Abstract
For the past decade, migratory beekeepers who provide honey bees for pollination services have experienced substantial colony losses on a recurring basis that have been attributed in part to exposure to insecticides, fungicides, or their combinations applied to crops. The phytochemicals p-coumaric acid and quercetin, which occur naturally in a wide variety of bee foods, including beebread and many types of honey, can enhance adult bee longevity and reduce the toxicity of certain pesticides. How variation in concentrations of natural dietary constituents affects interactions with xenobiotics, including synthetic pesticides, encountered in agroecosystems remains an open question. We tested the effects of these two phytochemicals at a range of natural concentrations on impacts of consuming propiconazole and chlorantraniliprole, a triazole fungicide and an insecticide frequently applied as a tank mix to almond trees during bloom in California’s Central Valley. Propiconazole, even at low field concentrations, significantly reduced survival and longevity when consumed by adult bees in a sugar-based diet. The effects of propiconazole in combination with chlorantraniliprole enhanced mortality risk. The detrimental effects of the two pesticides were for the most part reduced when either or both of the phytochemicals were present in the diet. These findings suggest that honey bees may depend on non-nutritive but physiologically active phytochemical components of their natural foods for ameliorating xenobiotic stress, although only over a certain range of concentrations; particularly at the high end of the natural range, certain combinations can incur additive toxicity. Thus, efforts to develop nectar or pollen substitutes with phytochemicals to boost insecticide tolerance or immunity or to evaluate toxicity of pesticides to pollinators should take concentration-dependent effects of phytochemicals into consideration.
Introduction
Many fungal diseases initiate their disease cycles during plant host flowering. For management of these fungal pathogens, many orchard crops are treated with fungicides during bloom [1], when honey bees (Apis mellifera), the principal managed pollinators in U.S. agriculture, are brought in to provide pollination services. Fungicides thus are among the pesticides most frequently found as contaminants in hives [2]. Even though most fungicides are considered “bee-safe”, low levels of exposure can cause sublethal health effects that lead to weaker colonies or even colony losses, reducing pollination efficiency and decreasing yields [3]. The intermittency of honey bee losses experienced by beekeepers after delivery of pollination services in California’s Central Valley, however, suggests factors other than fungicides alone influence the magnitude of their impact on bees. Fungicides are generally ingested by bees along with dietary phytochemicals present naturally in nectar and pollen as well as with insecticides applied by growers for pest management; these chemicals can all interact to influence bee health. In fact, more than 95% of insecticide applications to almond orchards are made concurrently with fungicide applications [4].
In general, honey bees use nectar from a variety of plant species as the foundation for making honey, a supplementary food for worker and drone grubs and an energy source for adult workers [5]. Honeys thus contain a diversity of phytochemicals, the identities of which depend on floral sources. Among the most commonly occurring phytochemicals in nectar are phenolic acids and flavonols, which are also found in pollen, the principal source of dietary protein, as well as propolis, a material manufactured by bees from plant resins mixed with salivary secretions and beeswax and used as an antimicrobial hive sealant [6]. Composition and concentrations of phenolic acids and flavonols in floral resources, and thus in bee foods, can vary both inter- and intra-specifically [e.g., 7], depending on factors such as genetics [e.g., 8], geography [e.g., 9] and agricultural management [e.g., 10,11].
Among phenolic acids and flavonols, p-coumaric acid and quercetin respectively stand out in having demonstrable impacts on honey bee physiology and health. When consumed by adult bees, they enhance longevity [12,13]. Moreover, the presence of p-coumaric acid in the diet can reduce spore-load in adult bees infected with the fungal pathogen Nosema ceranae [13], which may be due to its ability to upregulate genes encoding antimicrobial peptides [14]. Both p-coumaric acid and quercetin increased the survival rate of bees when ingested along with the pyrethroids bifenthrin and β-cyfluthrin [12], the in-hive acaricide coumaphos [15], or the neonicotinoid imidacloprid [16]. The protective effect of p-coumaric acid and quercetin against dietary toxins in adult bees is likely due to the ability of both compounds to upregulate specific detoxification genes in both larval and adult stages, particularly those in the CYP9Q subfamily encoding enzymes that metabolize pesticides.
In a study examining impacts of commonly used insecticides and fungicides during almond bloom on honey bee health, Wade et al. [4] determined that the combination of the insecticide chlorantraniliprole and the triazole fungicide propiconazole was seven times more toxic to adult bees than chlorantraniliprole alone. Chlorantraniliprole was the first commercialized member of the anthranilic diamide class of insecticides, which target insect ryanodine receptors, a class of intracellular calcium channels [17]. Altacor®, one of chlorantraniliprole’s many formulated products, is applied on almonds for control of peach twig borer (Anarsia lineatella) [18]. Propiconazole is a broad-spectrum triazole fungicide used for management of brown rot blossom blight (Monilinia laxa) and various pathogenic fungi in almonds. Like chlorantraniliprole, it is frequently applied during bloom. These two pesticides thus are often combined and applied as a tank mix in almond orchards from bud break to bloom [19].
Fungicides in the triazole class have long been known to act as synergists that enhance toxicity of insecticides to honey bees [reviewed in 4]. Collectively known as ergosterol-biosynthesis inhibitor (EBI) fungicides, they owe their fungicidal activity to their ability to interfere with the CYP51 (sterol 14-alpha-demethylase) enzyme involved in ergosterol synthesis in pathogenic fungi. Molecular modeling and in silico docking studies of honey bee CYP9Q1 have shown that propiconazole and other triazole fungicides dock in the active pocket of the catalytic site and thus are potential competitive inhibitors of insecticide detoxification [20]. Such triazole-mediated inhibition of honey bee P450-mediated insecticide detoxification likely accounts for the synergistic enhancement by these fungicides of insecticide toxicity.
Because both p-coumaric acid and quercetin upregulate CYP9Q P450s, they could potentially ameliorate synergistic interactions between insecticides and fungicides if ingested concurrently with these pesticides. However, the limited capacity of honey bees to manage detoxification of combinations of chemicals, possibly associated with their reduced inventory of detoxification P450s [21], suggests that phytochemicals may also interfere with xenobiotic detoxification. In fact, consumption of high concentrations of quercetin by adult bees can have adverse impacts on their behavior [22] and physiology [23]. In order to determine whether the optimal range of concentration for amelioration of xenobiotic toxicity by honey phytochemicals corresponds to the typical range of concentrations naturally encountered by adult bees, we first surveyed the literature to document the range of reported concentrations of p-coumaric acid and quercetin in nectar, honey, pollen and beebread. We then examined the effects of consuming these two phytochemicals, alone and in combination, across the reported range of natural concentrations on adult bee longevity in the presence and absence of chlorantraniliprole and propiconazole in their diet.
Materials and methods
Literature review
We conducted a survey of reported ranges of phytochemical concentrations searching PubMed and Google Scholar databases with the search terms “quercetin” or “p-coumaric acid” combined with either “honey”, “pollen”, “beebread”, “propolis” or “royal jelly” and “concentration” for papers published up to and including 2015. The reported phytochemical concentrations (means and/or ranges), type of bee products, botanical origin, and geographic sources of samples from previous literature in the papers produced by the search were recorded. If phytochemical concentrations were reported in a paper as a range, the maximum value of each sample was recorded on a separate column (Max). The reports with no quantitative phytochemical data or with only relative concentrations were not included in our final statistical analyses and tables. When the reported phytochemical concentration was lower than the detection limit of their method, we considered it as zero. All units of concentration in literature were converted into μM for cross-comparison. Pivot tables based on the bee product, botanical source, and geographic source were created in Excel (Version 16.21.1; Microsoft Corp., Redmond, WA) to sort and summarize the results of natural phytochemical concentrations of in forms of descriptive statistics.
Chemicals
Commercial granulated cane sugar (Domino Foods, Inc., West Palm Beach, FL, and Great Value, Walmart Inc., Bentonville, AR) was purchased at local grocery stores. Dimethyl sulfoxide (DMSO; D128) was obtained from Fisher Scientific International, Inc., Pittsburgh, PA, USA. All other chemicals were purchased from Sigma Aldrich, St. Louis, MI, USA, including casein (C3400), chlorantraniliprole (32510), propiconazole (45642), p-coumaric acid (C9008), and quercetin (Q4951).
General protocol for longevity bioassays
To examine diet effects on longevity, we followed the general protocols and the basic diet (comprising 50% sugar water, 0.25% DMSO, with casein added to bring the protein/carbohydrate ratio to 1:12) used in previous studies [12,16]. Colonies in apiaries maintained by the University of Illinois at Urbana-Champaign in Urbana, IL, were used as sources of bees during the fall of 2016 (the first pesticide-phytochemical interaction assay) and summer of 2017 (all remaining assays). All colonies providing bees for bioassays were strong and showed no signs of disease (with either no mites or very low mite counts on nurse bees, healthy brood patterns and no sign of dysentery around the entrance of hive) when we pulled the brood frames. For each bioassay, brood frames were obtained from three unrelated hives and kept in an incubator (34°C, 50% relative humidity). Emerging one-day-old adult bees were collected daily. Each replicate (with all concentrations of phytochemicals and pesticides) was carried out with one-day-old bees from each of the three colonies on the same day. Three to four replicates per each hive of three hives were carried out in all experiments within a week, with one exception; due to the seasonal constraints, the first pesticide-phytochemical interaction assay (30 ppm propiconazole and/or 2ppm chlorantraniliprole), carried out in October 2016, comprised six replicates with one-day-old adults pooled from three unrelated hives. Observation cages were constructed and provisioned with a basic defined diet differentially amended with test compounds to assess impacts on longevity. The assays were carried out in 9 oz. (266 ml) observation cages (made from disposable PET plastic cups, catalogue #:500CC9, WebstaurantStore, Lancaster, PA) containing 25 bees; each cage contained one 2-ml microcentrifuge tube with fresh sugar water diet (changed daily) and one with distilled water (changed weekly) for access ad libitum.
Effective range of phytochemicals on adult honey bee survival
Two phytochemicals in four concentrations, p-coumaric acid (5, 50, 500, 1000 μM) and quercetin (12.5, 25, 250, 1000 μM), based on levels in natural concentrations in honey, pollen, or propolis (Table 1), were tested, along with a phytochemical-free control, in order to identify phytochemical concentrations with beneficial effects on honey bees. Mortality of bees within each cage was recorded in 8-hr intervals (8:00 am, 4:00 pm, 12:00 am) for approximately one week.
Table 1. Naturally occurring concentrations of quercetin and p-coumaric acid in bee products1.
| mean concentration (μM)2 | Max3 (μM) | Ref. | ||||
|---|---|---|---|---|---|---|
| Min | Average | Max | ||||
| Quercetin | royal jelly | 0.7 | [28] | |||
| Honey | 0.0 | 26.0 | 228.3 | n/a | [9,28–44] | |
| Pollen | 5.8 | 1 190.8 | 3 250.0 | n/a | [45–51] | |
| Propolis | 0.0 | 4 227.5 | 27 193.6 | 54 648.74 | [28,52–61] | |
| p-coumaric acid | Honey | 0.0 | 42.5 | 1 914.1 | n/a | [29–31,35,36,41–43,62–64] |
| Pollen | 4.9 | 680.8 | 2 499.3 | n/a | [45,46,50,51,65] | |
| Propolis | 0.0 | 14 413.9 | 69 492.2 | 82 293.35 | [52,53,55,57–59,66–75] | |
1 Upper and lower limits of each group of bee products overlap each other. Phytochemical concentrations varied according to botanical origin (S1 Table) and geographic source (S2 Table).
2 The data, originally reported as a mean value in the cited paper, reported here.
3 This column shows the maximum value of the concentration of the phytochemical reported in the cited paper as a range. Studies in which values were reported as lower than the maximum mean concentration (sleft-hand column) were entered here as “n/a”.
4 Aliyazıcıoglu et al. [52] show this maximum value of quercetin in propolis.
5 Bertelli et al. [53] show this maximum value of p-coumaric acid in propolis.
Pesticide-phytochemical interaction assay
The two phytochemicals (p-coumaric acid and quercetin) in four combinations (control, 500 μM p-coumaric acid, 250 μM quercetin and 500 μM p-coumaric acid + 250 μM quercetin) and three pesticide combinations (2 ppm chlorantraniliprole, 30 ppm propiconazole, and 2 ppm chlorantraniliprole + 30 ppm propiconazole) were tested. The concentration of chlorantraniliprole used in this experiment was based on previous reports of maximum field residues [24] and the concentration of propiconazole was based on the assumed daily dosage with its LD50 [25] over the average lifespan and the average daily sugar water consumption per caged bee (25 μl as determined previously [12]).
The assay aimed at comparing a range of pesticide concentrations was performed in late summer 2016. Due to seasonal constraints, six replicates were carried out with a three-hive-mixed population of bees emerging within a four-day period. To obtain sufficient numbers of individuals for all replicates for each day of testing and minimize the genomic variation caused by hive identity, equivalent quantities (by weight) of one-day-old bees from each colony were intermingled in a container and then assigned randomly in lots of 25 to cages, the same group size used in earlier assays. The number of surviving bees in each cage was recorded daily until overall mortality reached 80% (about 30 days). In previous longevity assays we have conducted with honey bees [12,16], survival curves with endpoints of 80% mortality revealed treatment effects; accordingly, we used the endpoint of 80% mortality for this experiment.
Propiconazole: Chlorantraniliprole 9:4 tank-mix ratio assay
We chose for bioassay the ratio 9:4 propiconazole: chlorantraniliprole suggested for field tank-mixed applications in almond orchards and examined by Wade et al. [4] in larval bioassays and adult topical toxicity assays. This ratio was tested at two concentrations: 0.9 ppm propiconazole + 0.4 ppm chlorantraniliprole and 90 ppm propiconazole + 40 ppm chlorantraniliprole. These concentrations were previously determined to be sublethal by Wade et al. [4]. For the first ten days of the assay, survivorship was evaluated every 8 hours; for the remainder of the assay, survivorship was monitored once daily until overall mortality reached 80% (about 10 days on diets with high pesticide concentrations and 25 days on diets with low pesticide concentrations).
Diets containing p-coumaric acid (5, 50, 500, 1000 μM) and quercetin (12.5, 25, 250, 1000 μM) as well as a phytochemical-free control diet were tested as described earlier with chlorantraniliprole and propiconazole individually and in combination at two concentrations (low concentration: 0.9 ppm propiconazole, 0.4 ppm chlorantraniliprole, and 0.9 ppm propiconazole + 0.4 ppm chlorantraniliprole, high concentration 90 ppm propiconazole + 40 ppm chlorantraniliprole). We tested 36 combination diets in total. For ten days, mortality was recorded at 8-hour intervals; for the remainder of the assay, mortality was recorded once daily until overall mortality reached 80% (about 10 days on diets with high pesticide concentrations and 25 days on diets with low pesticide concentrations).
Statistical analysis of bioassays
Statistical analyses were conducted using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA) and OriginPro 2016 software (OriginLab Corporation, Northampton, MA, USA). OriginPro was used to plot Kaplan-Meier survival curves with the estimated means, and the medians with the Kaplan-Meier estimator. Significant differences between treatment and control were determined through the Tarone-Ware test [26]. The hazard of death according to dietary phytochemicals and pesticide concentrations was evaluated with Cox's proportional hazards regression models [27]. Data were adjusted for hive identity as a covariate stratum if available.
Results
Effective range of phytochemicals on adult honey bee survival
In general, across bee products, quercetin and p-coumaric acid concentrations decreased in abundance in the order propolis > pollen > honey > royal jelly (Table 1), with the highest mean concentration of quercetin and p-coumaric acid concentrations in propolis averaging 4 227.5 ± 2 214.5 and 14 413.9 ± 6 433.6 μM, respectively, and with the lowest quercetin concentration at trace levels (0.7 μM) in royal jelly (which lacked even traces of p-coumaric acid). Mean concentrations of quercetin (26.0 ± 4.7 μM) and p-coumaric acid (42.5 ± 27.2 μM) in honey were lower than mean concentrations in pollen, with quercetin at 1 190.8 ± 687.4 μM and p-coumaric acid at 680.8 ± 606.9 μM.
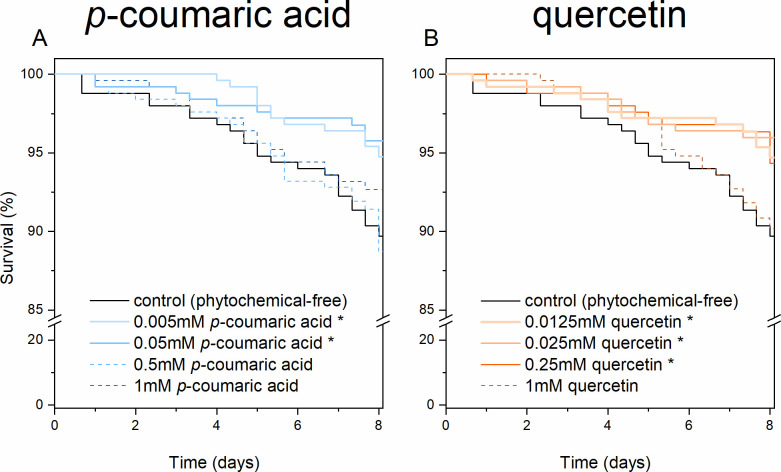
Consuming p-coumaric acid at 0.005 mM (χ2 = 4.49, p = 0.03; Cox model, p = 0.04, hazard ratio (HR): 0.48) and 0.05 mM (χ2 = 6.68, p = 0.01; Cox model, p = 0.01, HR: 0.40; Fig 1A) results in enhanced survival rates of adult bees, as does quercetin at 0.0125 mM (χ2 = 4.45, p = 0.04; Cox model, p = 0.04, HR: 0.48), 0.025 mM (χ2 = 6.49, p = 0.01; Cox model, p = 0.01, HR: 0.40) and 0.25 mM (χ2 = 4.55, p = 0.03; Cox model, p = 0.04, HR: 0.48; Fig 1B). The effective range of both phytochemicals reduced the hazard ratio (HR) by approximately 60% and encompassed the natural range concentrations in honey extending into the lower end of the range of concentrations in pollen.
Fig 1. Kaplan–Meier plots of honey bee longevity with dietary amendments at a range of concentrations.
(A) p-coumaric acid and (B) quercetin. (n = 1 250 for each phytochemical group and n = 250 for each phytochemical concentration subgroup, solid line and * = p < 0.05, Tarone-Ware test between the treatment and control).
Pesticide-phytochemical interaction assay
Overall, according to the Cox regression model, the diet containing pesticides, 2- ppm chlorantraniliprole and 30 ppm propiconazole, decreased the probability of survival (HR = 1.37, p < 0.001, propiconazole; HR = 1.21, p < 0.01, chlorantraniliprole); and they also showed a synergistic effect (HR = 1.37, p < 0.001, combination vs chlorantraniliprole; S1 Fig). Consumption of phytochemicals influenced survival probabilities; diets containing p-coumaric acid reduced mortality risk whereas diets containing quercetin enhanced mortality risk compared to the phytochemical-free control diet (HR = 0.87 and 1.17, p < 0.05, respectively). The negative effects of quercetin were due to its interaction with chlorantraniliprole and propiconazole in these specific concentrations.
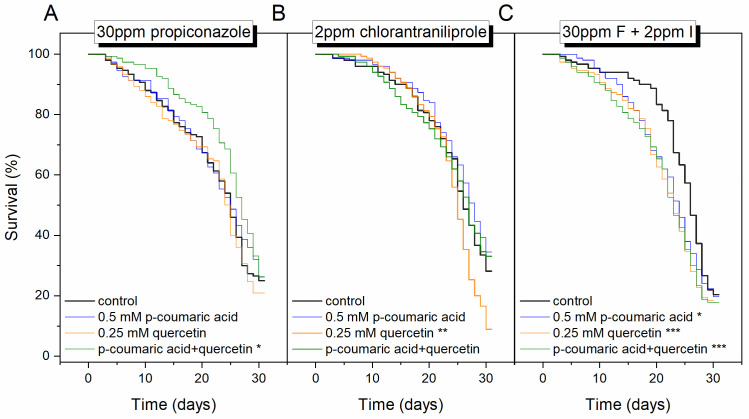
Based on the Kaplan–Meier survival analysis, ingesting 0.25 mM quercetin + 0.5 mM p-coumaric improved tolerance of propiconazole in the diet at 30 ppm (χ2 = 6.49, p = 0.01, Tarone-Ware test; Fig 2A); however, when these two phytochemicals were ingested individually with 30 ppm propiconazole, the survival curve was unaffected. Phytochemicals also did not improve survival on diets containing 2 ppm chlorantraniliprole. Quercetin with 2 ppm chlorantraniliprole even show a negative effect (χ2 = 6.97, p < 0.01, Tarone-Ware test; Fig 2B). Moreover, on the diet amended with 2 ppm chlorantraniliprole + 30 ppm propiconazole, consuming phytochemicals either individually or together significantly increased worker mortality (χ2 = 6.12, 13.39 and 12.95, p < 0.05, p-coumaric acid, quercetin, and combine respectively, Tarone-Ware test; Fig 2C).
Fig 2. Kaplan–Meier plots of honey bee longevity on diets varying in phytochemical and pesticide content.
(A) 30 ppm fungicide propiconazole (B) 2 ppm insecticide chlorantraniliprole, (C) 30 ppm propiconazole + 2 ppm chlorantraniliprole (F: fungicide propiconazole, I: insecticide chlorantraniliprole; n = 600 for each pesticide group and n = 150 for each phytochemical subgroup; *** = p < 0.001, ** = p < 0.01, * = p < 0.05, Tarone-Ware test between the treatment and control).
Chlorantraniliprole and propiconazole tank-mix 9:4 ratio assay
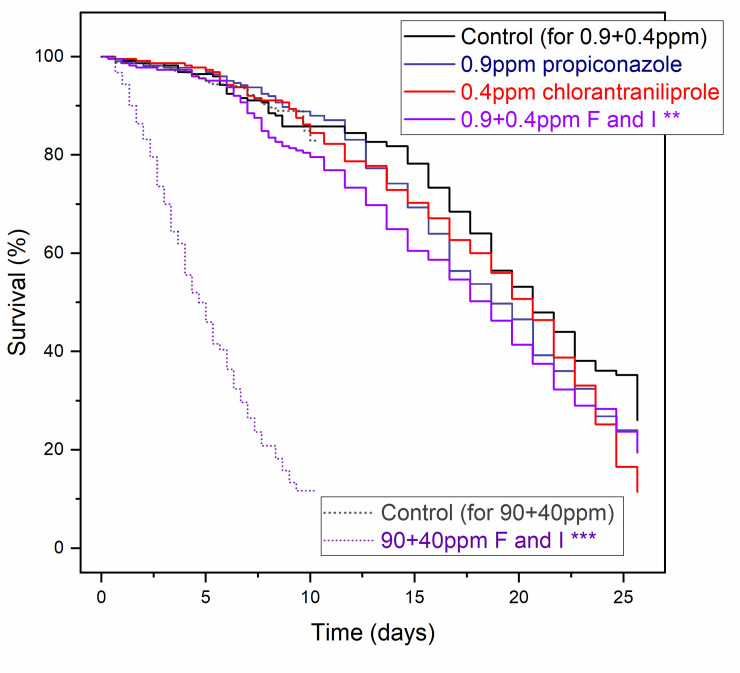
Tank-mixed fungicide and insecticide (propiconazole + chlorantraniliprole) at both low (0.9+0.4 ppm) and high (90+40 ppm) concentrations decreased survival (χ2 = 9.23 and 262.46, p = 0.002 and < 0.001, respectively, Tarone-Ware test, Fig 3). The high-concentration combination of fungicide and insecticide increased the risk of death 14-fold (HR: 14.06, p < 0.001, Cox model) compared to control; the low-concentration combination increased the risk of death by 40% (HR: 1.40, p = 0.003 <0.01, Cox model), while the risk of consuming propiconazole or chlorantraniliprole alone at these low concentrations was not significantly different from the control (p > 0.05, Kaplan–Meier and Cox model).
Fig 3. Kaplan–Meier survival curves of honey bees on tank-mix ratio pesticide treatments.
Honey bees were fed with low [0.9 ppm propiconazole (blue solid line), 0.4 ppm chlorantraniliprole (red solid line), and 0.9 ppm propiconazole + 0.4ppm chlorantraniliprole (purple solid line)] or high [90 ppm propiconazole + 40 ppm chlorantraniliprole(purple dashed line)] concentrations of tank-mixed pesticides. Bees consuming combinations of pesticides experienced reduced longevity relative to bees consuming unamended control diet and relative to bees consuming chlorantraniliprole or propiconazole alone (F: fungicide propiconazole, I: insecticide chlorantraniliprole; n = 900 in 0.9+0.4 ppm and 500 in 90+40ppm subgroup, *** = p < 0.001, ** = p < 0.01, Tarone-Ware test between the treatment and control).
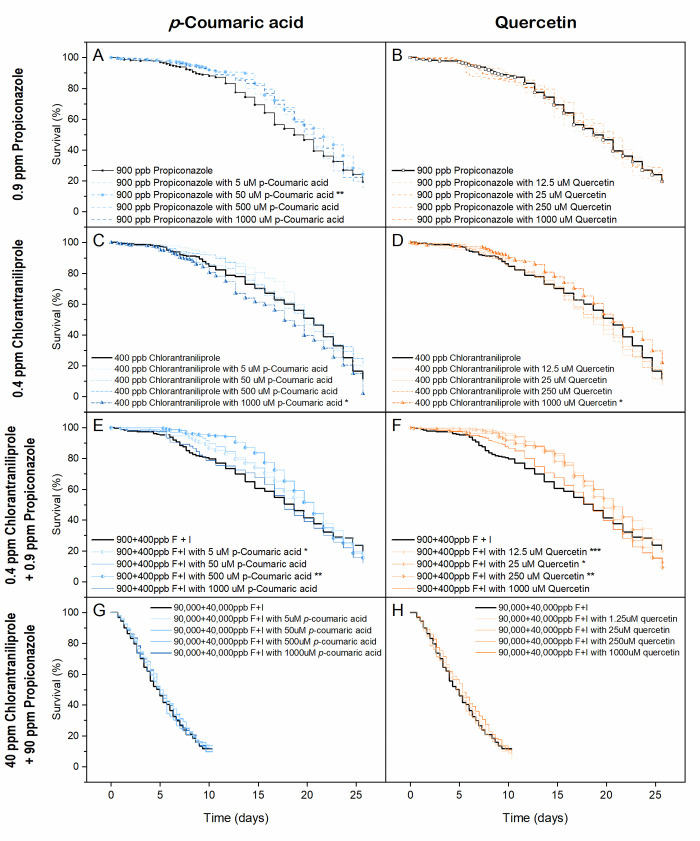
When consumed in diets containing low concentrations of pesticides (0.9 ppm propiconazole and 0.4 ppm chlorantraniliprole; Fig 4A–4F), lifespan extension occurred on the 50 μM p-coumaric acid + 0.9 ppm propiconazole diets (χ2 = 9.54, p = 0.002, Tarone-Ware test; Fig 4A) and 1000 μM quercetin + 0.4 ppm chlorantraniliprole diets (χ2 = 4.24, p = 0.04, Tarone-Ware test; Fig 4D). Similarly, consumption of either p-coumaric acid at 5 or 500 μM (χ2 = 5.55 and 7.97, p = 0.02 and 0.005, Tarone-Ware test; Fig 4E) or quercetin at 12.5, 25 or 250 μM (χ2 = 12.00, 3.98 and 8.28, p < 0.001, <0.05 and <0.01, respectively, Tarone-Ware test; Fig 4F) decreased the toxicity of the 0.9 ppm propiconazole + 0.4 ppm chlorantraniliprole diet, extending mean lifespan by 9–15% (39.3–60.3 h) and 12–16%, (49.5–68.1 h), respectively. Overall, p-coumaric acid at concentrations of 5, 50, and 500 μM decreased the risk of death by 12%-13% (HR: 0.87–0.88, p < 0.05, Cox model) compared to the phytochemical-free diet.
Fig 4. Kaplan–Meier survival curves of honey bees on dietary tank-mix pesticides and phytochemicals.
Diet treatments included: (A) 0.9 ppm propiconazole amendment with p-coumaric acid at a range of concentrations, (B) 0.9 ppm propiconazole amendment with quercetin at a range of concentrations, (C) 0.4 ppm chlorantraniliprole amendment with p-coumaric acid at a range of concentrations, (E) 0.9 ppm propiconazole + 0.4 ppm chlorantraniliprole amendment + p-coumaric acid at a range of concentrations, (F) 0.9 ppm propiconazole + 0.4 ppm chlorantraniliprole amendment + with quercetin at a range of concentrations, (G) 90 ppm propiconazole + 40 ppm chlorantraniliprole amendment with p-coumaric acid at a range of concentrations, and (H) 90 ppm propiconazole + 40 ppm chlorantraniliprole amendment with p-coumaric acid at a range of concentrations (F: fungicide propiconazole, I: insecticide chlorantraniliprole; n = 1 125 for each pesticide group in Fig 4A-4F and n = 1 250 for each pesticide group in Fig 4G and 4H; *** = p < 0.001, ** = p < 0.01, * = p < 0.05, Tarone-Ware test).
Phytochemical amelioration of toxicity, however, did not occur when pesticides were present at high concentrations. Consumption of diets containing 90 ppm propiconazole + 40 ppm chlorantraniliprole reduced lifespan significantly (Fig 3), but phytochemical consumption did not affect this outcome (Fig 4G and 4H). Additionally, a synergistic effect was observed between p-coumaric acid and 0.4 ppm chlorantraniliprole, whereby diets containing 1 000 μM p-coumaric acid and chlorantraniliprole reduced survival relative to diets containing the insecticide alone (χ2 = 6.47, p = 0.01, Tarone-Ware test; Fig 4C), causing a 7% reduction in lifespan (31 hours) compared to the control group.
Discussion
The beneficial effects of two routinely ingested phytochemicals, p-coumaric acid and quercetin, on adult honey bees appear upon consumption of concentrations naturally encountered in floral foods by adult bees over their lifetimes, suggesting that the detoxification system of A. mellifera reflects evolutionary specialization for a phenolic-rich diet. We determined the range of concentrations of phytochemicals most effective at extending survival in the presence and absence of pesticides. In the absence of pesticides, we found that the phytochemical concentrations tested that correspond to those found in many honeys and some pollens (5–50 μM p-coumaric acid and 12.5–250 μM quercetin) (Table 1) significantly promoted bee survival. Similarly, phytochemicals within this range could, depending on the pesticide and its concentration, ameliorate adverse effects. At the highest concentrations tested, which exceed the natural levels in nectar and honey but which are within the range found in pollen and propolis, phytochemicals could exacerbate toxicity in some circumstances. Because propolis is ubiquitous on surfaces throughout the hive, its constituents might be ingested by honey bees via a number of routes—e.g. as a consequence of absorption of its constituents into honey and subsequent ingestion and of social grooming—but propolis itself is not a food and therefore it is unlikely that adult bees ingest quantities of phytochemicals in proportion to their abundance in propolis.
In addition, these two phytochemicals can not only increase the longevity of bees consuming a sugar-based diet, as previously demonstrated, but also enhance survival by alleviating the adverse impacts of fungicides and insecticides frequently found in tank mixes, such as propiconazole and chlorantraniliprole, in a concentration-dependent manner. These two pesticides interact synergistically, most likely due to the P450-inhibiting effects of the triazole fungicide; the related fungicide myclobutanil, e.g., inhibits P450-mediated detoxification of the phytochemical quercetin [20]. Although the mechanism of detoxification of chlorantraniliprole has not yet been determined, cytochrome P450s have been implicated in its detoxification in other insects, such as the diamondback moth Plutella xylostella [76]. In examining transcriptional responses of honey bees to dietary pesticide exposure, Christen and Fent [77] reported that consumption of chlorantraniliprole led to upregulation of CYP9Q2 after 24 hours, which is suggestive of P450-involvement in its metabolism, but these investigators also demonstrated down-regulation of CYP9Q1 at all concentrations tested and CYP9Q3 after 48 hours at concentrations of 10 and 100 ng/bee.
The ability of p-coumaric acid and quercetin to ameliorate the synergistically enhanced effects of the tank-mixed pesticides in this study is consistent with rescue through enhancement of P450-mediated metabolism. Both of these phytochemicals, abundantly represented in the natural diet of the honey bee, upregulate expression of a diversity of cytochrome P450 genes [20], including all three CYP9Q genes in honey bees, which are involved in the metabolism of many pesticides, including pyrethroids, organophosphates [78] and neonicotinoids [21]. Moreover, the triazole fungicides interact synergistically with neonicotinoids [79,80] and pyrethroids [81] in A. mellifera. Finally, Mao et al. [20] demonstrated that a number of triazole fungicides, include propiconazole, dock in the CYP9Q catalytic site. These results collectively suggest an important role of CYP450s in honey bee detoxification of propiconazole. Upregulation of these cytochrome P450 genes by phytochemicals may thus compensate, at least in part, for the inhibitory impacts of the triazole fungicides and possibly the anthranilic diamide insecticide chlorantraniliprole.
Other properties of quercetin and p-coumaric acid may also contribute to reducing insecticide toxicity and prolonging survival. Quercetin may ameliorate the effects of chlorantraniliprole via inhibiting Ca2+-ATPase activity and thereby altering calcium ion fluxes [82–84] or via modulating ryanodine receptors [85,86]. Because chlorantraniliprole [87,88] and propiconazole [89] are both known to induce oxidative stress in other insects, phytochemicals may enhance longevity by attenuating pesticide-induced stress [90]. Quercetin [91,92] and p-coumaric acid [93–95] are both known for their antioxidant properties, which may also contribute to a mechanism by which they enhances survival in the presence of pesticides.
Flavonols and phenolic acids are collectively universal constituents of pollens, all of which contain the flavonol quercetin and/or kaempferol as a pollen tube germination signaling compound [96] and screening pigment against ultraviolet light [97–99] and p-coumaric acid as the monomeric subunit of the sporopollenin exine capsules [100–103]. Similarly, quercetin is ubiquitous in nectars utilized by honey bees; p-coumaric acid is present in many honeys, likely due to their content of pollen grains [14] and has been used as a marker for identifying floral sources in certain honeys [41,104]. Their reliable association with bee food may account for their apparent acquisition of a regulatory function in detoxification of ingested substances. The fact that the ability of these two phytochemicals to extend lifespan and alleviate pesticide toxicity is concentration-dependent should be taken into account in any efforts to incorporate them as additives to food substitutes to improve their quality for beekeeping applications and pollinator health.
Supporting information
(Cox regression model).
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Terry Harrison and Alison Sankey at the University of Illinois Bee Research Facility for assistance with colony establishment; Dr. Gene Robinson for access to the UIUC apiaries, and Dr. Maminirina Randrianandrasana for comments on an early draft of the manuscript. We also thank Dr. Chia-Hua Lin and Dr. Reed Johnson from Ohio State University for advice on pesticide tank-mix ratios and for helpful discussion. Finally, we thank Dr. Nathan Schroeder and Dr. C. Britt Carlson, from the Phenotypic Plasticity Research Experience for Community College Students Program, for their encouragement and support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was funded by the Almond Board of California (16.POLL17.Berenbaum and 18.POLL19.Berenbaum), the National Honey Board, USDA AFRI (USDA AFRI AG 2017-67013-26533 to MRB) and Phenotypic Plasticity Research Experience for Community College Student Program (NSF REU 1559908/1559929).
References
- 1.Adaskaveg JE, Gubler D, Michailides T. Fungicides, bactericides, and biologicals for deciduous tree fruit, nut, strawberry, and vine crops2017:[66 p.]. Available from: http://ipm.ucanr.edu/PDF/PMG/fungicideefficacytiming.pdf.
- 2.Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE. 2010;5(3):e9754 10.1371/journal.pone.0009754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodrich BK. Do more bees imply higher fees? Honey bee colony strength as a determinant of almond pollination fees. Food Policy. 2019. 10.1016/j.foodpol.2018.12.008 [DOI] [Google Scholar]
- 4.Wade A, Lin C-H, Kurkul C, Regan E, Johnson RM. Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects. 2019;10(1):20 Epub 2019/01/11. 10.3390/insects10010020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winston ML. The Biology of the Honey Bee Cambridge: Harvard University Press; 1987. 1987. 294 p. [Google Scholar]
- 6.Johnson RM, Harpur BA, Dogantzis KA, Zayed A, Berenbaum MR. Genomic footprint of evolution of eusociality in bees: floral food use and CYPome “blooms”. Insectes Soc. 2018;65(3):445–54. 10.1007/s00040-018-0631-x [DOI] [Google Scholar]
- 7.Palmer‐Young EC, Farrell IW, Adler LS, Milano NJ, Egan PA, Junker RR, et al. Chemistry of floral rewards: intra‐ and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol Monogr. 2019;89(1). 10.1002/ecm.1335 [DOI] [Google Scholar]
- 8.Guffa B, Nedić NM, Dabić Zagorac DČ, Tosti TB, Gašić UM, Natić MM, et al. Characterization of sugar and polyphenolic diversity in floral nectar of different ‘Oblačinska’ sour cherry clones. Chem Biodivers. 2017;14(9):e1700061 Epub 2017/07/13. 10.1002/cbdv.201700061 . [DOI] [PubMed] [Google Scholar]
- 9.Karabagias IK, Vavoura MV, Badeka A, Kontakos S, Kontominas MG. Differentiation of Greek thyme honeys according to geographical origin based on the combination of phenolic compounds and conventional quality parameters using chemometrics. Food Anal Methods. 2014;7(10):2113–21. 10.1007/s12161-014-9851-5 WOS:000343707100018. [DOI] [Google Scholar]
- 10.Mitchell AE, Hong Y-J, Koh E, Barrett DM, Bryant DE, Denison RF, et al. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J Agric Food Chem. 2007;55(15):6154–9. Epub 2007/06/26. 10.1021/jf070344+ . [DOI] [PubMed] [Google Scholar]
- 11.Consonni R, Bernareggi F, Cagliani LR. NMR-based metabolomic approach to differentiate organic and conventional Italian honey. Food Control. 2019;98(Carbohydrate Research 347 2012):133–40. 10.1016/j.foodcont.2018.11.007 [DOI] [Google Scholar]
- 12.Liao L-H, Wu W-Y, Berenbaum MR. Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees (Apis mellifera). Insects. 2017;8(1):22 Epub 2017/02/22. 10.3390/insects8010022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernklau E, Bjostad L, Hogeboom A, Carlisle A, Seshadri AH. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects. 2019;10(1):14 Epub 2019/01/11. 10.3390/insects10010014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao W, Schuler MA, Berenbaum MR. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc Natl Acad Sci USA. 2013;110(22):8842–6. 10.1073/pnas.1303884110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RM, Dahlgren L, Siegfried BD, Ellis MD. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE. 2013;8(1):e54092 10.1371/journal.pone.0054092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong MJ, Liao L-H, Berenbaum MR. Biphasic concentration-dependent interaction between imidacloprid and dietary phytochemicals in honey bees (Apis mellifera). PLoS ONE. 2018;13(11):e0206625 10.1371/journal.pone.0206625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattelle DB, Cordova D, Cheek TR. Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invertebr Neurosci. 2008;8(3):107–19. Epub 2008/08/13. 10.1007/s10158-008-0076-4 . [DOI] [PubMed] [Google Scholar]
- 18.E. I. du Pont de Nemours and Company. Altacor product label. Wilmington, DE, USA2017.
- 19.Adaskaveg JE, Duncan RA, Gubler WD, Hanson B, Haviland DR, Hembree KJ, et al. UC IPM pest management guidelines: Almond Oakland, CA: UC ANR Publication; 2019. [cited 2019 10 February 2019]. Available from: http://ipm.ucanr.edu/PMG/C003/m003yi01.html#BLOOM. [Google Scholar]
- 20.Mao W, Schuler MA, Berenbaum MR. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc Natl Acad Sci USA. 2017;114(10):2538–43. 10.1073/pnas.1614864114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manjon C, Troczka BJ, Zaworra M, Beadle K, Randall E, Hertlein G, et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr Biol. 2018;28(7):1137–43. 10.1016/j.cub.2018.02.045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao L-H, Wu W-Y, Berenbaum MR. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci Rep. 2017;7(1):15924 10.1038/s41598-017-15066-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenbaum MR, Liao L-H. Honey bees and environmental stress: Toxicologic pathology of a superorganism. Toxicol Pathol. 2019;47(8):1076–81. Epub 2019/10/05. 10.1177/0192623319877154 . [DOI] [PubMed] [Google Scholar]
- 24.Dinter A, Brugger KE, Frost N-M, Woodward MD, editors. Chlorantraniliprole (Rynaxypyr): A novel DuPont™ insecticide with low toxicity and low risk for honey bees (Apis mellifera) and bumble bees (Bombus terrestris) providing excellent tools for uses in integrated pest management 10th International Symposium of the ICP-Bee Protection Group; 2009 8–10 October, 2008; Bucharest, Romania: Julius-Kühn Institut; 2009.
- 25.Databank HS. Propiconazole (CASRN: 60207-90-1) Toxicology data network, U.S National library of Medicine; 2006. [cited 2019 20 May 2019]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+6731. [Google Scholar]
- 26.Tarone RE, Ware J. On distribution-free tests for equality of survival distributions. Biometrika. 1977;64(1):156–60. 10.1093/biomet/64.1.156. [DOI] [Google Scholar]
- 27.Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Stat Method). 1972;34(2):187–220. 10.1007/978-1-4612-4380-9_37 [DOI] [Google Scholar]
- 28.Campillo N, Viñas P, Férez-Melgarejo G, Hernández-Córdoba M. Dispersive liquid–liquid microextraction for the determination of flavonoid aglycone compounds in honey using liquid chromatography with diode array detection and time-of-flight mass spectrometry. Talanta. 2015;131:185–91. Epub 2014/10/05. 10.1016/j.talanta.2014.07.083 . [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Suarez JM, González-Paramás AM, Santos-Buelga C, Battino M. Antioxidant characterization of native monofloral Cuban honeys. J Agric Food Chem. 2010;58(17):9817–24. Epub 2010/08/13. 10.1021/jf1018164 . [DOI] [PubMed] [Google Scholar]
- 30.Escriche I, Kadar M, Juan-Borrás M, Domenech E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res Int. 2011;44(5):1504–13. 10.1016/j.foodres.2011.03.049 WOS:000296797900051. [DOI] [Google Scholar]
- 31.Escriche I, Kadar M, Juan-Borrás M, Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014;142:135–43. Epub 2013/09/05. 10.1016/j.foodchem.2013.07.033 . [DOI] [PubMed] [Google Scholar]
- 32.Ferreres F, García‐Viguera C, Tomás‐Lorente F, Tomás‐Barberán FA. Hesperetin: A marker of the floral origin of citrus honey. J Sci Food Agric. 1993;61(1):121–3. 10.1002/jsfa.2740610119 WOS:A1993KQ34500018. [DOI] [Google Scholar]
- 33.Fiorani M, Accorsi A, Blasa M, Diamantini G, Piatti E. Flavonoids from italian multifloral honeys reduce the extracellular ferricyanide in human red blood cells. J Agric Food Chem. 2006;54(21):8328–34. Epub 2006/10/13. 10.1021/jf061602q . [DOI] [PubMed] [Google Scholar]
- 34.Hamdy AA, Ismail HM, Al-Ahwal AE-M, Gomaa NF. Determination of flavonoid and phenolic acid contents of clover, cotton and citrus floral honeys. J Egypt Public Health Assoc. 2009;84(3–4):245–59. Epub 2009/11/06. . [PubMed] [Google Scholar]
- 35.Kečkeš S, Gašić U, Veličković T, Milojković-Opsenica D, Natić M, Tešić Ž. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013;138(1):32–40. Epub 2012/12/26. 10.1016/j.foodchem.2012.10.025 . [DOI] [PubMed] [Google Scholar]
- 36.Kurtagić H, Barudanović S, Durmić V. Determination of rutin, quercetin, naringenin and hesperetin in the honey from Bosnia and Herzegovina (B & H) in relation to the composition of pollen. J Environ Sci Eng A. 2015;4(12):615–22. 10.17265/2162-5298/2015.12.001 [DOI] [Google Scholar]
- 37.Liu H, Guo Y, Wang X, Liang X, Liu X. Preparation of an Al2O3/SiO2 core–shell composite material for solid phase extraction of flavonoids. Analytical Methods. 2015;7(8):3486–92. 10.1039/C5AY00271K [DOI] [Google Scholar]
- 38.Michalkiewicz A, Biesaga M, Pyrzynska K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J Chromatogr A. 2008;1187(1–2):18–24. Epub 2008/02/20. 10.1016/j.chroma.2008.02.001 . [DOI] [PubMed] [Google Scholar]
- 39.Petrus K, Schwartz H, Sontag G. Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal Bioanal Chem. 2011;400(8):2555–63. Epub 2011/01/14. 10.1007/s00216-010-4614-7 . [DOI] [PubMed] [Google Scholar]
- 40.Soler C, Gil MI, Garcia-Viguera C, Tomás-Barberán FA. Flavonoid patterns of French honeys with different floral origin. Apidologie. 1995;26(1):53–60. 10.1051/apido:19950107 WOS:A1995QG82700007. [DOI] [Google Scholar]
- 41.Tomás‐Barberán FA, Martos I, Ferreres F, Radovic BS, Anklam E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J Sci Food Agric. 2001;81(5):485–96. 10.1002/jsfa.836 [DOI] [Google Scholar]
- 42.Trautvetter S, Koelling-Speer I, Speer K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie. 2009;40(2):140–50. 10.1051/apido/2008072 WOS:000264935200006. [DOI] [Google Scholar]
- 43.Yao L, Datta N, Tomás-Barberán FA, Ferreres F, Martos I, Singanusong R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003;81(2):159–68. 10.1016/S0308-8146(02)00388-6 WOS:000181666800001. [DOI] [Google Scholar]
- 44.Yao L, Jiang Y, D'Arcy B, Singanusong R, Datta N, Caffin N, et al. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J Agric Food Chem. 2004;52(2):210–4. 10.1021/jf034990u . [DOI] [PubMed] [Google Scholar]
- 45.Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol. 2013;55:234–40. Epub 2012/12/04. 10.1016/j.fct.2012.11.022 . [DOI] [PubMed] [Google Scholar]
- 46.Fanali C, Dugo L, Rocco A. Nano-liquid chromatography in nutraceutical analysis: Determination of polyphenols in bee pollen. J Chromatogr A. 2013;1313:270–4. Epub 2013/07/25. 10.1016/j.chroma.2013.06.055 . [DOI] [PubMed] [Google Scholar]
- 47.Freire KRL, Lins ACS, Dórea MC, Santos FAR, Camara CA, Silva TMS. Palynological origin, phenolic content, and antioxidant properties of honeybee-collected pollen from Bahia, Brazil. Molecules. 2012;17(2):1652–64. Epub 2012/02/09. 10.3390/molecules17021652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park YK, Koo MH, Abreu J, Ikegaki M, Cury JA, Rosalen PL. Antimicrobial activity of propolis on oral microorganisms. Curr Microbiol. 1998;36(1):24–8. Epub 1998/02/28. 10.1007/s002849900274 . [DOI] [PubMed] [Google Scholar]
- 49.Šarić A, Balog T, Sobočanec S, Kušić B, Šverko V, Rusak G, et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem Toxicol. 2009;47(3):547–54. Epub 2009/01/07. 10.1016/j.fct.2008.12.007 . [DOI] [PubMed] [Google Scholar]
- 50.Serra Bonvehí J, Soliva Torrentó M, Centelles Lorente E. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J Agric Food Chem. 2001;49(4):1848–53. 10.1021/jf0012300 [DOI] [PubMed] [Google Scholar]
- 51.Ulusoy E, Kolayli S. Phenolic composition and antioxidant properties of Anzer bee pollen. J Food Biochem. 2014;38(1):73–82. 10.1111/jfbc.12027 WOS:000330901700009. [DOI] [Google Scholar]
- 52.Aliyazıcıoglu R, Sahin H, Erturk O, Ulusoy E, Kolayli S. Properties of phenolic composition and biological activity of propolis from Turkey. Int J Food Prop. 2013;16(2):277–87. 10.1080/10942912.2010.551312 [DOI] [Google Scholar]
- 53.Bertelli D, Papotti G, Bortolotti L, Marcazzan GL, Plessi M. 1H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem Anal. 2012;23(3):260–6. Epub 2011/08/20. 10.1002/pca.1352 . [DOI] [PubMed] [Google Scholar]
- 54.Daugsch A, Moraes CS, Fort P, Park YK. Brazilian red propolis—chemical composition and botanical origin. Evid-Based Complement Alternat Med. 2008;5(4):435–41. Epub 2008/10/29. 10.1093/ecam/nem057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jasprica I, Smolčić-Bubalo A, Mornar A, Medić-Šarić M. Investigation of the flavonoids in Croatian propolis by thin-layer chromatography. J Planar Chromat. 2004;17(2):95–101. 10.1556/JPC.17.2004.2.3 WOS:000221981300003. [DOI] [Google Scholar]
- 56.Medana C, Carbone F, Aigotti R, Appendino G, Baiocchi C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochem Anal. 2008;19(1):32–9. Epub 2007/07/27. 10.1002/pca.1010 . [DOI] [PubMed] [Google Scholar]
- 57.Medić-Šarić M, Jasprica I, Mornar A, Smolčić-Bubalo A, Golja P. Quantitative analysis of flavonoids and phenolic acids in propolis by two-dimensional thin layer chromatography. J Planar Chromat. 2004;17(100):459–63. 10.1556/JPC.17.2004.6.12 [DOI] [Google Scholar]
- 58.Papotti G, Bertelli D, Bortolotti L, Plessi M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J Agric Food Chem. 2012;60(11):2852–62. Epub 2012/03/01. 10.1021/jf205179d . [DOI] [PubMed] [Google Scholar]
- 59.Pellati F, Orlandini G, Pinetti D, Benvenuti S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J Pharm Biomed Anal. 2011;55(5):934–48. Epub 2011/04/19. 10.1016/j.jpba.2011.03.024 . [DOI] [PubMed] [Google Scholar]
- 60.Sulaiman GM, Sammarrae KW, Ad’hiah AH, Zucchetti M, Frapolli R, Bello E, et al. Chemical characterization of Iraqi propolis samples and assessing their antioxidant potentials. Food Chem Toxicol. 2011;49(9):2415–21. Epub 2011/07/05. 10.1016/j.fct.2011.06.060 . [DOI] [PubMed] [Google Scholar]
- 61.Sun Y-M, Wu H-L, Wang J-Y, Liu Z, Zhai M, Yu R-Q. Simultaneous determination of eight flavonoids in propolis using chemometrics-assisted high performance liquid chromatography-diode array detection. J Chromatogr B. 2014;962:59–67. Epub 2014/06/08. 10.1016/j.jchromb.2014.05.027 . [DOI] [PubMed] [Google Scholar]
- 62.Dimitrova B, Gevrenova R, Anklam E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem Anal. 2007;18(1):24–32. Epub 2007/01/31. 10.1002/pca.948 . [DOI] [PubMed] [Google Scholar]
- 63.Gambacorta E, Simonetti A, Garrisi N, Intaglietta I, Perna A. Antioxidant properties and phenolic content of sulla (Hedysarum spp.) honeys from Southern Italy. Int J Food Sci Tech. 2014;49(10):2260–8. 10.1111/ijfs.12541 WOS:000343818500014. [DOI] [Google Scholar]
- 64.Habib HM, Meqbali FT, Kamal H, Souka UD, Ibrahim WH. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem. 2014;153:28–34. Epub 2014/02/05. 10.1016/j.foodchem.2013.12.044 . [DOI] [PubMed] [Google Scholar]
- 65.Kao Y-T, Lu M-J, Chen C. Preliminary analyses of phenolic compounds and antioxidant activities in tea pollen extracts. J Food Drug Anal. 2011;19(4):470–7. WOS:000298774100012. [Google Scholar]
- 66.Ahn M-R, Kumazawa S, Hamasaka T, Bang K-S, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52(24):7286–92. Epub 2004/11/26. 10.1021/jf048726s . [DOI] [PubMed] [Google Scholar]
- 67.Barbarić M, Mišković K, Bojić M, Lončar M, Smolčić-Bubalo A, Debeljak Ž, et al. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J Ethnopharmacol. 2011;135(3):772–8. Epub 2011/04/26. 10.1016/j.jep.2011.04.015 . [DOI] [PubMed] [Google Scholar]
- 68.de Funari CS, de Oliveira Ferro V, Mathor MB. Analysis of propolis from Baccharis dracunculifolia DC. (Compositae) and its effects on mouse fibroblasts. J Ethnopharmacol. 2007;111(2):206–12. Epub 2007/01/09. 10.1016/j.jep.2006.11.032 . [DOI] [PubMed] [Google Scholar]
- 69.de Sousa JPB, Bueno PCP, Gregório LE, da Silva Filho AA, Furtado NAJC, de Sousa ML, et al. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. J Sep Sci. 2007;30(16):2656–65. Epub 2007/09/21. 10.1002/jssc.200700228 . [DOI] [PubMed] [Google Scholar]
- 70.Erdogan S, Ates B, Durmaz G, Yilmaz I, Seckin T. Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem Toxicol. 2011;49(7):1592–7. Epub 2011/05/03. 10.1016/j.fct.2011.04.006 . [DOI] [PubMed] [Google Scholar]
- 71.Fernandes-Silva CC, Salatino A, Salatino MF, Breyer EDH, Negri G. Chemical profiling of six samples of Brazilian propolis. Química Nova. 2013;36(2):237–40. 10.1590/S0100-40422013000200006 WOS:000316766400006. [DOI] [Google Scholar]
- 72.Kumazawa S, Ahn M-R, Fujimoto T, Kato M. Radical-scavenging activity and phenolic constituents of propolis from different regions of Argentina. Nat Prod Res. 2010;24(9):804–12. 10.1080/14786410802615270 [DOI] [PubMed] [Google Scholar]
- 73.Kumazawa S, Bonvehí JS, Torres C, Mok‐Ryeon A, Bermejo FJO. Chemical and functional characterisation of propolis collected from East Andalusia (southern Spain). Phytochem Anal. 2013;24(6):608–15. Epub 2013/05/15. 10.1002/pca.2439 . [DOI] [PubMed] [Google Scholar]
- 74.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50(9):2502–6. Epub 2002/04/18. 10.1021/jf011432b . [DOI] [PubMed] [Google Scholar]
- 75.Teixeira ÉW, Negri G, Meira RMSA, Message D, Salatino A. Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evid-Based Complement Alternat Med. 2005;2(1):85–92. Epub 2005/04/21. 10.1093/ecam/neh055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Z, Lin Q, Chen H, Li Z, Yin F, Feng X. Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bull Entomol Res. 2014;104(6):716–23. Epub 2014/09/12. 10.1017/S0007485314000510 . [DOI] [PubMed] [Google Scholar]
- 77.Christen V, Fent K. Exposure of honey bees (Apis mellifera) to different classes of insecticides exhibit distinct molecular effect patterns at concentrations that mimic environmental contamination. Environ Pollut. 2017;226(Ecotoxicol. Environ. Saf. 114 2015):48–59. Epub 2017/04/14. 10.1016/j.envpol.2017.04.003 . [DOI] [PubMed] [Google Scholar]
- 78.Mao W, Schuler MA, Berenbaum MR. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc Natl Acad Sci USA. 2011;108(31):12657–62. 10.1073/pnas.1109535108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwasa T, Motoyama N, Ambrose JT, Roe RM. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Protect. 2004;23(5):371–8. 10.1016/j.cropro.2003.08.018 [DOI] [Google Scholar]
- 80.Sgolastra F, Medrzycki P, Bortolotti L, Renzi MT, Tosi S, Bogo G, et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manage Sci. 2017;73(6):1236–43. Epub 2016/09/30. 10.1002/ps.4449 . [DOI] [PubMed] [Google Scholar]
- 81.Thompson H, Wilkins S. Assessment of the synergy and repellency of pyrethroid/fungicide mixtures. Bull Insectol. 2003;56(1):131–4. [Google Scholar]
- 82.Shoshan V, Campbell KP, MacLennan DH, Frodis W, Britt BA. Quercetin inhibits Ca2+ uptake but not Ca2+ release by sarcoplasmic reticulum in skinned muscle fibers. Proc Natl Acad Sci USA. 1980;77(8):4435–8. Epub 1980/08/01. 10.1073/pnas.77.8.4435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fewtrell CMS, Gomperts BD. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature. 1977;265(5595):635–6. Epub 1977/02/17. 10.1038/265635a0 . [DOI] [PubMed] [Google Scholar]
- 84.Ontiveros M, Rinaldi D, Marder M, Espelt MV, Mangialavori I, Vigil M, et al. Natural flavonoids inhibit the plasma membrane Ca2+-ATPase. Biochem Pharmacol. 2019;166:1–11. Epub 2019/05/10. 10.1016/j.bcp.2019.05.004 . [DOI] [PubMed] [Google Scholar]
- 85.Lee E, Meissner G, Kim D. Effects of quercetin on single Ca2+ release channel behavior of skeletal muscle. Biophys J. 2002;82(3):1266–77. 10.1016/S0006-3495(02)75483-0 WOS:000174170700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kadala A, Charreton M, Charnet P, Collet C. Honey bees long-lasting locomotor deficits after exposure to the diamide chlorantraniliprole are accompanied by brain and muscular calcium channels alterations. Sci Rep. 2019;9(1):2153 10.1038/s41598-019-39193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu Y, Bai Q, Zheng X, Lu Z. Expression and enzyme activity of catalase in Chilo suppressalis (Lepidoptera: Crambidae) is responsive to environmental stresses. J Econ Entomol. 2017;110(4):1803–12. Epub 2017/04/19. 10.1093/jee/tox117 . [DOI] [PubMed] [Google Scholar]
- 88.Franeta F, Mircic D, Todorovic D, Milovac Z, Granica N, Obradovic S, et al. Effects of different insecticides on the antioxidative defense system of the European Corn Borer (Ostrinia nubilalis Hübner) (Lepidoptera: Crambidae) larvae. Arch Biol Sci. 2018;70(4):765–73. 10.2298/ABS180701042F WOS:000452187000019. [DOI] [Google Scholar]
- 89.Han W, Yang Y, Gao J, Zhao D, Ren C, Wang S, et al. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology. 2019;28(4):399–411. Epub 2019/03/16. 10.1007/s10646-019-02030-4 . [DOI] [PubMed] [Google Scholar]
- 90.Poljšak B, Fink R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid Med Cell Longev. 2014;2014:671539 Epub 2014/08/21. 10.1155/2014/671539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Phcog Rev. 2016;10(20):84–9. Epub 2017/01/14. 10.4103/0973-7847.194044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061–80. 10.1016/0278-6915(95)00077-1 [DOI] [PubMed] [Google Scholar]
- 93.Ekinci-Akdemir FN, Albayrak M, Çalik M, Bayir Y, Gülçin İ. The protective effects of p-coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines. 2017;5(2):e18 Epub 2017/05/26. 10.3390/biomedicines5020018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ekinci-Akdemir FN, Gülçin I, Gürsul C, Alwasel SH, Bayir Y. Effect of p-coumaric acid against oxidative stress induced by cisplatin in brain tissue of rats. J Anim Plant Sci. 2017;27(5):1560–4. . [Google Scholar]
- 95.Pei K, Ou J, Huang J, Ou S. p‐Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96(9):2952–62. 10.1002/jsfa.7578 [DOI] [PubMed] [Google Scholar]
- 96.Ylstra B, Touraev A, Moreno R, Stöger E, van Tunen AJ, Vicente O, et al. Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol. 1992;100(2):902–7. Epub 1992/10/01. 10.1104/pp.100.2.902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan KG, Swinny EE, Markham KR, Winefield C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry. 2002;59(1):23–32. Epub 2002/01/05. 10.1016/s0031-9422(01)00404-6 . [DOI] [PubMed] [Google Scholar]
- 98.Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005;7(6):581–91. 10.1055/s-2005-873009 [DOI] [PubMed] [Google Scholar]
- 99.Treutter D. Significance of flavonoids in plant resistance: a review. Environ Chem Lett. 2006;4(3):147–57. 10.1007/s10311-006-0068-8. WOS:000239950400006. [DOI] [Google Scholar]
- 100.Wehling K, Niester C, Boon JJ, Willemse MT, Wiermann R. p-Coumaric acid—a monomer in the sporopollenin skeleton. Planta. 1989;179(3):376–80. Epub 1989/10/01. 10.1007/BF00391083 . [DOI] [PubMed] [Google Scholar]
- 101.Blokker P, Boelen P, Broekman R, Rozema J. The occurrence of p-coumaric acid and ferulic acid in fossil plant materials and their use as UV-proxy. Plant Ecol. 2006;182(1–2):197 10.1007/s11258-005-9026-y [DOI] [Google Scholar]
- 102.Nierop KGJ, Versteegh GJM, Filley TR, de Leeuw JW. Quantitative analysis of diverse sporomorph-derived sporopollenins. Phytochemistry. 2019;162:207–15. Epub 2019/04/06. 10.1016/j.phytochem.2019.03.023 . 30952081. [DOI] [PubMed] [Google Scholar]
- 103.Li F-S, Phyo P, Jacobowitz J, Hong M, Weng J-K. The molecular structure of plant sporopollenin. Nat Plants. 2019;5(1):41–6. Epub 2018/12/19. 10.1038/s41477-018-0330-7 . [DOI] [PubMed] [Google Scholar]
- 104.Haroun MI, Poyrazoglu ES, Konar N, Artik N. Phenolic acids and flavonoids profiles of some Turkish honeydew and floral honeys. J Food Technol. 2012;10(2):39–45. 10.3923/jftech.2012.39.45 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Cox regression model).
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.