within 5–9 months post-treatment (Hoopes et al., 2018). Hydrogels have been engineered to facilitate the local delivery of combination immunotherapy-radiotherapy. In one example, Chao et al. engineered a radioactive sodium alginate formulation capable of in situ gelation to facilitate the local delivery of therapeutic 131I radioisotopes along with immunostimulatory CpG oligonucleotides (Chao et al., 2018).
Funding Information
This work was supported by the UCLA startup package to Z.G and the NIH–NCI R01 CA234343–01A1 grant.
Footnotes
P.A. declares no competing interests
Z.W. declares no competing interests
Q.C. declares no competing interests.
A.C. declares no competing interests.
D.R.Z declares no competing interests.
V.G. declares no competing interests.
Z.G. is a scientific co-founder of ZenCapsule Inc.
References
- Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, . . . Sadelain M (2014). Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science Translational Medicine, 6(261), 261ra151–261ra151. doi: 10.1126/scitranslmed.3010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali OA, Emerich D, Dranoff G, & Mooney DJ (2009). In Situ Regulation of DC Subsets and T Cells Mediates Tumor Regression in Mice. Science Translational Medicine, 1(8), 8ra19–18ra19. doi: 10.1126/scitranslmed.3000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C, Her S, & Jaffray DA (2017). Radiotherapy for Cancer: Present and Future. Advanced Drug Delivery Reviews, 109, 1–2. doi: 10.1016/j.addr.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Almutairi AR, Alkhatib NS, Oh M, Curiel-Lewandrowski C, Babiker HM, Cranmer LD, . . . Abraham I. (2019). Economic Evaluation of Talimogene Laherparepvec Plus Ipilimumab Combination Therapy vs Ipilimumab Monotherapy in Patients With Advanced Unresectable Melanoma. JAMA Dermatology, 155(1), 22–28. doi: 10.1001/jamadermatol.2018.3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, & Iyer AK (2017). PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Frontiers in Pharmacology, 8, 561. doi: 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M, Yu C, Xi J, Reyes J, Mao G, Wei WZ, & Liu H (2018). Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale, 10(19), 9311–9319. doi: 10.1039/c8nr01376d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, . . . Zitvogel L (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Medicine, 13(9), 1050–1059. doi: 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria J-C, Zitvogel L, & Marabelle A (2016). Rationale for anti-OX40 cancer immunotherapy. European Journal of Cancer, 52, 50–66. doi: 10.1016/j.ejca.2015.08.021 [DOI] [PubMed] [Google Scholar]
- Aznar MA, Tinari N, Rullan AJ, Sanchez-Paulete AR, Rodriguez-Ruiz ME, & Melero I (2017). Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. Journal of Immunology, 198(1), 31–39. doi: 10.4049/jimmunol.1601145 [DOI] [PubMed] [Google Scholar]
- Bae KH, & Kurisawa M (2016). Emerging hydrogel designs for controlled protein delivery. Biomaterials Science, 4(8), 1184–1192. doi: 10.1039/c6bm00330c [DOI] [PubMed] [Google Scholar]
- Barker HE, Paget JT, Khan AA, & Harrington KJ (2015). The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature Reviews Cancer, 15(7), 409–425. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee MY, Dunlap KM, & Bartee E (2017). Tumor-Localized Secretion of Soluble PD1 Enhances Oncolytic Virotherapy. Cancer Research, 77(11), 2952–2963. doi: 10.1158/0008-5472.Can-16-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, & Mempel TR (2014). Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. Journal of Clinical Investigation, 124(6), 2425–2440. doi: 10.1172/jci66375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Li Y, & Long KB (2017). Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Review of Anticancer Therapy, 17(2), 175–186. doi: 10.1080/14737140.2017.1270208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, & O’Hara M (2016). Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacology & Therapeutics, 166, 30–39. doi: 10.1016/j.pharmthera.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsch F, Knoblock DM, Liu A, McAllister F, & Beatty GL (2017). CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunology, Immunotherapy, 66(12), 1609–1617. doi: 10.1007/s00262-017-2053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, . . . Melero I. (2019). Cytokines in clinical cancer immunotherapy. British Journal of Cancer, 120(1), 6–15. doi: 10.1038/s41416-018-0328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M, Trepel JB, Pastan I, Stroncek DF, Morris JC, & Wood LV (2018). Cancer vaccine strategies: translation from mice to human clinical trials. Cancer Immunology, Immunotherapy, 67(12), 1863–1869. doi: 10.1007/s00262-017-2084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommareddy PK, Aspromonte S, Zloza A, Rabkin SD, & Kaufman HL (2018). MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Science Translational Medicine, 10(471), 0417. doi: 10.1126/scitranslmed.aau0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommareddy PK, & Kaufman HL (2018). Unleashing the therapeutic potential of oncolytic viruses. Journal of Clinical Investigation, 128(4), 1258–1260. doi: 10.1172/jci120303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommareddy PK, Shettigar M, & Kaufman HL (2018). Integrating oncolytic viruses in combination cancer immunotherapy. Nature Reviews Immunology, 18(8), 498–513. doi: 10.1038/s41577-018-0014-6 [DOI] [PubMed] [Google Scholar]
- Bourgeois-Daigneault M-C, Roy DG, Aitken AS, El Sayes N, Martin NT, Varette O, . . . Bell JC (2018). Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Science Translational Medicine, 10(422), eaao1641. doi: 10.1126/scitranslmed.aao1641 [DOI] [PubMed] [Google Scholar]
- Broos S, Sandin LC, Apel J, Totterman TH, Akagi T, Akashi M, . . . Lindstedt M (2012). Synergistic augmentation of CD40-mediated activation of antigen-presenting cells by amphiphilic poly(gamma-glutamic acid) nanoparticles. Biomaterials, 33(26), 6230–6239. doi: 10.1016/j.biomaterials.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, . . . Badie B (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. doi: 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, . . . Nair SK (2017). Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Science Translational Medicine, 9(408). doi: 10.1126/scitranslmed.aan4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, . . . Fernandes R (2017). Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine, 13(2), 771–781. doi: 10.1016/j.nano.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z, . . . Liu Z (2018). Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nature Biomedical Engineering, 2(8), 611–621. doi: 10.1038/s41551-018-0262-6 [DOI] [PubMed] [Google Scholar]
- Chen CY, Hutzen B, Wedekind MF, & Cripe TP (2018). Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virotherapy, 7, 65–77. doi: 10.2147/ov.S145532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen Z, Wen D, Wang Z, Li H, Zeng Y, . . . Gu Z (2020). Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proceedings of the National Academy of Sciences of the United States of America, 117(7), 3687–3692. doi: 10.1073/pnas.1917891117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Chen G, Chen J, Shen J, Zhang X, Wang J, . . . Gu Z (2019). Bioresponsive Protein Complex of aPD1 and aCD47 Antibodies for Enhanced Immunotherapy. Nano Letters, 19(8), 4879–4889. doi: 10.1021/acs.nanolett.9b00584 [DOI] [PubMed] [Google Scholar]
- Chen Q, Chen J, Yang Z, Xu J, Xu L, Liang C, . . . Liu Z (2019). Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Advanced Materials, 31(10), e1802228. doi: 10.1002/adma.201802228 [DOI] [PubMed] [Google Scholar]
- Chen Q, Chen M, & Liu Z (2019). Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chemical Society Reviews. doi: 10.1039/C9CS00271E [DOI] [PubMed] [Google Scholar]
- Chen Q, Ci T, & Gu Z (2019). Sprayable gel for postsurgical immunotherapy. Immuno-Oncology Technology, 2, 11–13. doi: 10.1016/j.iotech.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, . . . Gu Z (2019). Photothermal Therapy Promotes Tumor Infiltration and Antitumor Activity of CAR T Cells. Advanced Materials, 31(23), 1900192. doi: 10.1002/adma.201900192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wang C, Chen G, Hu Q, & Gu Z (2018). Delivery Strategies for Immune Checkpoint Blockade. Advanced Healthcare Materials, 7(20), 1800424. doi: 10.1002/adhm.201800424 [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, . . . Gu Z (2019). In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nature Nanotechnology, 14(1), 89–97. doi: 10.1038/s41565-018-0319-4 [DOI] [PubMed] [Google Scholar]
- Chen Q, Xu L, Liang C, Wang C, Peng R, & Liu Z (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature Communications, 7, 13193. doi: 10.1038/ncomms13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Chang MC, & Cheng WF (2017). Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Letters, 400, 282–292. doi: 10.1016/j.canlet.2017.01.040 [DOI] [PubMed] [Google Scholar]
- Chen Z, Hu Q, & Gu Z (2018). Leveraging Engineering of Cells for Drug Delivery. Accounts of Chemical Research, 51(3), 668–677. doi: 10.1021/acs.accounts.7b00526 [DOI] [PubMed] [Google Scholar]
- Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, . . . Kaufman HL (2018). Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. Journal of Clinical Oncology, 36(17), 1658–1667. doi: 10.1200/jco.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AS, & Mooney DJ (2015). Engineered Materials for Cancer Immunotherapy. Nano Today, 10(4), 511–531. doi: 10.1016/j.nantod.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AS, Zhang DKY, Koshy ST, & Mooney DJ (2018). Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nature Biotechnology, 36, 160. doi: 10.1038/nbt.4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CYX, Jain P, Susnjar A, Rhudy J, Folci M, Ballerini A, . . . Grattoni A (2018). Nanofluidic drug-eluting seed for sustained intratumoral immunotherapy in triple negative breast cancer. Journal of Controlled Release, 285, 23–34. doi: 10.1016/j.jconrel.2018.06.035 [DOI] [PubMed] [Google Scholar]
- Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM, . . . Breitbach CJ (2015). Phase 1 Study of Intratumoral Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, in Pediatric Cancer Patients. Molecular Therapy, 23(3), 602–608. doi: 10.1038/mt.2014.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, & Allison JP (2010). PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America, 107(9), 4275–4280. doi: 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Coleman CN, & Formenti SC (2016). Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer, 2(6), 286–294. doi: 10.1016/j.trecan.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, & Fu YX (2014). Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. Journal of Clinical Investigation, 124(2), 687–695. doi: 10.1172/jci67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaby V, Tawk R, Sanogo V, Xiao H, & Montero AJ (2015). A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Research and Treatment, 151(1), 27–40. doi: 10.1007/s10549-015-3383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Liang J, Yang A, Qian Z, Kong D, & Lv F (2019). Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials, 209, 111–125. doi: 10.1016/j.biomaterials.2019.04.024 [DOI] [PubMed] [Google Scholar]
- Elias PZ, Liu GW, Wei H, Jensen MC, Horner PJ, & Pun SH (2015). A functionalized, injectable hydrogel for localized drug delivery with tunable thermosensitivity: synthesis and characterization of physical and toxicological properties. Journal of Controlled Release, 208, 76–84. doi: 10.1016/j.jconrel.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Fadel TR, Sharp FA, Vudattu N, Ragheb R, Garyu J, Kim D, . . . Fahmy TM (2014). A carbon nanotube–polymer composite for T-cell therapy. Nature Nanotechnology, 9, 639. doi: 10.1038/nnano.2014.154 [DOI] [PubMed] [Google Scholar]
- Fakhari A, & Anand Subramony J (2015). Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. Journal of Controlled Release, 220(Pt A), 465–475. doi: 10.1016/j.jconrel.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Fan Y, & Moon JJ (2015). Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel), 3(3), 662–685. doi: 10.3390/vaccines3030662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Bluestone JA, & Lim WA (2013). Cell-Based Therapeutics: The Next Pillar of Medicine. Science Translational Medicine, 5(179), 179ps177–179ps177. doi: 10.1126/scitranslmed.3005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti SC, & Demaria S (2013). Combining radiotherapy and cancer immunotherapy: a paradigm shift. Journal of the National Cancer Institute, 105(4), 256–265. doi: 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster TH, Stoffel F, & Gasser TC (2002). Hormone therapy in advanced prostate cancer. Frontiers of Radiation Therapy and Oncology, 36, 49–65. doi: 10.1159/000061329 [DOI] [PubMed] [Google Scholar]
- Francis DM, & Thomas SN (2017). Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Advanced Drug Delivery Reviews, 114, 33–42. doi: 10.1016/j.addr.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen MF, Sluijter M, Morreau H, Arens R, & Melief CJ (2011). Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clinical Cancer Research, 17(8), 2270–2280. doi: 10.1158/1078-0432.Ccr-10-2888 [DOI] [PubMed] [Google Scholar]
- Fransen MF, van der Sluis TC, Ossendorp F, Arens R, & Melief CJ (2013). Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clinical Cancer Research, 19(19), 5381–5389. doi: 10.1158/1078-0432.Ccr-12-0781 [DOI] [PubMed] [Google Scholar]
- Furumoto K, Soares L, Engleman EG, & Merad M (2004). Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. Journal of Clinical Investigation, 113(5), 774–783. doi: 10.1172/jci19762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti-Mays ME, Redman JM, Collins JM, & Bilusic M (2017). Cancer vaccines: Enhanced immunogenic modulation through therapeutic combinations. Human Vaccines & Immunotherapeutics, 13(11), 2561–2574. doi: 10.1080/21645515.2017.1364322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary SM, Lemke CD, Lubaroff DM, & Salem AK (2011). Tumor immunotherapy using adenovirus vaccines in combination with intratumoral doses of CpG ODN. Cancer Immunology, Immunotherapy, 60(9), 1309. doi: 10.1007/s00262-011-1038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Mailankody S, Giralt SA, Landgren CO, Smith EL, & Brentjens RJ (2018). CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leukemia & Lymphoma, 59(9), 2056–2067. doi: 10.1080/10428194.2017.1393668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Maus MV, & Porter DL (2016). Chimeric antigen receptor T cell therapy: 25years in the making. Blood Reviews, 30(3), 157–167. doi: 10.1016/j.blre.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, . . . Wargo JA (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science, 359(6371), 97–103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, & Mooney DJ (2016). Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nature Reviews Cancer, 16(1), 56–66. doi: 10.1038/nrc.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, & Sailor MJ (2012). Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Advanced Materials, 24(29), 3981–3987. doi: 10.1002/adma.201200776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch J, Muth CA, Diemer J, Riahinezhad H, & Spatz JP (2017). Integrin-Assisted T-Cell Activation on Nanostructured Hydrogels. Nano Letters, 17(10), 6110–6116. doi: 10.1021/acs.nanolett.7b02636 [DOI] [PubMed] [Google Scholar]
- Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, . . . Lu W (2014). Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano, 8(6), 5670–5681. doi: 10.1021/nn5002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Wang X, Cheng D, Xia Z, Luan M, & Zhang S (2014). PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One, 9(2), e89350. doi: 10.1371/journal.pone.0089350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemichael Y, Woods A, Fu T, He Q, Nielsen MC, Hasan F, . . . Overwijk WW (2018). Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. Journal of Clinical Investigation, 128(4), 1338–1354. doi: 10.1172/jci93303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HD, Byeon Y, Kang TH, Jung ID, Lee JW, Shin BC, . . . Park YM (2016). Toll-like receptor 3-induced immune response by poly(d,l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. International Journal of Nanomedicine, 11, 5729–5742. doi: 10.2147/ijn.S109001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T, . . . Shin BC (2008). A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. International Journal of Pharmaceutics, 350(1–2), 27–34. doi: 10.1016/j.ijpharm.2007.08.014 [DOI] [PubMed] [Google Scholar]
- Hewitt SL, Bai A, Bailey D, Ichikawa K, Zielinski J, Karp R, . . . Frederick JP (2019). Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Science Translational Medicine, 11(477), eaat9143. doi: 10.1126/scitranslmed.aat9143 [DOI] [PubMed] [Google Scholar]
- Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, . . . Fiering SN (2018). Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Molecular Pharmaceutics, 15(9), 3717–3722. doi: 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Stern PJ, Hynes RO, & Irvine DJ (2009). Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials, 30(35), 6757–6767. doi: 10.1016/j.biomaterials.2009.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotaling NA, Tang L, Irvine DJ, & Babensee JE (2015). Biomaterial Strategies for Immunomodulation. Annual Review of Biomedical Engineering, 17, 317–349. doi: 10.1146/annurev-bioeng-071813-104814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Sun W, Wang C, & Gu Z (2016). Recent advances of cocktail chemotherapy by combination drug delivery systems. Advanced Drug Delivery Reviews, 98, 19–34. doi: 10.1016/j.addr.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li X, Zhang C, Yang Y, Jiang J, & Wu C (2016). Tumor-associated macrophages in cancers. Clinical and Translational Oncology, 18(3), 251–258. doi: 10.1007/s12094-015-1373-0 [DOI] [PubMed] [Google Scholar]
- Hu ZI, McArthur HL, & Ho AY (2017). The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Current Breast Cancer Reports, 9(1), 45–51. doi: 10.1007/s12609-017-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh V, Jesmer AH, Shoaib MM, D’Angelo AD, Rullo AF, & Wylie RG (2019). Improved Efficacy of Antibody Cancer Immunotherapeutics through Local and Sustained Delivery. ChemBioChem, 20(6), 747–753. doi: 10.1002/cbic.201800579 [DOI] [PubMed] [Google Scholar]
- Hwang WL, Pike LRG, Royce TJ, Mahal BA, & Loeffler JS (2018). Safety of combining radiotherapy with immune-checkpoint inhibition. Nature Reviews Clinical Oncology, 15(8), 477–494. doi: 10.1038/s41571-018-0046-7 [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Hanson MC, Rakhra K, & Tokatlian T (2015). Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews, 115(19), 11109–11146. doi: 10.1021/acs.chemrev.5b00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara J, Fukunaga K, Ishihara A, Larsson HM, Potin L, Hosseinchi P, . . . Hubbell JA (2017). Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Science Translational Medicine, 9(415). doi: 10.1126/scitranslmed.aan0401 [DOI] [PubMed] [Google Scholar]
- Ishihara J, Ishihara A, Potin L, Hosseinchi P, Fukunaga K, Damo M, . . . Hubbell JA (2018). Improving Efficacy and Safety of Agonistic Anti-CD40 Antibody Through Extracellular Matrix Affinity. Molecular Cancer Therapeutics, 17(11), 2399–2411. doi: 10.1158/1535-7163.Mct-18-0091 [DOI] [PubMed] [Google Scholar]
- Ishii S, Kaneko J, & Nagasaki Y (2016). Development of a long-acting, protein-loaded, redox-active, injectable gel formed by a polyion complex for local protein therapeutics. Biomaterials, 84, 210–218. doi: 10.1016/j.biomaterials.2016.01.029 [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, . . . Weissman IL (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell, 138(2), 271–285. doi: 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes JM, Sable R, Ronzetti M, Bautista W, Knotts Z, Abisoye-Ogunniyan A, . . . Rudloff U (2020). Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Science Translational Medicine, 12(530), eaax6337. doi: 10.1126/scitranslmed.aax6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanbart L, & Swartz MA (2015). Engineering opportunities in cancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America, 112(47), 14467–14472. doi: 10.1073/pnas.1508516112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T, Zhao Y, Ding Y, & Nie G (2013). Using functional nanomaterials to target and regulate the tumor microenvironment: diagnostic and therapeutic applications. Advanced Materials, 25(26), 3508–3525. doi: 10.1002/adma.201300299 [DOI] [PubMed] [Google Scholar]
- Jiang L, Ding Y, Xue XL, Zhou SS, Li C, Zhang XK, & Jiang XQ (2018). Entrapping multifunctional dendritic nanoparticles into a hydrogel for local therapeutic delivery and synergetic immunochemotherapy. Nano Research, 11(11), 6062–6073. doi: 10.1007/s12274-018-2123-8 [DOI] [Google Scholar]
- Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng Y, . . . Yang K (2018). Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano, 12(4), 3295–3310. doi: 10.1021/acsnano.7b08148 [DOI] [PubMed] [Google Scholar]
- Johnson DB, Puzanov I, & Kelley MC (2015). Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy, 7(6), 611–619. doi: 10.2217/imt.15.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimi K, Karasaki T, Matsushita H, & Sugie T (2017). Advances in personalized cancer immunotherapy. Breast Cancer, 24(1), 16–24. doi: 10.1007/s12282-016-0688-1 [DOI] [PubMed] [Google Scholar]
- Kalbasi A, June CH, Haas N, & Vapiwala N (2013). Radiation and immunotherapy: a synergistic combination. Journal of Clinical Investigation, 123(7), 2756–2763. doi: 10.1172/JCI69219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, . . . Schellhammer PF (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England Journal of Medicine, 363(5), 411–422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- Katz SC, Point GR, Cunetta M, Thorn M, Guha P, Espat NJ, . . . Junghans RP (2016). Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Therapy, 23(5), 142–148. doi: 10.1038/cgt.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, & Bommareddy PK (2019). Two roads for oncolytic immunotherapy development. Journal for ImmunoTherapy of Cancer, 7(1), 26. doi: 10.1186/s40425-019-0515-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, & Hämmerling GJ (2001). NK- and CD8+ T Cell-Mediated Eradication of Established Tumors by Peritumoral Injection of CpG-Containing Oligodeoxynucleotides. Journal of Immunology, 167(9), 5247–5253. doi: 10.4049/jimmunol.167.9.5247 [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Rosenberg SA, & Restifo NP (2016). Prospects for gene-engineered T cell immunotherapy for solid cancers. Nature Medicine, 22(1), 26–36. doi: 10.1038/nm.4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komohara Y, Fujiwara Y, Ohnishi K, & Takeya M (2016). Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Advanced Drug Delivery Reviews, 99(Pt B), 180–185. doi: 10.1016/j.addr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Kono H, & Rock KL (2008). How dying cells alert the immune system to danger. Nature Reviews Immunology, 8(4), 279–289. doi: 10.1038/nri2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy ST, & Mooney DJ (2016). Biomaterials for enhancing anti-cancer immunity. Current Opinion in Biotechnology, 40, 1–8. doi: 10.1016/j.copbio.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong B, Gai SA, Elkhader J, Wittrup KD, & Irvine DJ (2013). Localized immunotherapy via liposome-anchored Anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Research, 73(5), 1547–1558. doi: 10.1158/0008-5472.Can-12-3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong B, Liu H, & Irvine DJ (2011). Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials, 32(22), 5134–5147. doi: 10.1016/j.biomaterials.2011.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert LH, Goebrecht GKE, De Leo SE, O’Connor RS, Nunez-Cruz S, Li T-D, . . . Kam LC (2017). Improving T Cell Expansion with a Soft Touch. Nano Letters, 17(2), 821–826. doi: 10.1021/acs.nanolett.6b04071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane P, Deshmukh R, Brown JA, Jakubski S, Parajuli P, Nolan T, . . . Lamichhane N (2019). Novel Delivery Systems for Checkpoint Inhibitors. Medicines, 6(3), 74. doi: 10.3390/medicines6030074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca CJ, & Warner SG (2018). Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clinical and Translational Medicine, 7(1), 35. doi: 10.1186/s40169-018-0214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, . . . Hartgerink JD (2018). STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials, 163, 67–75. doi: 10.1016/j.biomaterials.2018.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DG, Young S, & Hartgerink JD (2019). Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomaterialia, 88, 15–31. doi: 10.1016/j.actbio.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy AB, Elgarten CW, Grupp SA, Maude SL, & Teachey DT (2018). Tisagenlecleucel for the treatment of B-cell acute lymphoblastic leukemia. Expert Review of Anticancer Therapy, 18(10), 959–971. doi: 10.1080/14737140.2018.1512411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel M-È, Chartrand K, Tarrab E, Savard P, Leclerc D, & Lamarre A (2016). Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Letters, 16(3), 1826–1832. doi: 10.1021/acs.nanolett.5b04877 [DOI] [PubMed] [Google Scholar]
- Lee KL, Murray AA, Le DHT, Sheen MR, Shukla S, Commandeur U, . . . Steinmetz NF (2017). Combination of Plant Virus Nanoparticle-Based in Situ Vaccination with Chemotherapy Potentiates Antitumor Response. Nano Letters, 17(7), 4019–4028. doi: 10.1021/acs.nanolett.7b00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemhuis M, van Nostrum CF, Kruijtzer JAW, Zhong ZY, ten Breteler MR, Dijkstra PJ, . . . Hennink WE (2006). Functionalized Poly(α-hydroxy acid)s via Ring-Opening Polymerization: Toward Hydrophilic Polyesters with Pendant Hydroxyl Groups. Macromolecules, 39(10), 3500–3508. doi: 10.1021/ma052128c [DOI] [Google Scholar]
- Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, . . . Hellstrom KE (2010). Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. Journal of the American Chemical Society, 132(20), 6906–6907. doi: 10.1021/ja102414t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C, Shin Y, Magnuson JK, Fryxell G, Lasure LL, Elliott DC, . . . Ackerman EJ (2006). Characterization of functionalized nanoporous supports for protein confinement. Nanotechnology, 17(22), 5531–5538. doi: 10.1088/0957-4484/17/22/001 [DOI] [PubMed] [Google Scholar]
- Lemdani K, Seguin J, Lesieur C, Al Sabbagh C, Doan BT, Richard C, . . . Mignet N (2019). Mucoadhesive thermosensitive hydrogel for the intra-tumoral delivery of immunomodulatory agents, in vivo evidence of adhesion by means of non-invasive imaging techniques. International Journal of Pharmaceutics, 567, 118421. doi: 10.1016/j.ijpharm.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Levy A, Massard C, Soria JC, & Deutsch E (2016). Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. European Journal of Cancer, 68, 156–162. doi: 10.1016/j.ejca.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Li J, & Mooney DJ (2016). Designing hydrogels for controlled drug delivery. Nature Reviews Materials, 1(12), 16071. doi: 10.1038/natrevmats.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yang S, Song L, Zeng Y, He T, Wang N, . . . Gong C (2018). An Endogenous Vaccine Based on Fluorophores and Multivalent Immunoadjuvants Regulates Tumor Micro-Environment for Synergistic Photothermal and Immunotherapy. Theranostics, 8(3), 860–873. doi: 10.7150/thno.19826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Naylor MF, Le H, Nordquist RE, Teague TK, Howard CA, . . . Chen WR (2010). Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biology & Therapy, 10(11), 1081–1087. doi: 10.4161/cbt.10.11.13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, . . . Wang L (2016). Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology, 5(2), e1074374. doi: 10.1080/2162402x.2015.1074374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X, Doughty A, West C, Wang L, Zhou F, . . . Chen WR (2019). Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomedicine, 18, 44–53. doi: 10.1016/j.nano.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Xu L, Song G, & Liu Z (2016). Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chemical Society Reviews, 45(22), 6250–6269. doi: 10.1039/C6CS00458J [DOI] [PubMed] [Google Scholar]
- Lichty BD, Breitbach CJ, Stojdl DF, & Bell JC (2014). Going viral with cancer immunotherapy. Nature Reviews Cancer, 14(8), 559–567. doi: 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- Lin H, Li Q, Wang O, Rauch J, Harm B, Viljoen HJ, . . . Lei Y (2018). Automated Expansion of Primary Human T Cells in Scalable and Cell-Friendly Hydrogel Microtubes for Adoptive Immunotherapy. Advanced Healthcare Materials, 7(15), e1701297. doi: 10.1002/adhm.201701297 [DOI] [PubMed] [Google Scholar]
- Lindqvist C, Sandin LC, Fransson M, & Loskog A (2009). Local AdCD40L gene therapy is effective for disseminated murine experimental cancer by breaking T-cell tolerance and inducing tumor cell growth inhibition. Journal of Immunotherapy, 32(8), 785–792. doi: 10.1097/CJI.0b013e3181acea69 [DOI] [PubMed] [Google Scholar]
- Liu H, Kwong B, & Irvine DJ (2011). Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angewandte Chemie International Edition, 50(31), 7052–7055. doi: 10.1002/anie.201101266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, & Fiering S (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature Nanotechnology, 11(3), 295–303. doi: 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmueller J, & Finn OJ (2017). Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacology & Therapeutics, 178, 31–47. doi: 10.1016/j.pharmthera.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu X, Liao Y-P, Salazar F, Sun B, Jiang W, . . . Nel AE (2017). Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nature Communications, 8(1), 1811. doi: 10.1038/s41467-017-01651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, & Lin W (2016). Chlorin-Based Nanoscale Metal-Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. Journal of the American Chemical Society, 138(38), 12502–12510. doi: 10.1021/jacs.6b06663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KD, He CB, Guo NN, Chan C, Ni KY, Lan GX, . . . Lin WB (2018). Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nature Biomedical Engineering, 2(8), 600-+. doi: 10.1038/s41551-018-0203-4 [DOI] [PubMed] [Google Scholar]
- Lv Q, He C, Quan F, Yu S, & Chen X (2018). DOX/IL-2/IFN-gamma co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioactive Materials, 3(1), 118–128. doi: 10.1016/j.bioactmat.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi S, Zeinali Sehrig F, Samiei M, Milani M, Abbasi E, Dadashzadeh K, & Akbarzadeh A (2016). Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artificial Cells, Nanomedicine, and Biotechnology, 44(4), 1186–1193. doi: 10.3109/21691401.2015.1014093 [DOI] [PubMed] [Google Scholar]
- Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, & Totterman TH (2010). Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. Journal of Immunotherapy, 33(3), 225–235. doi: 10.1097/CJI.0b013e3181c01fcb [DOI] [PubMed] [Google Scholar]
- Manome Y, Suzuki D, Mochizuki A, Saito E, Sasa K, Yoshimura K, . . . Kamijo R (2018). The inhibition of malignant melanoma cell invasion of bone by the TLR7 agonist R848 is dependent upon pro-inflammatory cytokines produced by bone marrow macrophages. Oncotarget, 9(52), 29934–29943. doi: 10.18632/oncotarget.25711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Kohrt H, Caux C, & Levy R (2014). Intratumoral immunization: a new paradigm for cancer therapy. Clinical Cancer Research, 20(7), 1747–1756. doi: 10.1158/1078-0432.Ccr-13-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Kohrt H, & Levy R (2013). Intratumoral anti-CTLA-4 therapy: enhancing efficacy while avoiding toxicity. Clinical Cancer Research, 19(19), 5261–5263. doi: 10.1158/1078-0432.Ccr-13-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Tselikas L, de Baere T, & Houot R (2017). Intratumoral immunotherapy: using the tumor as the remedy. Annals of Oncology, 28(suppl_12), xii33–xii43. doi: 10.1093/annonc/mdx683 [DOI] [PubMed] [Google Scholar]
- Meng Z, Zhou X, Xu J, Han X, Dong Z, Wang H, . . . Liu Z (2019). Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Advanced Materials, 31(24), e1900927. doi: 10.1002/adma.201900927 [DOI] [PubMed] [Google Scholar]
- Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, . . . Fox BA (2017). Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clinical Cancer Research, 23(20), 6165–6177. doi: 10.1158/1078-0432.Ccr-16-2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Lenz LL, & Harris RA (2016). A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Research, 76(3), 513–516. doi: 10.1158/0008-5472.Can-15-1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, . . . Wang AZ (2017). Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nature Nanotechnology, 12(9), 877–882. doi: 10.1038/nnano.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, & Wherry EJ (2016). Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell, 165(2), 272–275. doi: 10.1016/j.cell.2016.03.031 [DOI] [PubMed] [Google Scholar]
- Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, . . . Wittrup KD (2019). Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Science Translational Medicine, 11(498). doi: 10.1126/scitranslmed.aaw2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monette A, Ceccaldi C, Assaad E, Lerouge S, & Lapointe R (2016). Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies. Biomaterials, 75, 237–249. doi: 10.1016/j.biomaterials.2015.10.021 [DOI] [PubMed] [Google Scholar]
- Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, . . . Irvine DJ (2016). Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nature Medicine, 22(12), 1402–1410. doi: 10.1038/nm.4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Hunt CR, Pandita RK, Ferrari M, Krishnan S, Cooke JP, . . . Pandita TK (2018). Harnessing and Optimizing the Interplay between Immunotherapy and Radiotherapy to Improve Survival Outcomes. Molecular Cancer Research, 16(8), 1209–1214. doi: 10.1158/1541-7786.Mcr-17-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH (2012). Blocking IDO activity to enhance anti-tumor immunity. Frontiers in Bioscience, 4, 734–745. doi: 10.2741/414 [DOI] [PubMed] [Google Scholar]
- Murad JP, Kozlowska AK, Lee HJ, Ramamurthy M, Chang WC, Yazaki P, . . . Priceman SJ (2018). Effective Targeting of TAG72(+) Peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells. Frontiers in Immunology, 9, 2268. doi: 10.3389/fimmu.2018.02268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka D, Seo N, Hayashi T, Tahara Y, Fujii K, Tawara I, . . . Shiku H (2019). Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. Journal of Clinical Investigation, 129(3), 1278–1294. doi: 10.1172/jci97642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AA, Wang C, Fiering S, & Steinmetz NF (2018). In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Molecular Pharmaceutics, 15(9), 3700–3716. doi: 10.1021/acs.molpharmaceut.8b00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, . . . Nakamura T (2020). Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Science Translational Medicine, 12(526), eaax7992. doi: 10.1126/scitranslmed.aax7992 [DOI] [PubMed] [Google Scholar]
- Nam J, Son S, Park KS, Zou W, Shea LD, & Moon JJ (2019). Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews Materials, 4(6), 398–414. doi: 10.1038/s41578-019-0108-1 [DOI] [Google Scholar]
- Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, . . . Lee DW (2018). Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. Journal for ImmunoTherapy of Cancer, 6(1), 30. doi: 10.1186/s40425-018-0340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurauter AA, Bonyhadi M, Lien E, Nokleby L, Ruud E, Camacho S, & Aarvak T (2007). Cell isolation and expansion using Dynabeads. Advances in Biochemical Engineering/Biotechnology, 106, 41–73. doi: 10.1007/10_2007_072 [DOI] [PubMed] [Google Scholar]
- Ni K, Lan G, Chan C, Quigley B, Lu K, Aung T, . . . Lin W(2018). Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nature Communications, 9(1), 2351. doi: 10.1038/s41467-018-04703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Chen J, Huang D, Yao Y, Chen J, Ding L, . . . Song E (2017). Tumor-Associated Macrophages Promote Malignant Progression of Breast Phyllodes Tumors by Inducing Myofibroblast Differentiation. Cancer Research, 77(13), 3605–3618. doi: 10.1158/0008-5472.Can-16-2709 [DOI] [PubMed] [Google Scholar]
- Oh E, Oh JE, Hong J, Chung Y, Lee Y, Park KD, . . . Yun CO (2017). Optimized biodegradable polymeric reservoir-mediated local and sustained co-delivery of dendritic cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for cancer immunotherapy. Journal of Controlled Release, 259, 115–127. doi: 10.1016/j.jconrel.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Oliva N, Conde J, Wang K, & Artzi N (2017). Designing Hydrogels for On-Demand Therapy. Accounts of Chemical Research, 50(4), 669–679. doi: 10.1021/acs.accounts.6b00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XC, Li L, Mao JJ, Yao W, Zheng JN, Liu M, & Fu JJ (2013). Synergistic effects of soluble PD-1 and IL-21 on antitumor immunity against H22 murine hepatocellular carcinoma. Oncology Letters, 5(1), 90–96. doi: 10.3892/ol.2012.966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, van Schalkwyk M, & Maher J (2015). Clinical Evaluation of ErbB-Targeted CAR T-Cells, Following Intracavity Delivery in Patients with ErbB-Expressing Solid Tumors. Methods in Molecular Biology, 1317, 365–382. doi: 10.1007/978-1-4939-2727-2_21 [DOI] [PubMed] [Google Scholar]
- Pardoll DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, . . . Kirn DH (2008). Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. The Lancet Oncology, 9(6), 533–542. doi: 10.1016/s1470-2045(08)70107-4 [DOI] [PubMed] [Google Scholar]
- Park CG, Hartl CA, Schmid D, Carmona EM, Kim HJ, & Goldberg MS (2018). Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Science Translational Medicine, 10(433). doi: 10.1126/scitranslmed.aar1916 [DOI] [PubMed] [Google Scholar]
- Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, . . . Fahmy TM (2012). Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature Materials, 11(10), 895–905. doi: 10.1038/nmat3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R, Winnicki M, Liu E, & Chu WM (2019). Immune checkpoints and cancer in the immunogenomics era. Briefings in Functional Genomics, 18(2), 133–139. doi: 10.1093/bfgp/ely027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, Restrepo-Perdomo CA, Mato-Berciano A, Ottaviani D, . . . Carcaboso AM (2019). Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Science Translational Medicine, 11(476), eaat9321. doi: 10.1126/scitranslmed.aat9321 [DOI] [PubMed] [Google Scholar]
- Patel R, Czapar AE, Fiering S, Oleinick NL, & Steinmetz NF (2018). Radiation Therapy Combined with Cowpea Mosaic Virus Nanoparticle in Situ Vaccination Initiates Immune-Mediated Tumor Regression. ACS Omega, 3(4), 3702–3707. doi: 10.1021/acsomega.8b00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri SN, Clune J, Ariyan S, & Narayan D (2016). Malignant Melanoma: Beyond the Basics. Plastic and Reconstructive Surgery, 138(2), 330e–340e. doi: 10.1097/prs.0000000000002367 [DOI] [PubMed] [Google Scholar]
- Phuengkham H, Song C, Um SH, & Lim YT (2018). Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Advanced Materials, 30(18), e1706719. doi: 10.1002/adma.201706719 [DOI] [PubMed] [Google Scholar]
- Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, & Gandhi V (1995). Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Seminars in Oncology, 22(4 Suppl 11), 3–10. [PubMed] [Google Scholar]
- Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, . . . Brown CE (2018). Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2(+) Breast Cancer Metastasis to the Brain. Clinical Cancer Research, 24(1), 95–105. doi: 10.1158/1078-0432.Ccr-17-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwada A, Tian YF, Huang W, Rohrbach KM, Deol S, August A, & Singh A (2016). Self-Assembly Protein Nanogels for Safer Cancer Immunotherapy. Advanced Healthcare Materials, 5(12), 1413–1419. doi: 10.1002/adhm.201501062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, & Pollard JW (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141(1), 39–51. doi: 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G, Wang X, Li X, Ito A, Sogo Y, & Ye J (2019). An immuno-potentiating vehicle made of mesoporous silica-zinc oxide micro-rosettes with enhanced doxorubicin loading for combined chemoimmunotherapy. Chemical Communications, 55(7), 961–964. doi: 10.1039/c8cc09044k [DOI] [PubMed] [Google Scholar]
- Rahimian S, Fransen MF, Kleinovink JW, Amidi M, Ossendorp F, & Hennink WE (2015). Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer. Biomaterials, 61, 33–40. doi: 10.1016/j.biomaterials.2015.04.043 [DOI] [PubMed] [Google Scholar]
- Reynders K, Illidge T, Siva S, Chang JY, & De Ruysscher D (2015). The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treatment Reviews, 41(6), 503–510. doi: 10.1016/j.ctrv.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, . . . Long GV (2017). Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell, 170(6), 1109–1119.e1110. doi: 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, & Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science, 359(6382), 1350–1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti B, Leonardi GC, Puccetti P, Fallarino F, Bianconi V, Sahebkar A, . . . Pirro M (2019). Targeting indoleamine-2,3-dioxygenase in cancer: Scientific rationale and clinical evidence. Pharmacology & Therapeutics, 196, 105–116. doi: 10.1016/j.pharmthera.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Riley RS, June CH, Langer R, & Mitchell MJ (2019). Delivery technologies for cancer immunotherapy. Nature Reviews Drug Discovery, 18(3), 175–196. doi: 10.1038/s41573-018-0006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivankar S (2014). An overview of doxorubicin formulations in cancer therapy. Journal of Cancer Research and Therapeutics, 10(4), 853–858. doi: 10.4103/0973-1482.139267 [DOI] [PubMed] [Google Scholar]
- Roberts ZJ, Better M, Bot A, Roberts MR, & Ribas A (2018). Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leukemia & Lymphoma, 59(8), 1785–1796. doi: 10.1080/10428194.2017.1387905 [DOI] [PubMed] [Google Scholar]
- Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, . . . Weissleder R (2018). TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nature Biomedical Engineering, 2, 578–588. doi: 10.1038/s41551-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA (2014). IL-2: the first effective immunotherapy for human cancer. Journal of Immunology, 192(12), 5451–5458. doi: 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Bu L, Hu Q, Cheng H, Lu W, & Gu Z (2019). Strategies of Combination Drug Delivery for Immune Checkpoint Blockades. Advanced Healthcare Materials, 8(4), 1801099. doi: 10.1002/adhm.201801099 [DOI] [PubMed] [Google Scholar]
- Ruan H, Hu Q, Wen D, Chen Q, Chen G, Lu Y, . . . Gu Z (2019). A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Advanced Materials, e1806957. doi: 10.1002/adma.201806957 [DOI] [PubMed] [Google Scholar]
- Sabel MS, Arora A, Su G, Mathiowitz E, Reineke JJ, & Chang AE (2007). Synergistic effect of intratumoral IL-12 and TNF-alpha microspheres: systemic anti-tumor immunity is mediated by both CD8+ CTL and NK cells. Surgery, 142(5), 749–760. doi: 10.1016/j.surg.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, & Levy R (2018). Eradication of spontaneous malignancy by local immunotherapy. Science Translational Medicine, 10(426). doi: 10.1126/scitranslmed.aan4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Martuza RL, & Rabkin SD (2018). Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy, 10(9), 779–786. doi: 10.2217/imt-2018-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Barcelo EJ, & Mediavilla MD (2014). Recent patents on light based therapies: photodynamic therapy, photothermal therapy and photoimmunotherapy. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery, 8(1), 1–8. doi: 10.2174/1872214807666131229103707 [DOI] [PubMed] [Google Scholar]
- Sandin LC, Eriksson F, Ellmark P, Loskog AS, Tötterman TH, & Mangsbo SM (2014). Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology, 3(1), e27614. doi: 10.4161/onci.27614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Totterman TH, & Mangsbo SM (2014). Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunology Research, 2(1), 80–90. doi: 10.1158/2326-6066.Cir-13-0067 [DOI] [PubMed] [Google Scholar]
- Schluck M, Hammink R, Figdor CG, Verdoes M, & Weiden J (2019). Biomaterial-Based Activation and Expansion of Tumor-Specific T Cells. Frontiers in Immunology, 10, 931–931. doi: 10.3389/fimmu.2019.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, & Schreiber RD (2015). Neoantigens in cancer immunotherapy. Science, 348(6230), 69–74. doi: 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- Seo SH, Han HD, Noh KH, Kim TW, & Son SW (2009). Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity. Clinical & Experimental Metastasis, 26(3), 179–187. doi: 10.1007/s10585-008-9228-5 [DOI] [PubMed] [Google Scholar]
- Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, & Santamaria P (2015). Nanoparticle-Based Immunotherapy for Cancer. ACS Nano, 9(1), 16–30. doi: 10.1021/nn5062029 [DOI] [PubMed] [Google Scholar]
- Sharifzadeh G, & Hosseinkhani H (2017). Biomolecule-Responsive Hydrogels in Medicine. Advanced Healthcare Materials, 6(24). doi: 10.1002/adhm.201700801 [DOI] [PubMed] [Google Scholar]
- Sheen MR, & Fiering S (2019). In situ vaccination: Harvesting low hanging fruit on the cancer immunotherapy tree. Wiley Interdisciplinary Reviews - Nanomedicine and Nanobiotechnology, 11(1), e1524. doi: 10.1002/wnan.1524 [DOI] [PubMed] [Google Scholar]
- Shekarian T, Sivado E, Jallas A-C, Depil S, Kielbassa J, Janoueix-Lerosey I, . . . Marabelle A (2019). Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Science Translational Medicine, 11(515), eaat5025. doi: 10.1126/scitranslmed.aat5025 [DOI] [PubMed] [Google Scholar]
- Shi Y, & Lammers T (2019). Combining Nanomedicine and Immunotherapy. Accounts of Chemical Research, 52(6), 1543–1554. doi: 10.1021/acs.accounts.9b00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Tourkova IL, Kaneno R, & Shurin MR (2009). Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. Journal of Immunology, 183(1), 137–144. doi: 10.4049/jimmunol.0900734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AD, Moskalenko M, Creson J, Fang J, Yi S, VanRoey MJ, . . . Jooss K (2008). Local secretion of anti-CTLA-4 enhances the therapeutic efficacy of a cancer immunotherapy with reduced evidence of systemic autoimmunity. Cancer Immunology, Immunotherapy, 57(8), 1263–1270. doi: 10.1007/s00262-008-0451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GR, Relph K, Harrington K, Melcher A, & Pandha H (2016). Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virotherapy, 5, 1–13. doi: 10.2147/OV.S66083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, & Peppas NA (2014). Hydrogels and scaffolds for immunomodulation. Advanced Materials, 26(38), 6530–6541. doi: 10.1002/adma.201402105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, . . . Gajewski TF (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science, 350(6264), 1084–1089. doi: 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, . . . Stephan MT (2017). Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. Journal of Clinical Investigation, 127(6), 2176–2191. doi: 10.1172/JCI87624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Ngiow SF, Ribas A, & Teng MW (2016). Combination cancer immunotherapies tailored to the tumour microenvironment. Nature Reviews Clinical Oncology, 13(3), 143–158. doi: 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- Song C, Phuengkham H, Kim YS, Dinh VV, Lee I, Shin IW, . . . Lim YT (2019). Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nature Communications, 10(1), 3745. doi: 10.1038/s41467-019-11730-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Yang P, Huang P, Zhang C, Kong D, & Wang W (2019). Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics, 9(8), 2299–2314. doi: 10.7150/thno.30577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Yin Y, Shang L, Wu T, Zhang D, Kong M, . . . Zhang Z (2017). Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy. Nano Letters, 17(10), 6366–6375. doi: 10.1021/acs.nanolett.7b03186 [DOI] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, . . . Roberts DD (2014). CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Research, 74(23), 6771–6783. doi: 10.1158/0008-5472.CAN-14-0037-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto M, Fu YX, & Weichselbaum RR (2016). The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Science Immunology, 1(3). doi: 10.1126/sciimmunol.aag1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, & Stephan MT (2015). Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nature Biotechnology, 33(1), 97–101. doi: 10.1038/nbt.3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, . . . Shi Y (2019). Nanomedicine and macroscale materials in immuno-oncology. Chemical Society Reviews, 48(1), 351–381. doi: 10.1039/c8cs00473k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, & Albelda SM (2005). Gemcitabine Selectively Eliminates Splenic Gr-1+/CD11b+ Myeloid Suppressor Cells in Tumor-Bearing Animals and Enhances Antitumor Immune Activity. Clinical Cancer Research, 11(18), 6713–6721. doi: 10.1158/1078-0432.Ccr-05-0883 [DOI] [PubMed] [Google Scholar]
- Tahara Y, & Akiyoshi K (2015). Current advances in self-assembled nanogel delivery systems for immunotherapy. Advanced Drug Delivery Reviews, 95, 65–76. doi: 10.1016/j.addr.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Matsushima H, Mizumoto N, & Takashima A (2009). Classification of Chemotherapeutic Agents Based on Their Differential In vitro Effects on Dendritic Cells. Cancer Research, 69(17), 6978–6986. doi: 10.1158/0008-5472.Can-09-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ju E, Ren J, & Qu X (2014). Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials, 35(37), 9963–9971. doi: 10.1016/j.biomaterials.2014.08.036 [DOI] [PubMed] [Google Scholar]
- Thambi T, Li Y, & Lee DS (2017). Injectable hydrogels for sustained release of therapeutic agents. Journal of Controlled Release, 267, 57–66. doi: 10.1016/j.jconrel.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Thomas S, & Prendergast GC (2016). Cancer Vaccines: A Brief Overview. Methods in Molecular Biology, 1403, 755–761. doi: 10.1007/978-1-4939-3387-7_43 [DOI] [PubMed] [Google Scholar]
- Tsai H-C, Chou H-Y, Chuang S-H, Lai J-Y, Chen Y-S, Wen Y-H, . . . Lo C-L (2019). Preparation of Immunotherapy Liposomal-Loaded Thermal-Responsive Hydrogel Carrier in the Local Treatment of Breast Cancer. Polymers, 11(10), 1592. doi: 10.3390/polym11101592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, & Zhang M (2014). Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules, 15(7), 2656–2662. doi: 10.1021/bm500502n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JM, Mihalcioiu C, & Cury FL (2018). Abscopal Effect in a Stage IV Melanoma Patient who Progressed on Pembrolizumab. Cureus, 10(2), e2238. doi: 10.7759/cureus.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, . . . Minn AJ (2015). Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature, 520(7547), 373–377. doi: 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uger R, & Johnson L (2020). Blockade of the CD47-SIRPα axis: a promising approach for cancer immunotherapy. Expert Opinion on Biological Therapy, 20(1), 5–8. doi: 10.1080/14712598.2020.1685976 [DOI] [PubMed] [Google Scholar]
- Umeki Y, Mohri K, Kawasaki Y, Watanabe H, Takahashi R, Takahashi Y, . . . Nishikawa M (2015). Induction of Potent Antitumor Immunity by Sustained Release of Cationic Antigen from a DNA-Based Hydrogel with Adjuvant Activity. Advanced Functional Materials, 25(36), 5758–5767. doi: 10.1002/adfm.201502139 [DOI] [Google Scholar]
- Van De Voort TJ, Felder MA, Yang RK, Sondel PM, & Rakhmilevich AL (2013). Intratumoral delivery of low doses of anti-CD40 mAb combined with monophosphoryl lipid a induces local and systemic antitumor effects in immunocompetent and T cell-deficient mice. Journal of Immunotherapy, 36(1), 29–40. doi: 10.1097/CJI.0b013e3182780f61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooren L, Sandin LC, Moskalev I, Ellmark P, Dimberg A, Black P, . . . Mangsbo SM (2017). Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. European Journal of Immunology, 47(2), 385–393. doi: 10.1002/eji.201646583 [DOI] [PubMed] [Google Scholar]
- van Schalkwyk MC, Papa SE, Jeannon JP, Guerrero Urbano T, Spicer JF, & Maher J (2013). Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Human Gene Therapy Clinical Development, 24(3), 134–142. doi: 10.1089/humc.2013.144 [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, & Hennink WE (2007). Biodegradable dextran hydrogels for protein delivery applications. Expert Review of Medical Devices, 4(2), 147–164. doi: 10.1586/17434440.4.2.147 [DOI] [PubMed] [Google Scholar]
- Velcheti V, & Schalper K (2016). Basic Overview of Current Immunotherapy Approaches in Cancer American Society of Clinical Oncology Educational Book, 35, 298–308. doi: 10.14694/edbk_15657210.1200/edbk_156572 [DOI] [PubMed] [Google Scholar]
- Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, . . . Zitvogel L (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 350(6264), 1079–1084. doi: 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH (2015). CD47 blockade as another immune checkpoint therapy for cancer. Nature Medicine, 21(10), 1122–1123. doi: 10.1038/nm.3965 [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, & Glennie MJ (2013). Agonistic CD40 antibodies and cancer therapy. Clinical Cancer Research, 19(5), 1035–1043. doi: 10.1158/1078-0432.Ccr-12-2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Beiss V, & Steinmetz NF (2019). Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. Journal of Virology, 93(21). doi: 10.1128/jvi.00129-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, & Steinmetz NF (2019). CD47 Blockade and Cowpea Mosaic Virus Nanoparticle In Situ Vaccination Triggers Phagocytosis and Tumor Killing. Advanced Healthcare Materials, e1801288. doi: 10.1002/adhm.201801288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sun W, Wright G, Wang AZ, & Gu Z (2016). Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Advanced Materials, 28(40), 8912–8920. doi: 10.1002/adma.201506312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, . . . Gu Z (2018). In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Science Translational Medicine, 10(429). doi: 10.1126/scitranslmed.aan3682 [DOI] [PubMed] [Google Scholar]
- Wang C, Xu L, Liang C, Xiang J, Peng R, & Liu Z (2014). Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Advanced Materials, 26(48), 8154–8162. doi: 10.1002/adma.201402996 [DOI] [PubMed] [Google Scholar]
- Wang C, Ye Y, & Gu Z (2017). Local delivery of checkpoints antibodies. Human Vaccines & Immunotherapeutics, 13(1), 245–248. doi: 10.1080/21645515.2016.1223000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ye Y, Hochu GM, Sadeghifar H, & Gu Z (2016). Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Letters, 16(4), 2334–2340. doi: 10.1021/acs.nanolett.5b05030 [DOI] [PubMed] [Google Scholar]
- Wang C, Ye Y, Hu Q, Bellotti A, & Gu Z (2017). Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Advanced Materials, 29(29), 1606036. doi: 10.1002/adma.201606036 [DOI] [PubMed] [Google Scholar]
- Wang H, & Mooney DJ (2018). Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nature Materials, 17(9), 761–772. doi: 10.1038/s41563-018-0147-9 [DOI] [PubMed] [Google Scholar]
- Wang M, Song J, Zhou F, Hoover AR, Murray C, Zhou B, . . . Chen WR (2019). NIR-Triggered Phototherapy and Immunotherapy via an Antigen-Capturing Nanoplatform for Metastatic Cancer Treatment. Advanced Science, 6(10), 1802157. doi: 10.1002/advs.201802157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Li X, Wang J, Gao D, Li Y, Li H, . . . Wang Y (2017). Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nature Communications, 8(1), 1395. doi: 10.1038/s41467-017-01385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Shigdar S, Gantier MP, Hou Y, Wang L, Li Y, . . . Duan W (2015). Cancer stem cell targeted therapy: progress amid controversies. Oncotarget, 6(42), 44191–44206. doi: 10.18632/oncotarget.6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, . . . Li Y (2018). A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nature Communications, 9(1), 1532. doi: 10.1038/s41467-018-03915-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Ito A, Yoshiyuki K, Sogo Y, Watanabe Y, . . . Tsuji NM (2016). Hollow Structure Improved Anti-Cancer Immunity of Mesoporous Silica Nanospheres In Vivo. Small, 12(26), 3510–3515. doi: 10.1002/smll.201600677 [DOI] [PubMed] [Google Scholar]
- Weber JS, & Mule JJ (2015). Cancer immunotherapy meets biomaterials. Nature Biotechnology, 33(1), 44–45. doi: 10.1038/nbt.3119 [DOI] [PubMed] [Google Scholar]
- Weiden J, Voerman D, Dölen Y, Das RK, van Duffelen A, Hammink R, . . . Figdor CG (2018). Injectable Biomimetic Hydrogels as Tools for Efficient T Cell Expansion and Delivery. Frontiers in Immunology, 9(2798). doi: 10.3389/fimmu.2018.02798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Chen G, Chen Q, Li PY, Cheng H, & Gu Z (2019). Engineering Protein Delivery Depots for Cancer Immunotherapy. Bioconjugate Chemistry, 30(3), 515–524. doi: 10.1021/acs.bioconjchem.9b00061 [DOI] [PubMed] [Google Scholar]
- Wen D, Wang J, Van Den Driessche G, Chen Q, Zhang Y, Chen G, . . . Gu Z (2019). Adipocytes as Anticancer Drug Delivery Depot. Matter, 1(5), 1203–1214. doi: 10.1016/j.matt.2019.08.007 [DOI] [Google Scholar]
- West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, . . . Ahmed R (2013). PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. Journal of Clinical Investigation, 123(6), 2604–2615. doi: 10.1172/jci67008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, & Kim YJ (2018). Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine: Nanotechnology, Biology and Medicine, 14(2), 237–246. doi: 10.1016/j.nano.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, Ganguly S, Wang TL, Anderson CW, Sadtler K, Narain R, . . . Elisseeff JH (2019). A biologic scaffold–associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Science Translational Medicine, 11(477), eaat7973. doi: 10.1126/scitranslmed.aat7973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, He C, Wu Y, Chen X, & Cheng J (2015). Nanogel-Incorporated Physical and Chemical Hybrid Gels for Highly Effective Chemo–Protein Combination Therapy. Advanced Functional Materials, 25(43), 6744–6755. doi: 10.1002/adfm.201502742 [DOI] [Google Scholar]
- Wu X, Wu Y, Ye H, Yu S, He C, & Chen X (2017). Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. Journal of Controlled Release, 255, 81–93. doi: 10.1016/j.jconrel.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, . . . Liu Z (2017). Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano, 11(5), 4463–4474. doi: 10.1021/acsnano.7b00715 [DOI] [PubMed] [Google Scholar]
- Xu X, Li T, Shen S, Wang J, Abdou P, Gu Z, & Mo R (2019). Advances in Engineering Cells for Cancer Immunotherapy. Theranostics, 9(25), 7889–7905. doi: 10.7150/thno.38583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zeng X, Tang Y. a., Liu B-F, Wang Y, & Liu X (2019). Activating Antitumor Immunity and Antimetastatic Effect Through Polydopamine-Encapsulated Core–Shell Upconversion Nanoparticles. Advanced Materials, 0(0), 1905825. doi: 10.1002/adma.201905825 [DOI] [PubMed] [Google Scholar]
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, . . . Rosenberg SA (2007). Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. Journal of Immunotherapy, 30(8), 825–830. doi: 10.1097/CJI.0b013e318156e47e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Yang C, Tao Y, Tang J, Huang Q, Guo W, . . . Liu Y (2018). Combination therapy with F5/35 fiber chimeric conditionally replicative adenoviruses expressing IL-24 enhances the antitumor effect of temozolomide against melanoma. Cancer Medicine, 7(12), 5928–5942. doi: 10.1002/cam4.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y (2015). Cancer immunotherapy: harnessing the immune system to battle cancer. Journal of Clinical Investigation, 125(9), 3335–3337. doi: 10.1172/jci83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R, . . . Ngwa W (2018). Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer. Frontiers in Immunology, 9, 2030. doi: 10.3389/fimmu.2018.02030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, . . . Nishikawa M (2017). DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials, 146, 136–145. doi: 10.1016/j.biomaterials.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Ye X, Liang X, Chen Q, Miao Q, Chen X, Zhang X, & Mei L (2019). Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano, 13(3), 2956–2968. doi: 10.1021/acsnano.8b07371 [DOI] [PubMed] [Google Scholar]
- Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, & Gu Z (2016). Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano, 10(9), 8956–8963. doi: 10.1021/acsnano.6b04989 [DOI] [PubMed] [Google Scholar]
- Yi M, Jiao D, Qin S, Chu Q, Wu K, & Li A (2019). Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Molecular Cancer, 18(1), 60. doi: 10.1186/s12943-019-0974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa A, Dutt S, Chester C, Kim J, & Kohrt HE (2015). Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clinical Cancer Research, 21(14), 3113–3120. doi: 10.1158/1078-0432.Ccr-15-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi H, Yuan J, Keshavarz-Fathi M, Murphy JF, & Rezaei N (2017). Immunotherapy of cancers comes of age. Expert Review of Clinical Immunology, 13(10), 1001–1015. doi: 10.1080/1744666x.2017.1366315 [DOI] [PubMed] [Google Scholar]
- Yu S, Wang C, Yu J, Wang J, Lu Y, Zhang Y, . . . Gu Z (2018). Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade. Advanced Materials, 30(28), 1801527. doi: 10.1002/adma.201801527 [DOI] [PubMed] [Google Scholar]
- Zaharoff DA, Hance KW, Rogers CJ, Schlom J, & Greiner JW (2010). Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. Journal of Immunotherapy, 33(7), 697–705. doi: 10.1097/CJI.0b013e3181eb826d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, . . . Allison JP. (2014). Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Science Translational Medicine, 6(226), 226ra232–226ra232. doi: 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek RM, De Jong E, Chin WL, Schuster IS, Fear VS, Casey TH, . . . Lesterhuis WJ (2019). Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Science Translational Medicine, 11(501), eaav7816. doi: 10.1126/scitranslmed.aav7816 [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhai Y, Cai Y, Zhao Y, & Li Y (2019). Nanomedicine-Based Immunotherapy for the Treatment of Cancer Metastasis. Advanced Materials, 0(0), 1904156. doi: 10.1002/adma.201904156 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu F, Men K, Huang R, Zhou B, Zhang R, . . . Yang L (2018). Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy. Nanoscale Research Letters, 13(1), 240. doi: 10.1186/s11671-018-2661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Li Y, Wei D, & Luo H (2018). The Application of Nanoparticle-Based Drug Delivery Systems in Checkpoint Blockade Cancer Immunotherapy. Journal of Immunology Research, 2018, 3673295. doi: 10.1155/2018/3673295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A, Carrillo MA, & Kitchen SG (2017). Chimeric antigen receptor engineered stem cells: a novel HIV therapy. Immunotherapy, 9(5), 401–410. doi: 10.2217/imt-2016-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, & Savai R (2017). Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget, 8(29), 48436–48452. doi: 10.18632/oncotarget.17061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Wu S, Song S, Chen WR, Resasco DE, & Xing D (2012). Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials, 33(11), 3235–3242. doi: 10.1016/j.biomaterials.2011.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Liu Y, Yang X, Kim YH, Zhang H, Jia R, . . . Chen X (2016). DNA-inorganic hybrid nanovaccine for cancer immunotherapy. Nanoscale, 8(12), 6684–6692. doi: 10.1039/c5nr08821f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, & Chen L (2016). PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science Translational Medicine, 8(328), 328rv324. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]