Abstract
Objectives
We report on the key clinical predictors of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and present a clinical decision rule that can risk stratify patients for COVID-19.
Design, participants and setting
A prospective cohort of patients assessed for COVID-19 at a screening clinic in Melbourne, Australia. The primary outcome was a positive COVID-19 test from nasopharyngeal swab. A backwards stepwise logistic regression was used to derive a model of clinical variables predictive of a positive COVID-19 test. Internal validation of the final model was performed using bootstrapped samples and the model scoring derived from the coefficients, with modelling performed for increasing prevalence.
Results
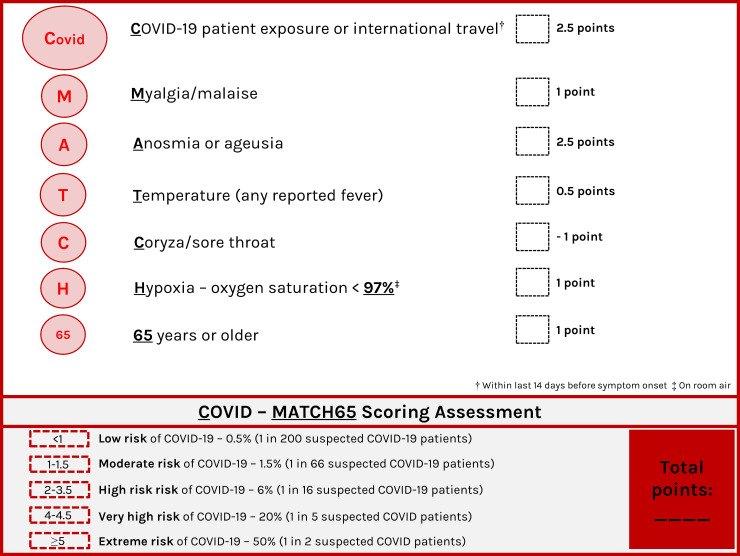
Of 4226 patients with suspected COVID-19 who were assessed, 2976 patients underwent SARS-CoV-2 testing (n = 108 SARS-CoV-2 positive) and were used to determine factors associated with a positive COVID-19 test. The 7 features associated with a positive COVID-19 test on multivariable analysis were: COVID-19 patient exposure or international travel, Myalgia/malaise, Anosmia or ageusia, Temperature, Coryza/sore throat, Hypoxia–oxygen saturation < 97%, 65 years or older—summarized in the mnemonic COVID-MATCH65. Internal validation showed an AUC of 0.836. A cut-off of ≥ 1.5 points was associated with a 92.6% sensitivity and 99.5% negative predictive value (NPV) for COVID-19.
Conclusions
From the largest prospective outpatient cohort of suspected COVID-19 we define the clinical factors predictive of a positive SARS-CoV-2 test. The subsequent clinical decision rule, COVID-MATCH65, has a high sensitivity and NPV for SARS-CoV-2 and can be employed in the pandemic, adjusted for disease prevalence, to aid COVID-19 risk-assessment and vital testing resource allocation.
Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in China and has now infected over 9 million people globally [1]. A range of clinical symptoms and syndromes have been reported in confirmed COVID-19 [2–4]. However, there have been limited prospective reports of the clinical and epidemiological predictors of COVID-19 infection [5]. We report on the clinical and epidemiological predictors of COVID-19 from a uniquely derived prospective database and present a point-of-care ready COVID-19 clinical decision tool.
Methods
A COVID-19 rapid assessment screening clinic was established at Austin Health on 11 March 2020 with prospective electronic medical record (EMR; S1 Data and S1 File) data of patients presenting to the clinic systematically collected by medical staff from 11 March to 22 April 2020. Patients were predominantly adults—children over 6 months were seen at clinician discretion. Modifications to the EMR were made during the study period to align with the Victorian Department of Health and Human Services (DHHS) testing criteria [6] (S1 Data and S1 File). Only those patients that met the DHHS criteria for SARS-CoV-2 testing had nasopharyngeal swab collected for SARS-Cov-2 nucleic acid detection by polymerase chain reaction (PCR), the platforms utilized outlined in S1 Data and S1 File. Patients with swabs that had SARS-CoV-2 nucleic acid detected were termed “COVID-19 test positive”; those with swabs where SARS-CoV-2 nucleic acid was not detected were termed “COVID-19 test negative”. This study was approved by the Austin Health Human Research and Ethics Committee (Austin HREC Audit/20/Austin/37). Participants were not recruited and did not required to provide consent for this study as this was an audit of routine clinical practice and standardised data collection.
Derivation and internal validation cohort
Clinical data from the data collection tool (baseline demographics, clinical symptoms, clinical observations) and COVID-19 testing results were extracted from Austin Health EMR platform (Cerner®) by the Data Analytics Research and Evaluation (DARE) Centre (Austin Health/University of Melbourne).
Statistical analysis
All results are presented according to TRIPOD guidelines [7]. Categorical variables are presented as frequency (percentage) and continuous variables as median (interquartile range [IQR]). Fisher’s exact test or rank sum test were used to compare characteristics between tested and not tested patients. To determine the predictors of a positive COVID-19 test, a multivariable logistic regression with backward stepwise procedure was used, eliminating variables with p>0.10 and re-inclusion of variables with p<0.05. Bootstrapping was used for internal validation. Further details on variable selection, model development and performance, internal validation and score derivation are outlined in eMethods 3 of S1 File.
Results
Study population and setting
During the study period 4359 assessments were performed in 4226 patients (S1 Table and S1 Fig of S1 File). For those with multiple presentations (n = 118) only their first testing date was used (for patients that were not tested, their first assessment was taken). Median (IQR) number of daily assessments was 96 (71, 134) with an average of 51% of patients being tested each day (S2 Fig of S1 File).
The characteristics of those with suspected COVID-19 that presented to a COVID-19 testing service, stratified by testing performed status, is outlined in S2 Table of S1 File. The most frequently reported symptoms in both groups were any fever (reported or documented), cough, sore throat and coryza as outlined in S2 Table of S1 File. SARS-CoV-2 testing was undertaken in 2935patients (70%).
COVID-19 test positivity
Of the 2976 patients that were tested, 41 were excluded from the analysis due to pending results (n = 38) or indeterminate results (n = 3) (eFig 1 of S1 File). The prevalence of a positive COVID-19 test in the final cohort was 3.7% (108/2935). Characteristics of those patients with a positive COVID-19 test are shown in Table 1.
Table 1. Characteristics of patients who underwent testing for COVID-19.
| Factor | Overall | Not detected | Detected | |
|---|---|---|---|---|
| N | 2935 | 2827 | 108 | |
| Age, years, median (IQR) | 39 (29, 53) | 38 (29, 52) | 51 (33, 62) | |
| Sex—male | 1071 (36.5%) | 1016 (35.9%) | 55 (50.9%) | |
| Comorbidities | ||||
| Cardiovascular disease | 105 (3.6%) | 101 (3.6%) | 4 (3.7%) | |
| Diabetes | 85 (2.9%) | 84 (3.0%) | 1 (0.9%) | |
| Hypertension | 262 (8.9%) | 245 (8.7%) | 17 (15.7%) | |
| ACEI/ARB treatment | 98 (3.3%) | 89 (3.1%) | 9 (8.3%) | |
| Smoking | 259 (8.8%) | 256 (9.1%) | 3 (2.8%) | |
| Chronic renal or liver disease | 21 (0.7%) | 21 (0.7%) | 0 (0.0%) | |
| Immunosuppressed | 90 (3.1%) | 87 (3.1%) | 3 (2.8%) | |
| Chronic respiratory disease | 343 (11.7%) | 339 (12.0%) | 4 (3.7%) | |
| Pregnancy | 38 (1.3%) | 38 (1.3%) | 0 (0.0%) | |
| Overseas health facility exposure | 114 (3.9%) | 112 (4.0%) | 2 (1.9%) | |
| Australian health facility exposure | 902 (30.7%) | 890 (31.5%) | 12 (11.1%) | |
| Any contact or overseas travel | 1182 (40.3%) | 1093 (38.7%) | 89 (82.4%) | |
| Contact with known COVID-19 positive patient | 508 (17.3%) | 446 (15.8%) | 62 (57.4%) | |
| Overseas travel (incl. cruise) | 723 (24.6%) | 684 (24.2%) | 39 (36.1%) | |
| Days from arrival to symptom onset, median (IQR) | 2 (-1, 6) | 2 (-1, 6) | 1 (-1, 3) | |
| Number of symptoms | ||||
| 0 | 49 (1.7%) | 45 (1.6%) | 4 (3.7%) | |
| 1 | 243 (8.3%) | 240 (8.5%) | 3 (2.8%) | |
| 2 | 540 (18.4%) | 526 (18.6%) | 14 (13.0%) | |
| 3 | 669 (22.8%) | 646 (22.9%) | 23 (21.3%) | |
| 4 | 646 (22.0%) | 623 (22.0%) | 23 (21.3%) | |
| 5 or more | 788 (26.8%) | 747 (26.4%) | 41 (38.0%) | |
| Symptoms | ||||
| Any fever | 1119 (38.1%) | 1063 (37.6%) | 56 (51.9%) | |
| Fever > 38 C | 274 (9.3%) | 260 (9.2%) | 14 (13.0%) | |
| Fever subjective | 905 (30.8%) | 859 (30.4%) | 46 (42.6%) | |
| Sore throat | 2038 (69.4%) | 1983 (70.1%) | 55 (50.9%) | |
| Sinusitis | 14 (0.5%) | 13 (0.5%) | 1 (0.9%) | |
| Cough | 2042 (69.6%) | 1956 (69.2%) | 86 (79.6%) | |
| Shortness of breath | 897 (30.6%) | 868 (30.7%) | 29 (26.9%) | |
| Chest pain | 71 (2.4%) | 68 (2.4%) | 3 (2.8%) | |
| Anosmia | 75 (2.6%) | 64 (2.3%) | 11 (10.2%) | |
| Ageusia | 81 (2.8%) | 69 (2.4%) | 12 (11.1%) | |
| Anosmia or ageusia | 126 (4.3%) | 109 (3.9%) | 17 (15.7%) | |
| Coryza | 1606 (54.7%) | 1559 (55.1%) | 47 (43.5%) | |
| Diarrhoea | 483 (16.5%) | 457 (16.2%) | 26 (24.1%) | |
| Other GI symptoms | 63 (2.1%) | 62 (2.2%) | 1 (0.9%) | |
| Malaise/myalgia/arthralgia | 1410 (48.0%) | 1339 (47.4%) | 71 (65.7%) | |
| Headache | 402 (13.7%) | 381 (13.5%) | 21 (19.4%) | |
| Asymptomatic | 25 (0.9%) | 23 (0.8%) | 2 (1.9%) | |
| Days since symptom onset, median (IQR) | 3 (1, 6) | 3 (1, 6) | 4 (2, 7) | |
| Clinical signs | ||||
| SPO2, median (IQR) | 98 (97, 99) | 98 (97, 99) | 98 (96, 99) | |
| Temperature Tympanic, median (IQR) | 36.6 (36.3, 36.9) | 36.6 (36.3, 36.9) | 36.7 (36.3, 37.1) | |
| Systolic Blood Pressure, median (IQR) | 133 (121, 147) | 132 (121, 147) | 134 (122, 146) | |
| Diastolic Blood Pressure, median (IQR) | 82 (75, 89) | 81 (75, 89) | 83.5 (78, 88) | |
| Respiratory Rate, median (IQR) | 18 (16, 18) | 18 (16, 18) | 18 (17, 18) | |
| Pulse Rate, median (IQR) | 83 (73, 94) | 84 (73, 94) | 82 (73, 93.5) | |
| Discharge destination | ||||
| Discharged | 1895 (64.6%) | 1802 (63.7%) | 93 (86.1%) | |
| Transferred to ED | 18 (0.6%) | 18 (0.6%) | 0 (0.0%) | |
| Transferred to ward | 1 (<0.1%) | 1 (<0.1%) | 0 (0.0%) | |
| Unknown | 1021 (34.8%) | 1006 (35.6%) | 15 (13.9%) | |
Abbreviations: N, number; IQR, interquartile range; SPO2, oxygen saturation; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; GI, gastrointestinal; ED, emergency department.
Demographic, epidemiological and clinical factors associated with a positive COVID-19 test
The characteristics associated with a positive COVID-19 test in univariate and multivariable analysis are shown in Table 2. The seven features associated with a COVID-19 test on multivariable analysis were summarized in the mnemonic COVID-MATCH65 (Fig 1). The model showed good discrimination (AUC = 0.843, Hosmer-Lemeshow chi2 = 4.96, p = 0.762) and calibration (calibration slope = 1.00, Brier score = 0.03, product-moment correlation between observed and predicted probability = 0.35). Internal validation showed minimal mean optimism of 0.007 with internally validated AUC of 0.836 (S3 & S4 Figs of S1 File). The resulting score ranges from -1 to 6.5 points with score ≤ 1 representing low risk of a positive test (<1%) and scores above 4 having beyond 20% probability of a positive test (Fig 1).
Table 2. Univariate & multivariable analysis of features associated with a positive COVID-19 test (SARS-CoV-2 nucleic acid detected).
| Variables considered for inclusion | Overall tested (n = 2935) | COVID-19 positive test (n = 108) | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | Beta coefficient (95% CI) | p-value | Presence in 1000 bootstrap replications, %* | Points | |||
| Age 65+ | 254 (8.7%) | 19 (17.6%) | 2.35 (1.41, 3.93) | 0.001 | 2.80 (1.56, 5.04) | 1.03 (0.45, 1.62) | 0.001 | 75 | 1 |
| Male sex | 1071 (36.5%) | 55 (50.9%) | 1.85 (1.26, 2.72) | 0.002 | 50 | ||||
| Hypertension | 262 (8.9%) | 17 (15.7%) | 1.97 (1.15, 3.36) | 0.013 | 50 | ||||
| Contact with known COVID-19 positive patient or overseas travel | 1182 (40.3%) | 89 (82.4%) | 7.43 (4.50, 12.27) | <0.001 | 14.24 (7.92, 25.63) | 2.66 (2.07, 3.24) | <0.001 | 100 | 2.5 |
| Any fever (documented or reported) | 1119 (38.1%) | 56 (51.9%) | 1.79 (1.22, 2.63) | 0.003 | 1.59 (1.03, 2.43) | 0.46 (0.03, 0.89) | 0.035 | 71 | 0.5 |
| Coryza or sore throat | 2455 (83.6%) | 73 (67.6%) | 0.39 (0.26, 0.59) | <0.001 | 0.36 (0.23, 0.58) | -1.01 (-1.48, -0.55) | <0.001 | 99 | -1 |
| Cough | 2042 (69.6%) | 86 (79.6%) | 1.74 (1.08, 2.80) | 0.022 | 52 | ||||
| Shortness of breath* | 897 (30.6%) | 29 (26.9%) | 0.83 (0.54, 1.28) | 0.394 | |||||
| Anosmia or ageusia | 126 (4.3%) | 17 (15.7%) | 4.66 (2.68, 8.09) | <0.001 | 13.67 (6.89, 27.13) | 2.62 (1.93, 3.30) | <0.001 | 100 | 2.5 |
| Diarrhoea | 483 (16.5%) | 26 (24.1%) | 1.64 (1.05, 2.58) | 0.031 | 26 | ||||
| Myalgia or Malaise | 1410 (48.0%) | 71 (65.7%) | 2.13 (1.42, 3.19) | <0.001 | 2.20 (1.41, 3.44) | 0.79 (0.45, 1.35) | 0.001 | 96 | 1 |
| Headache | 402 (13.7%) | 21 (19.4%) | 1.55 (0.95, 2.53) | 0.079 | 36 | ||||
| SPO2 <97% | 473 (16.1%) | 36 (33.3%) | 2.73 (1.81, 4.13) | <0.001 | 2.46 (1.57, 3.87) | 0.90 (0.45, 1.35) | <0.001 | 93 | 1 |
| Temperature ≥37.5 C | 174 (5.9%) | 11 (10.2%) | 1.85 (0.97, 3.53) | 0.060 | 15 | ||||
| Systolic blood pressure >140 mmHg* | 1082 (36.9%) | 43 (39.8%) | 1.14 (0.77, 1.69) | 0.518 | |||||
| Diastolic blood pressure >80 mmHg | 1623 (55.3%) | 72 (66.7%) | 1.65 (1.10, 2.47) | 0.016 | 54 | ||||
| Respiratory rate <16/min or >20/min* | 196 (6.7%) | 7 (6.5%) | 0.97 (0.44, 2.11) | 0.934 | |||||
| Pulse rate <60/min or >100/min | 486 (16.6%) | 11 (10.2%) | 0.56 (0.30, 1.06) | 0.073 | 51 | ||||
*Not considered for inclusion due to p<0.200.
Abbreviations: OR, odds ratio; CI, confidence interval; SPO2, oxygen saturation.
Fig 1. COVID-19 clinical decision rule–COVID-MATCH65.
The positive and negative results for each COVID-MATCH65 score are outlined in S3 Table of S1 File. A score of at least 1.5 was shown to have 92.6% (95% CI 85.9%, 96.7%) sensitivity, 51.3% (49.4, 53.1) specificity, 6.8% (5.5, 8.2) positive predictive value and 99.5% (98.9, 99.8) negative predictive value of identifying a patient who was COVID-19 test positive (S4 Table of S1 File). COVID-MATCH65 also retains a high NPV with increasing prevalence of COVID-19 (30% prevalence) (S3 Table of S1 File).
Admission to hospital
A total of 15 COVID-19 positive patients (14%) were admitted to hospital. Median (IQR) COVID-MATCH65 score in admitted was 3.5 (2.5, 4.5) while in non-admitted it was 3 (2.5, 4). Score was not predictive of admission (OR 1.04, 95%CI: 0.70, 1.53, p = 0.852). Variables predictive of admission were oxygen saturation (SpO2) < 97%, shortness of breath, male gender and not being exposed to confirmed case/international travel (S5 Table of S1 File).
Discussion
Whilst the clinical features of COVID-19 have been reported, robust prospective data from patients presenting for COVID-19 assessment (SARS-CoV-2 positive and negative) remains absent. Therefore, the clinical predictors associated with a positive SARS-CoV-2 test have remained ill defined. Whilst fever has been the predominant presenting feature of confirmed COVID-19 cases from published inpatient populations [4], it was in fact observed less frequently (36.5%) in our outpatient cohort, potentially the result of earlier presentation (5 days[median] from symptom onset). Bajema et al. [5] reported fever in 68% in a retrospective cohort study (n = 210) from the USA with similar incidence rate of COVID-19 positive tests to our cohort (5% USA vs. 4.7% AUS). Whilst in the earliest reports from confirmed cases in China the figures were 83–98% [2, 3]. Coryza and sore throat were also frequently reported, the presence of either was in fact a negative predictor of COVID-19. Anosmia or ageusia as seen in other emerging studies was a strong predictor of a positive COVID-19 test [8]. Whilst contact and/or international travel was a predictor of COVID-19 infection in our model, as seen in US model from Challenger et al. [9], it may be less relevant in outbreak settings and during periods of travel bans, however these criteria alone are not required for a patient to be at high risk of COVID-19.
Our model has some limitations, including the single centre prospective data source, jurisdictional guided testing criteria, testing of symptomatic only patients and absence of external validation. However, only one small retrospective US cohort (n = 49 COVID-19 positive /n = 98 COVID-19 negative) [9] and two non-peer reviewed publications from China have examined the role of clinical decision rules from large datasets—Meng et al. (n = 620 outpatients; 48.7% positive) [10] and Song et al. [11] (n = 304 inpatients; 24.0% positive), both limited by requirement for clinical and laboratory parameters. COVID-MATCH65 uses readily available clinical information without laboratory test results, with a score of ≥ 1.5 associated with high sensitivity (92.6 [95% CI 85.9, 96.7]) and NPV (99.5 [95% CI 98.9, 99.8]), enabling application in the outpatient and potentially early inpatient setting. The model also retains a high NPV (99.3 [95% CI 98.9, 99.6]) with a score of ≥ 2 with only a slightly reduced sensitivity, which may appeal to some centres trying to reduce unnecessary testing. Further risk stratification can be made with the COVID-MATCH65 (lowest risk [< 1 in 100] to extreme risk [1 in 1]), aiding diagnostic approaches in patients with suspected COVID-19, such as additional testing or serological evaluation With ranging SARS-CoV-2 prevalence internationally, it is important to note that COVID-MATCH65 also performed well with increasing disease prevalence (Table 3). In a pandemic where diagnostic resources are limited in both low- and high-income settings, [12] risk stratification of those likely to have COVID-19 is urgently required and tools such as COVID-MATCH65 can aid the front-line clinician. We encourage readers to urgently employ and validate COVID-MATCH65 in their own datasets, as it is likely to aid clinicians at point-of-care especially via an open access web platform (http://COVID-MATCH65.austin.org.au).
Table 3. The sensitivity, specificity, positive predictive value and negative predictive value of COVID-MATCH65 with increasing prevalence of COVID-19.
| Sensitivity (%) | Specificity (%) | Prevalence (%) | PPV | NPV |
|---|---|---|---|---|
| 93 | 35 | 4 | 5.0 | 99.4 |
| 93 | 35 | 5 | 7.1 | 99.1 |
| 93 | 35 | 10 | 13.8 | 98.1 |
| 93 | 35 | 20 | 26.6 | 95.9 |
| 93 | 35 | 30 | 38.3 | 93.2 |
| 93 | 35 | 40 | 49.1 | 89.7 |
| 93 | 35 | 50 | 59.1 | 85.4 |
| 93 | 35 | 60 | 68.4 | 79.5 |
| 93 | 35 | 70 | 77.1 | 71.4 |
| 93 | 35 | 80 | 85.3 | 59.3 |
Supporting information
(XLSX)
(PDF)
Data Availability
Data is provided as supporting information files.
Funding Statement
Funding was received from the Austin Health Fundraising Department to undertake this work.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema KL, Oster AM, McGovern OL, Lindstrom S, Stenger MR, Anderson TC, et al. Persons Evaluated for 2019 Novel Coronavirus—United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(6):166–70. 10.15585/mmwr.mm6906e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DHHS. Currnt Victorian coronavirus disease (COVID-19) case-defintion and testing criteria 2020 [Available from: https://www.dhhs.vic.gov.au/health-services-and-general-practitioners-coronavirus-disease-covid-19].
- 7.Wynants L, Van Calster B, Bonten MMJ, Collins GS, Debray TPA, De Vos M, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trubiano JA, Vogrin S, Kwong JC, Homes N. Alterations in smell or taste—Classic COVID-19? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. 10.1093/cid/ciaa655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challener D, Challener G, Gow-Lee V, Fida M, Shah A, O'Horo J. Screening for COVID-19: Patient Factors Predicting Positive PCR Test. Infect Control Hosp Epidemiol. 2020:1–7. 10.1017/ice.2020.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng C, Huang Z, Wang LF. A novel triage tool of artificialintelligence assisted diagnosis aid system for suspected covid-19 pneumonia in fever clinics. medRxiv [Preprint]. 2020. [Google Scholar]
- 11.Qi X, Jiang Z, Yu Q. Machine learning-based CT radiomics model for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: a multicenter study. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus-2: A Narrative Review. Ann Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(PDF)
Data Availability Statement
Data is provided as supporting information files.