Abstract
Background:
The androgen receptor (AR) is a key prostate cancer drug target. Suppression of AR signaling mediated by the full-length AR (AR-FL) is the therapeutic goal of all existing AR-directed therapies. AR-targeting agents impart therapeutic benefit, but lead to AR aberrations that underlie disease progression and therapeutic resistance. Among the AR aberrations specific to castration-resistant prostate cancer (CRPC), AR variants (AR-Vs) have emerged as important indicators of disease progression and therapeutic resistance.
Methods:
We conducted a systemic review of the literature focusing on recent laboratory studies on AR-Vs following our last review article published in 2016. Topics ranged from measurement and detection, molecular origin, regulation, genomic function, and preclinical therapeutic targeting of AR-Vs. We provide expert opinions and perspectives on these topics.
Results:
Transcript sequences for 22 AR-Vs have been reported in the literature. Different AR-Vs may arise through different mechanisms, and can be regulated by splicing factors and dictated by genomic rearrangements, but a low-androgen environment is a prerequisite for generation of AR-Vs. The unique transcript structures allowed development of in-situ and in-solution measurement and detection methods, including mRNA and protein detection, in both tissue and blood specimens. AR variant-7 (AR-V7) remains the main measurement target and the most extensively characterized AR-V. Although AR-V7 co-exists with AR-FL, genomic functions mediated by AR-V7 do not require the presence of AR-FL. The distinct cistromes and transcriptional programs directed by AR-V7 and their co-regulators are consistent with genomic features of progressive disease in a low-androgen environment. Preclinical development of AR-V-directed agents currently focuses on suppression of mRNA expression and protein degradation as well as targeting of the amino-terminal domain.
Conclusions:
Current literature continues to support AR-Vs as biomarkers and therapeutic targets in prostate cancer. Laboratory investigations reveal both challenges and opportunities in targeting AR-Vs to overcome resistance to current AR-directed therapies.
I. Introduction
Prostate cancer is an androgen-dependent disease. Management of patients with advanced prostate cancer often involves androgen-deprivation therapies (ADT) established in 1941 1. Under ADT, castrate levels of androgens indicated by circulating testosterone (T) less than 50ng/dL are achieved. Castration-resistant prostate cancer (CRPC) defines disease progression under castrate levels of T. In CRPC, expression level of the androgen receptor (AR) is often elevated, leading to AR activity under reduced androgen levels. In addition, the AR gene on the X chromosome may undergo genomic alterations including structural changes and point mutations. These CRPC-specific AR alterations provided a mechanistic explanation for continued dependence of CRPC on AR signaling 2–4. This important concept in CRPC biology has guided and resulted in successful clinical development of second-generation AR-targeting therapies to treat CRPC, including agents that antagonize AR (enzalutamide, apalutamide, darolutamide) or further suppress extragonadal androgen synthesis (abiraterone, orteronel) 5–17. The next-generation AR antagonists bind to the AR ligand-binding domain (LBD) with higher affinity than first-generation anti-androgens 6,8, while abiraterone inhibits CYP17A1, a rate-limiting enzyme in the synthesis of adrenal and intra-tumoral androgens 5,7. Recently, clinical use of these next-generation AR-targeting therapies has been extended to castration-sensitive prostate cancer (CSPC) 9,18,19 and non-metastatic CRPC (nmCRPC) 10–12,20–22.
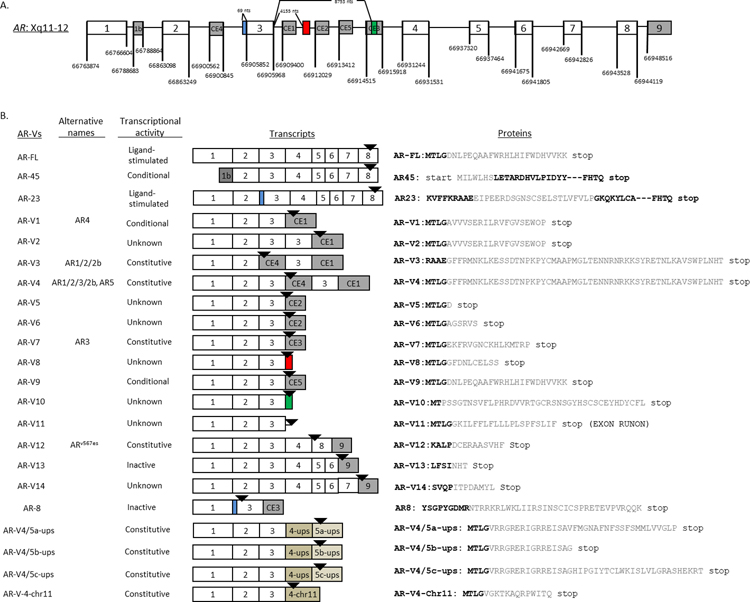
Androgen receptor variants (AR-Vs) have mRNA sequences that are structurally different from the canonical full-length AR (AR-FL). A total of 22 AR-Vs have been cloned and reported in the literature (Figure 1). The majority of these AR-Vs lack the ligand-binding domain (LBD), the therapeutic target of all existing AR-targeting agents. In preclinical models, some but not all of these AR-Vs mediate constitutively active AR signaling, i.e., their activity is not dependent of the presence of androgens or AR-FL 23. Among the AR-Vs described to date, AR-V7 remains to be the most extensively evaluated and characterized, and several blood-based tests for AR-V7 have been developed (see companion review). General topics on AR-Vs have been reviewed extensively in the past 23–26. The intent of the current review is to provide a sequel to a previous review article published in 2016 24. Specifically, we will highlight recent preclinical studies covering topics ranging from measurement and detection, molecular origin, regulation, genomic function, and preclinical therapeutic targeting of AR-Vs. We will provide expert opinions and perspectives on these topics. Readers are directed to a companion review focusing on clinical studies related to AR-Vs.
Figure 1.
Decoding the androgen receptor splice variant transcripts. (A) AR gene structure with canonical and cryptic exon splice junctions marked according to GRCh37/hg19 human genome sequences (not drawn to scale); (B) Nomenclature, functional annotation, exon compositions, and variant-specific mRNA (color matched to Figure 1A) and peptide sequences (in gray). Modified from reference #70.
II. Advances in AR-V measurement and detection methods.
Accurate, reliable, and reproducible measurement of AR-Vs is a key requirement for inferring functional and clinical relevance. A variety of detection methods have been developed for the measurement of AR-Vs. These methods differ according to the method of sampling and specific measurement target. Some methods developed for blood-based AR-V7 detection have been analytically validated and implemented for clinical use (see companion review).
Detection by CTC mRNA.
Blood-based detection of AR-V7 in the treatment setting was first reported in 2014 27. In this initial report, CTC enrichment was achieved by AdnaTest, followed by RT-PCR-based detection of AR-FL and AR-V7. Following analytical validation, a slightly modified version of the laboratory-developed test was implemented in a CLIA- and CAP-certified laboratory for clinical use 28,29, and another modified version was implemented as a clinical trial test 30. The AdnaTest employs a simple workflow enabling fast turnaround time at low cost, but with the drawback of requiring sample processing within 24 hours of blood collection. Nevertheless, with careful management this drawback can be overcome. For example, a global biomarker selection trial was conducted by Tokai after implementing three central laboratories in three continents 30. A recent prospective study further validated the feasibility of conducting the test in the multi-institutional setting involving overnight shipping 13. While analytical and clinical validity are the two key requirements for clinical implementation, further improvement of the CTC-based test should take into consideration many factors that may also impede or facilitate clinical implementation, including cost and ease of use. Because the number of CTCs detected is always the limiting factor, new technologies to improve efficiency/sensitivity of CTC enrichment may further improve the test. For example, novel microfluidic apparatus or in vivo CTC collection methods were designed for CTC isolation and molecular analysis. Using negative depletion microfluidics (CTC-iChip) 31 (NCT01961843) or positive selection microfluidic chip (e.g., IsoFlux) 32, CTC mRNA analysis including analysis of AR-FL/AR-V7 were conducted with digital droplet PCR (ddPCR). An intravascular CTC collection rod with antibody-coated surface (CellCollector, Gilupy, Germany) 33–35, as well as antibody-coated magnetic wire designed for CTC collection directly from blood flow 36, may also help to address the limitation of low CTC numbers. With regard to the limitation posed by low amount of CTC RNA, multiplexing may be the solution. For example, a 27-gene panel (iGene panel) was tested with high-throughput qPCR on Biomark platform (Fluidigm, San Francisco, CA) from CRPC patients receiving docetaxel treatment 37. These technologies may be used for biomarker development, including the detection of AR-Vs. However, it is challenging to conduct direct comparison of various CTC-based mRNA detection platforms.
Detection in whole blood.
AR-Vs may be detected in whole blood without CTC enrichment. Using blood collection tube containing additives for the purpose of stabilizing intracellular RNA (e.g. PAXgene Blood RNA tube), total RNA from peripheral blood were prepared for AR-V7 detection 38–41. However, mainly due to RNA contamination from large amount of leukocytes, the tumor-cell origin of AR-V7 will need to be validated especially when the measured signals are low. In addition, it is important to note that measurement of AR-FL is no longer possible in whole blood samples because non-CTC cells such as regulatory T-cells and macrophages have low-level of AR expression 42,43.
Detection in exosomes.
Exosomal RNA from extracellular vesicles in plasma represents another source for AR-V detection. Using exosomes, AR-V7 was detected by ddPCR using TaqMan probe with high sensitivity (at 2 copies/mL blood) and shown to be a strong predictive marker for enzalutamide/abiraterone resistance in mCRPC patients 44. In another report, extracellular vehicles (EVs) from urine were used in detecting AR-V7 45. Again, studies designed to conduct head-to-head comparisons are important but challenging to implement in the treatment setting.
Tissue Detection by RISH.
PCR-based detection methods described above are in-solution methods that generally do not capture cellular heterogeneity and the morphological context in tumors. In-situ detection methods such as RNA in situ hybridization (RISH) and immunohistochemistry (IHC) (see below) have been developed. The use of RISH for AR-V7 detection was first reported in 2014 27. Subsequently, different types of probes, including padlock probes, modified branched DNA probe, or junction-specific probes with enhanced sensitivity and specificity were developed to evaluate AR-FL and AR-Vs in FFPE tissue biopsy and isolated CTCs 34,46–48. In these studies, higher AR-V7 levels were associated with poorer response to AR-targeting therapies in mCRPC 47,48.
Tissue Detection by IHC.
Recent studies on in-situ detection of AR-V7 protein by IHC focused on newly developed and validated antibodies 48–53. Using matched CSPC and CRPC tissue samples, nuclear AR-V7 protein detected by a rabbit AR-V7 monoclonal antibody (EPR15656; Abcam, Burlingame, CA) was associated with poor prognosis of CRPC patients after abiraterone or enzalutamide treatment 51. Following detailed characterization in clinical cohorts 13,49,54, the EPIC CTC AR-V7 IHC test (Epic Sciences, San Diego, CA) initially developed using the same anti-AR-V7 antibody was recently implemented for clinical use (see companion review). In tissue-based studies, the use of the RM7 (RevMab) antibody further established CRPC-specific expression AR-V7 48,50,55. Interestingly, AR-V7 protein expression was detected using this well-validated antibody in a subset of cells in small cell prostate carcinoma and some salivary ductal carcinoma specimens from untreated female patients 52,56. These recent studies on AR-V7 protein expression further supported that AR-V7 expression arises specifically in a low androgen environment.
Tissue Detection by NGS.
AR-Vs can be detected in clinical specimens by RNA-seq 27. In Antonarakis et al, two autopsy specimens from mCRPC patients with positive CTC AR-V7 underwent RNA-seq analysis. Using number of reads spanning the exon 3/cryptic exon (CE3) and exon 7/exon 8 junctions as surrogate expression values for AR-V7 and AR-FL, respectively, the AR-V7/AR-FL ratios in the two samples were 25.8% (139/539) and 12.1% (151/1248), in line with AR-V7/AR-FL ratios (median 21%, range 1.8% to 208%) estimated from RT-PCR-based CTC AR-V7 test reported in the same study. Using a slightly different method, the median AR-V7/AR-FL ratio from a larger-scale study of CRPC tissues was estimated at ~5% 57. The lower % reported in tissue-based studies is expected and does not contradict with our initial report in unselected CRPC tissues 58 or the ratios reported in CTC AR-V7 positive cases, because higher ratios reported in the Antonarakis study 27 excluded CTC-AR-V7 negative cases (no ratios can be calculated in AR-V7 negative cases). In addition, RNA-seq in primary hormone naïve prostate cancers also detected AR-V7 at slightly lower AR-V7/AR-FL ratio 59. This is not unexpected either, as primary PC tissues are known to have low levels of AR-V7 detected by RT-PCR 58. However, we previously posited 24 that since AR-FL is also overexpressed in CRPC, a ratio of AR-V7/AR-FL at 10%, under the assumption of 10-fold overexpression of AR-FL in CRPC tissues, would bring the absolute level of AR-V7 equivalent to the level of AR-FL detected in primary hormone naïve prostate cancers tissues. This interpretation also explained why AR-V7 protein is not detected in primary tissues 50.
Recent AR-V studies using whole-genome sequencing.
Recent studies have used whole-genome NGS to examine AR for DNA structural alterations and RNA expression. Henzler et. al. 60 evaluated 30 soft tissue metastases from 15 rapid autopsies for AR structural changes and AR-V expression. In this study, diverse AR-genomic structural rearrangements (AR-GSRs) including deletion, duplication, inversion and translocation were observed in 10/30 metastases (6/15 patients). This study discovered many novel AR-Vs driven by AR-GSR. Cloned AR-Vs are depicted in Figure 1. A pilot study by De Laere et. al. 61 applied NGS to CTC and cell-free DNA (cfDNA) from mCRPC patients. In this study, cfDNA was evaluated by low-pass whole-genome sequencing and targeted sequencing of 112 genes including all coding exons and non-repetitive intronic regions of AR, and expression levels of AR-FL and AR variant (AR-FL, AR45, AR-V1, AR-V2, AR-V3, AR-V5, and AR-V7) was interrogated by amplicon sequencing from multiplex junction–specific PCR using cDNA derived from CTCs. In 30 CTC samples from 26 mCRPC patients, 15/26 (57.7%) patients were AR-V-positive with AR-V7 being the most frequently detected variant (12/15 patients), followed by AR-V3 (11/15), AR45 (10/15), AR-V9 (6/15), AR-V1 (5/15), AR-V2 (3/15), and AR-V5 (3/15). In this study, 50% (15/30) of cfDNA samples had an intra-AR structural variation, and 14 of these 15 samples were positive for AR-Vs. Within the small cohort of patients treated with abiraterone or enzalutamide, 15/26 (57.7%) were AR-V-positive, and positive patients were either resistant (13/15) or moderately responsive (2/15) to abiraterone or enzalutamide.
Kallio et. al. also applied targeted AR DNA-seq and RNA-seq to examine AR-Vs and other AR aberrations in CSPC, CRPC, mCRPC, BPH, and noncancerous tissues 62. AR-V3, AR-V7, and AR-V9 were most frequently detected and co-expressed AR mRNA variants in mCRPC, and these variants were also detected in BPH and hormone-naïve primary tumors with lower abundance and frequency, although corresponding protein expression (in BPH and hormone-naïve samples) was not shown. AR mutations and copy number changes were only detected in locally recurrent and metastatic CRPC specimens but not in untreated patients. Non-recurring AR-GSRs (i.e., breakpoints are unique to each sample) were also specifically detected in 5/30 mCRPC. In this study, no AR-GSR co-existed with AR missense mutations in mCRPC 62. In addition, no definitive association was found between these AR-GSRs and AR-Vs, in line with the finding by Henzler et al 60.
Due to limitation of short-read NGS sequencing by Illumina, the contribution of AR GSR to AR-V generation remains to be thoroughly investigated. To date, only one study used a long-read sequencing method (PacBio sequencing platform) to characterize AR variants. In this prospective biomarker study (PROMOTE trial, NCT01953640), Kohli et. al. performed targeted AR RNA-seq, whole transcriptome sequence, and long-read sequencing of AR 3’ RACE (rapid amplification of cDNA ends) products in cell lines, patient-derived xenograft, and metastatic CRPC biopsy tissue samples 63. Expression of AR and AR-Vs was also measured by qRT-PCR in CTC samples. This study found that the 3’ UTR of AR-V9 included cryptic exon 3 (CE3) of AR-V7, and high-level of baseline AR-V9 was associated with resistance to abiraterone 63.
III. Molecular origin and regulation of AR-V expression
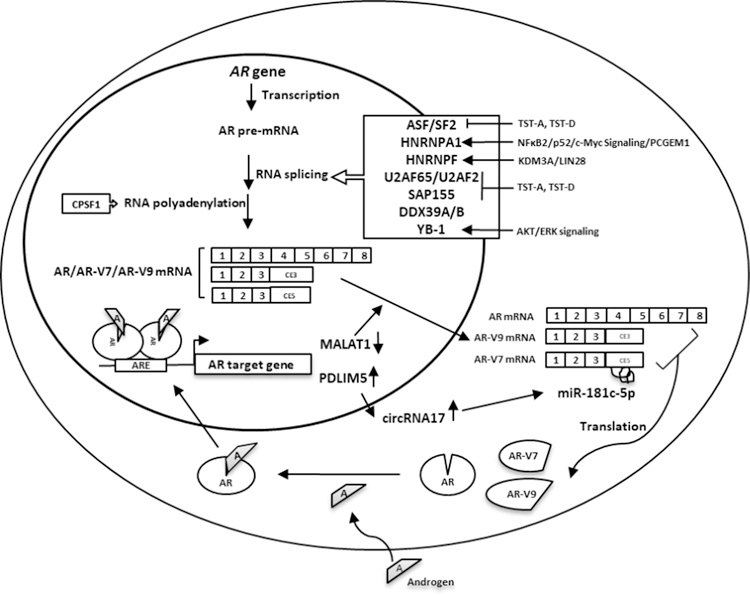
It is well established that expression of AR-Vs are regulated by androgens 64–69. Since our last review in 2016 24, many studies have been published that are relevant to the topic of molecular origin and regulation of AR-V expression. We will summarize the current literature on this topic in three general categories: 1) studies focusing on the role of genomic alterations; 2) studies focusing on the role of RNA splicing; and 3) studies demonstrating the role of other co-factors and signaling pathways on regulating AR-V expression. The findings from studies focusing on splicing factors and related regulators are summarized in Figure 2. Readers are directed to a recent review 70 for further reading on the specific topic of AR-V regulation, the role of genomic structural alterations, and the requirement of low androgens for AR-V7 generation.
Figure 2.
Regulatory mechanisms involved in AR-V expression. Splicing factors involved in AR-V generation include U2AF65, ASF/SF2, HNRNPA1 (NFκB2/p52- and c-Myc-signaling related), and SAP155. LncRNAs involved in splice factor binding to AR pre-mRNA include PCGEM1 (p54/nrb signaling related) and MALAT1. Epigenetic modifier KDM3A may bind AR pre-mRNA and recruit HNRNPF and U2AF265. RNA helicase DDX39A & DDX39B and RNA-binding protein LIN28. Inhibition of CPSF1 by siRNA with morpholinos targeting a single polyadenylation signal (PAS) in AR CE3 suppresses AR-V7/V9 expression. Several protein kinase pathways, e.g. AKT/ERK/YB-1, and non-coding RNAs including circRNA17 and miR-181c-5p may also be involved in AR-V expression. Refer to text descriptions for details.
Role of genomic alterations.
AR genomic structural rearrangements (GSRs) underlie the generation of some of the AR-Vs. As discussed in the section “Recent AR-V studies using whole-genome sequencing”, AR GSRs may also contribute to the generation of AR variants 60–63. In an interesting model proposed by Henzler el. al., successive structural alterations may occur on the same AR allele, leading to generation of AR-Vs. The study presented a case in which tumors from multiple metastatic sites in the same patient showed similar complex patterns of deletion and duplication within the AR LBD that explained the dominant expression of ARv567es 60. However, due to the limitation of short-read sequencing and the involvement of target enrichment, this model may need further validation with long-read genomic sequencing.
Role of RNA splicing.
Although CRPC-specific splicing factors involved in the process have not been definitively identified, studies focusing on factors that regulate AR splicing have identified several key factors related to the RNA spliceosome. On the basis of predicted intronic/exonic splicing enhancer sequences in cryptic exon 3 (CE3) of the AR gene, ChIP and RNA-pull down assays were performed and identified U2AF65 (splicing factor U2AF 65 kDa subunit, aka U2AF2, U2 small nuclear RNA auxiliary factor 2) and ASF/SF2 (alternative splicing factor 1/splicing factor 2) as two key factors mediating AR-V7 splicing 65. A general splicing factor HNRNPA1 (heterogeneous nuclear ribonucleoprotein A1) was also reported to regulate AR-V7 expression in prostate cancer cells. Activation of NFκB2/p52 and c-Myc signaling resulted in recruitment of HNRNPA1 to the splice site of AR pre-mRNA to promote AR-V7 expression 71.
The ADT-induced lncRNA PCGEM1 (prostate cancer gene expression marker 1) was also implicated in AR-V7 generation. PCGEM1 regulates the binding of splice factors HNRNPA1 and U2AF65 to AR pre-mRNA 72. In addition, p54/nrb (nuclear RNA-binding protein, aka NONO, non-POU domain-containing octamer-binding protein), a nuclear protein with multiple functions in RNA splicing and gene regulation positively regulates PCGEM1 expression, as revealed by DNA-pull down assay using PCGEM1 gene promoter in prostate cancer cells 73. Suppression of p54/nrb by siRNA or a natural compound from cruciferous vegetables that interferes with p54/nrb-DNA binding resulted in reduced expression of PCGEM1 and AR-V7 73.
Natural spliceosome inhibitors, thailanstatins (TST-A or TST-D), were used to investigate the relationship between AR-V7 and splice factors SAP155 (spliceosome-associated protein 155, aka SF3B1, splicing factor 3b subunit 1) and U2AF65. TSTs interrupts the interaction between U2AF65 and SAP155 leading to reduced binding to the polypyrimidine tract located between the branch point and the 3’ splice site, and consequentially reduced AR-V7 expression 74.
AR-V7 may be regulated by Jumonji domain containing 1A (JMJD1A, aka KDM3A), a histone demethylase that removes the repressive H3K9 methylation marks (H3K9me1 or H3K9me2) 75. KDM3A also interacts with AR as an AR coactivator to regulate prostate cancer cell proliferation and survival by altering AR transcriptional program and elevating c-Myc levels 76–78. KDM3A promotes alternative splicing of AR-V7 by binding to guanosine(G)-tract sequences in cryptic exon 3 (CE3) of the AR gene leading to recruitment of heterogeneous nuclear ribonucleoprotein F (HNRNPF) and other splice factors such as U2AF265 75.
DDX39 [DEAD (Asp-Glu-Ala-Asp) box polypeptide 39] is an ATP-dependent RNA helicase implicated in RNA splicing, mRNA export, and telomere structure integrity. Using a shRNA library focused on 88 spliceosome-related genes, DDX39B was found to be a regulator of AR-V7 expression 79. After Knockdown of DDX39B and its paralogue DDX39A in AR variant-expressed cells, the AR-V7 mRNA was selectively downregulated 79.
LIN28 is an RNA-binding protein involved in AR and c-Myc signaling in prostate cancer. Overexpression of LIN28 in PCa cell lines resulted in increased AR splice variant expression and resistance to anti-androgens. Downregulation of LIN28 can re-sensitize PCa cells to enzalutamide treatment 80.
The long non-coding RNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) was also implicated in regulating AR-V7 activity. Using enzalutamide-resistant prostate cancer cell lines, AR-V7 and MALAT1 were both elevated when AR signaling is suppressed. AR negatively regulates MALAT1 by direct binding to AREs in the promoter of MALAT1. Increased MALAT1 during AR suppression by enzalutamide could elevate AR-V7 transcription through interaction between MALAT1 and serine/arginine rich splicing factor 1 (SRSF1, aka ASF/SF2). CRPC progression in animal models was suppressed following MALAT1 knockdown 81.
Other co-factors regulating AR-V expression.
Protein kinase pathways may regulate AR-V expression. The most relevant is the AKT pathway, which modulates AR function and mediates survival signaling in CRPC. In a screen of kinase inhibitors involving a 145 small‐molecule compound library and a high-throughput siRNA-kinome library, several kinases including Akt, Abl, and Src family kinases (SFK) were found to regulate AR-V7 mRNA expression and protein nuclear translocation. Following treatment of CWR22Rv1 cells with a Src/Abl dual kinase inhibitor PD180970, AR-V7 protein was decreased and cell proliferation inhibited in the absence of DHT. In the presence of DHT (when AR-FL is activated), the potency of PD180970 was decreased. Pre-treatment with AR-FL antagonist bicalutamide re-sensitized CWR22Rv1 cells to PD180970 with a substantial EC50 drop (from 184.2 to 12.6 nM) in the presence of DHT 82, suggesting that PD180970 reduced AR-V7 protein level and CWR22Rv1 growth in AR-independent manner. Tyrosine kinases such as ACK1/TNK2 regulate AR gene transcription through epigenetic modification. ACK1/TNK2 phosphorylates histone H4 at tyrosine 88 upstream of the AR transcription start site, and treatment with an ACK1 inhibitor (R)-9bMS reduced AR and AR-V7 levels and suppressed CRPC tumor growth 83.
Y-box-binding protein 1 (YB-1) is a transcription factor that binds Y-box (5′-ATTGG-3′), and also a RNA-binding protein involved in RNA splicing. In a study involving kinome arrays, Akt, RSK (ribosomal S6 kinase), ERK (extracellular signal-regulated kinases, p42/44 MAPK) were found to phosphorylate YB-1 and affect AR-V7 expression 84. Treatment of CWR22Rv1 cells with kinase inhibitors against Akt, MEK (MAPK/ERK kinase, an upstream activator of MAPK/ERK) resulted in reduced levels of phosphorylated-YB-1, AR-FL, and AR-V7. However, an inhibitor of RSK specifically downregulated AR-V7 but not AR-FL. How RSK/YB-1 signaling affects AR splicing remains largely uncharacterized 84.
The relationship of AKT signaling and prostate cancer was evaluated by a synthetic AKT inhibitor alkyl-lysophospholipid edelfosine (ET-18-O-CH3). Edelfosine interacts with lipid rafts on plasma membrane, and induce endoplasmic reticulum (ER) stress following inhibition of phosphatidylcholine biosynthesis. Treatment of LNCaP and VCaP cells with Edelfosine resulted in suppression of AR-V7 expression and cell apoptosis that was enhanced by ATF3 (activating transcription factor 3), a corepressor of AR 85.
Other nuclear receptors may regulate AR-FL/AR-V7 expression. For example, ROR-γ (RAR-related orphan receptor gamma) recruits nuclear receptor coactivator 1 and 3 [NCOA1 and NCOA3, also known as steroid receptor coactivator (SRC)-1 and SRC-3] to an AR-ROR response element (RORE) to stimulate AR gene transcription 86. ROR-γ antagonists suppress the expression of both AR and its variant AR-V7 in PCa cell lines and tumors 86. Given that many ROR-γ inhibitors are approved for treating autoimmune disease, it may be possible to repurpose these drugs to suppress AR-V7-driven prostate cancer.
Non-coding circular RNA (circRNA) or miRNA could also regulate AR-V7 expression. By in silico analysis, a circular RNA 17 (circRNA17, hsa_circ_0001427) was found to interact with microRNA 181c-5p (miR-181c-5p) leading to suppression of AR-V7 expression. By interacting with and stabilizing miR-181c-5p, circular RNA 17 increased the relative level of miR-181c-5p, which bound to the 3’UTR of AR-V7 transcript to downregulate AR-V7 expression. Castration can suppress circRNA17 expression by inhibiting its host gene PDLIM5 (PDZ and LIM domain 5) expression via androgen response element (ARE) in the gene promoter. In this study, the level of circRNA17 was downregulated in clinical prostate cancer specimens especially in higher Gleason score prostate cancer specimens 87.
IV. Genomic functions mediated by AR-Vs.
As transcription factors, AR-Vs may mediate their downstream genomic functions by DNA binding and interaction with co-regulators following nuclear localization. While AR-FL and many AR-Vs may share common chromatin binding sites, genomic binding sites and transcriptional programs specific to AR-V7 have been reported 64,88–91. In studies relevant to this topic, it is critical to take into consideration a few premises: 1) AR-V7 protein expression is specific to CRPC and negatively regulated by androgen signaling mediated by AR-FL 64,68; 2) AR-V7 expression is often associated with a progressive phenotype under low androgen conditions 64,92; and 3) AR-V7 often co-exists with AR-FL, and whether AR-V7/AR-FL forms heterodimer under low androgen conditions will affect interpretation of data 68,89,93–95.
Since our last review in 2016 24, a few important factors mediating downstream functional effects of AR-V7 have been characterized. Chen et. al. revealed diverse AR-V7 cistromes and transcriptomes in different CRPC cell lines and clinical specimens 89. Employing a high-resolution ChIP-exonuclease sequencing (ChIP-exo) approach using an AR-V7-specific antibody, HOXB13 was found to co-localize with AR-V7 and function as an essential co-activator mediating AR-V7 function. By inhibiting HOXB13 in AR-V7-expressing cells, the oncogenic function of AR-V7 was suppressed 89. These results implicated that HOXB13, which is very specifically expressed in tissues of prostatic origin, could be an alternative target in suppressing the development of AR-V7-driven CRPC. Cai et. al. used specific antibodies against either AR-FL or AR-V7 for ChIP-seq. In this study, 15,162 out of a total of 17,409 binding sites were shared by both ARs, and only a small proportion of binding sites (about 12.8%, 2,221 out of 17,409 spots) were specifically bound by AR-V7. In these AR-V7-specific binding sites, ZFX (zinc finger protein X-linked) was found to exclusively colocalize with AR-V7. The AR-V7-specific binding sites are mainly located at the gene promoter and these AR-V7 targeted genes were mainly involved in MYC-bound genes or genes related to cell cycles and autophagy 88.
V. Preclinical and early clinical development of agents targeting AR/AR-Vs
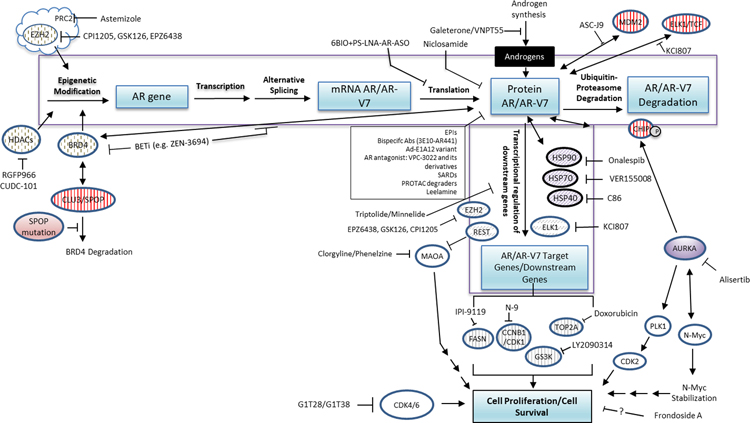
Given the availability of blood-based detection methods and the general poor prognosis of patients having positive CTC AR-V7 testing results, recent efforts have focused on development of agents that may overcome drug resistance and poor prognosis by targeting AR-V7. Several compounds with in vitro anti-AR-V7 activity have already been evaluated in clinical trials, while many others are in various stages of preclinical evaluation and development (see companion review). A comprehensive review of the current literature on possible preclinical agents and strategies returned many studies. Due to space limitations, we summarized these studies in Figure 3 and Table I (with references).
Figure 3.
Novel preclinical agents that suppress AR/AR-Vs by regulating gene expression, degradation, AR transcriptional activity, and downstream signaling. The following broad categories are summarized. Refer to Table I for details.
(1) Targeting AR/AR-Vs translation: 6BIO+PS-LNA-AR-ASO
(2) Novel AR antagonists: EPIs, 3E10-AR411, VPC-3022, SARDs, Ad-E1A12 variant,
(3) Enhancing AR or AR-Vs degradation: Niclosamide, ASC-J9, alisertib, PROTAC degrader, leelamine
(4) Targeting AR chaperones: C86, VER155008, onalespib
(5) Targeting molecules involved in epigenetic modification: BETi, HDACi, CUDC-101, EZH2i, astemizole
(6) Targeting AR/AR-Vs co-regulators or transcriptional activity: KCI807, triptolide/minnelide, clorgyline/phenelzine, IPI-9119, BETi
(7) Targeting AR/AR-Vs downstream signaling molecules: LY2090314, N9 + doxorubicin, G1T28/G1T38, alisertib, FrA
Table I.
Novel agents targeting AR/AR-V signaling
| Name of the agents | Description of agents and their background | Action mechanisms |
|---|---|---|
| Agents inhibit androgen synthesis/AR antagonist/AR or AR-V degradation | ||
| Galeterone and its derivatives VNPT55 | Galeterone (TOK-001) and its analog VNPT55‚a 3β-carbamate analog | • Multi-targeted steroidal agents that
function as 17α-hydroxylase/17, 20-lyase A1 (CYP17A1)
inhibitor •AR/AR-V antagonist • Post-translation modulators of AR degradation by enhancing ubiquitination 96 |
| EPI-001 compound family | Derived from endocrine disruptor bisphenol A, including EPI-001, EPI-002, EPI-506 (a prodrug of EPI-002, aka ralaniten acetate), EPI-7386 | • Interact with the transcriptional
activation domains TAU1 and TAU5 in AR N-terminal domain (NTD) to
exhibit anti-androgen effects in preclinical studies 97. • Selectively activate peroxisome proliferator-activated receptor-gamma (PPAR-γ) in prostate cancer cells 98. • EPI-002 was able to inhibit proliferation of prostate cancer cell lines resistant to enzalutamide and docetaxel with unknown mechanisms 99. • EPI-7386 is a more potent, orally bioavailable and metabolically stable AR NTD inhibitor that appears to be a better drug candidate than its predecessors, and possess antitumor activity in several AR-V7-positive CRPC cell lines and xenograft models including in enzalutamide-refractory models 100. |
| 3E10-AR441 | A bispecific single-chain variable antibody contains an anti-DNA monoclonal antibody (3E10) to facilitate cell penetrance, and an anti-AR N-terminal domain mAb (AR441) generated with epitope aa299–315 of the AR protein. | 3E10-AR441 may enter cell nucleus and bind to AR and AR-V7 and block their function 101. |
| AR degradation enhancer ASC-J9 | Aka dimethylcurcumin, 5-hydroxy-1,7-bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one | • Promoting AR degradation by
increasing its association with MDM2 and phosphorylation of AKT and MDM2
to promote the proteasome-dependent AR degradation 102. • Increasing the dissociation of AR with selective co-regulators protein 55 (ARA55) and ARA70, which subsequently resulted in suppression of AR transactivation and AR-mediated cell growth 102. • Decreasing CD133(+)-stem/progenitor PCa cell population in cell culture and suppress CD133(+)-PCa cell invasion via inhibition of known AKT-enhancer of zeste 2-signal transducer-activator of transcription 3 (AKT-EZH2-STAT3) axis involved in regulating stem-like tumor cells 103. • Overcome the resistance of enzalutamide in prostate cancer cells by decreasing MALAT-1-induced AR-V7 protein level 81. |
| Novel AR antagonists: VPC-3022 and its derivatives VPC-3033 and VPC-3045 | VPC-3022: 10-benzylidene-10H-anthracen-9-one, and its derivatives (e.g. VPC-3033, VPC-3045) | • VPC-3022 docks at the hormone binding
site of AR. • May induce AR/AR-V7 degradation though the unknown mechanism 104,105. |
| Selective AR degraders (SARDs): UT-69, UT-155, and (R)-UT-155; modified indolyl and indolinyl propanamides (series II and III); bisphenol AP | A series of modified AR antagonist molecules (including UT-69, UT-155, (R)-UT-155, indolyl (series II) and indolinyl (series III) propanamides) | • Targeting AR as antagonist and
functioning as AR/AR-V degrader. • The SARD activity may be mediated through a binding site in the NTD of AR/AR-Vs, implicating its capability in overcoming drug resistance mediated by AR LBD point mutation or AR-Vs 106,107. |
| Proteolysis targeting chimeras (PROTAC) degraders: ARV-110, ARD-69, ARCC-4, ARV-771 | Bifunctional small molecules which mainly
combine a target-binding moiety and an E3 ligase recruiter to bring a
target protein into contact with an E3 ligase for ubiquitylation and
subsequent protein degradation by proteasome 108. ARV-110, ARD-69, ARCC-4: Function against AR 109–112. ARV-771: Function against AR co-regulators of transcriptional activity bromodomain and extra-terminal (BET) family proteins BRD2/3/4 113. |
• Inducing AR or its co-regulator
degradation. • Inhibiting AR signaling including suppression of AR-FL and AR-V7 expression 113. • On March 2019, the first phase I clinical trial on AR PROTAC degrader ARV-110 has been launched by Arvinas Inc. in multiple clinical institutes with primary objective in evaluating the safety and tolerability in men with mCRPC who have progressed on at least 2 prior systemic therapies for their castrate resistant disease (one of which must be enzalutamide or abiraterone) (NCT03888612). |
| Antisense oligonucleotide enhancer: 6BIO | 6-bromo-indirubin-3’-oxime (6BIO), a derivative of indirubin, was found to enhance the activity of antisense oligonucleotides (ASOs) | • Repressing AR-FL and AR-V7 expression
through the simultaneous inhibition of GSK-3α and GSK-3β.
• The combination of 6BIO with an anti-AR phosphorothioate (PS) modified locked nucleic acids containing ASO (PS LNA AR-ASO) virtually eliminated all AR expression in PCa cells 114. |
| Reluzole | An anti‐glutamatergic agent and FDA‐approved treatment for amyotrophic lateral sclerosis (ALS) | Promoting protein degradation of AR‐FL, mutant ARs, and AR‐V7 via activating transcription factor 6 alpha (ATF6α)/inositol requiring kinase enzyme 1 alpha (IRE1α)‐mediated endoplasmic reticulum stress (ERS) pathway and downstream selective autophagy 115. |
| Leelamine (LLM) | A component extracted from pine tree bark | Non-covalently interacting with Y739 in AR and inhibits AR activity 116. |
| Targeting AR chaperones | ||
| Heat shock protein (HSP40/70/90) inhibitors | HSP40 inhibitor: C86
(3-nitro-2’,4’,6’-trimethoxychalcone) HSP70 inhibitors: Quercetin and VER155008 HSP90 Inhibitor: onalespib (AT13387) |
• C86: identified by a compound screen
for perturbing the AR-FL/AR-V7 transcriptional program in 22Rv1 cells.
Decreased protein levels of AR-FL and AR-V7 with suppressed FL-AR- and
AR-V7-mediated transcriptional activity. The inhibition effect of C86 on
tumor growth was enhanced by combination administration of HSP70
inhibitor JG98 in a 22Rv1 CRPC xenograft model 117. • HSP70 inhibitors: quercetin and VER155008 decreased LNCaP95 cell proliferation, and the levels of AR-FL and AR-V7. VER155008 decreased AR-FL and AR-V7 in mRNA level indirectly via down-regulation of Y-box binding protein 1 (YB-1) phosphorylation and nuclear localization 118. VER155008 was also reported to destabilize AR-V7 protein by promoting AR-V7 dissociation from HSP70 and degradation via co-chaperone/E3 ubiquitin ligase STIP1 homology and U-box containing protein 1 (STUB1)-related ubiquitination 118. • HSP90 Inhibitor onalespib (AT13387): this second generation HSP90 inhibitor potently inhibited the growth of AR-V7-containing cell lines VCaP, 22Rv1 and LNCaP95 in vitro. Onalespib also decreased protein levels of AR and AR-V7, together with other client proteins. Onalespib also down-regulated mRNA level of AR-V7 but not AR-FL via possible disruption of AR splicing following HSP90 inhibition 119. However, this agent did not demonstrate efficacy in a Phase I/II trial when combined with abiraterone in patients progressing on abiraterone (NCT01685268). |
| Targeting molecules involved in epigenetic modification | ||
| BET/BRD4 inhibitors: GSK525762, GS5829, OTX015, JQ1, ABBV-075, ZEN-3694, PFI-1 | The bromodomain and extra-terminal (BET) domain containing protein 4 (BRD4), an epigenetic adapter, interacts with the acetylated histones 3 and 4 through its bromodomain, and regulates gene transcription by recruiting and activating the essential transcription elongation complex, the positive transcription elongation factor b (P-TEFb). BRD4 is also an AR transcriptional coregulator. BRD4 interacts with the AR NTD to facilitate transcriptional activity 120,121. BET inhibitors (BETi) (e.g. GSK525762, GS5829, OTX015, JQ1, ABBV-075, ZEN-3694, PFI-1) targeting BET-containing protein family members including BRD2, BRD3, and BRD4 were under development for CRPC treatment. | • Disrupting BRD4-AR interaction and
prevent DNA binding of AR-FL or AR variants. • Decreasing AR-V7 expression by regulating splicing factors required for its generation 120,121. • Enhancing the response of homologous recombination-proficient cancer cells to PARP inhibition (PARPi)-induced DNA damage by decreasing transcription of critical proteins BRCA1 and RAD5 involved in homologous recombination of double-strand DNA repair 122. • BRD4 protein degradation was depended on cullin 3-speckle-type POZ protein (CUL3-SPOP)-mediated ubiquitination. In PCa cells with SPOP mutation, BETi treatment may lack efficacy due to the dominant-negative effect of mutant SPOP and an increased level of BRD4, implicating that SPOP mutation detection may be considered as a treatment selection biomarker for BETi treatment 123,124. ZEN-3694 is a BETi that has entered clinical trials either as a single agent Phase I study in CRPC (NCT02705469) or a Phase 1b/2a safety and tolerability study in combination with enzalutamide in mCRPC patients (NCT02711956). |
| HDAC3-selective inhibitor: RGFP966 | A slow-on/slow-off, competitive, tight-binding inhibitor targeting class I HDACs (HDAC1, 2, 3, 8) with the greatest inhibition of HDAC3 125. | Epigenetically suppressing AR signaling without induction of epithelial-mesenchymal transition (EMT) in PCa cells 126. |
| EZH2 inhibitors: GSK126, GSK343, GSK503,
NPD13668, EPZ6438 and PRC2 inhibitor: astemizole |
Enhancer of zeste 2 (EZH2), the enzymatic subunit of polycomb repressive complex 2 (PRC2), catalyzes histone H3 lysine 27 trimethylatiion (H3K27me3) to trigger gene silencing. In prostate cancer, EZH2 was found to be overexpressed and quantitatively associated with progression and poor prognosis 127. In addition to its methyltransferase activities, EZH2 could also act as transcriptional coactivator in gene activation including AR 128. | • EZH2 binds to AR gene exon 1 around
~1.7–2.5 kb downstream of the transcriptional start site
(TSS) to elicit its transactivity in a PRC2-independent manner, and thus
mediates dual methylation-dependent and -independent transcription
programs in PCa 129.
• Preclinical study showed that using EZH2 inhibitors (such as GSK126, GSK343, GSK503, NPD13668) can effectively inhibit the growth of prostate cancer cells 130,131. • EZH2 inhibitors GSK126 and EPZ6438 could also help to restore the expression of AR and re-sensitize tumor response to enzalutamide in neuroendocrine prostate cancer (NEPC) mouse models with Pten+Rb1 KO or Pten+Rb1+Trp53 KO 132. Together with docetaxel, the resistance of EZH2 inhibitor could be overcome in PTEN-mutated cancer cells via releasing EZH2 suppression on PTEN effector FOXO1 gene expression and sustaining FOXO1 nuclear localization to induce cell death 133. • EZH2 and embryonic ectoderm development (EED) (another component of PRC2) could also affect AR signaling by directly interacting with AR to target AR downstream genes. Using a newly identified PRC2 inhibitor astemizole, the AR-EZH2-EED interaction can be disrupted and led to protein degradation of AR and EZH2; thus astemizole exerted a potent therapeutic effect on prostate cancer preclinically 134. • Currently, the EZH2 inhibitor CPI-1205 (Constellation Pharmaceuticals) is under Phase 1b/2 study in mCRPC patients to determine the maximum tolerated dose and safety in combination with enzalutamide or abiraterone/prednisone (NCT03480646) and AR-V7 expression in CTC was included as a monitoring variable for drug response. |
| CUDC-101 | CUDC-101 exerts inhibition effect on multiple targets including histone deacetylase, the receptor kinases epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) in lung and breast cancer cells 135. | Suppressing PCa cell growth and downregulated AR-FL and AR-V7 via HDAC5 and HDAC10 inhibition 136. |
| CBP/p300 inhibitors: GNE049, CCS1477, NEO2734 | The highly homologous histone acetyltransferases CBP [cAMP response element binding protein (CREB) binding protein] and p300 are both coactivators of AR, especially the p300 is widely required for androgen-induced gene regulation and histone acetylation and methylation 137. CBP and p300 were both highly expressed during androgen deprivation in prostate cancer 138. | • Several CBP/p300 bromodomain
inhibitors (e.g. GNE049, CCS1477), and a novel dual inhibitor of both
BET and CBP/p300, NEO2734 (Epigene Therapeutics Inc.) had been suggested
in suppression of prostate cancer growth in models of in
vitro and in vivo
139–141. • In preclinical study, CCS1477 led to protein reduction of AR-FL, AR-Vs, and c-Myc, AR-targeted gene expression, and in vivo tumor growth of bicalutamide-resistant LNCaP cells either as monotherapy or in combination with enzalutamide 140. • CCS1477 is currently in a Phase I/IIa study for CRPC by CellCentric Ltd (NCT03568656) 142. |
| Targeting AR/AR-V co-regulators or related downstream signaling | ||
| Cell cycle CDK4/6 inhibitors: G1T28 and G1T38 | G1T28 and G1T38 were two new CDK4/6 inhibitors
that were previously investigated in lung and breast cancers (e.g.
NCT02978716, NCT02514447). PCa is also a cancer with upregulated expression of cyclin D1 and activation of the G1 cyclin-dependent kinases CDK4/6 [124]. |
Stice et. al. tested G1T28 and G1T38 in preclinical in vitro and in vivo models of PCa as single drug and compared them to docetaxel and enzalutamide. Results showed these inhibitors were as effective as docetaxel in mouse models of enzalutamide-resistant LNCaP-AR-F876L+ xenograft or AR-V7+ CWR22Rv1 xenograft with less toxicity 143. |
| Aurora kinase A inhibitor: alisertib | Aurora kinase A (AURKA) is a serine/threonine kinase with a major role in promoting mitosis by phosphorylating polo like kinase 1 (PLK1) for CDK1 activation. AURKA could also stabilize the transcription factor N-MYC by preventing its proteasomal degradation, and thereby promotes G1–S progression. | • Inhibition of AURKA suppressed the
growth of CRPC cells that had high AR expression, and AURKA also
indirectly regulated the expression of AR-V7 in PCa cells 144. • Inhibition of AURKA reduced AR-V7 protein level and AR target gene expression and suppressed CWR22Rv1 cell proliferation in vitro 145. • AURKA inhibition decreased AR proteasome degradation via decreasing phosphorylation of E3 ubiquitin-protein ligase CHIP (carboxyl terminus of Hsc70 interacting protein) 146. • Alisertib is under a phase II trial for mCRPC patients with neuroendocrine/small cell PCa feature (NCT01799278). Eight patients (13.4%) were radiographic progression free at 6 months as primary endpoint. Eighteen patients (30%) had stable disease or better at cycle 3 scans with exceptional responders were identified including 2 with complete remission of liver metastases on therapy and 2 with prolonged stable disease for14 months and 3.8 years. Although the study did not meet its primary endpoint, a subset of patients of mCRPC with activated signaling in Aurora-A and N-myc achieved promising clinical response 147. |
| Triptolides and its pro-drug minnelide | Triptolide (TPL) is a diterpene triepoxide isolated from a Chinese herb Tripterygium wilfordii | Triptolide inhibited the proliferation of prostate cancer cells via variable mechanisms, most notably through inhibition of gene transcription 148. However, due to its poor aqueous solubility, its clinical application was limited and a water-soluble pro-drug minnelide was developed 149. Preclinically, minnelide showed sufficient effect on suppressing 22Rv1 tumors growth in mice 150. |
| ETS Like-1 protein (ELK1)-AR interrupter: KCI807 | ELK1 is a member of the E26 transformation-specific (ETS) family of transcription factors and of the ternary complex factor (TCF) subfamily. Phosphorylation of ELK1 by MAPKs activates TCF and enhances ELK1-containing TCF binding to the corresponding DNA response element. ELK1 could also interact with AR and activate some AR targeted genes 151. Using cell-based systems, KCI807 was discovered as the top hit of 92 compounds screened out from a total of 18,270 compounds. | • Disrupting AR interaction with
ELK1 • Reducing chromatin recruitment mediated by ELK1 • Suppressing growth of enzalutamide-resistant prostate cancer cells overexpressing AR-V7 152. |
| Monoamine oxidase A (MAOA) inhibitors: clorgyline and phenelzine |
Clorgyline: a selective,
irreversible MAOA inhibitor Phenelzine: a non-selective, irreversible MAOA inhibitor |
• Clorgyline and phenelzine decreased
growth and proliferation of androgen‐sensitive LNCaP cells and
castration‐resistant prostate cancer cells C4‐2B, and
22Rv1. • Clorgyline also inhibited expression of AR‐FL and AR‐V7 expression and decreases growth of an enzalutamide‐resistant, AR-V7-positive C4‐2B cell line 153. |
| Fatty acid synthase (FASN) inhibitor: IPI-9119 | IPI-9119 irreversibly inhibited FASN | Reducing FASN activity by IPI-9119 inhibited PCa cell growth with cell apoptosis and altered metabolome. The altered metabolome might subsequently induced reticulum endoplasmic stress response that led to protein translation inhibition including AR/AR-V7 protein 154. |
| GSK3 inhibitor LY-2090314 | LY‐2090314, a GSK3 inhibitor, identified as AR-V7 transcription inhibitor using a PSA-luciferase reporter system with AR-V7 co-expression | LY‐2090314 inhibited AR-V7 action partially via the activation of β-catenin signaling. Reciprocally, AR-V7 signaling also negatively regulated β-catenin signaling, and therefore, GSK3 inhibition can repress AR-V7 transcriptional activity by inducing a positive feedback to maintain the activation of β-catenin signaling 155. |
| CCNB1/CDK1 inhibitor: N9-isopropylolomoucine (N-9) | With an integrated and unbiased data mining and experimental strategy, Magani el. al. identified a 7-gene set [(kinesin family member 20A (KIF20A), kinesin family member 23 (KIF23), topoisomerase DNA II alpha (TOP2A), cyclin B1 (CCNB1), cyclin B2 (CCNB2), BUB1 mitotic checkpoint serine/threonine kinase (BUB1)]. These genes were upregulated with association of higher levels of AR‐V7 in prostate cancer patients. | By combined treatment of doxorubicin (DOX), a TOP2A inhibitor, and N9‐isopropylolomoucine (N‐9), AR-V7 downstream cell cycle genes were targeted and led to the reduction of cell proliferation in CRPC cell line 22Rv1 156. |
| GHR antagonist: pegvisomant | Growth hormone (GH)-insulin like growth factor 1 (IGF-1) axis signaling is associated with prostate cancer. The source of GH in PCa might come from pituitary or tumor itself as autocrine/paracrine since GH was detected in PCa cells. In LnCaP and enzalutamide-resistant C4–2B cells, GH promoted cell growth with increased expression of AR-V7 and IGF1. | GHR antagonist pegvisomant blocked GH action and reversed the effect of GH on PCa cells, which also implicating the promoting role of GH in PCa progression during castration 157. |
| VNLG-152 | VNLG-125, a novel retinamide as retinoic acid metabolism blocking agent (RAMBA) with multi-function in anti-tumor therapy. | • Suppressing PCa tumor growth via
reducing AR/AR-V7 protein levels and AR signaling pathway 158. • Inhibiting PCa invasiveness by reducing epithelial-mesenchymal transition (EMT) and mitogen‐activated protein kinase‐interacting kinase 1/2 (MNK1/2)–eukaryotic initiation factor 4E (eIF4E) pathway 159. |
| Adenovirus 12 E1A (Ad-E1A12) | A protein identified in adenovirus contains a conserved region (CR3) that could compete with co-repressors for binding to nuclear receptors, thereby promoting nuclear receptor activation | The full length of E1A12 (266aa) preferentially binds to AR, while the shorter E1A12 variant (235 aa) interacts stronger with AR-V7 to promote AR nuclear translocation, trigger apoptosis and these functions were enhanced by PI3K-AKT-mTOR signaling pathway inhibition 160. |
| Artesunate | Artemisinin derivatives (ADs), such as artesunate, are semi-synthetic compounds derived from Artemisia annua and used for treating malarial infection | Artesunate (AS) was reported by Nunes et. al. that in combination with bicalutamide (Bic), it exhibited inhibition effect on prostate cancer cells by suppressing nuclear factor (NF)-κB signaling, reducing AR and AR-V7 levels via ubiquitin-mediated proteasomal degradation, and inducing oxidative stress and apoptosis 161. |
| Frondoside A (FrA) | A triterpene glycoside compound from sea cucumber | • Inducing PCa cell cycle arrest and
apoptosis in caspase-dependent or -independent manner via up-regulating
several pro-apoptotic proteins (Bax, Bad, PTEN), and down-regulating of
anti-apoptotic proteins (survivin and Bcl-2). • Inhibiting pro-survival autophagy in PCa cells to inhibit growth of prostate cancer cells with high AR-V7 activity 162. |
VI. Conclusions
Laboratory investigations and findings reported since 2016 continue to support AR-Vs as biomarkers and therapeutic targets in prostate cancer. These new findings further validate the importance of AR-Vs, AR-V7 in particular, in castration-resistant prostate cancer. Development of mature measurement methods have enabled detection of the AR-V targets in both liquid and tissue specimens, and quantitative measurement data on AR-V7 support its functional and clinical relevance. While AR-Vs (e.g. ARv567es) driven by complex AR GSRs, as well as dominant expression of AR-V7 in isolated cases provide compelling examples of clonal expansion consistent with a driver role for AR-Vs in castration resistance, in most cases AR-FL continue to co-exist with AR-Vs, justifying further investigations to dissect the distinct roles of the different AR molecules. In this regard, distinct genomic functions mediated by AR-FL and AR-Vs have been defined in greater details now, further supporting the therapeutic relevance of AR-Vs. Given the feasibility of conducting blood-based detection for AR-V7, the hope is that clinical development of agents possessing anti-AR-V activity can be accelerated, even in the presence of many competing mechanism that may co-exist. Going forward, both challenges and opportunities exist in targeting AR-Vs to overcome resistance to current AR-directed therapies. Development of agents with specific anti-AR-V7 activity remains a top priority in prostate cancer research.
Acknowledgements/Funding:
ESA has received funding from the Prostate Cancer Foundation, the Patrick C. Walsh Fund, and NIH grants R01 CA185297 and P30 CA006973. AJA has received funding from a Prostate Cancer Foundation and Movember Global Treatment Sciences Challenge Award and the NIH under a P30 CA014236 and 1R01CA233585–01 grant. JL is currently funded by a Prostate Cancer Foundation grant, NIH grant R01 CA185297, and US Department of Defense Prostate Cancer Research Program grant W81XWH-19–1-0686.
Disclosures/Conflicts of Interest: ESA has served as a paid consultant/advisor for Janssen, Pfizer, Sanofi, Dendreon, Essa, Merck, Bristol-Myers Squibb, AstraZeneca, Clovis, Eli Lilly and Amgen; has received research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Merck, Bristol-Myers Squibb, AstraZeneca and Constellation; and is a co-inventor of an AR-V7 biomarker technology that has been licensed to Qiagen. AJA has served as a paid consultant for AstraZeneca, Merck, Dendreon, Janssen, Clovis, Bayer, and Medivation/Astellas; is on the speaker’s bureau for Bayer and Dendreon; and receives research funding to his institution from Janssen, Medivation/Astellas, Sanofi-Aventis, Active Biotech, Bayer, Dendreon, Merck, AstraZeneca, Genentech/Roche, BMS, Constellation, Novartis, and Pfizer. JL has served as a paid consultant/advisor for Sun Pharma, Janssen, Tolero, and Sanofi; has received research funding to his institution from Orion, Mirati, Astellas, Sanofi, Constellation, Calibr, Pandomedx, and Gilead; and is a co-inventor of a technology that has been licensed to Tokai, Qiagen, and A&G. CL is a co-inventor of a technology that has been licensed to Tokai and Qiagen.
References:
- 1.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002; 168(1): 9–12. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol 2008; 8(4): 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell 2009; 16(6): 458–462. [DOI] [PubMed] [Google Scholar]
- 4.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324(5928): 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364(21): 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367(13): 1187–1197. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368(2): 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371(5): 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017; 377(4): 352–360. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018; 378(26): 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2019; 380(13): 1235–1246. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018; 378(15): 1408–1418. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J Clin Oncol 2019; 37(13): 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 2019. [DOI] [PubMed]
- 15.Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J Clin Oncol 2016; 34(18): 2098–2106. [DOI] [PubMed] [Google Scholar]
- 16.Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G et al. Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort. Eur Urol 2016; 70(6): 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fizazi K, Massard C, Bono P, Kataja V, James N, Tammela TL et al. Safety and Antitumour Activity of ODM-201 (BAY-1841788) in Castration-resistant, CYP17 Inhibitor-naive Prostate Cancer: Results from Extended Follow-up of the ARADES Trial. European urology focus 2017; 3(6): 606–614. [DOI] [PubMed] [Google Scholar]
- 18.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 2017; 377(4): 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. The Lancet Oncology 2019; 20(5): 686–700. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CJ, Crawford ED, Shore ND, Underwood W 3rd, Taplin ME, Londhe A et al. The IMAAGEN Study: Effect of Abiraterone Acetate and Prednisone on Prostate Specific Antigen and Radiographic Disease Progression in Patients with Nonmetastatic Castration Resistant Prostate Cancer. J Urol 2018; 200(2): 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small EJ, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Annals of Oncology 2019. [DOI] [PMC free article] [PubMed]
- 22.Tombal B, Saad F, Penson D, Hussain M, Sternberg CN, Morlock R et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology 2019; 20(4): 556–569. [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol 2013; 2(3): 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis 2016; 19(3): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Attard G, Balk SP, Bevan C, Burnstein K, Cato L et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur Urol 2018; 73(5): 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr Relat Cancer 2014; 21(4): T87–T103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol 2015; 1(5): 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lokhandwala PM, Riel SL, Haley L, Lu C, Chen Y, Silberstein J et al. Analytical Validation of Androgen Receptor Splice Variant 7 Detection in a Clinical Laboratory Improvement Amendments (CLIA) Laboratory Setting. J Mol Diagn 2017; 19(1): 115–125. [DOI] [PubMed] [Google Scholar]
- 29.Markowski MC, Silberstein JL, Eshleman JR, Eisenberger MA, Luo J, Antonarakis ES. Clinical Utility of CLIA-Grade AR-V7 Testing in Patients With Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taplin ME, Antonarakis ES, Ferrante KJ, Horgan K, Blumenstein B, Saad F et al. Androgen Receptor Modulation Optimized for Response-Splice Variant: A Phase 3, Randomized Trial of Galeterone Versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer. Eur Urol 2019; 76(6): 843–851. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto DT, Lee RJ, Kalinich M, LiCausi JA, Zheng Y, Chen T et al. An RNA-Based Digital Circulating Tumor Cell Signature Is Predictive of Drug Response and Early Dissemination in Prostate Cancer. Cancer Discov 2018; 8(3): 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Luk A, Young FP, Lynch D, Chua W, Balakrishnar B et al. Droplet Digital PCR Based Androgen Receptor Variant 7 (AR-V7) Detection from Prostate Cancer Patient Blood Biopsies. Int J Mol Sci 2016; 17(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markou A, Lazaridou M, Paraskevopoulos P, Chen S, Swierczewska M, Budna J et al. Multiplex Gene Expression Profiling of In Vivo Isolated Circulating Tumor Cells in High-Risk Prostate Cancer Patients. Clin Chem 2018; 64(2): 297–306. [DOI] [PubMed] [Google Scholar]
- 34.El-Heliebi A, Hille C, Laxman N, Svedlund J, Haudum C, Ercan E et al. In Situ Detection and Quantification of AR-V7, AR-FL, PSA, and KRAS Point Mutations in Circulating Tumor Cells. Clin Chem 2018; 64(3): 536–546. [DOI] [PubMed] [Google Scholar]
- 35.Theil G, Fischer K, Weber E, Medek R, Hoda R, Lucke K et al. The Use of a New CellCollector to Isolate Circulating Tumor Cells from the Blood of Patients with Different Stages of Prostate Cancer and Clinical Outcomes - A Proof-of-Concept Study. PLoS One 2016; 11(8): e0158354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermesh O, Aalipour A, Ge TJ, Saenz Y, Guo Y, Alam IS et al. An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo. Nat Biomed Eng 2018; 2(9): 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skerenova M, Mikulova V, Capoun O, Svec D, Kolostova K, Soukup V et al. Gene Expression Analysis of Immunomagnetically Enriched Circulating Tumor Cell Fraction in Castration-Resistant Prostate Cancer. Mol Diagn Ther 2018; 22(3): 381–390. [DOI] [PubMed] [Google Scholar]
- 38.Seitz AK, Thoene S, Bietenbeck A, Nawroth R, Tauber R, Thalgott M et al. AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide. Eur Urol 2017; 72(5): 828–834. [DOI] [PubMed] [Google Scholar]
- 39.Todenhofer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME et al. AR-V7 Transcripts in Whole Blood RNA of Patients with Metastatic Castration Resistant Prostate Cancer Correlate with Response to Abiraterone Acetate. J Urol 2017; 197(1): 135–142. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Ledet E, Li D, Dotiwala A, Steinberger A, Feibus A et al. A Whole Blood Assay for AR-V7 and AR(v567es) in Patients with Prostate Cancer. J Urol 2016; 196(6): 1758–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeuchi T, Okuno Y, Hattori-Kato M, Zaitsu M, Mikami K. Detection of AR-V7 mRNA in whole blood may not predict the effectiveness of novel endocrine drugs for castration-resistant prostate cancer. Res Rep Urol 2016; 8: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walecki M, Eisel F, Klug J, Baal N, Paradowska-Dogan A, Wahle E et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell 2015; 26(15): 2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest 2002; 110(5): 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur Urol 2017; 71(4): 680–687. [DOI] [PubMed] [Google Scholar]
- 45.Woo HK, Park J, Ku JY, Lee CH, Sunkara V, Ha HK et al. Urine-based liquid biopsy: non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip 2018; 19(1): 87–97. [DOI] [PubMed] [Google Scholar]
- 46.Guedes LB, Morais CL, Almutairi F, Haffner MC, Zheng Q, Isaacs JT et al. Analytic Validation of RNA In Situ Hybridization (RISH) for AR and AR-V7 Expression in Human Prostate Cancer. Clin Cancer Res 2016; 22(18): 4651–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saylor PJ, Lee RJ, Arora KS, Deshpande V, Hu R, Olivier K et al. Branched Chain RNA In Situ Hybridization for Androgen Receptor Splice Variant AR-V7 as a Prognostic Biomarker for Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res 2017; 23(2): 363–369. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Sharp A, Anderson CM, Silberstein JL, Taylor M, Lu C et al. Novel Junction-specific and Quantifiable In Situ Detection of AR-V7 and its Clinical Correlates in Metastatic Castration-resistant Prostate Cancer. Eur Urol 2018; 73(5): 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scher HI, Graf RP, Schreiber NA, McLaughlin B, Lu D, Louw J et al. Nuclear-specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-resistant Prostate Cancer. Eur Urol 2017; 71(6): 874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest 2019; 129(1): 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R et al. Analytical Validation and Clinical Qualification of a New Immunohistochemical Assay for Androgen Receptor Splice Variant-7 Protein Expression in Metastatic Castration-resistant Prostate Cancer. Eur Urol 2016; 70(4): 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao P, Zhu Y, Cheng L, Luo J. Detection of androgen receptor (AR) and AR-V7 in small cell prostate carcinoma: Diagnostic and therapeutic implications. Asian J Urol 2019; 6(1): 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, Wang Z, Xiao W, Yan L, Guan W, Hu Z et al. Androgen-receptor splice variant-7-positive prostate cancer: a novel molecular subtype with markedly worse androgen-deprivation therapy outcomes in newly diagnosed patients. Mod Pathol 2018; 31(1): 198–208. [DOI] [PubMed] [Google Scholar]
- 54.Scher HI, Graf RP, Schreiber NA, Jayaram A, Winquist E, McLaughlin B et al. Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer. JAMA Oncol 2018; 4(9): 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp A, Welti JC, Lambros MBK, Dolling D, Rodrigues DN, Pope L et al. Clinical Utility of Circulating Tumour Cell Androgen Receptor Splice Variant-7 Status in Metastatic Castration-resistant Prostate Cancer. Eur Urol 2019. [DOI] [PubMed]
- 56.Yang RK, Zhao P, Lu C, Luo J, Hu R. Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naive salivary duct carcinomas. Hum Pathol 2019; 84: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015; 162(2): 454. [DOI] [PubMed] [Google Scholar]
- 58.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 2009; 69(1): 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015; 163(4): 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henzler C, Li Y, Yang R, McBride T, Ho Y, Sprenger C et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun 2016; 7: 13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Laere B, van Dam PJ, Whitington T, Mayrhofer M, Diaz EH, Van den Eynden G et al. Comprehensive Profiling of the Androgen Receptor in Liquid Biopsies from Castration-resistant Prostate Cancer Reveals Novel Intra-AR Structural Variation and Splice Variant Expression Patterns. Eur Urol 2017; 72(2): 192–200. [DOI] [PubMed] [Google Scholar]
- 62.Kallio HML, Hieta R, Latonen L, Brofeldt A, Annala M, Kivinummi K et al. Constitutively active androgen receptor splice variants AR-V3, AR-V7 and AR-V9 are co-expressed in castration-resistant prostate cancer metastases. Br J Cancer 2018; 119(3): 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohli M, Ho Y, Hillman DW, Van Etten JL, Henzler C, Yang R et al. Androgen Receptor Variant AR-V9 Is Coexpressed with AR-V7 in Prostate Cancer Metastases and Predicts Abiraterone Resistance. Clin Cancer Res 2017; 23(16): 4704–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res 2012; 72(14): 3457–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene 2014; 33(24): 3140–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakata D, Nakayama K, Masaki T, Tanaka A, Kusaka M, Watanabe T. Growth Inhibition by Testosterone in an Androgen Receptor Splice Variant-Driven Prostate Cancer Model. Prostate 2016; 76(16): 1536–1545. [DOI] [PubMed] [Google Scholar]
- 67.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol 2018; 19(1): 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A 2010; 107(39): 16759–16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res 2014; 20(6): 1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y, Luo J. Regulation of androgen receptor variants in prostate cancer. Asian Journal of Urology 2020. 10.1016/j.ajur.2020.01.001. [DOI] [PMC free article] [PubMed]
- 71.Nadiminty N, Tummala R, Liu C, Lou W, Evans CP, Gao AC. NF-kappaB2/p52:c-Myc:hnRNPA1 Pathway Regulates Expression of Androgen Receptor Splice Variants and Enzalutamide Sensitivity in Prostate Cancer. Mol Cancer Ther 2015; 14(8): 1884–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Zhou N, Huang J, Ho TT, Zhu Z, Qiu Z et al. Regulation of androgen receptor splice variant AR3 by PCGEM1. Oncotarget 2016; 7(13): 15481–15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho TT, Huang J, Zhou N, Zhang Z, Koirala P, Zhou X et al. Regulation of PCGEM1 by p54/nrb in prostate cancer. Sci Rep 2016; 6: 34529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang B, Lo UG, Wu K, Kapur P, Liu X, Huang J et al. Developing new targeting strategy for androgen receptor variants in castration resistant prostate cancer. Int J Cancer 2017; 141(10): 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan L, Zhang F, Xu S, Cui X, Hussain A, Fazli L et al. Histone demethylase JMJD1A promotes alternative splicing of AR variant 7 (AR-V7) in prostate cancer cells. Proc Natl Acad Sci U S A 2018; 115(20): E4584–E4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 2006; 125(3): 483–495. [DOI] [PubMed] [Google Scholar]
- 77.Fan L, Peng G, Sahgal N, Fazli L, Gleave M, Zhang Y et al. Regulation of c-Myc expression by the histone demethylase JMJD1A is essential for prostate cancer cell growth and survival. Oncogene 2016; 35(19): 2441–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson S, Fan L, Sahgal N, Qi J, Filipp FV. The histone demethylase KDM3A regulates the transcriptional program of the androgen receptor in prostate cancer cells. Oncotarget 2017; 8(18): 30328–30343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakata D, Nakao S, Nakayama K, Araki S, Nakayama Y, Aparicio S et al. The RNA helicase DDX39B and its paralog DDX39A regulate androgen receptor splice variant AR-V7 generation. Biochem Biophys Res Commun 2017; 483(1): 271–276. [DOI] [PubMed] [Google Scholar]
- 80.Tummala R, Nadiminty N, Lou W, Evans CP, Gao AC. Lin28 induces resistance to anti-androgens via promotion of AR splice variant generation. Prostate 2016; 76(5): 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang R, Sun Y, Li L, Niu Y, Lin W, Lin C et al. Preclinical Study using Malat1 Small Interfering RNA or Androgen Receptor Splicing Variant 7 Degradation Enhancer ASC-J9((R)) to Suppress Enzalutamide-resistant Prostate Cancer Progression. Eur Urol 2017; 72(5): 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szafran AT, Stephan C, Bolt M, Mancini MG, Marcelli M, Mancini MA. High-Content Screening Identifies Src Family Kinases as Potential Regulators of AR-V7 Expression and Androgen-Independent Cell Growth. Prostate 2017; 77(1): 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahajan K, Malla P, Lawrence HR, Chen Z, Kumar-Sinha C, Malik R et al. ACK1/TNK2 Regulates Histone H4 Tyr88-phosphorylation and AR Gene Expression in Castration-Resistant Prostate Cancer. Cancer Cell 2017; 31(6): 790–803 e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shiota M, Fujimoto N, Imada K, Yokomizo A, Itsumi M, Takeuchi A et al. Potential Role for YB-1 in Castration-Resistant Prostate Cancer and Resistance to Enzalutamide Through the Androgen Receptor V7. J Natl Cancer Inst 2016; 108(7). [DOI] [PubMed] [Google Scholar]
- 85.Udayakumar TS, Stoyanova R, Shareef MM, Mu Z, Philip S, Burnstein KL et al. Edelfosine Promotes Apoptosis in Androgen-Deprived Prostate Tumors by Increasing ATF3 and Inhibiting Androgen Receptor Activity. Mol Cancer Ther 2016; 15(6): 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Zou JX, Xue X, Cai D, Zhang Y, Duan Z et al. ROR-gamma drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat Med 2016; 22(5): 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu G, Sun Y, Xiang Z, Wang K, Liu B, Xiao G et al. Preclinical study using circular RNA 17 and micro RNA 181c-5p to suppress the enzalutamide-resistant prostate cancer progression. Cell Death Dis 2019; 10(2): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai L, Tsai YH, Wang P, Wang J, Li D, Fan H et al. ZFX Mediates Non-canonical Oncogenic Functions of the Androgen Receptor Splice Variant 7 in Castrate-Resistant Prostate Cancer. Mol Cell 2018; 72(2): 341–354 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Z, Wu D, Thomas-Ahner JM, Lu C, Zhao P, Zhang Q et al. Diverse AR-V7 cistromes in castration-resistant prostate cancer are governed by HoxB13. Proc Natl Acad Sci U S A 2018; 115(26): 6810–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cato L, de Tribolet-Hardy J, Lee I, Rottenberg JT, Coleman I, Melchers D et al. ARv7 Represses Tumor-Suppressor Genes in Castration-Resistant Prostate Cancer. Cancer Cell 2019. [DOI] [PMC free article] [PubMed]
- 91.He Y, Lu J, Ye Z, Hao S, Wang L, Kohli M et al. Androgen receptor splice variants bind to constitutively open chromatin and promote abiraterone-resistant growth of prostate cancer. Nucleic Acids Res 2018; 46(4): 1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cato L, de Tribolet-Hardy J, Lee I, Rottenberg JT, Coleman I, Melchers D et al. ARv7 Represses Tumor-Suppressor Genes in Castration-Resistant Prostate Cancer. Cancer Cell 2019; 35(3): 401–413 e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011; 20(4): 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao B, Qi Y, Zhang G, Xu D, Zhan Y, Alvarez X et al. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget 2014; 5(6): 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu D, Zhan Y, Qi Y, Cao B, Bai S, Xu W et al. Androgen Receptor Splice Variants Dimerize to Transactivate Target Genes. Cancer Res 2015; 75(17): 3663–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Purushottamachar P, Kwegyir-Afful AK, Martin MS, Ramamurthy VP, Ramalingam S, Njar VC. Identification of Novel Steroidal Androgen Receptor Degrading Agents Inspired by Galeterone 3beta-Imidazole Carbamate. ACS Med Chem Lett 2016; 7(7): 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang YC, Banuelos CA, Mawji NR, Wang J, Kato M, Haile S et al. Targeting Androgen Receptor Activation Function-1 with EPI to Overcome Resistance Mechanisms in Castration-Resistant Prostate Cancer. Clin Cancer Res 2016; 22(17): 4466–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brand LJ, Olson ME, Ravindranathan P, Guo H, Kempema AM, Andrews TE et al. EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer. Oncotarget 2015; 6(6): 3811–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimizu Y, Tamada S, Kato M, Hirayama Y, Takeyama Y, Iguchi T et al. Androgen Receptor Splice Variant 7 Drives the Growth of Castration Resistant Prostate Cancer without Being Involved in the Efficacy of Taxane Chemotherapy. J Clin Med 2018; 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moigne RL, Banuelos CA, Mawji NR, Tam T, Wang J, Jian K et al. 503PEPI-7386 is a novel N-terminal domain androgen receptor inhibitor for the treatment of prostate cancer. Annals of Oncology 2019; 30(Supplement_5). [Google Scholar]
- 101.Goicochea NL, Garnovskaya M, Blanton MG, Chan G, Weisbart R, Lilly MB. Development of cell-penetrating bispecific antibodies targeting the N-terminal domain of androgen receptor for prostate cancer therapy. Protein Eng Des Sel 2017; 30(12): 785–793. [DOI] [PubMed] [Google Scholar]
- 102.Lai KP, Huang CK, Chang YJ, Chung CY, Yamashita S, Li L et al. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am J Pathol 2013; 182(2): 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng MA, Chou FJ, Wang K, Yang R, Ding J, Zhang Q et al. Androgen receptor (AR) degradation enhancer ASC-J9((R)) in an FDA-approved formulated solution suppresses castration resistant prostate cancer cell growth. Cancer Lett 2018; 417: 182–191. [DOI] [PubMed] [Google Scholar]
- 104.Li H, Hassona MD, Lack NA, Axerio-Cilies P, Leblanc E, Tavassoli P et al. Characterization of a new class of androgen receptor antagonists with potential therapeutic application in advanced prostate cancer. Mol Cancer Ther 2013; 12(11): 2425–2435. [DOI] [PubMed] [Google Scholar]
- 105.Dalal K, Morin H, Ban F, Shepherd A, Fernandez M, Tam KJ et al. Small molecule-induced degradation of the full length and V7 truncated variant forms of human androgen receptor. Eur J Med Chem 2018; 157: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 106.Ponnusamy S, Coss CC, Thiyagarajan T, Watts K, Hwang DJ, He Y et al. Novel Selective Agents for the Degradation of Androgen Receptor Variants to Treat Castration-Resistant Prostate Cancer. Cancer Res 2017; 77(22): 6282–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang DJ, He Y, Ponnusamy S, Mohler ML, Thiyagarajan T, McEwan IJ et al. New Generation of Selective Androgen Receptor Degraders: Our Initial Design, Synthesis, and Biological Evaluation of New Compounds with Enzalutamide-Resistant Prostate Cancer Activity. J Med Chem 2019; 62(2): 491–511. [DOI] [PubMed] [Google Scholar]
- 108.Neklesa TK, Winkler JD, Crews CM. Targeted protein degradation by PROTACs. Pharmacol Ther 2017; 174: 138–144. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez-Gonzalez A, Cyrus K, Salcius M, Kim K, Crews CM, Deshaies RJ et al. Targeting steroid hormone receptors for ubiquitination and degradation in breast and prostate cancer. Oncogene 2008; 27(57): 7201–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang CY et al. Discovery of ARD-69 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Androgen Receptor (AR) for the Treatment of Prostate Cancer. J Med Chem 2019; 62(2): 941–964. [DOI] [PubMed] [Google Scholar]
- 111.Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol 2018; 1: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neklesa T, Snyder LB, Willard RR, Vitale N, Pizzano J, Gordon DA et al. ARV-110: An oral androgen receptor PROTAC degrader for prostate cancer. Journal of Clinical Oncology 2019; 37(7_suppl): 259–259. [Google Scholar]
- 113.Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AM et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2016; 113(26): 7124–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Castanotto D, Nam S, Horne D, Stein C. 6BIO Enhances Oligonucleotide Activity in Cells: A Potential Combinatorial Anti-androgen Receptor Therapy in Prostate Cancer Cells. Mol Ther 2017; 25(1): 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wadosky KM, Shourideh M, Goodrich DW, Koochekpour S. Riluzole induces AR degradation via endoplasmic reticulum stress pathway in androgen-dependent and castration-resistant prostate cancer cells. Prostate 2019; 79(2): 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh KB, Ji X, Singh SV. Therapeutic Potential of Leelamine, a Novel Inhibitor of Androgen Receptor and Castration-Resistant Prostate Cancer. Mol Cancer Ther 2018; 17(10): 2079–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moses MA, Kim YS, Rivera-Marquez GM, Oshima N, Watson MJ, Beebe KE et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res 2018; 78(14): 4022–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kita K, Shiota M, Tanaka M, Otsuka A, Matsumoto M, Kato M et al. Heat shock protein 70 inhibitors suppress androgen receptor expression in LNCaP95 prostate cancer cells. Cancer Sci 2017; 108(9): 1820–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferraldeschi R, Welti J, Powers MV, Yuan W, Smyth T, Seed G et al. Second-Generation HSP90 Inhibitor Onalespib Blocks mRNA Splicing of Androgen Receptor Variant 7 in Prostate Cancer Cells. Cancer Res 2016; 76(9): 2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Welti J, Sharp A, Yuan W, Dolling D, Nava Rodrigues D, Figueiredo I et al. Targeting Bromodomain and Extra-Terminal (BET) Family Proteins in Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res 2018; 24(13): 3149–3162. [DOI] [PubMed] [Google Scholar]
- 121.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014; 510(7504): 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang L, Zhang Y, Shan W, Hu Z, Yuan J, Pi J et al. Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci Transl Med 2017; 9(400). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P, Wang D, Zhao Y, Ren S, Gao K, Ye Z et al. Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation. Nat Med 2017; 23(9): 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dai X, Gan W, Li X, Wang S, Zhang W, Huang L et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat Med 2017; 23(9): 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 2013; 110(7): 2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLeod AB, Stice JP, Wardell SE, Alley HM, Chang CY, McDonnell DP. Validation of histone deacetylase 3 as a therapeutic target in castration-resistant prostate cancer. Prostate 2018; 78(4): 266–277. [DOI] [PubMed] [Google Scholar]
- 127.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419(6907): 624–629. [DOI] [PubMed] [Google Scholar]
- 128.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 2012; 338(6113): 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q et al. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell Rep 2018; 25(10): 2808–2820 e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu C, Jin X, Yang J, Yang Y, He Y, Ding L et al. Inhibition of EZH2 by chemo- and radiotherapy agents and small molecule inhibitors induces cell death in castration-resistant prostate cancer. Oncotarget 2016; 7(3): 3440–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murashima A, Shinjo K, Katsushima K, Onuki T, Kondoh Y, Osada H et al. Identification of a chemical modulator of EZH2-mediated silencing by cell-based high-throughput screening assay. J Biochem 2019; 166(1): 41–50. [DOI] [PubMed] [Google Scholar]
- 132.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017; 355(6320): 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma L, Yan Y, Bai Y, Yang Y, Pan Y, Gang X et al. Overcoming EZH2 Inhibitor Resistance by Taxane in PTEN-Mutated Cancer. Theranostics 2019; 9(17): 5020–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Q, Wang G, Li Q, Jiang W, Kim JS, Wang R et al. Polycomb group proteins EZH2 and EED directly regulate androgen receptor in advanced prostate cancer. Int J Cancer 2019; 145(2): 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lai CJ, Bao R, Tao X, Wang J, Atoyan R, Qu H et al. CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res 2010; 70(9): 3647–3656. [DOI] [PubMed] [Google Scholar]
- 136.Sun H, Mediwala SN, Szafran AT, Mancini MA, Marcelli M. CUDC-101, a Novel Inhibitor of Full-Length Androgen Receptor (flAR) and Androgen Receptor Variant 7 (AR-V7) Activity: Mechanism of Action and In Vivo Efficacy. Horm Cancer 2016; 7(3): 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ianculescu I, Wu DY, Siegmund KD, Stallcup MR. Selective roles for cAMP response element-binding protein binding protein and p300 protein as coregulators for androgen-regulated gene expression in advanced prostate cancer cells. J Biol Chem 2012; 287(6): 4000–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM et al. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res 2007; 67(7): 3422–3430. [DOI] [PubMed] [Google Scholar]
- 139.Jin L, Garcia J, Chan E, de la Cruz C, Segal E, Merchant M et al. Therapeutic Targeting of the CBP/p300 Bromodomain Blocks the Growth of Castration-Resistant Prostate Cancer. Cancer Res 2017; 77(20): 5564–5575. [DOI] [PubMed] [Google Scholar]
- 140.Pegg N, Brooks N, Worthington J, Young B, Prosser A, Lane J et al. Characterisation of CCS1477: A novel small molecule inhibitor of p300/CBP for the treatment of castration resistant prostate cancer. Journal of Clinical Oncology 2017; 35(15_suppl): 11590–11590. [Google Scholar]
- 141.Yan Y, Ma J, Wang D, Lin D, Pang X, Wang S et al. The novel BET-CBP/p300 dual inhibitor NEO2734 is active in SPOP mutant and wild-type prostate cancer. EMBO Molecular Medicine 2019; 11(11): e10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Butler L, Irani S, Centenera M, Ryan N, Pegg N, Brooks AN. Preclinical investigation of a small molecule inhibitor of p300/CBP reveals efficacy in patient-derived prostate tumor explants. Journal of Clinical Oncology 2019; 37(15_suppl): e16534–e16534. [Google Scholar]
- 143.Stice JP, Wardell SE, Norris JD, Yllanes AP, Alley HM, Haney VO et al. CDK4/6 Therapeutic Intervention and Viable Alternative to Taxanes in CRPC. Mol Cancer Res 2017; 15(6): 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kivinummi K, Urbanucci A, Leinonen K, Tammela TLJ, Annala M, Isaacs WB et al. The expression of AURKA is androgen regulated in castration-resistant prostate cancer. Sci Rep 2017; 7(1): 17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones D, Noble M, Wedge SR, Robson CN, Gaughan L. Aurora A regulates expression of AR-V7 in models of castrate resistant prostate cancer. Sci Rep 2017; 7: 40957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarkar S, Brautigan DL, Larner JM. Aurora Kinase A Promotes AR Degradation via the E3 Ligase CHIP. Mol Cancer Res 2017; 15(8): 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin Cancer Res 2019; 25(1): 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol 2011; 7(3): 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S et al. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 2012; 4(156): 156ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Isharwal S, Modi S, Arora N, Uhlrich C, 3rd, Giri B, Barlass U et al. Minnelide Inhibits Androgen Dependent, Castration Resistant Prostate Cancer Growth by Decreasing Expression of Androgen Receptor Full Length and Splice Variants. Prostate 2017; 77(6): 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosati R, Patki M, Chari V, Dakshnamurthy S, McFall T, Saxton J et al. The Amino-terminal Domain of the Androgen Receptor Co-opts Extracellular Signal-regulated Kinase (ERK) Docking Sites in ELK1 Protein to Induce Sustained Gene Activation That Supports Prostate Cancer Cell Growth. J Biol Chem 2016; 291(50): 25983–25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosati R, Polin L, Ducker C, Li J, Bao X, Selvakumar D et al. Strategy for Tumor-Selective Disruption of Androgen Receptor Function in the Spectrum of Prostate Cancer. Clin Cancer Res 2018; 24(24): 6509–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gaur S, Gross ME, Liao CP, Qian B, Shih JC. Effect of Monoamine oxidase A (MAOA) inhibitors on androgen-sensitive and castration-resistant prostate cancer cells. Prostate 2019; 79(6): 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2019; 116(2): 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakata D, Koyama R, Nakayama K, Kitazawa S, Watanabe T, Hara T. Glycogen synthase kinase-3 inhibitors suppress the AR-V7-mediated transcription and selectively inhibit cell growth in AR-V7-positive prostate cancer cells. Prostate 2017; 77(9): 955–961. [DOI] [PubMed] [Google Scholar]
- 156.Magani F, Bray ER, Martinez MJ, Zhao N, Copello VA, Heidman L et al. Identification of an oncogenic network with prognostic and therapeutic value in prostate cancer. Molecular Systems Biology 2018; 14(8): e8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Recouvreux MV, Wu JB, Gao AC, Zonis S, Chesnokova V, Bhowmick N et al. Androgen Receptor Regulation of Local Growth Hormone in Prostate Cancer Cells. Endocrinology 2017; 158(7): 2255–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramamurthy VP, Ramalingam S, Gediya LK, Njar VCO. The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK-eIF4E signaling pathways to suppress EMT and castration-resistant prostate cancer xenograft growth. FEBS J 2018; 285(6): 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ramamurthy VP, Ramalingam S, Gediya L, Kwegyir-Afful AK, Njar VC. Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines. Oncotarget 2015; 6(5): 3195–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li D, Tian G, Wang J, Zhao LY, Co O, Underill ZC et al. Inhibition of androgen receptor transactivation function by adenovirus type 12 E1A undermines prostate cancer cell survival. Prostate 2018; 78(15): 1140–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nunes JJ, Pandey SK, Yadav A, Goel S, Ateeq B. Targeting NF-kappa B Signaling by Artesunate Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 2017; 19(4): 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dyshlovoy SA, Menchinskaya ES, Venz S, Rast S, Amann K, Hauschild J et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int J Cancer 2016; 138(10): 2450–2465. [DOI] [PubMed] [Google Scholar]