Abstract
The chronic social defeat stress (CSDS) model has been instrumental in shaping our understanding of neurobiology relevant to affect-related illnesses, including major depressive disorder (MDD). However, the classic CSDS procedure is limited by its exclusive application to adult male rodents. We have recently developed a novel vicarious social defeat stress (VDS) procedure wherein one mouse witnesses the physical defeat bout of a conspecific from the safety of an adjacent compartment. This witness mouse develops a similar behavioral phenotype to that of the mouse that physically experiences social defeat stress, modeling multiple aspects of MDD. Importantly, this new procedure allows researchers to perform VDS in males or females, and in juvenile mice – which typically are excluded from classic social defeat experiments. Here we discuss several recent advances made using this procedure, and how its application provides a new preclinical approach to study the neurobiology of psychological stress-induced phenotypes.
Keywords: Adolescent, animal model, anxiety, depression, female, psychological stress, social defeat, witness defeat
Introduction
Depression is among the most prevalent mental illnesses, with 3.6% of the global population suffering from mood-related disorders (1), and depression is currently the leading worldwide cause of disability (2). Although there is a strong genetic component to Major Depressive Disorder (MDD) and other psychiatric illnesses, the effects of individual genes and polymorphisms are weak, suggesting a strong environmental contribution to the manifestation of these diseases (3, 4). A variety of environmental factors can increase risk for MDD, and exposure to stress, both chronic and traumatic, is associated with a higher incidence of mood disorders, including MDD (5–7). Because of the connection between exposure to stress and affective illnesses, an array of procedures expose rodents to stress to model aspects of mood-related symptoms (8, 9).
Chronic social defeat stress (CSDS) is a widely adopted preclinical stress procedure that produces a persistent suite of behavioral changes (10) including deficits in social interaction, preference for rewarding stimuli like sucrose, and body weight (11, 12). CSDS-induced behavioral alterations can be reversed by chronic (>10 days), but not acute, exposure to traditional antidepressant drugs (11, 13–16) as well as by acute administration of the rapidly acting antidepressant drug ketamine (17–19). Moreover, CSDS produces a subgroup of “resilient” animals (~30%) that fail to develop these behavioral deficits after stress (10–12), providing face validity to the model as not all people exposed to stress develop mood disorders. Therefore, CSDS has been used to study resilience to the effects of stress on mood. Resilience is an active process involving changes in gene expression, neuronal activity, and neurotransmitter function in multiple brain regions that can be targeted for novel therapeutics (20–22). Thus, CSDS is a valid and productive model for the study of resilience and susceptibility to stress-induced affective illnesses in adult males. However, stress exposure and mood disorders are not limited to adult males in human populations.
Women in the United States are twice as likely as men to be diagnosed with MDD (23). Although cultural pressures and perceived norms likely contribute to this disparity, depression in women is higher than in men across the world (24, 25), indicating that cultural biases are unlikely to be the only factor in this difference. Moreover, both human and animal studies have revealed sex differences in brain regions and molecular pathways central to mood disorders (26–28). This suggests that physiological distinctions between the sexes drive differences in stress responses and/or the manifestation of affective illnesses. In addition, exposure to trauma or chronic stress can also occur in childhood and adolescence, and there are clear links between exposure to trauma at an early age and mood disorders occurring soon after or much later in life (29–31). Although women and young populations are particularly vulnerable to stress-induced mood disorders, most preclinical mechanistic studies of these diseases have exclusively involved adult male animals. While CSDS has been a valuable model, its reliance on adult male territorial aggression prevents its application to these understudied populations, and new models are needed to expand the impact of mechanistic studies. In this review, we discuss a novel vicarious defeat stress (VDS) procedure wherein one mouse witnesses the physical defeat bout of a conspecific from the safety of an adjacent compartment and the potential impact of this new model on our understanding of mood disorders across all affected populations.
Overview of CSDS and VDS
Procedures
Although CSDS has been used across several animal species, this review focuses on findings from the mouse procedure introduced by Berton et al. (11). We focus on this version of CSDS because it serves as the basis for VDS and allows for straightforward comparison of the effects of each stressor (32). In CSDS, a male C57BL/6 intruder mouse intrudes into the home cage of a larger retired breeder male CD-1 resident mouse for 10 minutes per day for a total of 10 days. During this time, the resident repeatedly confronts and overpowers the intruder. At the end of this interaction, the resident and intruder are separated by a perforated divider, allowing visual, olfactory, and auditory interaction without further physical defeat for 24-hours. The intruder is exposed to a novel aggressor each day, and then typically single housed. Within 24-hours of the last social defeat session, CSDS-susceptible mice display aberrant mood- and reward-related behaviors (10–12).
VDS (sometimes called emotional or witness social defeat stress) uses a similar protocol (Figure 1) wherein a CD-1 resident mouse physically defeats a C57BL/6 intruder. However, a separate experimental VDS mouse is placed on the other side of the perforated divider during this encounter, allowing visual, olfactory, and auditory perception of the confrontation (33). The VDS mouse is then housed (separated by a divider) with an aggressor CD-1 for 24-hours, and the process is repeated for 10 days. Behavioral effects of VDS begin as early as 24-hours after witnessing the last defeat, though some effects can take several weeks to emerge (32, 34–36). While specific effects are immediately evident, others develop over time (i.e. incubate), such as decreases of sucrose preference or social avoidance. The mechanisms behind this incubation period are unknown, but multiple circuits are likely involved. An interesting possibility is that the delayed emergence of VDS-induced social avoidance (37) and/or anhedonia-like behavior (32), may represent resilience processes tapering off, leading to the emergence of latent behavioral effects (38, 39).
Figure 1: Vicarious Social Defeat Stress Procedure.
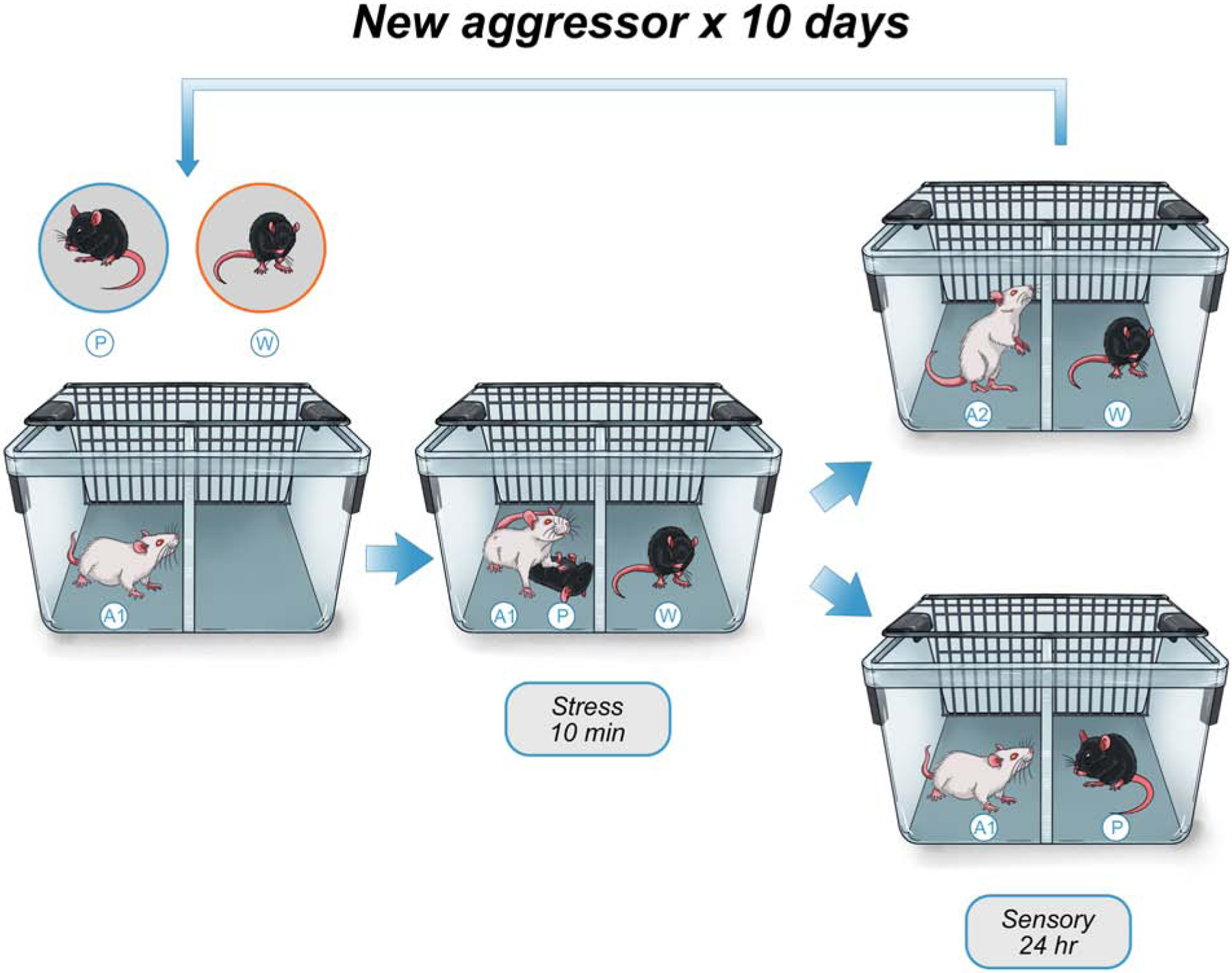
The adult male C57BL/6 physical target intruder mouse (P) is placed into the divided cage with a resident adult male retired breeder CD1 aggressor mouse (A1), while the witness intruder mouse (W) is placed across the divider. Critically, the witness mouse can be either sex and adolescent or adult. After a 10-minute stress interaction involving physical aggression of the resident (A1) against the physical target (P), the witness mouse (W) is moved to a new cage, where it is housed across a divider from a different resident male retired breeder CD1 aggressor mouse (A2), while the physical target intruder mouse (P) is placed across the divider from the original aggressor mouse (A1), and the mice are co-housed for 24-hours. This process then repeats for a total of 10 consecutive days.
Behavioral effects of male CSDS and VDS
Social interaction (SI).
Mice exposed to CSDS typically spend less time interacting with a novel social target (11, 12, 40). This is considered a maladaptive response modeling social withdrawal relevant to many mood disorders because defeated mice avoid social interactions with all mice, including those derived from their own background (11). Chronic, but not acute, treatment with traditional antidepressants reverses this phenotype (11, 15) as does a single injection of the rapidly-acting antidepressant ketamine (17, 41). This behavioral test is also used to differentiate defeated mice into subgroups based on their resilience to CSDS. Mice that spend more time interacting with a social target are termed “resilient,” while those spending less time with the social target are called “susceptible” (12). Similarly, VDS in adult male mice decreases SI both 24-hour and 30 days after the last stress session (32, 37). Although significant, the decrease in SI is lower in magnitude after VDS than CSDS (41), precluding the differentiation of resilient vs. susceptible phenotypes using SI ratios. Importantly, while SI is used to categorize mice as “susceptible” or “resilient” to CSDS, resilient mice are not equivalent to non-stressed controls. Resilient mice display differences in other behavioral endpoints (Table 1), neuronal activity (42) and gene expression (12, 43) from controls – demonstrating that stress exposure impacts them, albeit differently from SI behavior specifically.
Table 1.
Acute behavioral and physiological outcomes to VDS and CSDS in male rodents
| Behavioral/Physiological Syndrome | VDS | CSDS | |||
|---|---|---|---|---|---|
| Reference | Susceptible | Resilient | Reference | ||
| Social interaction | ↓ | 32 | ↓ | = | 12 |
| Forced Swim Test (immobility) | ↑ | 32 | = | = | 12 |
| Anxiogenic behavior | ↑ | 32 | ↑ | ↑ | 12 |
| Sucrose preference | ↓ | 32 | ↓ | = | 12 |
| Drug reward sensitivity | ↑ | 67 | ↑ | = | 12 |
| Memory performance | ↓ | 108 | ↓ | = | 43 |
| Locomotor activity | = | 32 | = | 12 | |
| Weight change | ↓ | 32 | ↓ | = | 12 |
| Serum corticosterone | ↑ | 32 | ↑ | ↑ | 37 |
| Stress-induced Polydipsia | ↑ | 108 | ↑ | ↑ | 12 |
| Proinflammatory markers | ↑ | 37 | ↑ | ↑ | 37 |
| Cardiovascular-related factors | ↑ | 93 | = | ↑ | 12 |
↑, significantly higher; ↓, significantly lower; =, no change from control. VDS, vicarious social defeat stress; CSDS, chronic social defeat stress
Forced Swim Test (FST).
This test is a model for studying despair (44, 45) and involves placing mice into water in an inescapable situation. Acute treatment with virtually all antidepressants increases the time spent struggling and decreases time spent immobile. Surprisingly, CSDS does not reliably influence immobility in FST (12, 46–49), and in studies dividing mice into CSDS-susceptible and CSDS-resilient categories, no group differences are seen (12). This finding underscores the necessity for a multifaceted approach to assessing mood in rodent models. By contrast, VDS increases total immobility in adult male mice within 24-hour of the last stress session, persisting up to 30 days later (32). Similarly, VDS increases FST immobility in male rats (34), a behavioral response that is prevented by prior exercise exposure (50), highlighting how naturalistic experiences, in addition to pharmacological treatments, reverse VDS-induced behavioral endpoints.
Elevated plus-maze (EPM).
EPM is a putative measure of anxiety-like behavior (51). Mice initially prefer the safety of the closed arms but will eventually explore the open arms of the EPM. Anxiolytic drugs decrease the latency to enter and total time spent in the open arms. CSDS increases time spent in the closed arms of the maze in both CSDS-susceptible and - resilient mice (52), and this effect persists up to 30 days after the last defeat session (12, 32). Similarly, VDS in adult male mice increases closed-arm time within 24-hours of the last VDS session, and persists for at least one month (32).
Natural reward.
The sucrose preference test assesses hedonic response to natural rewards, allowing a mouse to choose to drink either water or a sweet sucrose solution. Typically, rodents exposed to CSDS consume less sucrose than unstressed controls, suggesting anhedonia (12, 46, 53–58), with some exceptions (59). Initial studies found that while VDS-exposed adult male mice do not initially show decreases in sucrose preference, an anhedonia-like response is apparent 30-days after the last VDS session (32). However, a recent report found that VDS decreases sucrose preference at both early and late time-points (36).
Drug responses.
Conditioned Place Preference (CPP) is used to assess hedonic responses to drugs (60). CPP for cocaine is increased following CSDS in mice (12, 61–63) and rats (64), and the same is true of alcohol and benzodiazepines (61, 65), suggesting increased sensitivity to the rewarding effects of drugs. However, the effect of VDS on CPP has not been elucidated and should be tested. Mice exposed to CSDS voluntarily consume more alcohol (66) and morphine (67) and may self-administer more cocaine, but this effect is variable between studies (59, 68). Similarly, VDS increases voluntary consumption of morphine (67). The effect of CSDS on drug self-administration has been well-described in rats (69). Defeated rats self-administer cocaine more readily (70, 71) and will work harder for cocaine (72, 73). Furthermore, CSDS can reinstate drug-seeking behavior after extinction (74, 75), mimicking stress-induced drug seeking in human addicts. The contrast between CSDS-induced decrease in sucrose preference with this increase in drug preference/seeking suggests complex effects of CSDS on the brain circuitry underlying reward processing.
Neurobiological effects of CSDS on reward related circuitry
Changes in the function of reward-related circuitry, specifically nucleus accumbens (NAc) and ventral tegmental area (VTA), underlie behavioral responses to CSDS. Altered activity of NAc- projecting VTA dopamine (DA) neurons or of their target medium spiny neurons (MSNs) in NAc is necessary and sufficient to produce CSDS-associated behavioral effects (76). Since direct modulation of these neurons can promote and prevent CSDS behavioral phenotypes, these data indicate that CSDS alters the physiology of these neurons to produce depressive-like behavior. However, the underlying neurobiological changes that promote these effects are not understood. Recent efforts have sought to link molecular and cellular changes in the reward circuitry to depressive-like behavior (reviewed in (76)), and here we highlight key neurobiological changes in NAc and VTA after CSDS.
NAc:
Brain-derived neurotrophic factor (BDNF) levels in the NAc are increased following CSDS, and KO of VTA-BDNF reversed both CSDS-induced BDNF increases in the NAc and social avoidance (11), consistent with BDNF release from DA neuron terminals (77). Further, the increase in NAc BDNF was limited to mice exhibiting social avoidance, as resilient mice displayed NAc BDNF levels equivalent to controls (12). These data implicated BDNF in the NAc as a key biological substrate for CSDS (78, 79). Other neuropeptides, kinases, transcription factors, and epigenetic modifiers (76) have also been implicated in NAc-mediated CSDS effects, and whole genome gene expression and epigenetic studies indicate CSDS induces differential expression of hundreds or even thousands of genes (7), suggesting myriad players in NAc- associated stress phenotypes.
Many of the molecules implicated in these studies are involved in synapse structure and function, and indeed, CSDS changes the synaptic morphology of NAc MSNs. CSDS-susceptible mice had increased MSN dendritic spine density compared to control or CSDS-resilient mice (80, 81). Analysis of single spine dynamics support changes in functional glutamatergic input onto NAc MSNs, although, more prominent changes were observed in resilient compared to susceptible mice (82). Critically, there seem to be opposing adaptations induced in dopamine receptor type 1 (D1)- vs. dopamine receptor type 2 (D2)-containing MSNs, as CSDS-susceptible mice display decreased and increased excitatory input to D1-MSNs and D2-MSNs, respectively (83). Consistent with the activity findings, D1-MSNs in CSDS-susceptible mice exhibit decreased dendritic length, with no change in dendritic spine density, following CSDS (84–86), suggesting that stress-induced increases in spine density are driven by D2-MSNs. Moreover, altering cytoskeletal signaling dynamics is sufficient to prevent changes in spine plasticity and to reverse social-avoidance phenotypes (81, 84). These data suggest that altered NAc connectivity is critical for CSDS effects, consistent with the NAc’s role as a key integrator of cortical and limbic signals.
VTA:
Activity of VTA DA neurons is increased in CSDS-susceptible (but not resilient) mice, and optogenetic activation or inhibition of these neurons is sufficient to promote susceptibility and resilience to CSDS, respectively (12, 87, 88). Moreover, these effects are driven specifically by VTA DA neurons that project to NAc (and not prefrontal cortex) suggesting cell type-specific adaptations are also critical in the VTA (87). In contrast to the NAc, the underlying signaling and molecular mechanisms have been understudied in the VTA. VTA BDNF plays a critical role in CSDS phenotypes, as VTA BDNF KO prevents CSDS susceptibility (11), and modulation of kinases associated with neurotrophic factor signaling in the VTA has been linked to CSDS responses (89–91), but molecular mechanisms of CSDS responses in VTA remain an ongoing research focus for many groups.
Neurobiological effects of VDS in adult male mice
In comparison to the numerous studies elucidating the neurobiological underpinnings of traditional CSDS, less is known about the mediators of VDS. Notably, studies have yet to determine whether increased VTA DA neuronal activity and output to the NAc are necessary for VDS susceptibility, highlighting the critical need for electrophysiological and optogenetic studies in this area. However, VDS and CSDS similarly decreased the number of Fos-positive cells in the striatum and dorsal hippocampus (67). In contrast, regulation of another immediate early gene, ΔFosB, was more complex, as both VDS and CSDS increased the number of ΔFosB- positive cells in the dorsal hippocampus, but only CSDS increased ΔFosB-positive cells in the prefrontal cortex and dorsal and ventral striatum (67). While tentative, these data suggest that VDS and CSDS may rely on overlapping, but not identical, circuits and mechanisms.
To compare mechanisms contributing to CSDS vs. VDS, gene expression changes in the VTA induced by VDS were examined (32). RNA-sequencing showed considerable overlap between CSDS and VDS with 312 and 349 transcripts similarly upregulated and downregulated, respectively (32). One of these transcripts was serum- and glucocorticoid-regulated kinase 1 (SGK1), which was increased in the VTA following CSDS and VDS in a follow-up study (67). We observed that transcription of ERK2 is increased and ΔFosB decreased in NAc following both VDS and CSDS (92). However, we noted differences between the models in this study as well, as CSDS induced an increase in NAc dendritic spine density, consistent with previous studies, while no change in spine density was observed in mice exposed to VDS (92). Overall, the mechanisms supporting VDS phenotypes are largely unexplored, and given the results suggesting potential divergence from CSDS studies, identification and characterization of the molecular and cellular changes induced in the reward circuitry by VDS should become an active area of study.
Use of VDS where physical stress could serve as a confound
There are multiple advantages to VDS that allow completion of studies that are not compatible with CSDS. While established CSDS protocols attempt to minimize serious injury to the stressed mouse (10), aggression-mediated wounding is unavoidable. Wounding is not correlated with susceptibility to CSDS (12, 37), thus disconnecting physical injury with behavioral adaptations to stress such as social avoidance. However, this wounding (and subsequent pain and inflammation) is a biological difference that is not typically controlled for, adding a caveat to many studies, particularly those investigating the role of inflammatory signaling. Therefore, studies investigating inflammatory mechanisms contributing to depressive-like phenotypes have recently utilized VDS to avoid confound of physical injury. For example, Hodes et al. found that IL-6 was increased in mice following their first exposure to CSDS, an effect that predicted their susceptibility to CSDS at the 11-day timepoint (37). However, this increase in IL-6 was also observed following VDS, indicating the IL-6 increase was not due to an acute injury response, but the psychological stress induced in both models. Moreover, bone marrow chimeras from VDS were sufficient to induce susceptibility, indicating that witnessing CSDS produced a peripheral immunogenic response that induced social avoidance. In this case, VDS and CSDS produced similar inflammatory responses. However, a recent study comparing CSDS and VDS in rats observed differences between the two models (93). Here, increased peripheral inflammation (via blood cytokine levels) was evident in CSDS, but not VDS, rats re-exposed to the stress context, despite both CSDS and VDS rats exhibiting increased mean arterial pressure and heart rate and increased epinephrine and corticosterone levels.
A related promising avenue for VDS studies is in modeling co-morbidity of depression with pain disorders, which are also often linked to inflammation. Traditional CSDS promotes hyperalgesia and changes in nociception and pain sensitivity (94–96) and exacerbates post-surgical pain (97). VDS models might be beneficial in pain studies as they lack any potential confound of pain or inflammation induced by physical insult. A similar approach has been used recently, wherein mice that repeatedly witnessed foot-shock stress developed hyperalgesia and long-lasting pain sensitivity in a variety of nociception tests (mechanical, thermal, chemical) (98). VDS offers the additional benefit of utilizing an ethologically relevant stressor to potentially sensitize or promote pain sensitivity and hyperalgesia.
Another benefit of VDS is the ability to evaluate intake of drugs of abuse during stress exposure. These studies can be completed using traditional CSDS models, but caution must be taken that drug intake does not interfere with the subsequent physical defeat and escape behavior. Additionally, assessment of operant drug intake, typically via intravenous self-administration, is difficult to assess during CSDS due to potential damage to the catheter/harness. Thus, VDS offers the potential benefit of investigating drug intake both during and following social stress. Given the consistent findings that repeated social stress sensitizes post-stress drug responses (69), delineating the role of these processes during stress, or whether access to drugs during stress affects the development of depressive-like behaviors are fertile grounds for study. Likewise, VDS would confer a similar benefit to studies attempting to record in vivo electrophysiological, imaging, or microdialysis measurements by allowing measurements to be taken from harnessed animals during a stress session.
Importantly, VDS could be beneficial in evaluating the connection between stress and opioid use disorders. Since most stress models involve physical insult that could result in pain, it may be difficult to separate the analgesic from rewarding aspects of opioid intake following stress. We took advantage of this approach recently, as we determined whether voluntary morphine consumption was altered following CSDS and VDS (67). We found that morphine preference and intake were similarly increased in CSDS- and VDS-exposed mice, suggesting vicarious stress was sufficient to promote increased morphine reward. This encourages use of the VDS procedure for examining stress mechanisms that alter opioid reward and intake, which may help inform our understanding of risk factors for opioid addiction without the confound of analgesia.
Use of VDS in populations typically excluded from CSDS
Traditional CSDS is mostly limited to male subjects, since male and female rodents typically do not attack female conspecifics – some exceptions include Syrian hamsters, prairie voles, and California mice (99–103). In mice, researchers have encouraged physical aggressive behavior from a male resident to a female intruder by stimulating the ventromedial hypothalamus of male resident aggressors or by spraying male urine onto the female intruder (104, 105). While such studies have demonstrated that CSDS-female mice display a depressive-like phenotype, these approaches induce artificial stress situations; since in mice male-to-female aggression is unlikely under normal circumstances. Consequently, the female CSDS-model may display low external and ethological validity for the study of stress-induced disorders. Critically, VDS can be applied to vulnerable populations that are traditionally excluded from CSDS studies, under ethologically relevant conditions. A growing body of work indicates that witnessing social-related stressors induces depression-related outcomes in male and female mice (41) when observed/experienced at different stages of development (92, 106, 107) as well as across species (108–111).
Females.
Female C57BL/6 mice that have experienced VDS display behavioral and physiologic outcomes indicative of a depressive-like phenotype: decreased sucrose preference (anhedonia), lower sociability, and increased immobility in the tail suspension test (despair), along with increases in plasma corticosterone (HPA activation) and decreased body weight (41). Acute administration of ketamine reverses VDS-induced reductions in sociability, providing pharmacological validity to this model and offering a platform for future studies to screen for novel therapeutic agents. More recently, a complementary behavioral and physiologic profile has been reported in female Sprague Dawley rats witnessing male-to-male social aggression. Here, VDS-exposed female rats exhibited a depressive- and anxiogenic-like behavioral profile, along with a sensitized plasma corticosterone, epinephrine, and pro-inflammatory cytokine response (112). Additionally, this study demonstrates that VDS increases heart rate and blood pressure, as well as corticotropin releasing factor (CRF) protein and interleukin-1β within the central amygdala, highlighting a potential biological mechanism by which psychological stress precipitates the development of depression-related behavior in the female population. This study also found that ovarian hormones are necessary for the development of the VDS-induced outcomes, although they occur in any stage of the estrous cycle (112). Collectively, these behavioral, pharmacological, cardiac, and neurobiological indices provide strong evidence of the translational validity of VDS for the study of sex differences in mood-related illnesses.
Adolescents.
Male juvenile rodents display neurobehavioral alterations after CSDS exposure (58, 113, 114), a depressive-like phenotype that is sustained in later adulthood (115), but less is known about the effects of VDS on affect-related behavior within adolescent or younger populations. We demonstrated that male C57BL/6 adolescent mice exposed to VDS during postnatal days 35–44 had decreased social behavior (92), a depression-related endpoint that social buffering ameliorates (114). Furthermore, CSDS and VDS mediate unique alterations in NAc ERK2, ΔFosB, and CREB-signaling as a function of age (92). Interestingly, CSDS and VDS similarly decrease NAc ΔFosB gene expression in adolescent and adult mice. In contrast, when compared to age-matched controls, CSDS and VDS increase NAc ERK2 gene expression in adult mice, but decrease their expression in adolescents. These findings highlight similar neurobiological effects induced by both stress models, while displaying differential age- dependent effects in NAc mRNA expression. Further, CSDS and VDS reduced NAc-CREB phosphorylation in adolescent but not adult mice. Because adolescence is a period of increased synaptic plasticity, and alterations in ERK2, ΔFosb, and CREB influence spine plasticity, we further evaluated total spine densities within this brain region. VDS increased spine density in the adolescent, but not adult, NAc of male mice, suggesting that juvenile populations are more sensitive to the detrimental effects of psychological stress. This age-dependent alteration in NAc spines is intriguing, since the first episode of MDD is most commonly reported in adolescence (116), thus highlighting the strength and applicability of the VDS model for the study of juvenile affect-related illnesses. Moreover, VDS allows the inclusion of younger experimental populations to examine the long-term effects of witnessing parental maltreatment (106), a psychological stressor frequently reported in the pediatric population (117).
Conclusions
Procedures using social stress to model aspects of affective illnesses are providing critical insights into the neurobiology of depression and anxiety disorders. Most rodent studies using social stress have relied on territorial aggression, necessitating a focus on adult male subjects. Here, we demonstrated that models using vicarious stress are adaptable to underrepresented populations and remove confounds caused by physical interactions, such as pain and inflammatory responses. The behavioral and neurobiological sequelae of VDS and physical CSDS are similar but not identical (Table 2). VDS primarily alters behavioral endpoints that are associated with despair, anhedonia, and sociability, while not consistently altering anxiogenic-like behavior. Collectively, this profile not only lends validity to vicarious stress models, but also allows the dissection of cellular and behavioral responses specific to either physical or psychosocial stress. We anticipate that vicarious models will continue to grow in popularity as they are further refined and characterized, and that they will contribute to our developing understanding and potential treatment of stress-related psychiatric disorders in all populations at all ages.
Table 2.
Behavioral and physiological responses to VDS.
| Species/Strain | Sex | Age | Stress Protocol | Behavioral Findings | Physiological Response | Reference |
|---|---|---|---|---|---|---|
| C57BL/6 mice | Male | Adult (8 week old) | VDS or CSDS: 10 min episodes / 10 days | VDS and CSDS mediated depressive/anxiety-like behaviors 24 h post stress exposure (SI, FST, SPT, EPM). Both stressors decreased SI 1-month later. Fluoxetine (20 mg/kg) reversed SI deficits after 30, but not 1, days of antidepressant exposure. | VDS and CSDS increased plasma CORT, decreased body weight, and dysregulated gene expression in the ventral tegmental area. | 32 |
| C57BL/6 mice | Male | Adolescent (PD 35) | VDS or CSDS: 10 min episodes / 10 days | VDS and CSDS decreased sociability (SI) 24 h post last stress exposure. | Adolescent VDS and CSDS increased nucleus accumbens spine density, and decreased ERK2 and DeltaFosB mRNA. CSDS increased pERK2 and DeltaFosB protein. Both VDS and CSDS decreased pCREB protein within this brain region. | 92 |
| Adult (8 week old) | VDS or CSDS: 10 min episodes / 10 days | VDS and CSDS decreased sociability (SI) 24 h post last stress exposure. | Adult CSDS increased nucleus accumbens spine density. Both VDS and CSDS increased ERK2, while decreasing DeltaFosB mRNA.Only CSDS increased pERK2 protein in nucleus accumbens. | |||
| CD45.1+/CD45.2+C57BL/6 | Male | Adult (7–8 week old) | VDS: 10 min episodes / 10 days | VDS decreases sociability (SI) 30-days post VDS. | Increases in IL-6 30-days after VDS. | 37 |
| Sprague-Dawley rats | Male | Adult (225–250 g) | VDS or CSDS: 30 min per day (across 3 separate episodes) / 7 days | Depressive/anxiety-like phenotype (OFT, LDB, EPM, FST) and memory impairment (RAWM) up to 8 days post VDS or CSDS exposure. Group housing (social buffering) ameliorates CSDS-induced alterations in LDB performance. | Both VDS and CSDS decreased body weight gain, and increased water intake 24 h post stress exposure. Also, both stressors increased plasma CORT, and decreased adrenal/thymus weight up to 9 days post VDS or CSDS. Group housing also prevents CSDSand VDS-induced decreases in thymus weight. | 108 |
| Sprague-Dawley rats | Male | Adult (225–250 g) | VDS or CSDS: 30 min per day (across 3 separate episodes) / 7 days | Depressive/anxiety-like behaviors (LDB, EPM, OFT, FST) and memory impairment (RAWM) up to 6 weeks after VDS. | None reported. | 34 |
| Sprague-Dawley rats | Male | Adult (225–250 g) | VDS or CSDS: 30 min per day (across 3 separate episodes) / 7 days | Depressive/anxiety-like phenotype (LDB, EPM, OFT, FST) along with memory impairment (RAWM) up to 1-week post VDS. 14 days of exercise prior to VDS exposure prevents the development of the depressive/anxiety-like phenotype. | VDS increased plasma CORT 1-week post stress. | 50 |
| Sprague-Dawley rats | Male and Female | Periadolescent(PD 21) | VDS (of mother); 30 min per day (across 3 separate episodes) / 7 days | Despair-like behavior (FST), without changes in anxiogenic-like behavior (EPM, OF) or memory performance (RAWM) 1 month after viewing their mother be socially defeated. | Periadolescent exposure to maternal VDS did not alter weight, food intake, or water intake in adulthood (PD 60). | 106 |
| Sprague-Dawley rats | Male | Adult (225–250 g) | VDS or CSDS: 15 min episodes / 5 days | Depressive-like behavior (SPT) in VDS, but not CSDS, rats. | Both VDS and CSDS increased heart rate and mean arterial pressure. Both stressors also increased plasma CORT and epinephrine. Only CSDS increased plasma inflammatory-related proteins. | 93 |
| Sprague-Dawley rats | Female | Adult (175–200 g) | VDS: 15 min episodes / 5 days | VDS mediated depressive/anxiety-like phenotype (FST, SPT, stressevoked burying behavior). | VDS increased heart rate and mean arterial pressure. VDS elevated plasma CORT, epinephrine, and inflammatory-related proteins, along with corticotropin releasing factor and IL-1Beta protein in the central amygdala. | 112 |
| C57BL/6 mice | Female | Adult (8 week old) | VDS: 10 min episodes / 10 days | VDS-mediated depressive-like behavior (SI, SPT, TST). Acute ketamine exposure (20 mg/kg) reversed VDS-induced SI deficits. | VDS increased plasma CORT and decreased body weight. | 41 |
| C57BL/6 mice | Male | Adolescent (PD 28) | VDS or CSDS: 15 min episodes / 10 randomized defeats within one-week. | Only CSDS reduced sociability (SI). Social buffering ameliorated, but did not reverse, the CSDS-induced avoidance. Social buffering increased general locomotor activity in juvenile VDS mice. | Juvenile VDS and CSDS decreases body weight under grouped, but not isolated, housing conditions. | 114 |
| C57BL/6 mice | Female | Adult (8 week/pregnant) | VDS (of male partner) 5 min episodes / 17 days | Witnessing the defeat bout of a male parther, during pregnancy, induces anhedonia-like behavior (SPT). VDS-exposed females further display anxiety-like behavior (EPM, LDB) 21-days post lactation. | 5-weeks post VDS (of male partner), females display alterations in miR-206–3p and BDNF protein levels in the hippocampus, medial prefrontal cortex, and amygdala. | 35 |
| C57BL/6 mice | Male | Adolescent (PD 49) | VDS or CSDS: 10 min episodes / 10 days | VDS and CSDS mediated depressive-like behavior (SI, FST, SPT) up to 6-days post stress exposure. Fasudil (10 mg/kg) prior to each stress exposure normalized immobility behavior (FST) in CSDS, but not VDS, mice. | Juvenile CSDS increased body weight during stress exposure. One month after CSDS or VDS plasma chemokines (CXCL16) were decreased. | 36 |
CORT, corticosterone; CSDS, chronic social defeat stress; EPM, elevated plus maze; FST, forced swim test; LDB, light/dark box; OFT, open field test; PD, postnatal day; RAWM, radial arm water maze; SI, social interaction; SPT, sucrose preference test; TST, tail suspension test; VDS, vicarious social defeat stress.
Acknowledgements
BLW acknowledges support from the National Institutes of Drug Abuse (DA042102) and the Brain and Behavior Research Foundation. AJR acknowledges support from the National Institutes of Mental Health (MH111604), the National Institutes of Neurological Disease and Stroke (NS085171), the National Institutes of Drug Abuse (DA040621, and DA040621-03S1) and the Avielle Foundation. MMR acknowledges support from the National Institute of Drug Abuse (DA039895). SDI acknowledges support from the National Institutes of General Medical Sciences (SC3M130467) and a STARs (Faculty Science and Technology Acquisition and Retention program) award from the State of Texas. Lastly, the authors thank Elizabeth J. Flores and Samuel A. Castillo for the artistic work that led to the development of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Rehm J, Shield KD (2019): Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep. 21:10. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH (2017): Depression and Other Common Mental Disorders: Global Health Estimates.
- 3.Gonda X, Petschner P, Eszlari N, Baksa D, Edes A, Antal P, et al. (2019): Genetic variants in major depressive disorder: From pathophysiology to therapy. Pharmacol Ther. 194:22–43. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh AM, Sullivan PF, Lewis CM (2019): Uncovering the Genetic Architecture of Major Depression. Neuron. 102:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodes GE, Epperson CN (2019): Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Powers A, Bradley B, Ressler KJ (2016): Gene x Environment Determinants of Stress- and Anxiety-Related Disorders. Annu Rev Psychol. 67:239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pena CJ, Nestler EJ (2018): Progress in Epigenetics of Depression. Prog Mol Biol Transl Sci. 157:41–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson ESJ (2018): Translational new approaches for investigating mood disorders in rodents and what they may reveal about the underlying neurobiology of major depressive disorder. Philos Trans R Soc Lond B Biol Sci. 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soderlund J, Lindskog M (2018): Relevance of Rodent Models of Depression in Clinical Practice: Can We Overcome the Obstacles in Translational Neuropsychiatry? Int J Neuropsychopharmacol. 21:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 311:864–868. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 13.Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, et al. (2014): Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology. 39:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN, et al. (2010): DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006): Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 9:519–525. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald PJ (2014): Forbearance for fluoxetine: do monoaminergic antidepressants require a number of years to reach maximum therapeutic effect in humans? Int J Neurosci. 124:467–473. [DOI] [PubMed] [Google Scholar]
- 17.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr. (2014): Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, et al. (2017): Rapid and Sustained Antidepressant Action of the mGlu2/3 Receptor Antagonist MGS0039 in the Social Defeat Stress Model: Comparison with Ketamine. Int J Neuropsychopharmacol. 20:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. (2017): Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol Psychiatry. 81:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, et al. (2016): KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 7:11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. (2014): Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 344:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ (2019): Neurobiology of Resilience: Interface Between Mind and Body. Biol Psychiatry. 86:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walter EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 24.Auerbach RP, Mortier P, Bruffaerts R, Alonso J, Benjet C, Cuijpers P, et al. (2018): WHO World Mental Health Surveys International College Student Project: Prevalence and distribution of mental disorders. J Abnorm Psychol. 127:623–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler RC, Sampson NA, Berglund P, Gruber MJ, Al-Hamzawi A, Andrade L, et al. (2015): Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 24:210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeGates TA, Kvarta MD, Thompson SM (2019): Sex differences in antidepressant efficacy. Neuropsychopharmacology. 44:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma L, Xu Y, Wang G, Li R (2019): What do we know about sex differences in depression: A review of animal models and potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 89:48–56. [DOI] [PubMed] [Google Scholar]
- 28.Laman-Maharg A, Trainor BC (2017): Stress, sex, and motivated behaviors. J Neurosci Res. 95:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodden DHM, Stikkelbroek Y, Dirksen CD (2018): Societal burden of adolescent depression, an overview and cost-of-illness study. J Affect Disord. 241:256–262. [DOI] [PubMed] [Google Scholar]
- 30.Johnson D, Dupuis G, Piche J, Clayborne Z, Colman I (2018): Adult mental health outcomes of adolescent depression: A systematic review. Depress Anxiety. 35:700–716. [DOI] [PubMed] [Google Scholar]
- 31.Vibhakar V, Allen LR, Gee B, Meiser-Stedman R (2019): A systematic review and meta-analysis on the prevalence of depression in children and adolescents after exposure to trauma. J Affect Disord. 255:77–89. [DOI] [PubMed] [Google Scholar]
- 32.Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. (2013): Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 73:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sial OK, Warren BL, Alcantara LF, Parise EM, Bolaños-Guzman CA (2016): Vicarious social defeat stress: Bridging the gap between physical and emotional stress. J Neurosci Methods. 258:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patki G, Salvi A, Liu H, Salim S (2015): Witnessing traumatic events and post-traumatic stress disorder: Insights from an animal model. Neurosci Lett. 600:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao Z, Mao F, Liang J, Szyf M, Wang Y, Sun ZS (2018): Anxiety-Related Behaviours Associated with microRNA-206–3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Mol Neurobiol. 55:1097–1111. [DOI] [PubMed] [Google Scholar]
- 36.Nakatake Y, Furuie H, Yamada M, Kuniishi H, Ukezono M, Yoshizawa K, et al. (2019): The effects of emotional stress are not identical to those of physical stress in mouse model of social defeat stress. Neurosci Res. [DOI] [PubMed] [Google Scholar]
- 37.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012): Neurobiology of resilience. Nat Neurosci. 15:1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutter M (2012): Resilience as a dynamic concept. Dev Psychopathol. 24:335–344. [DOI] [PubMed] [Google Scholar]
- 40.Avgustinovich DF, Kovalenko IL, Kudryavtseva NN (2005): A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol. 35:917–924. [DOI] [PubMed] [Google Scholar]
- 41.Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. (2018): Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol Psychiatry. 83:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao JL, Covington HE 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. (2010): Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 30:16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jianhua F, Wei W, Xiaomei L, Shao-Hui W (2017): Chronic social defeat stress leads to changes of behaviour and memory-associated proteins of young mice. Behav Brain Res. 316:136–144. [DOI] [PubMed] [Google Scholar]
- 44.Porsolt RD, Bertin A, Jalfre M (1977): Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 229:327–336. [PubMed] [Google Scholar]
- 45.Porsolt RD, Le Pichon M, Jalfre M (1977): Depression: a new animal model sensitive to antidepressant treatments. Nature. 266:730–732. [DOI] [PubMed] [Google Scholar]
- 46.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A (2014): Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 76:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keeney AJ, Hogg S (1999): Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol. 10:753–764. [DOI] [PubMed] [Google Scholar]
- 48.Razzoli M, Carboni L, Andreoli M, Ballottari A, Arban R (2011): Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav Brain Res. 216:100–108. [DOI] [PubMed] [Google Scholar]
- 49.Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A, Arban R (2011): Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol Biochem Behav. 97:566–576. [DOI] [PubMed] [Google Scholar]
- 50.Kochi C, Liu H, Zaidi S, Atrooz F, Dantoin P, Salim S (2017): Prior treadmill exercise promotes resilience to vicarious trauma in rats. Prog Neuropsychopharmacol Biol Psychiatry. 77:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montgomery KC, Monkman JA (1955): The relation between fear and exploratory behavior. J Comp Physiol Psychol. 48:132–136. [DOI] [PubMed] [Google Scholar]
- 52.Duque A, Vinader-Caerols C, Monleon S (2017): Indomethacin counteracts the effects of chronic social defeat stress on emotional but not recognition memory in mice. PLoS One. 12:e0173182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. (2011): A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 31:9084–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, et al. (2014): Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry. 76:231–238. [DOI] [PubMed] [Google Scholar]
- 55.Hollis F, Duclot F, Gunjan A, Kabbaj M (2011): Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Horm Behav. 59:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, et al. (2019): Lack of dopamine D1 receptors in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- 57.Carnevali L, Mastorci F, Graiani G, Razzoli M, Trombini M, Pico-Alfonso MA, et al. (2012): Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol Behav. 106:142–150. [DOI] [PubMed] [Google Scholar]
- 58.Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. (2014): Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 17:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arena DT, Covington HE 3rd, DeBold JF, Miczek KA (2019): Persistent increase of I.V. cocaine self-administration in a subgroup of C57BL/6J male mice after social defeat stress. Psychopharmacology (Berl). 236:2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bardo MT, Bevins RA (2000): Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl). 153:31–43. [DOI] [PubMed] [Google Scholar]
- 61.Macedo GC, Morita GM, Domingues LP, Favoretto CA, Suchecki D, Quadros IMH (2018): Consequences of continuous social defeat stress on anxiety- and depressive-like behaviors and ethanol reward in mice. Horm Behav. 97:154–161. [DOI] [PubMed] [Google Scholar]
- 62.Montagud-Romero S, Aguilar MA, Maldonado C, Manzanedo C, Minarro J, Rodriguez-Arias M (2015): Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol Biochem Behav. 135:1–12. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C (2006): Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 31:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stelly CE, Pomrenze MB, Cook JB, Morikawa H (2016): Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. eLife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riad-Allen L, van der Kooy D (2013): Social defeat stress switches the neural system mediating benzodiazepine conditioned motivation. Behav Neurosci. 127:515–523. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andres A, Aguilar MA, et al. (2016): Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict Biol. 21:87–97. [DOI] [PubMed] [Google Scholar]
- 67.Cooper SE, Kechner M, Caraballo-Perez D, Kaska S, Robison AJ, Mazei-Robison MS (2017): Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Sci Rep. 7:8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yap JJ, Miczek KA (2007): Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl). 192:261–273. [DOI] [PubMed] [Google Scholar]
- 69.Miczek KA, Yap JJ, Covington HE 3rd (2008): Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 120:102–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H (2001): Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl). 158:382–387. [DOI] [PubMed] [Google Scholar]
- 71.Tidey JW, Miczek KA (1996): Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 721:140–149. [DOI] [PubMed] [Google Scholar]
- 72.Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, et al. (2014): Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci. 34:6659–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covington HE 3rd, Miczek KA (2005): Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl). 183:331–340. [DOI] [PubMed] [Google Scholar]
- 74.Funk D, Harding S, Juzytsch W, Le AD (2005): Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl). 183:341–349. [DOI] [PubMed] [Google Scholar]
- 75.Manvich DF, Stowe TA, Godfrey JR, Weinshenker D (2016): A Method for Psychosocial Stress-Induced Reinstatement of Cocaine Seeking in Rats. Biol Psychiatry. 79:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fox ME, Lobo MK (2019): The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. (1997): Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 389:856–860. [DOI] [PubMed] [Google Scholar]
- 78.Miczek KA, Nikulina EM, Shimamoto A, Covington HE 3rd (2011): Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 31:9848–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fanous S, Terwilliger EF, Hammer RP Jr., Nikulina EM (2011): Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation. Neurosci Lett. 502:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. (2011): IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 31:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. (2013): Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 19:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, et al. (2016): Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens. Biol Psychiatry. 79:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. (2015): Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 77:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox ME, Chandra R, Menken MS, Larkin EJ, Nam H, Engeln M, et al. (2018): Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Francis TC, Chandra R, Gaynor A, Konkalmatt P, Metzbower SR, Evans B, et al. (2017): Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry. 22:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francis TC, Gaynor A, Chandra R, Fox ME, Lobo MK (2019): The Selective RhoA Inhibitor Rhosin Promotes Stress Resiliency Through Enhancing D1-Medium Spiny Neuron Plasticity and Reducing Hyperexcitability. Biol Psychiatry. 85:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. (2013): Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. (2014): Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 17:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, et al. (2010): Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 30:7652–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, et al. (2008): AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 64:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, et al. (2014): Fluoxetine Exposure during Adolescence Alters Responses to Aversive Stimuli in Adulthood. J Neurosci. 34:1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, et al. (2014): Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 36:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, et al. (2017): Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One. 12:e0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sawicki CM, Kim JK, Weber MD, Faw TD, McKim DB, Madalena KM, et al. (2019): Microglia Promote Increased Pain Behavior through Enhanced Inflammation in the Spinal Cord during Repeated Social Defeat Stress. J Neurosci. 39:1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pagliusi MOF Jr., Bonet IJM, Dias EV, Vieira AS, Tambeli CH, Parada CA, et al. (2018): Social defeat stress induces hyperalgesia and increases truncated BDNF isoforms in the nucleus accumbens regardless of the depressive-like behavior induction in mice. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- 96.Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, et al. (2010): Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 150:358–368. [DOI] [PubMed] [Google Scholar]
- 97.Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, et al. (2014): Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci. 34:13737–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ueda H, Neyama H (2017): LPA1 receptor involvement in fibromyalgia-like pain induced by intermittent psychological stress, empathy. Neurobiol Pain. 1:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, et al. (2003): Conditioned defeat in male and female Syrian hamsters. Horm Behav. 44:293–299. [DOI] [PubMed] [Google Scholar]
- 100.Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC (2015): Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci. 42:3081–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowler CM, Cushing BS, Carter CS (2002): Social factors regulate female-female aggression and affiliation in prairie voles. Physiol Behav. 76:559–566. [DOI] [PubMed] [Google Scholar]
- 102.Ophir AG, Crino OL, Wilkerson QC, Wolff JO, Phelps SM (2008): Female-directed aggression predicts paternal behavior, but female prairie voles prefer affiliative males to paternal males. Brain Behav Evol. 71:32–40. [DOI] [PubMed] [Google Scholar]
- 103.Scotti MA, Carlton ED, Demas GE, Grippo AJ (2015): Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster). Horm Behav. 70:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2018): A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology. 43:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. (2017): Establishment of a repeated social defeat stress model in female mice. Sci Rep. 7:12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu H, Patki G, Salvi A, Kelly M, Salim S (2018): Behavioral effects of early life maternal trauma witness in rats. Prog Neuropsychopharmacol Biol Psychiatry. 81:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lukkes JL, Meda S, Thompson BS, Freund N, Andersen SL (2017): Early life stress and later peer distress on depressive behavior in adolescent female rats: Effects of a novel intervention on GABA and D2 receptors. Behav Brain Res. 330:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patki G, Solanki N, Salim S (2014): Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol. 17:2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akinbo O, Wardwell JJ, Watanasriyakul WT, Normann MC, Cox M, Ciosek S, et al. (2018): Observing a sibling experience a stressor alters behavioral and endocrine stress reactivity in prairie voles. Society for Neuroscience. Poster [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bruchey AK, Jones CE, Monfils MH (2010): Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res. 214:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. (2010): Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 13:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, et al. (2018): Essential Role of Ovarian Hormones in Susceptibility to the Consequences of Witnessing Social Defeat in Female Rats. Biol Psychiatry. 84:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iñiguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, et al. (2016): Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 5:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li M, Xu H, Wang W (2018): An Improved Model of Physical and Emotional Social Defeat: Different Effects on Social Behavior and Body Weight of Adolescent Mice by Interaction With Social Support. Front Psychiatry. 9:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watt MJ, Burke AR, Renner KJ, Forster GL (2009): Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 123:564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kessler RC, Avenevoli S, Ries Merikangas K (2001): Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 49:1002–1014. [DOI] [PubMed] [Google Scholar]
- 117.McDonald R, Jouriles EN, Ramisetty-Mikler S, Caetano R, Green CE (2006): Estimating the number of American children living in partner-violent families. J Fam Psychol. 20:137–142. [DOI] [PubMed] [Google Scholar]


