Abstract
CXCL12 is abundantly expressed in reticular cells associated with the perivascular niches of the bone marrow (BM) and is indispensable for B-lymphopoiesis. Cxcl12 promotes osteoclastogenesis and has been implicated in pathologic bone resorption. We had shown earlier that estrogen receptor α deletion in osteoprogenitors and estrogen deficiency in mice increase Cxcl12 mRNA and protein levels in the BM plasma, respectively. We have now generated female and male mice with conditional deletion of a Cxcl12 allele in Prrx1 targeted cells (Cxcl12ΔPrrx1) and show herein that they have a 90% decrease in B lymphocytes, but increased erythrocytes and adipocytes in the marrow. Ovariectomy increased the expression of Cxcl12 and B cell number in the Cxcl12f/f control mice, but these effects were abrogated in the Cxcl12ΔPrrx1 mice. Cortical bone mass was not affected in Cxcl12ΔPrrx1 mice. Albeit, the cortical bone loss caused by ovariectomy was greatly attenuated. Most unexpectedly, the rate of bone turnover in sex steroid sufficient female or male Cxcl12ΔPrrx1 mice was dramatically increased, as evidenced by a more than two-fold increase in several osteoblast- and osteoclast- specific mRNAs, as well as increased mineral apposition and bone formation rate and increased osteoclast number in the endosteal surface. The magnitude of the Cxcl12ΔPrrx1 induced changes were much greater than those caused by ovariectomy or orchidectomy in the Cxcl12f/f mice. These results strengthen the evidence that CXCL12 contributes to the loss of cortical bone mass caused by estrogen deficiency. Moreover, they reveal for the first time that in addition to its effects on hematopoiesis, CXCL12 restrains bone turnover – without changing the balance between resorption and formation – by suppressing osteoblastogenesis and the osteoclastogenesis support provided by cells of the osteoblast lineage.
Keywords: Bone, estrogens, osteoblasts, osteoclasts
INTRODUCTION
CXCL12 is a C-X-C motif containing chemo-attractant with a seminal role in the bone marrow (BM) (1–3). Specifically, CXCL12 is a key recruiting signal for hematopoietic cells into the bone compartment and their subsequent homing in supportive stromal environmental niches, such as the endosteal surfaces. CXCL12 promotes hematopoietic cell interactions with vascular endothelial cells, transmigration through the matrix barrier of blood vessels, as well as the maintenance and repopulating activity of hematopoietic stem cells (HSC). In addition, CXCL12 stimulates survival, proliferation, and migration of mesenchymal stromal cells in vitro (4,5); and potentiates bone repair in vivo (6,7). The major source of CXCL12 are the CXCL12-Abundant Reticular (CAR) cells, a heterogeneous population of reticular cells closely associated with the perivascular niches of the BM, which can be targeted with Prrx1- or Osx1-cre (2,3). Deletion of Cxcl12 in mesenchymal progenitors using Prrx1-cre causes a decrease in HSC in mice (3).
The primary physiologic receptor for CXCL12 is the G-protein coupled receptor CXCR4 (8). CXCR4 is highly expressed by human and mouse monocyte populations and osteoclasts as well as other bone cells including osteoblasts (9). Studies with murine models of Cxcl12 or Cxcr4 deletion from cells of the osteoblast lineage have suggested that CXCL12 also promotes bone formation (10–12). Consistent with this evidence, antagonists of CXCR4 attenuate the pathologic bone loss at sites of inflammation in the synovium and bone in collagen-induced arthritis (13), osteoclastogenesis associated with multiple myeloma (14), and the skeletal effects of ovariectomy (OVX) in mice (15).
Estrogens contribute to the maintenance of bone mass by decreasing osteoclast numbers and bone resorption. These effects are the result of direct estrogen actions on osteoclast precursors of the myeloid lineage and mature osteoclasts, as well as estrogen actions on osteoblast precursors of the mesenchymal lineage that suppress the production of osteoclastogenic cytokines (16,17). Furthermore, genetic studies of the function of the estrogen receptor (ER) α in different bone cell types have elucidated that the protective effect of estrogens against loss of trabecular bone results from their direct actions on osteoclast precursors and mature osteoclasts. The protective effect of estrogens against endocortical resorption, on the other hand, results from estrogen actions on osteoprogenitors (18). Estrogens also decrease the number of B-lymphocytes in the BM via mechanisms that remain unclear (19).
We previously performed microarray analysis of ERα deficient Osx1-cre targeted cells and found that ERα downregulates the mRNA expression of Cxcl12. In line with this, CXCL12 protein levels are increased in the BM of mice lacking ERα in Prrx1-cre expressing cells or following OVX or orchidectomy (ORX) of wild-type mice (20). In addition, we and others have shown that CXCL12 stimulates osteoclastogenesis in cultures of BM derived macrophages; and stromal cell-derived CXCL12 promotes the recruitment, development, and survival of human osteoclasts in vitro (9,21–24). In line with these effects, CXCL12 administration to mice increases BM osteoclast precursors and osteoclasts number (25). Moreover, CXCL12 has been implicated in the pathologic bone resorption associated with rheumatoid arthritis (22,23), multiple myeloma (26), cancer metastases to bone (27) and glucocorticoid excess (28).
Taken together, these lines of evidence have suggested that the suppression of Cxcl12 by estrogens may represent at least one of the molecular mechanisms of their effects on the skeleton. In the present study we examined whether Cxcl12 contributes to the loss of cortical bone mass and/or the increase in B-cells with estrogen deficiency.
Materials and Methods
Animal Experimentation
Mice with conditional deletion of Cxcl12 in the mesenchymal lineage were generated by a two-step breeding strategy. Hemizygous Prrx1-cre transgenic mice (B6.Cg-Tg(Prrx1-cre)1Cjt/J; Jackson Laboratories, stock # 5584) were crossed with Cxcl12 floxed (f/f) mice (B6(FVB)-Cxcl12tm1.1Link/J, Jackson Laboratories, stock # 21773) to generate mice heterozygous for the Cxcl12 floxed allele with and without the Cre allele. These mice were intercrossed to generate Cxcl12f/f (used as control) and Cxcl12ΔPrrx1 mice. Offspring were genotyped by PCR using the following primer sequences: Cre‐forward 5′‐GCG ATT ATC TTC TAT ATC TTC AGG‐3′, Cre‐reverse 5′-GCC AAT ATG GAT TAA CAT TCT CCC‐3′, product size 400 bp; Cxcl12‐flox‐forward 5′‐ CTG CAC CAG GCA GAT AAT GA‐3′, Cxcl12‐flox‐reverse 5′-TTT GGA CAC CAG AAC CTT GA‐3′, product size 525 bp (mutant), product size 525 bp and 476 bp (heterozygous) and product size 476 bp (wild-type). Offspring from all genotypes were tail-clipped for DNA extraction at the time of weaning (21 days) and then group-housed with same sex littermates. Mice were maintained with a constant temperature of 23°C, a 12-hour light/dark cycle, and had access to food and water ad libitum. All mice used in these experiment were obtained from the same group of breeders in 2 consecutive breeding cycles (2 cohorts) separated by 1 month. Eighteen-week-old female and male Cxcl12ΔPrrx1 mice and Cxcl12f/f littermates were either gonadectomized or sham-operated after being stratified by body weight. Specifically, within each genotype, mice were sorted from low to high weight values. Mice were then assigned the numbers 1 and 2, successively. All animals with the same number were assigned to the same group. Weight means and SD for each group were calculated and compared by t-test to assure that means were similar. Twelve Cxcl12f/f and 15 Cxcl12ΔPrrx1 females were sham-operated, and 14 Cxcl12f/f and 13 Cxcl12ΔPrrx1 were OVX. Twelve Cxcl12f/f and 10 Cxcl12ΔPrrx1 male mice were sham-operated, and 12 Cxcl12f/f and 10 Cxcl12ΔPrrx1 were ORX. Surgeries were performed in the morning under sedation with 2% isoflurane, as previously described (29). Seven and 3 days before euthanasia mice were injected with tetracycline (15mg/kg body weight) to quantify bone-formation rates. Six weeks after surgery, animals were euthanized and the tissues dissected for further analyses. Uterine or seminal vesicle weights were obtained to confirm depletion of sex steroids. Body weight measurements were performed 2 days before surgery, before tetracycline injections and before euthanasia. Investigators were blinded during animal handling and endpoint measurements. No adverse events occurred during surgeries, tetracycline injections and harvest procedures. The Institutional Animal Care and Use Committees of UAMS and the Central Arkansas Veterans Healthcare System approved the animal protocols used in these studies.
Bone imaging
Femora were dissected, cleaned from adherent muscle and fixed in 10% Millonig’s formalin, dehydrated, and kept in 100% ethanol at 4°C. Femora length was measured with a micrometer followed by micro-CT analysis using a μCT40 (Scanco Medical, Bruttisellen, Switzerland). Specifically, bones were scanned at 12μm nominal isotropic voxel size, 500 projection (medium resolution, E=55kVp, I=72μA, 4W, integration time 150ms and threshold 200mg/cm3), integrated into 3-D voxel images (1024×1024 pixel matrices for each individual planar stack) from the distal epiphysis to the mid-diaphysis to obtain a number of slices variable between 650 and 690. Cortical thickness, total and medullary area were determined using the 18 slices at the femur mid-shafts. Cortical thickness at the proximal metaphysis was determined using 8 slices distal to the third trochanter. Cortical analysis were measured at a threshold of 200mg/cm3. Two-dimensional evaluation of trabecular bone was performed on contours of the cross sectional acquired images excluding the primary spongiosa and cortex. Contours were drawn starting 8–10 slices away from the growth plate from the distal metaphysis to the diaphysis of the femur to obtain 151 slices (12μm/slice). For all trabecular bone measurements contours were drawn every 10 to 20 slices. Voxel counting was used for bone volume per tissue volume measurements and sphere filling distance transformation indices were used for trabecular microarchitecture with a threshold value of 220mg/cm3, without pre-assumptions about the bone shape as a rod or plate. Micro-CT measurements were expressed in 3-D nomenclature as recommended by (30).
Histology
Freshly dissected femora were fixed for 24h in 10% Millonig’s formalin, transferred to ethanol and embedded undecalcified in methyl methacrylate. Tetracycline labels and osteoclasts were quantified on 5μm thick longitudinal/sagittal nondecalcified femur sections using the OsteoMeasure Analysis System (OsteoMetrics, Inc. Atlanta, GA). Osteoclasts were staining for tartrate-resistant acid phosphatase (Acp5) using napthol AS-MX and Fast Red TR salt (Sigma-Aldrich). Cortical bone analysis was performed on both endocortical surfaces of the proximal metaphysis and diaphysis. The following primary measurements were made: bone surface (BS), single label surface (sL.S), double label surface (dL.S), inter-label thickness (Ir.L.Th), osteoclast number (N.Oc,/μm), and osteoclast surface (Oc.S, %). The following derived indices were calculated: mineralizing surface (MS, %), mineral apposition rate (MAR, μm/d), and bone formation rate (BFR, μm3/μm2/d). The referent for Oc.S, MS and BFR was BS, and for N.Oc B.Pm. A total of 10 mice per group were analyzed. During our dynamic histomorphometric analysis we noticed that 4 mice were not injected with tetracycline since no double labels were observed. All histology measurements were made in a blinded fashion. The terminology used in this study is recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (31).
RNA isolation and qPCR assay
The right femur and both tibia shafts were flushed to remove the bone marrow, cleaned from adherent tissues and frozen in liquid N2. Frozen shafts were pulverized with a multi-well tissue pulverizer (BioSpec Products, Inc. Bartlesville, OK, USA) and frozen in Trizol at −80°C. Total RNA was isolated following the Trizol reagent method (Life Technologies, 15596). Seven samples from the female experiment and 3 samples from male experiment were discarded due to low RNA quality. RNA was reverse-transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA, USA). Taqman quantitative PCR was performed to determine mRNA levels using the following primers: Mm03413826_mH (Bglap), Mm00445553_m1 (Cxcl12), Mm00484039_m1 (Ctsk), Mm00475698_m1 (Acp5), Mm00435452_m1 (Tnfrsf1 1B), Mm00441908_m1 (Tnfsf11), Mm00470479_m1 (Sost), Mm01292123_m1 (Cxcr4), Mm02619632_s1 (Cxcr7) and Mm00475528_m1 (Mrps2) manufactured by the TaqMan Gene Expression Assay service (Applied Biosystems). The mRNA expression levels were normalized to the house-keeping gene mitochondrial ribosomal protein S2 (Mrsp2) using the ΔCt method (32).
Flow cytometry
BM cells were obtained by flushing the femur and tibia diaphysis and washed with PBS. One to two million cells were washed with anti-mouse CD16/CD32 (mouse BD Fc-block, BD Biosciences) for 5 min in a 3% FBS solution. The cells were then stained for 30 min using the following antibodies: anti-CD19-APC-Cy7 (2μg/mL) and anti-CD45R/B220-PE-Cy7 (2.5μg/mL) to identify B cells, anti-CD3-FITC (5μg/mL) to identify T cells, anti-CD11b-APC (0.5μg/mL) to identify monocytes-macrophages, and anti-TER-119-PerCP/Cy5.5 (0.5μg/mL) to identify erythroid cells. All antibodies were purchased from BD Biosciences (San Jose, CA). After washing to remove unbound antibodies, samples were analyzed by a BD FACS Aria flow cytometer (BD Biosciences). The data were analyzed using FlowJo Software (FlowJo, LLC, Ashland, OR). Appropriate gates for the cell populations were drawn with guidance by Fluorescence Minus One (FMO) controls (BD Biosciences).
Statistical analysis
Graph plots and statistical analysis were performed with GraphPad Prism 8 software. Data are presented as dot plots with the central line representing the mean and error bars representing SD. Group mean values were compared by two-way ANOVA with Holm-Sidak multiple comparison test, after determining that data were normally distributed and exhibited equivalent variances. Outliers were identified and removed from the data by the ROUTE method with a Q=1% or by the Grubbs test with an α=5%. Comparisons with a P value lower than 0.1 are shown in the graphs. All data analysis is presented in supplementary tables.
RESULTS
Cxcl12 deletion in mesenchymal progenitors alters hematopoiesis and increases adiposity in the bone marrow.
To elucidate the role of CXCL12 in bone homeostasis, we generated mice with conditional deletion of Cxcl12 in mesenchymal progenitors expressing Prrx1 (Cxcl12ΔPrrx1) and we used floxed mice (Cxcl12f/f) as control. The Prrx1-cre transgene targets early limb bud and a subset of craniofacial mesenchymal stem cells. We did not detect a skull phenotype and all our measurements were made in the femur. In the following description of the results, in addition to the pictorial representation of the data in the figures, the numerical values for the mean, the size effect of the change (%), the standard error, and the P value are provided in supplementary tables corresponding to each figure.
The expression of the Cxcl12 mRNA in femur shafts was dramatically decreased in the Cxcl12ΔPrrx1 mice (Figure 1A), establishing the effectiveness of the deletion. The mRNA levels of Cxcr4, the primary physiologic receptor for CXCL12, were lower in the Cxcl12ΔPrrx1 mice (Figure 1B), consistent with evidence that CXCL12 upregulates the expressions of its own receptor. The mRNA levels of Cxcr7, an alternative CXCL12 receptor, were more than two orders of magnitude lower than the Cxcr4 mRNA levels in both Cxcl12f/f and Cxcl12ΔPrrx1 mice (Figure 1C), making it unlikely that CXCR7 plays a role in the effects of CXCL12 on bone homeostasis. Body weight, uterine weight, and femur length were not affected by the Cxcl12 deletion (Figures 1D–F).
Figure 1. Cxcl12 deletion in mesenchymal progenitors alters hematopoiesis and increases adiposity in the bone marrow.
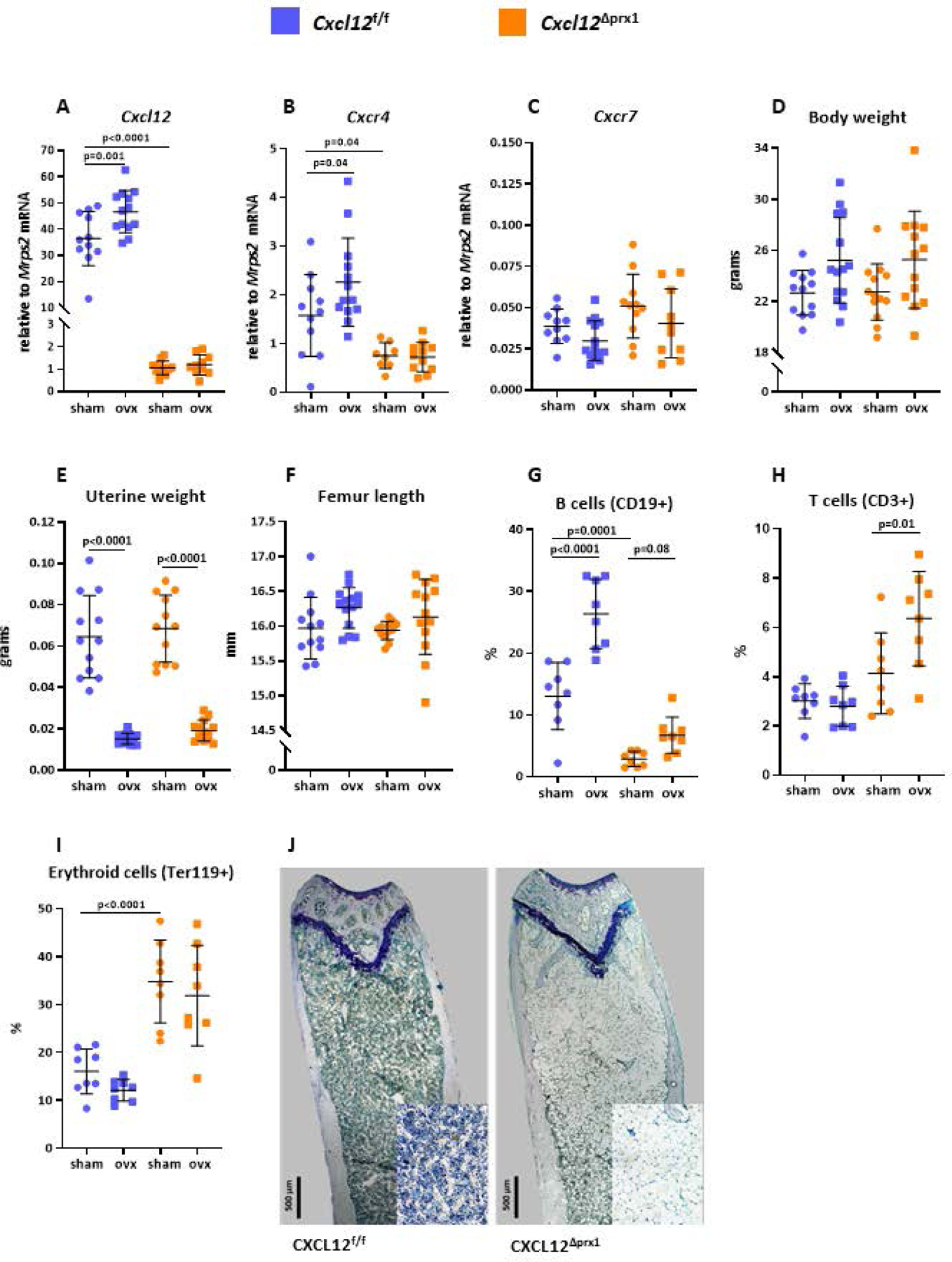
Female mice with Cxcl12 deletion in Prrx1 expressing cells (Cxcl12ΔPrrx1) and control littermates (Cxcl12f/f) were either ovariectomized (OVX) or sham-operated (Sham) at 4.5 months of age and euthanized six weeks later. (A) Cxcl12, (B) Cxcr4, and (C) Cxcr7 relative mRNA expression in femur shafts by qRT-PCR (n=11–14 mice/group). (D) Body weight, (E) uterine weight and (F) femur length (n=12–15mice/group). (G) Percentage of B lymphocytes (CD19+), (H) T lymphocytes (CD3+), and (I) erythroid cells (Ter11+) in bone marrow isolates, excluding adipocytes, from the femora quantified by flow cytometry (n=8 mice/group). (J) Representative photomicrographs (10x magnification) of longitudinal femur sections from a sham-operated Cxcl12f/f and Cxcl12ΔPrrx1 mice highlighting the extent of marrow fat in the latter; lower right insets depict 20x magnification. Data represent mean ± S.D.; p values by two-way ANOVA, followed by Holm-Sidak multiple comparison test.
Cxcl12 deletion caused the expected profound decrease in B lymphocyte number (CD19+) in the BM (Figure 1G and supplementary table 1), functionally confirming the effectiveness of the deletion. T cell number (CD3+) was minimal affected (Figures1H). Unexpectedly, however, Cxcl12 deletion increased erythroid cell number (Ter119+) (Figure 1I).
To determine whether CXCL12 plays a role in the adverse effects of loss of estrogens on bone mass, we gonadectomized both, Cxcl12f/f and Cxcl12ΔPrrx1 female or male mice, at 4.5-month-old and sacrificed them 6 weeks later. Consistent with previous work of ours showing an increase of CXCL12 protein levels in the BM of OVX mice (20), OVX increased the mRNA levels of Cxcl12 in the Cxcl12f/f mice, but it had no discernable effect in the Cxcl12ΔPrrx1 mice (Figure 1A). OVX also increased Cxcr4 mRNA in the Cxcl12f/f mice, but as in the case of Cxcl12, it had no effect in the Cxcl12ΔPrrx1 mice (Figure 1B). Cxcr7 mRNA was not affected by the OVX in either genotype (Figure 1C).
As expected, OVX increased body weight and decreased uterine weight in Cxcl12f/f mice; and the same changes were reproduced in the Cxcl12ΔPrrx1 mice (Figures 1D and 1E). In agreement with earlier evidence (19), OVX increased B cell numbers by two-fold (Figure 1G) in the Cxcl12f/f mice. The effect of OVX was, however, abrogated in the Cxcl12 deficient mice (interaction P=0.0037, supplementary table 1), suggesting that the increase in Cxcl12 following OVX contributes, at least in part, to the increase of B-lymphopoiesis that ensues upon estrogen deficiency in mice and humans (19).
Erythroid cell number was not affected by the OVX in either genotype (Figures 1I). The number of T cells was unaffected by the OVX in the Cxcl12f/f mice, but increased in the ovariectomized Cxcl12 deficient mice (interaction P=0.0181, supplementary table 1). Finally, Cxcl12 deletion caused a profound and striking increase in the number of adipocytes in the BM of female mice (Figure 1J).
Cxcl12 deletion has a mild effect on trabecular bone and attenuates the effects of estrogen deficiency on cortical bone
Cxcl12 deletion in Prrx1 cells had no effect on cortical thickness in the femur diaphysis or proximal metaphysis (Figures 2A–F). Cxcl12 deletion had also no effects on trabecular bone volume, but decreased trabecular number and increased trabecular separation and thickness (Figures 2G–J).
Figure 2. Cxcl12 deletion has a mild effect on trabecular bone and attenuates the effects of estrogen deficiency on cortical bone.

Femur cortical and trabecular bone were evaluated by micro-CT. (A) Cortical thickness, (B) cortical area/total area, (C) medullary area and (D) total area measured in mid-shaft region (n=12–15 mice/group). (E) Representative micro-CT images of femur mid-shafts. (F) Cortical thickness measured at proximal metaphysis (n=12–15 mice/group). (G) Trabecular bone volume (bone volume/tissue volume) and (H-J) microarchitecture of the trabeculae measured in distal metaphysis (n=12–15 mice/group). Data represent mean ± S.D.; p values by two-way ANOVA, followed by Holm-Sidak multiple comparison test.
OVX in the Cxcl12f/f mice caused a decrease in cortical thickness and cortical area at the diaphysis (Figures 2A and 2B) as well as a decrease in cortical thickness at the proximal metaphysis (Figure 1F). The effect of OVX in diaphysis was due to an increase in the medullary area (Figure 2C), consistent with the evidence that loss of estrogens leads to increases endocortical resorption in both rodents and humans. Total area in diaphysis was not affected (Figure 2D). Notably, the effects of OVX on cortical bone at diaphysis and metaphysis were attenuated in the Cxcl12ΔPrrx1 mice (Figures 2A–F and supplementary data table 2). Six-month-old female mice have very low trabecular bone mass at the distal metaphysis and no effect of OVX was detectable at this site in either genotype (Figures 2G–J). All together these data suggest that Cxcl12 deletion has no effects on femur bone mass but greatly attenuates the loss of cortical bone caused by estrogen deficiency.
Cxcl12 deletion increases osteoblast and osteoclast specific mRNAs in cortical bone.
Most surprisingly, Cxcl12 deletion greatly increased the expression of both osteoblast (Bglap) and osteoclast (Ctsk and Acp5) specific mRNAs in bone shafts, independently of the estrogen status (Figures 3A–C). Tnfrsf1 1B, Tnfsf11 and Sost mRNAs also increased with Cxcl12 deletion (Figures 3D–F). OVX caused an increase in all these mRNAs, with the exception of Sost, in Cxcl12f/f mice; the OVX-induced increases, however, were of much lower magnitude than the ones caused by the Cxcl12 deletion (supplementary table 3).
Figure 3. Cxcl12 deletion increases osteoblast and osteoclast specific mRNAs in cortical bone.

Expression levels of (A) Osteocalcin (Bglap), (B) Cathepsin K (Ctsk), (C) TRAP(Acp5), (D) Osteoprotegerin (Tnfrsf1 1B), (E) RANKL (Tnfsf11) and (F) Sclerostin (Sost) by qRT-PCR from bone shafts (n=11–14 mice/group). Data represent mean ± S.D.; p values by two-way ANOVA, followed by Holm-Sidak multiple comparison test.
Cxcl12 deletion increases bone formation as well as osteoclast number in cortical bone
To confirm the evidence suggesting that Cxcl12 deletion in mesenchymal progenitors increases the rate of bone turnover, we proceeded to perform histomorphometric analysis of the endocortical surface of the femora in estrogen sufficient female mice. We found that Cxcl12 deletion caused an increase of 22.5% (P=0.02) in MAR and 41.0% (P=0.15) in BFR, but only minor changes (8.4%) in MS (Figures 4A–D and supplementary table 4). More strikingly, Cxcl12 deletion caused an increase in N.Oc and Oc.S by more than 200% (P<0.0001) (Figures 4E–G).
Figure 4. Cxcl12 deletion increases bone formation as well as osteoclast number in cortical bone.

Dynamic histomorphometry measurements at the endocortical bone surface in longitudinal undecalcified femur sections from 6-month-old female mice. (A) Mineral apposition rate, (B) mineralizing surface and (C) bone formation rate determined by tetracycline labels (n=8–10 mice/group). (D) Representative photomicrographs of cortical bone labeled with tetracycline (fluorescent green) used to measure A to C. Ec, endocortical surface; head of arrows indicate the double labels; star indicates the medullar cavity; asterisk indicates adipocytes. (E) Osteoclast number per bone perimeter and (F) osteoclast surface per bone surface determined in sections stained with tartrate-resistant acid phosphatase (Acp5) (n=10 mice/group). (G) Representative microphotographs of undecalcified femur sections stained with Acp5 from a Cxcl12f/f (upper panel) and a Cxcl12ΔPrrx1 mice (two lower panels), both sham operated. Head of arrows indicate osteoclasts. In the lower panel the last image is a high-power image (40x) of the area denoted by the boxes in the central image. Data represent mean ± S.D.; p values by two-way ANOVA, followed by Holm-Sidak multiple comparison test.
In the Cxcl12f/f mice, we found no effect of OVX on MAR, MS and BFR, but as expected N.Oc and Oc.S increased 47.49% and 32.07%, respectively. Notably, OVX in Cxcl12ΔPrrx1 mice decreased MAR, MS, BFR and N.Oc and Oc.S as compared to estrogen sufficient Cxcl12ΔPrrx1 mice, suggesting that CXCL12 contributes to the OVX-induced increase in osteoclast number and resorption (interaction P=0.0027 and P=0.0144, supplementary table 4) in the endocortical surface and the resulting increase in the rate of bone remodeling. Because of the paucity of osteoblasts in the endosteal surface we were unable to reliably assess osteoblast number in this analysis.
Cxcl12 deletion in males has a mild effect on trabecular bone and dramatically increases osteoblast and osteoclast specific mRNAs
As expected, ORX decreased seminal vesicle weight in Cxcl12f/f and Cxcl12ΔPrrx1 mice, but did not affect total body weight (Figures 5A and 5B). ORX caused a decrease in the mRNA levels of Cxcl12 in Cxcl12f/f mice (Figure 5C); this effect was the opposite of the effect of OVX.
Figure 5. Cxcl12 deletion in males has a mild effect on trabecular bone and dramatically increases osteoblast and osteoclast specific mRNAs.

Male mice with deletion of Cxcl12 in Prrx1 expressing cells (Cxcl12ΔPrrx1) and control littermates (Cxcl12f/f) were generated, at 4.5-month-old they were either orchidectomized (ORX) or sham-operated (sham) and euthanized at 6-month-old. (A) Body weight and (B) vesicle seminal weight (n=10–12 mice/group). (C) Cxcl12 relative mRNA expression in femur shafts measured by qRT-PCR (n=10–12 mice/group). (D) Femur cortical thickness measured in the diaphysis, (E) trabecular bone volume (bone volume/tissue volume) and (F-H) microarchitecture at the distal metaphysis by micro-CT (n=10–12 mice/group). Expression levels of (I) Osteocalcin (Bglap), (J) Cathepsin K (Ctsk), (K) Tartrate-resistant acid phosphatase (Acp5), (L) Receptor activator of nuclear factor kappaB ligand (Tnfsf11) and (M) Osteoprotegerin (Tnfrsf1 1B) detected by qRT-PCR from bone shafts (n=8–12 mice/group). Data represent mean ± S.D.; p values by two-way ANOVA, followed by Holm-Sidak multiple comparison test.
Cxcl12 deletion in androgen sufficient mice had no effect on cortical thickness at the femur diaphysis and did not influence the decrease in cortical thickness caused by ORX (Figure 5D). As we observed in female mice, Cxcl12 deletion did not alter trabecular bone volume, but caused a decrease in trabecular number and an increase in trabecular separation (Figure 5E–H). The magnitude of the decrease in trabecular bone volume caused by ORX was similar between Cxcl12f/f and Cxcl12ΔPrrx1 mice (interaction P=0.46, supplementary table 5). Cxcl12 deletion had no effect on Bglap mRNA expression in the bone shafts of the gonad-intact mice, but it increased it in the ORX mice (Figure 5I). As in females, Cxcl12 deletion increased the mRNA expression of Ctsk, Acp5, Tnfsf11 and Tnfrsf1 1B, irrespective of gonadal status (Figures 5J–M). ORX had no effect on the expression of any of these mRNAs in the Cxcl12 sufficient Cxcl12f/f mice. In fact, both OVX and ORX in the Cxcl12 sufficient mice had no effect on the osteoblast- and osteoclast-specific mRNAs. However, in the Cxcl12ΔPrrx1 mice, OVX increased Acp5 and Tnfsf11 and ORX increased Acp5 and Tnfsf11 as well as Bglap, (but not Ctsk), over and above the increase in these mRNAs caused by the Cxcl12 deletion, perhaps because of an already higher number of osteoclast and osteoblast progenitors.
DISCUSSION
Prompted by preliminary evidence suggesting that CXCL12 is upregulated in estrogen deficiency (24), the goal of the work presented in this paper was to examine whether CXCL12 contributes to the loss of cortical bone mass caused by estrogen or androgen deficiency. To do this we generated mice with conditional deletion of Cxcl12 in mesenchymal progenitors using a Prrx1-cre transgene.
We found that Cxcl12 deletion in the Prrx1 targeted mesenchymal progenitors decreased not only the expression of Cxcl12 in femoral bone but also its primary receptor Cxcr4, suggesting that in bone cells the abundance of CXCR4 is controlled by its ligand. As it was shown previously by several other groups (2,3), Cxcl12 deletion dramatically decreased lymphopoiesis. Conversely, it increased erythrocyte as well as adipocyte cell number in the BM.
Cxcl12 deletion had no effect on the cortical or trabecular bone mass of the gonad-intact female and male mice. Most surprisingly Cxcl12 deletion greatly increased osteoblast and osteoclast specific mRNAs in femur bone shafts of both sexes, strongly suggesting an increase in the rate of bone remodeling, but without a change in the balance between resorption and formation. Moreover, Cxcl12 deletion increased bone formation as well as osteoclast number in the cortical bone of our female mice. Notably, all the indices of increased remodeling caused by the Cxcl12 deletion were much greater than those caused by estrogen deficiency and the unbalanced remodeling associated with the latter. These findings reveal for the first time that in addition to its potent effects on hematopoiesis, CXCL12 restrains the rate of bone turnover by suppressing osteoblastogenesis and the osteoclast support provided by cells of the osteoblast lineage, including the essential osteoclast differentiation factor Tnfsf11. This previously unrecognized effect of CXCL12 is opposite to the evidence that CXCL12 acts directly on myeloid progenitors to stimulate osteoclastogenesis and increase bone resorption in several disease states (22,23,26,27). In any case, unleashing osteoblastogenesis and the resulting indirect stimulation of osteoclast generation in the Cxcl12 deficient mice has likely overcompensated for the loss of the direct and relatively weaker stimulatory effect of CXCL12 on osteoclastogenesis. A schematic representation of these concepts is provided in figure 6.
Figure 6. Schematic representation of the effects of CXCL12 on osteoblastogenesis and osteoclastogenesis.

Green arrow depicts the pro-osteoclastogenic effect of CXCL12 exerted directly on cells of the osteoclast lineage. Red blocks depict the effects of the CXCL12 on osteoprogenitors and terminally differentiated osteocytes, causing suppression of osteoblastogenesis and indirectly suppression of osteoclastogenesis. The difference in the size of the width of the green and red lines are meant to indicate that the indirect effects of the chemokine on osteoclastogenesis are greater than the direct effects.
Shahnazari and colleagues have deleted Cxcr4 in osteoblasts and osteocytes (expressing Col2.3-cre) and showed that Cxcr4 signaling on these cells may suppress osteoprogenitor and osteoclast precursor populations, as well as their maturation and recruitment to the bone surface (11). Taken together with our own findings, the available evidence favors the idea that the suppressive effects of CXCL12 on bone remodeling result, at least in part, from actions on differentiated cells of the osteoblast lineage.
Tzeng and colleagues have previously investigated the bone phenotype of mice with Cxcl12 deletion using Prrx1-cre and in agreement with our observations found an increase in marrow adiposity (12). However, deletion of Cxcr4 in the same cell population in that study had no effect on adiposity. These results suggest that the suppressive effect of CXCL12 on adipogenesis is not exerted directly on adipocyte precursors and their progeny, but perhaps via the upregulation of pro-adipogenic factors in cell types other than cells of the mesenchymal lineage that are targeted by Prrx1. Tzeng and colleagues also found a decrease in trabecular bone mass in 3 to 4-month-old Cxcl12 deficient male mice, associated with decreased bone formation but unchanged osteoclasts (12).
Consistent with the evidence that estrogen deficiency upregulates Cxcl12 expression and the hypothesis that CXCL12 may be a culprit of the cortical bone loss caused by estrogen deficiency, the effects of estrogen deficiency on osteoclast number and cortical bone mass were abolished or attenuated in mice lacking Cxcl12. These findings are at first sight incongruent with the increased osteoclastogenesis in the Cxcl12 deficient mice. Intriguingly, however, bone formation and osteoclast numbers decreased rather than increased with OVX in the Cxcl12 deficient mice, perhaps as result of a decrease in the number of cells of the mesenchymal lineage secondary to the loss of the well-established anti-apoptotic effect of estrogens in this cell population (33). The still higher osteoblastogenesis in the Cxcl12 deficient mice, as compared to the Cxcl12 sufficient mice, could counterbalance the effect of resorption on bone mass and contribute, at least in part, to the attenuation of the cortical bone loss caused by OVX.
Cxcl12 deletion attenuated the OVX-induced increase in B lymphocyte number. This result suggests that CXCL12 may contribute, at least in part, to the increase of B-lymphopoiesis that ensues upon estrogen deficiency in mice and humans (19,34,35). Albeit, earlier work from our center has demonstrated that the effects of estrogens on B lymphopoiesis are exerted indirectly, as ERα deletion in B cells does not alter the effect of OVX on B cell number (36). But notably, Tnfsf11 produced by osteocytes is required for the OVX- induced increase in B cells. Furthermore, Tnfsf11 produced by B lymphocytes contributes to the OVX-induced loss of trabecular bone in mice, but plays no role in the OVX-induced loss of cortical bone (37). Be that as it may, the increase in osteoclast number, in spite of the dramatic decrease of B lymphocytes, in the Cxcl12 deficient mice of the present report suggests that the attenuation of the OVX-induced loss of cortical bone is mechanistically unrelated to the prevention of the OVX-induced increase in lymphopoiesis in the Cxcl12 deficient mice.
Based on the results of our earlier studies of mice with cell-specific deletion of the estrogen or androgen receptor, we had proposed that an ERα-mediated action on early mesenchymal progenitors may be responsible for the protection against endocortical bone resorption in both females and males – via the conversion androgen to estrogens in the latter (38). Support of this idea was provided by our earlier findings that both OVX and ORX increases CXCL12 levels in the BM and estrogen administration suppressed the effect of ORX on CXCL12 levels as well the loss of cortical thickness (24). However, in the present work Cxcl12 deletion attenuated the effects of OVX, but not ORX. This finding suggests that CXCL12 may not be a common mediator of the cortical bone loss caused by the loss of both ovarian and testicular function.
In closing, the results presented in this report reveal a potent and previously unrecognized restraining effect of CXCL12 on osteoblastogenesis and bone turnover. They also provide functional evidence in support of the notion that CXCL12 may contribute to the loss of cortical bone mass caused by estrogen deficiency. Additionally, they provide new insights into the intricacy of the BM niche and its modulation by systemic hormones. Future work with newer methodologies for the identification of distinct subsets of marrow stromal cells (e.g. mass cytometry cyTOF) in conjunction with cell-specific deletion of Cxcl12 and the CXCL12 receptor CXCR4 in osteoclast and osteoblast progenitors and their descendants will be necessary to test the working hypotheses generated by the findings of the present report.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Biomedical Laboratory Research and Development Service of the Veteran’s Administration Office of Research and Development [I01 BX001405 (SCM)], the NIH [R01 AR056679; P20 GM125503], and the University of Arkansas for Medical Sciences Tobacco Funds and Translational Research Institute (1UL1RR029884). We thank A Warren, S Berryhill, and J Crawford for technical assistance; and Katie Poe for help with the preparation of the manuscript.
Grant Supporters: This work was supported by the Department of Veterans Affairs (I01 BX001405), the National Institutes of Health (P01 AG013918, R01 AR056679, P20 GM125503), and the UAMS Tobacco Funds and Translational Research Institute (1UL1RR029884).
Footnotes
Supplemental Data: Supplemental data is attached to this manuscript.
DISCLOSURES
None
Conflict of Interest Statement: The authors declare no conflicts.
References
- 1.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7(4):333–7. [DOI] [PubMed] [Google Scholar]
- 2.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Duan B, Cheng Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herberg S, Shi X, Johnson MH, et al. Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8(3):e58207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herberg S, Kondrikova G, Hussein KA, et al. Mesenchymal stem cell expression of stromal cell-derived factor-1beta augments bone formation in a model of local regenerative therapy. J Orthop Res. 2015;33(2):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cipitria A, Boettcher K, Schoenhals S, et al. In-situ tissue regeneration through SDF-1alpha driven cell recruitment and stiffness-mediated bone regeneration in a critical-sized segmental femoral defect. Acta Biomater. 2017;60:50–63. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. [DOI] [PubMed] [Google Scholar]
- 9.Wright LM, Maloney W, Yu X, et al. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36(5):840–53. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Liang G, Huang Z, Doty SB, Boskey AL. Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem. 2011;286(30):26794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahnazari M, Chu V, Wronski TJ, Nissenson RA, Halloran BP. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27(9):3505–13. [DOI] [PubMed] [Google Scholar]
- 12.Tzeng YS, Chung NC, Chen YR, et al. Imbalanced Osteogenesis and Adipogenesis in Mice Deficient in the Chemokine Cxcl12/Sdf1 in the Bone Mesenchymal Stem/Progenitor Cells. J Bone Miner Res. 2018;33(4):679–90. [DOI] [PubMed] [Google Scholar]
- 13.De KB, Geboes L, Hatse S, et al. Pro-inflammatory properties of stromal cell-derived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res Ther. 2005;7(6):R1208–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey MK, Kale VP, Song C, et al. Gambogic acid inhibits multiple myeloma mediated osteoclastogenesis through suppression of chemokine receptor CXCR4 signaling pathways. Exp Hematol. 2014;42(10):883–96. [DOI] [PubMed] [Google Scholar]
- 15.Im JY, Min WK, Park MH, et al. AMD3100 improves ovariectomy-induced osteoporosis in mice by facilitating mobilization of hematopoietic stem/progenitor cells. BMB Rep. 2014;47(8):439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosla S Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95(8):3569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida M, Iyer S, Martin-Millan M, et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osteoimmunology Pacifici R. and its implications for transplantation. Am J Transplant. 2013;13(9):2245–54. [DOI] [PubMed] [Google Scholar]
- 20.Ucer S, Iyer S, Kim HN, et al. The Effects of Aging and Sex Steroid Deficiency on the Murine Skeleton Are Independent and Mechanistically Distinct. J Bone Miner Res. 2017;32(3):560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18(8):1404–18. [DOI] [PubMed] [Google Scholar]
- 22.Grassi F, Piacentini A, Cristino S, et al. Human osteoclasts express different CXC chemokines depending on cell culture substrate: molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem Cell Biol. 2003;120(5):391–400. [DOI] [PubMed] [Google Scholar]
- 23.Grassi F, Cristino S, Toneguzzi S, et al. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J Cell Physiol. 2004;199(2):244–51. [DOI] [PubMed] [Google Scholar]
- 24.Ucer S, Iyer S, Kim HN, et al. The Effects of Aging and Sex Steroid Deficiency on the Murine Skeleton Are Independent and Mechanistically Distinct. J Bone Miner Res. 2017;32(3):560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. [DOI] [PubMed] [Google Scholar]
- 26.Zannettino AC, Farrugia AN, Kortesidis A, et al. Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65(5):1700–9. [DOI] [PubMed] [Google Scholar]
- 27.Gronthos S, Zannettino AC. The role of the chemokine CXCL12 in osteoclastogenesis. Trends Endocrinol Metab. 2007;18(3):108–13. [DOI] [PubMed] [Google Scholar]
- 28.Tang Q, Su YW, Fan CM, et al. Release of CXCL12 From Apoptotic Skeletal Cells Contributes to Bone Growth Defects Following Dexamethasone Therapy in Rats. J Bone Miner Res. 2019;34(2):310–26. [DOI] [PubMed] [Google Scholar]
- 29.Bartell SM, Kim HN, Ambrogini E, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5:3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 31.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 33.Almeida M, Laurent MR, Dubois V, et al. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev. 2017;97(1):135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smithson G, Beamer WG, Shultz KL, et al. Increased B Lymphopoiesis in Genetically Sex Steroid-Deficient Hypogonadal (hpg) Mice. J Exp Med. 1994;180(1):717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuzawa T, Miyaura C, Onoe Y, et al. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara Y, Piemontese M, Liu Y, et al. RANKL produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J Biol Chem. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onal M, Xiong J, Chen X, et al. Receptor Activator of Nuclear Factor kappaB Ligand (RANKL) Protein Expression by B Lymphocytes Contributes to Ovariectomy-induced Bone Loss. J Biol Chem. 2012;287(35):29851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ucer S, Iyer S, Bartell SM, et al. The effects of androgens on murine cortical bone do not require AR or ERalpha signaling in osteoblasts and osteoclasts. J Bone Miner Res. 2015;30(7):1138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


