Abstract
Gulf War Illness (GWI) manifests a multitude of symptoms, including neurological and immunological, and approximately a third of the 1990-1991 Gulf War (GW) veterans suffer from it. This study sought to characterize the acute neurochemical (monoamine) and neuroinflammatory profiles of two established GWI animal models and examine the potential modulatory effects of the novel immunotherapeutic Lacto-N-fucopentaose III (LNFPIII). In Model 1, male C57BL/6J mice were treated for 10 days with pyridostigmine bromide (PB) and permethrin (PM). In Model 2, a separate cohort of mice were treated for 14 days with PB and N,N-Diethyl-methylbenzamide (DEET), plus corticosterone (CORT) via drinking water on days 8-14 and diisopropylfluorophosphate (DFP) on day 15. LNFPIII was administered concurrently with GWI chemicals treatments. Brain and spleen monoamines and hippocampal inflammatory marker expression were examined by, respectively, HPLC-ECD and qPCR, 6 h post treatment cessation. Serotonergic (5-HT) and dopaminergic (DA) dyshomeostasis caused by GWI chemicals was apparent in multiple brain regions, primarily in the nucleus accumbens (5-HT) and hippocampus (5-HT, DA) for both models. Splenic levels of 5-HT (both models) and norepinephrine (Model 2) were also disrupted by GWI chemicals. LNFPIII treatment prevented many of the GWI chemicals induced monoamine alterations. Hippocampal inflammatory cytokines were increased in both models, but the magnitude and spread of inflammation was greater in Model 2; LNFPIII was anti-inflammatory, more so in the apparently milder Model 1. Overall, in both models, GWI chemicals led to monoamine disbalance and neuroinflammation. LNFPIII co-treatment prevented many of these disruptions in both models, which is indicative of its promise as a potential GWI therapeutic.
Keywords: Gulf War Illness, LNFPIII, monoamines, neuroinflammation, pesticides
1. Introduction
Aberrant communication between the immune and the central nervous systems (CNS) has been implicated in the pathogenesis of various disease states. While controlled innate immune system activation and ensuing inflammation serves important protective and homeostatic functions in the body, if left unchecked, it can be detrimental (Karin et al., 2006). In the CNS, uncontrolled inflammation (neuroinflammation) can cause cognitive, memory, and mood deficits; it exacerbates, among others, Alzheimer’s disease (AD), Multiple Sclerosis (MS), and Parkinson’s disease (PD) pathologies (Capuron and Miller, 2011; Czirr and Wyss-Coray, 2012; Ferrari and Tarelli, 2011). Further, neuroinflammation can be initiated in the periphery and have destructive effects centrally. For example, peripheral inflammation exacerbated nigrostriatal degeneration and motor dysfunction in a PD model (Pott Godoy et al., 2010).
Recently, this neuroimmune crosstalk has been implicated in a complex multi-symptom disorder known as Gulf War Illness (GWI). GWI affects approximately 30% of the veterans from the 1990-1991 Gulf War (GW) and targets the immune, nervous, musculoskeletal, and gastrointestinal systems (DDGWIRP, 2018; White et al., 2016). Prominent symptoms of GWI include fatigue, impaired cognition, memory and motor function, and altered mood (White et al., 2016). The precise etiology of GWI remains undetermined, but suspected contributing factors, in combination with war theatre stress, include overexposures to chemicals, such as the nerve agent prophylactic pyridostigmine bromide (PB), and pesticides, like permethrin (PM), chlorpyrifos, and N-Diethyl-3-methylbenzamide (DEET). A subset of GW veterans were also exposed to the nerve agents sarin and cyclosarin (Boyd et al., 2003; Cherry et al., 2001; Steele et al., 2012; White et al., 2016). Due to their association with GWI, these chemicals are referred to as GWI chemicals.
In addition to the cognition, memory, motor function and mood deficits exhibited by GWI veterans, structural abnormalities, such as reduced hippocampal volumes and increased diffusivity in white matter connections, are reported (Chao et al., 2010; Rayhan et al., 2013; Toomey et al., 2009; White et al., 2016). Accumulating clinical and experimental evidence suggests that at least some neurological GWI deficits might be associated with immune dysfunction. For example, several studies have shown that GWI veterans exhibit systemic inflammation compared to matched veteran controls (Broderick et al., 2013; Broderick et al., 2018; Parkitny et al., 2015). Many of these neurological and immunological deficits are recapitulated in animal models of GWI; data show that post exposure to GWI chemicals, neuronal dysfunction and increases in neuroinflammatory markers throughout the brain are present (Carreras et al., 2018; Miller et al., 2018; O’Callaghan et al., 2015; Parihar et al., 2013). Further, rodent behavioral data mirror GWI symptomology in terms of impaired cognition, memory and motor function, as well as increased anxiety and depressive-like behaviors (Abdullah et al., 2011; Carreras et al., 2018; Hattiangady et al., 2014; Parihar et al., 2013; Zakirova et al., 2015).
Many of the neurological and behavioral deficits associated with GWI could stem from neurotransmitter dysfunction. Affect disorders, including sickness behavior and depression, have been linked to serotonin (5-HT) and dopamine (DA) dysregulation (Belujon and Grace, 2017; Korte-Bouws et al., 2018). Dopaminergic dysfunction, especially in the basal ganglia, is implicated in motor function impairments (Ferrari and Tarelli, 2011; Willard et al., 2015). Further, multiple studies show that inflammation can alter monoamine neurotransmitter balance and transmission in the brain, which subsequently influences behavior (Dunn, 2006). For example, inflammatory cytokine challenge alters central monoaminergic transmission, i.e., metabolism of 5-HT and norepinephrine (NE) is increased in response to IL-1 and DA homeostasis is disrupted by IFN-α (Dunn, 2006; Felger and Miller, 2012). In addition, peripheral monoamines can be immunomodulatory as the spleen, rich in 5-HT and NE, can modulate activation of T-cells and pro-inflammatory cytokines (Blandino et al., 2006; Wu et al., 2019).
While researchers have sifted through the immunological component of GWI, few have investigated the role neurotransmitters, monoamines in particular, play in GWI symptomology. Studies investigating neurochemical changes have largely focused on acetylcholinesterase (AchE) inhibition and cholinergic activation due to the mechanism of action of some GWI chemicals, such as PB, sarin, or its surrogate, DFP (Miller et al., 2018; Ojo et al., 2014; Zakirova et al., 2015). Others have found alterations in GABA-ergic activity in brain regions that regulate cognition (Carreras et al., 2018; Megahed et al., 2014). However, as indicated earlier, some of the neurological symptoms might be due to monoamine disbalance (Parihar et al., 2013; Zakirova et al., 2015).
Immunotherapies have shown promise in treating common neurological diseases such as PD, AD, and MS (Wisniewski and Goni, 2015; Zella et al., 2019; Ziemssen et al., 2016). Given that GWI has both immunological and neurological underpinnings, it is important to examine treatments that cater to both fields. Therapeutic intervention in GWI has been explored including trials with the antibiotic doxycycline, coenzyme Q10, and carnosine supplementation (Baraniuk et al., 2013; Donta et al., 2004; Golomb et al., 2014). However, like other GWI interventions, these treatments improved only some GWI symptoms and might not be a desirable option for long term treatment (DDGWIRP, 2018). Moreover, while viable treatment options to veterans suffering from GWI would be the ultimate goal, testing of new therapeutics with a strong safety profile in an acute (exposure-related) paradigm is also important, but such testing has not been done.
Lacto-N-fucopentaose III (LNFPIII) is an immunomodulatory glycan found in human breast milk and, when conjugated to a dextran carrier, it utilizes CD14/TLR-4 signaling to activate ERK-dependent production of anti-inflammatory mediators (Tundup et al., 2015). As a result, the inflammatory balance of the innate immune system is skewed in an anti-inflammatory direction (Tundup et al., 2015). LNFPIII, being a component of human milk, provides insight into its safety profile; so far, there have been no reported adverse reactions to it (Atochina et al., 2008; Bhargava et al., 2012; Srivastava et al., 2014; Tundup et al., 2015; Zhu et al., 2012). Studies have demonstrated LNFPIII’s ability to increase production of anti-inflammatory cytokines under chronic states of inflammation and to alternatively activate macrophages (Atochina et al., 2008; Bhargava et al., 2012; Srivastava et al., 2014). Further, in an animal model of MS, LNFPIII significantly reduced the severity of experimental autoimmune encephalomyelitis, in part by decreasing inflammation and infiltration of immune cells into the CNS (Zhu et al., 2012).
Given the lack of detailed neurochemical characterization of accepted models of GWI and the modulatory effects that inflammation plays on neurotransmitter homeostasis, the present study sought to characterize the acute neurochemical monoamine profiles in two established GWI animal models. In addition, we also aimed at further characterizing the hippocampal neuroinflammatory profiles of these two models, as this is a key structure associated with GWI-related cognitive dysfunction. Finally, we examined whether the immunotherapeutic LNFPIII had any modulatory effects on the aforementioned parameters.
2. Materials and Methods
2.1. Materials
The following chemicals were used for animal treatments: pyridostigmine bromide (PB; Sigma Aldrich, St. Louis, MO), permethrin (PM; 29.5% cis/69.5% trans isomer; Chem Service Inc., West Chester, PA), diisopropylfluorophosphate (DFP; Sigma Aldrich), N-Diethyl-3-methylbenzamide (DEET; Sigma Aldrich), and corticosterone (CORT; Steraloids, Newport, RI). Lacto-N-fucopentaose III (LNFPIII) dextran conjugate was produced as previously described (Tundup et al., 2015). All additional chemicals and reagents used in this study, unless otherwise noted, were of analytical or higher grade and were obtained from Sigma Aldrich or Fisher Scientific (Hampton, NH).
2.2. Animals
Male C57BL/6J mice (8-9 weeks old; Jackson Laboratories, Bar Harbor, ME) were housed 4 per cage in an environmentally controlled room (22-24° C) and maintained on a 12 h light/dark cycle (0700-1900 lights on) for one week of acclimation and throughout the study. Mice were handled daily prior to the start of the study to minimize experimenter induced stress. Food and water were available ad libitum. All procedures were approved in advance by the University of Georgia Institutional Animal Care and Use Committee and were in accordance with the latest National Institutes of Health and ARRIVE guidelines.
2.3. GWI Models
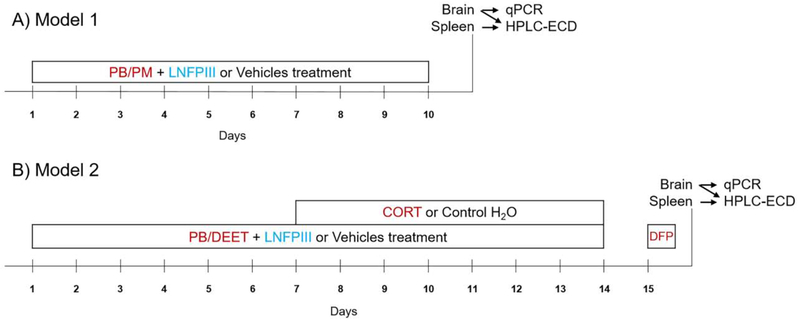
The present study followed two established, chemically different GWI animal models as outlined in Figure 1 (O’Callaghan et al., 2015; Zakirova et al., 2015). Following Zakirova et al. (2015), mice (N=24) were treated daily for 10 days with a combination of PB and PM (0.7 and 200 mg/kg, respectively) or DMSO vehicle IP, immediately followed by a SC injection of LNFPIII or dextran vehicle (both 35 μg/mouse) diluted in sterile saline. For this model, there were 4 treatment groups with 6 mice per group and were as follows: Vehicle-Vehicle (DMSO-Dextran), Vehicle-LNFPIII (DMSO-LNFPIII), PB/PM-Vehicle (PB/PM-Dextran), and PB/PM-LNFPIII. This model will be referred to as Model 1 throughout this manuscript (Fig. 1A).
Figure 1. Experimental design and description of the Gulf War Illness models.
A) GWI Model 1 (Zakirova et al. 2015) followed a 10 day treatment protocol of pyridostigmine bromide (PB; 0.7 mg/kg, IP) and permethrin (PM; 200 mg/kg, IP) or DMSO vehicle followed by concurrent administration of LNFPIII (35 μg, SC) or dextran vehicle (35 μg, SC). B) GWI Model 2 (O’Callaghan et al. 2015) induced GWI over a 15 day protocol in which PB (2 mg/kg, SC) and N,N-Diethyl-methylbenzamide (DEET;30 mg/kg, SC) or saline vehicle followed by concurrent LNFPIII (35 μg) or dextran vehicle (35 μg) were administered for 14 days. Corticosterone (CORT; 200 mg/L in 1.5% EtOH water) or vehicle were administered on days 7-14. A single injection of DFP (3.75 mg/kg) or saline vehicle was given on day 15. In both models, mice were sacrificed 6 h post the last treatment, and neurochemistry (HPLC-ECD) and qPCR analysis were then conducted on frozen brain and spleen tissues.
A separate cohort of mice (N=26), following O’Callaghan et al. (2015), were treated for 14 days with a combination of PB and DEET (2 and 30 mg/kg, respectively; SC), or saline vehicle and immediately followed by LNFPIII or dextran vehicle (both 35 μg/mouse) diluted in sterile saline. On days 8-14, these mice were also administered CORT via drinking water (200 mg/L in 1.5% EtOH tap water) ad libitum; control mice were given the 1.5% EtOH vehicle water during this period. On day 15, mice received a single IP injection of DFP (3.75 mg/kg) or saline vehicle. The four treatment groups were as follows: Vehicle-Vehicle (Saline/water/saline-Dextran), Vehicle-LNFPIII (Saline/water/saline-LNFPIII), PB/DEET/CORT/DFP-Vehicle (PB/DEET/CORT/DFP-Dextran), and PB/DEET/CORT/DFP-LNFPIII. There were 6 mice per treatment group, except in the PB/DEET/CORT/DFP-Vehicle group in which there were 8. This model will be referred to as Model 2 throughout this manuscript (Fig. 1B).
2.4. Tissue Collection
Six hours after the last treatment, mice were euthanized, blood was collected, and organs (brain, inguinal lymph nodes, spleen, thymus, liver and kidney) were weighed and frozen on dry ice. For the brain only, a sagittal cut was made and one half was quickly frozen on dry ice while the other half was immersed in 4% paraformaldehyde for fixation as in Krishna et al. (2016). All tissues were stored at −80° C until analysis.
2.5. Neurochemistry
Concentrations of brain and spleen monoamines and their metabolites were determined using high performance liquid chromatography with electrochemical detection (HPLC-ECD) as previously described (Coban and Filipov, 2007; Krishna et al., 2016). Briefly, brains were sectioned into 500-μm thick slices and micropunches (1.5 mm diameter) from the prefrontal cortex (PFC), nucleus accumbens (NAc), striatum (STR), amygdala (AMY), dorsal hippocampus (dHIP), and ventral hippocampus (vHIP) were homogenized in 100 μl of 0.2 N HClO4. A small portion (average 6.2 mg weight) of the spleen was cut, weighed and homogenized in 200 μl of 0.2 N HClO4. Samples were centrifuged (13,200 x G at 4° C for 10 min) and sample supernatant (20 μL) was injected into the HPLC-ECD for detection of: 1) norepinephrine (NE) and its metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG); 2) dopamine (DA) and its metabolite homovanillic acid (HVA); 3) serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). Prior to statistical analysis, neurotransmitters and metabolite levels were protein-normalized using the Bradford assay as previously described (Coban and Filipov, 2007; Krishna et al., 2016).
2.6. Real-time quantitative PCR (qPCR) for inflammatory markers
Total RNA from a single brain punch (1.5 mm diameter, 500 μm thick section, ventral hippocampus: vHip) was isolated by an E.Z.N.A total RNA isolation kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer’s directions. The RNA was quantified with a Take3 micro-volume plate and Epoch microplate spectrophotometer (BioTek, Winooski, VT). Seventy-five ng RNA/sample was used to synthesize cDNA with a Maxima first strand cDNA synthesis kit for RT-qPCR (Thermo Scientific, Waltham, MA) and a Peltier thermal cycler (Bio-Rad, Hercules, CA; 10 min at 25°C, 15 min at 50°C, 5 min at 85°C). Using 1 or 2 ng of cDNA per sample, expression of various inflammatory genes and the growth factors NGF and BDNF (Supplementary Table 1) were determined by a qPCR with mouse-specific primers (RealTimePrimers, Elkins Park, PA) and Maxima SYBR Green/lowRox qPCR Master Mix (2x) (Thermo Scientific). Amplifications were performed on Mx3005P qPCR machine (Stratagene, San Diego, CA) and treatment differences were calculated as a fold change by the ΔΔCt method with 18S as the house keeping gene, as described previously (Krishna et al., 2016; Lin et al., 2013).
2.7. Statistical Analysis
Two-way analysis of variance (ANOVA) was used to determine main effects of treatments or treatment interactions with each GWI model analyzed separately. If a two-way ANOVA was significant (p ≤ 0.05), treatment means were separated by Student-Newman-Keuls (SNK) post hoc test or pairwise comparisons (as appropriate). If normality failed, data were log transformed prior to statistical analyses, but the non-transformed values were used for graphical representation. Data were analyzed using SigmaPlot 12.5 (San Jose, CA), and graphs were generated using GraphPad Prism 5 (San Diego, CA).
3. Results
3.1. Brain Neurotransmitters
3.1.1. Serotonin (5-HT)
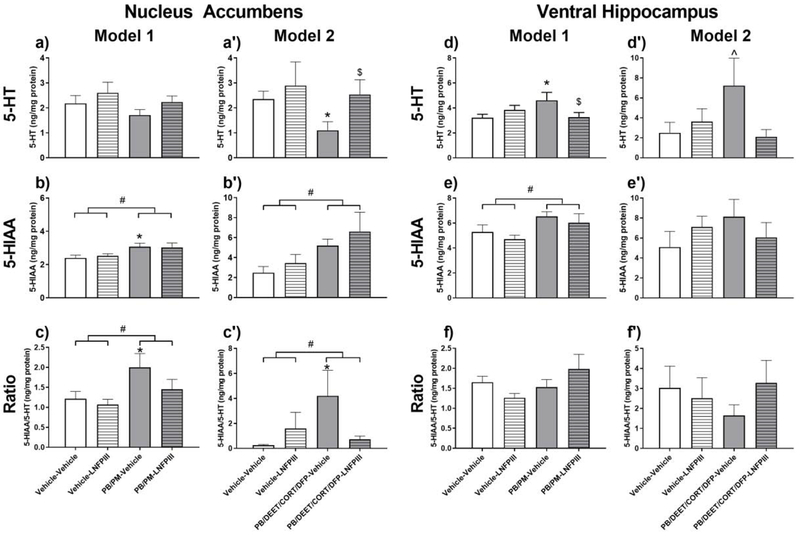
In the NAc, levels of the 5-HT metabolite 5-HIAA were increased in both models (Model 1, p ≤ 0.01, Fig. 2b and Model 2, p ≤ 0.05, Fig. 2b’) by GWI chemicals. This was accompanied by a numerical decrease of 5-HT in Model 1 (Fig. 2a) and significant decrease of 5-HT in Model 2 (p ≤ 0.05, Fig 2a’). LNFPIII treatment did not have a significant effect on NAc 5-HIAA, but it did prevent the numerical/significant decreases of 5-HT caused by GWI chemicals in both models (Fig. 2a and 2a’). As a result, 5-HT turnover (5-HIAA/5-HT ratio) was increased by GWI chemicals significantly in both models (Fig. 2c, p ≤ 0.05 and Fig. 2c’, p ≤ 0.05); this effect was prevented by LNFPIII (Fig. 2c’).
Figure 2. Serotonergic alterations in the Nucleus Accumbens and Ventral Hippocampus in two models of Gulf War Illness.
Data are presented as mean ± SEM; unit: ng/mg protein; n=6 per group/treatment except PB/DEET/CORT/DFP-Vehicle group, which was n=8. # indicates a significant main effect of GWI treatment (PB/PM (Model 1) or PB/DEET/CORT/DFP (Model 2)) after a two-way ANOVA (p ≤ 0.05). * indicates a significant difference compared to Vehicle-Vehicle group (p ≤ 0.05). ^ indicates trend compared to Vehicle-Vehicle group (p < 0.10). $ indicates significant difference compared to GWI-Vehicle group (p ≤ 0.05). Abbreviations: 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; acid; PB: pyridostigmine bromide; PM: permethrin; DEET: N,N-Diethyl-methylbenzamide; CORT: corticosterone; DFP: diisopropylfluorophosphate; LNFPIII: lacto-N-fucopentaose III.
Similarly, in the vHip, GWI chemicals increased levels of the 5-HT metabolite 5-HIAA in Model 1 (p ≤ 0.05, Fig. 2e) and numerically in Model 2. This was accompanied by a concomitant increase in 5-HT by GWI chemicals significantly in Model 1 (p ≤ 0.05, Fig. 2d) and trending in Model 2 (p = 0.07, Fig. 2d’). LNFPIII did not have an effect on the levels of 5-HIAA, however, it did prevent the increases in 5-HT in both models (Model 1, p ≤ 0.05, Fig. 2d and Model 2, p = 0.06, Fig. 2d’). Due to the increases in both 5-HIAA and 5-HT, the 5-HIAA/5-HT ratio in the vHip was not affected by GWI chemicals (Fig. 2f and 2f’).
Serotonergic effects of the GWI chemicals in other regions included the STR, AMY, and dHip. In particular, in Model 1 GWI chemicals increased levels of 5-HIAA in the STR, AMY, and dHip (p’s ≤ 0.05, Table 1) and these effects were not present in Model 2 (Table 2). There was a trend (p ≤ 0.10) for increases in 5-HT turnover by GWI chemicals in both models for the STR (data not shown) and in Model 2 for the AMY (data not shown). LNFPIII did not prevent GWI chemical effects on striatal 5-HIAA, but it did prevent the numerical decrease of STR 5-HT in Model 1 (p = 0.06, Table 1). As a result, LNFPIII prevented the increase in STR turnover in Model 1 (p ≤ 0.05), an effect not observed in Model 2 (data not shown).
Table 1.
Brain monoamines and their metabolites in male C57BL6/J mice 6 h post chemicals exposure in Model 1 of Gulf War Illness.
| Brain Region | Monoamine or Metabolite | Treatment |
|||||
|---|---|---|---|---|---|---|---|
| Vehicle –Vehicle | Vehicle –LNFPIII | PB/PM –Vehicle | PB/PM –LNFPIII | ||||
| PFC | 5-HT | 1.28 ± 0.30 | 2.23 ± 0.56 | 1.51 ± 0.28 | 1.37 ± 0.29 | ||
| 5-HIAA | 2.51 ± 0.12 | 2.46 ± 0.19 | 2.79 ± 0.31 | 2.64 ± 0.27 | |||
| DA | 0.37 ± 0.07 | 0.26 ± 0.13 | 0.52 ± 0.21 | 0.24 ± 0.11 | |||
| HVA | 0.70 ± 0.27 | 0.43 ± 0.16 | 1.40 ± 0.48 | 0.65 ± 0.21 | |||
| STR | 5-HT | 2.50 ± 0.48 | 3.02 ± 0.62 | 1.72 ± 0.39 | 3.05 ± 0.30 | ||
| 5-HIAA | 3.15 ± 0.47 | 2.55 ± 0.16 | 3.65 ± 0.46 a | 3.68 ± 0.32 a | |||
| DA | 97.62 ± 2.91 | 106.60 ± 3.82 | 102.23 ± 6.36 | 95.02 ± 3.95 | |||
| HVA | 9.03 ± 0.46 | 9.25 ± 0.53 | 11.63 ± 1.34a | 10.24 ± 0.53a | |||
| AMY | 5-HT | 3.80 ± 0.81 | 4.37 ± 0.37 | 4.30 ± 0.60 | 3.39 ± 0.75 | ||
| 5-HIAA | 3.65 ± 0.43 | 3.49 ± 0.34 | 4.91 ± 0.42a | 4.25 ± 0.44a | |||
| DA | 17.15 ± 3.79 | 25.01 ± 5.28 | 16.08 ± 4.44 | 19.02 ± 6.23 | |||
| HVA | 2.79 ± 0.71 | 2.87 ± 0.27 | 2.79 ± 0.44 | 2.84 ± 0.67 | |||
| dHip | 5-HT | 1.25 ± 0.33 | 1.69 ± 0.23 | 1.41 ± 0.22 | 1.59 ± 0.17 | ||
| 5-HIAA | 2.96 ± 0.22 | 3.32 ± 0.60 | 3.99 ± 0.44 a | 4.36 ± 0.59 a | |||
| DA | 0.24 ± 0.08 | 0.26 ± 0.05 | 0.20 ± 0.06 | 0.14 ± 0.04 | |||
| HVA | 0.15 ± 0.02 | 0.20 ± 0.04 | 0.25 ± 0.06 | 0.25 ± 0.08 | |||
Data are presented as mean ± SEM; unit: ng/mg protein; n= 6 per group.
a, b or c indicate significant main effect for GWI treatment (PB/PM), LNFPIII treatment or Treatment (GWI x LNFPIII) interaction, respectively, after a two-way ANOVA (p ≤ 0.05) and are bolded.
Italicized values indicate trend (p < 0.10) for main effect. Abbreviations: PFC: prefrontal cortex; NAc: nucleus accumbens; STR: striatum; AMY: amygdala; dHip: dorsal hippocampus; vHip: ventral hippocampus; 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; DA: dopamine; HVA: homovanillic acid; PB: pyridostigmine bromide; PM: permethrin; LNFPIII: lacto-N-fucopentaose III.
Table 2.
Brain monoamines and their metabolites in male C57BL6/J mice 6 h post chemicals exposure in Model 2 of Gulf War Illness.
| Brain Region | Monoamine or Metabolite | Treatment |
|||||
|---|---|---|---|---|---|---|---|
| Vehicle –Vehicle | Vehicle –LNFPIII | PB/DEET/CORT/DFP –Vehicle | PB/DEET/CORT/DFP-LNFPIII | ||||
| PFC | 5-HT | 1.55 ± 0.38 | 4.91 ± 3.80 | 4.46 ± 3.10 | 1.47 ± 0.33 | ||
| 5-HIAA | 2.64 ± 0.32 | 3.50 ± 0.62 | 2.52 ± 0.45 | 3.87 ± 0.95 | |||
| DA | 0.56 ± 0.22 | 0.45 ± 0.13 | 0.54 ± 0.14 | 0.58 ± 0.19 | |||
| HVA | 0.46 ± 0.12 | 1.35 ± 0.60 | 0.55 ± 0.15 | 1.50 ± 0.60 | |||
| STR | 5-HT | 2.47 ± 0.50 | 2.63 ± 0.48 | 1.64 ± 0.34 | 2.64 ± 0.59 | ||
| 5-HIAA | 2.64 ± 0.35 | 2.00 ± 0.58 | 2.81 ± 0..44 | 2.17 ± 0.75 | |||
| DA | 112.54 ± 8.75 | 105.11 ± 13.92 | 122.32 ± 6.93 | 102.28 ± 6.08 | |||
| HVA | 10.82 ± 1.40 | 9.26 ± 0.89 | 10.16 ± 1.16 | 11.73 ± 0.70 | |||
| AMY | 5-HT | 3.84 ± 0.87 | 4.59 ± 0.71 | 4.72 ± 1.06 | 4.39 ± 1.46 | ||
| 5-HIAA | 2.72 ± 0.64 | 2.86 ± 0.26 | 9.14 ± 3.57 | 3.42 ± 0.46 | |||
| DA | 12.87 ± 1.53 | 13.47 ± 3.54 | 13.63 ± 2.59 | 25.25 ± 6.76 | |||
| HVA | 4.10 ± 1.19 | 4.40 ± 2.06 | 3.27 ± 1.09 | 7.92 ± 2.35 | |||
| dHip | 5-HT | 1.17 ± 0.39 | 0.97 ± 0.19 | 0.74 ± 0.23 | 1.02 ± 0.32 | ||
| 5-HIAA | 3.41 ± 1.04 | 2.73 ± 0.49 | 5.66 ± 1.30 | 3.91 ± 1.04 | |||
| DA | 0.36 ± 0.12 | 0.27 ± 0.06 | 2.36 ± 1.84 a | 0.84 ± 0.27 a | |||
| HVA | 0.11 ± 0.02 | 0.27 ± 0.07 b | 0.78 ± 0.15 a | 1.45 ± 0.53ab | |||
Data are presented as mean ± SEM; unit: ng/mg protein; n=6 per group, except the PB/DEET/CORT/DFP-Vehicle group, which was n=8).
a and b indicate significant main effect for GWI treatment (PB/DEET/CORT/DFP) or LNFPIII treatment, respectively, after a two-way ANOVA (p ≤ 0.05) and are bolded.
Italicized values indicate trend (p < 0.10) for main effect. Abbreviations: PFC: prefrontal cortex; NAc: nucleus accumbens; STR: striatum; AMY: amygdala; dHip: dorsal hippocampus; vHip: ventral hippocampus; 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; DA: dopamine; HVA: homovanillic acid; PB: pyridostigmine bromide; DEET: N,N-Diethyl-methylbenzamide; DFP: diisopropylfluorophosphate; CORT: corticosterone; LNFPIII: lacto-N-fucopentaose III.
3.1.2. Dopamine (DA)
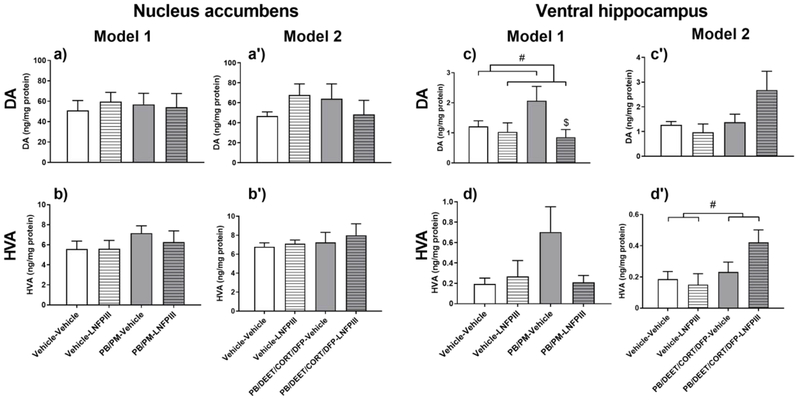
In the vHip, numerical and trending increases in DA by GWI chemicals were present in Model 1 (Fig. 3c) and Model 2 (p = 0.08, Fig. 3c’), respectively. These effects were accompanied by increases in the DA metabolite HVA in Model 1 (Fig. 3d) and Model 2 (p ≤ 0.05, Fig. 3d’). In Model 1, LNFPIII prevented the increase in vHip DA (p ≤ 0.05, Fig. 3c) and the numerical increase in HVA. Interestingly, LNFPIII further increased levels of both DA (Fig. 3c’) and HVA (p ≤ 0.05, Fig. 3d’) in Model 2, suggesting that the drive for GWI chemicals induced DA dyshomeostasis in the vHip and the modulatory effect of LNFPIII is model-specific.
Figure 3. Dopaminergic alterations in the Nucleus Accumbens and Ventral Hippocampus in two models of Gulf War Illness.
Data are presented as mean ± SEM; unit: ng/mg protein; n=6 per group/treatment except PB/DEET/CORT/DFP-Vehicle group, which was n=8. # (p ≤ 0.05) indicate a significant main effect of GWI treatment (PB/PM (Model 1) or PB/DEET/CORT/DFP (Model 2)) or LNFPIII treatment after a two-way ANOVA. $ indicates significant difference compared to GWI-vehicle group (p ≤ 0.05). Abbreviations: DA: dopamine; HVA: homovanillic acid; PB: pyridostigmine bromide; PM: permethrin; DEET: N,N-Diethyl-methylbenzamide; CORT: corticosterone; DFP: diisopropylfluorophosphate; LNFPIII: lacto-N-fucopentaose III.
In the dHip, trending decreases and significant increases of DA by GWI chemicals were apparent in Model 1 (p = 0.10, Table 1) and Model 2 (p ≤ 0.05, Table 2), respectively. This was accompanied by increases of the DA metabolite HVA in both models, with Model 2’s effects of HVA being significant (p ≤ 0.001, Table 2). LNFPIII did not prevent the effects observed in Model 1. However, similar to the vHip, LNFPIII further increased HVA in Model 2 (p ≤ 0.05, Table 2). As a result, trends (p < 0.10) for increased turnover (HVA/DA ratio) were apparent in both models (data not shown).
DA alterations by GWI chemicals were also observed in the PFC and STR in Model 1, NAc in Model 2, and AMY in both models. Increases in HVA by GWI chemicals were observed in the STR (p ≤ 0.05, Table 1) and PFC (Table 1) in Model 1. This was accompanied by a numerical increase in DA in the PFC (Table 1) in Model 1. As a result of the increased HVA by GWI chemicals in the STR of Model 1, there was an increase in turnover (p ≤ 0.001, data not shown). Similar trending increases in turnover were apparent in the AMY (p = 0.07, data not shown) in Model 1 and NAc (p = 0.09, data not shown) in Model 2. LNFPIII did not modulate the increases in DA, HVA, or turnover in these regions, except for the PFC, in which LNFPIII prevented the trending increases of DA and HVA in Model 1 (Table 1).
3.2. Spleen Neurotransmitters
In Model 1, a significant treatment interaction (p ≤ 0.05, Table 3) was seen on splenic 5-HT; LNFPIII prevented the 5-HT increase by GWI chemicals, but also increased 5-HT on its own (Table 3). No significant differences in splenic 5-HIAA were present in this model. In Model 2, GWI chemicals significantly increased levels of 5-HT (p ≤ 0.01, Table 3) and 5-HIAA (p ≤ 0.05, Table 3). LNFPIII did not prevent the 5-HT or 5-HIAA effects in Model 2. Splenic 5-HT turnover remained unaffected by treatments in both models.
Table 3.
Splenic monoamines and their metabolites in male C57BL6/J mice 6 h post chemicals exposure in two models of Gulf War Illness.
| Monoamine or metabolite | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle – Vehicle | Vehicle – LNFPIII | PB/PM – Vehicle | PB/PM – LNFPIII | Vehicle – Vehicle | Vehicle – LNFPIII | PB/DEET/CORT/DFP – Vehicle | PB/DEET/CORT/DFP-LNFPIII | |
| 5-HT | 80.29 ± 7.02 | 94.69 ± 4.46 b | 96.99 ± 6.78 b | 81.30 ± 6.60 | 80.29 ± 9.30 | 69.29 ± 5.78 | 107.44 ± 9.30 a | 100.87 ± 14.27 a |
| 5-HIAA | 0.66 ± 0.04 | 0.71 ± 0.13 | 0.74 ± 0.09 | 0.66 ± 0.06 | 0.66 ± 0.12 | 0.71 ± 0.09 | 1.02 ± 0.22 a | 1.42 ± 0.25 a |
| NE | 66.83 ± 8.12 | 70.10 ± 12.17 | 59.16 ± 5.43 | 62.21 ± 8.90 | 95.83 ± 6.65 | 93.08 ± 9.90 | 90.84 ± 3.67 | 110.32 ± 11.65 |
| MHPG | 43.21 ± 1.98 | 48.77 ± 3.10 | 41.90 ± 1.80 | 47.55 ± 5.96 | 34.96 ± 2.58 | 39.45 ± 4.81 | 43.15 ± 3.79 a | 51.82 ± 3.24a |
Data are presented as mean ± SEM; unit: ng/mg protein; n=6 per group, except the PB/DEET/CORT/DFP-Vehicle group, which was n=8).
a and b indicate significant main effect for PB/DEET/CORT/DFP or Treatment (PB/PM x LNFPIII) interaction, respectively, after a two-way ANOVA (p ≤ 0.05) and are bolded.
Italicized values indicate trend (p < 0.10) for main effect of LNFPIII. Abbreviations: 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; MHPG: 3-methoxy-4-hydroxyphenylglycol; NE: norepinephrine; PB: pyridostigmine bromide; PM: Permethrin; DEET: N,N-Diethyl-methylbenzamide; DFP: diisopropylfluorophosphate; CORT: corticosterone; LNFPIII: lacto-N-fucopentaose III.
In Model 1, no significant alterations in splenic NE, its metabolite MHPG or NE turnover (MHPG/NE ratio) were present (Table 3). However, in Model 2, LNFPIII (trending, p = 0.10) increased NE in the presence of GWI chemicals (Table 3). GWI chemicals significantly increased MHPG (p ≤ 0.05; Table 3) and LNFPIII also increased MHPG (trending, p = 0.10; Table 3). This increase in MHPG by GWI chemicals led to a trending increase in NE turnover (p = 0.06, data not shown) in Model 2.
3.3. Real-time qPCR for inflammatory markers in the vHip
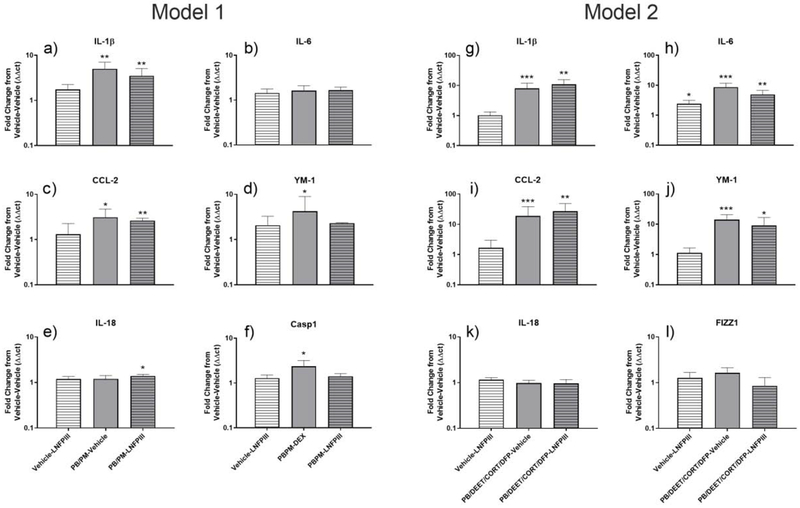
In Model 1, PB/PM caused significant upregulation of IL-1β (Fig. 5a), CCL-2 (Fig. 4c), YM-1 (Fig. 4f) and F4/80 (Table 4; p’s ≤ 0.05) in the vHip. Casp1 was also significantly increased (p ≤ 0.01, Fig. 4d), and there was a numerical increase of TNFα (p = 0.07, Table 4). In mice treated with LNFPIII, increases in Fizz1 (Table 4), Casp1 (Fig. 4d), and YM-1 (Fig. 4f) were prevented and there were similar trends for preventing the increases of IL-1β and CCL-2 (Fig. 4a and c). IL-18 was increased specifically by LNFPIII in the PB/PM + LNFPIII group (p ≤ 0.01, Fig. 4e).
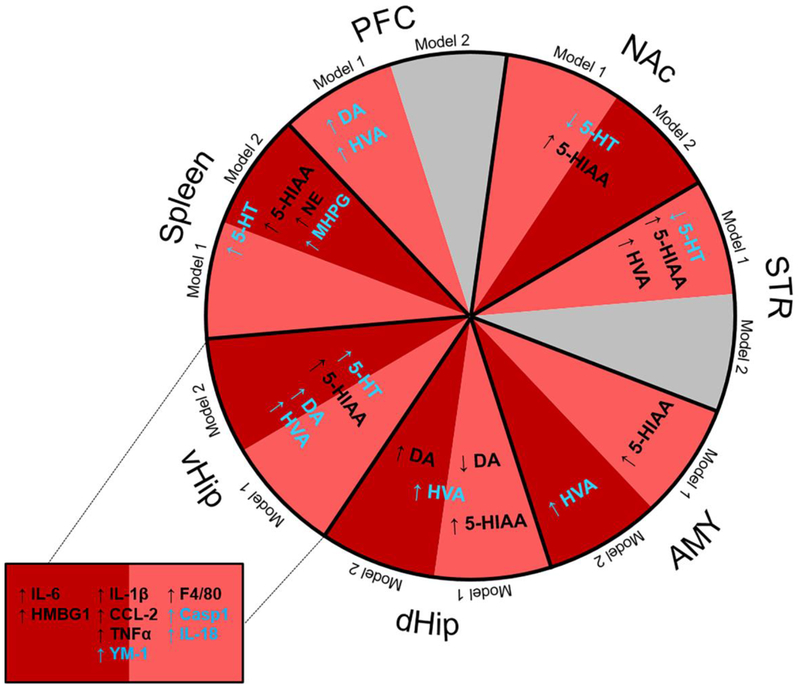
Figure 5. Summary of the major neurochemical (monoamines) and neuroinflammatory (vHip) effects in the two GWI models and their modulation by LNFPIII.
Effects of Model 1 (PB/PM; light red) and Model 2 (PB/DEET/CORT/DFP; dark red) on 5-HT, DA, NE and their metabolites in the spleen and brain regions (PFC, NAc, STR, AMY, dHip, vHip), as well as, inflammatory gene expression in the vHip are indicated. Modulation of these effects by LNFPIII is represented in blue color. Abbreviations: PFC: prefrontal cortex; NAc: nucleus accumbens; STR: striatum; AMY: amygdala; dHip: dorsal hippocampus; vHip: ventral hippocampus; 5-HT: serotonin; 5-HIAA: 5-hydroxyindoleacetic acid; DA: dopamine; HVA: homovanillic acid; MHPG: 3-methoxy-4-hydroxyphenylglycol; NE: norepinephrine; PB: pyridostigmine bromide; PM: permethrin; DEET: N,N-Diethyl-methylbenzamide; DFP: diisopropylfluorophosphate; CORT: corticosterone LNFPIII: lacto-N-fucopentaose III.
Figure 4. Inflammatory markers in the ventral hippocampus in two models of Gulf War Illness.
Data were analyzed by the ΔΔCt method with 18S as the HKG and are presented as fold change from vehicle-vehicle control. *, **, *** indicate significant difference from control (p ≤ 0.05, ≤ 0.01, and ≤ 0.001, respectively). n=6/group for Model 1 and n=5–7/group for Model 2. Abbreviations: PB: pyridostigmine bromide; PM: permethrin; DEET: N,N-Diethyl-methylbenzamide; DFP: diisopropylfluorophosphate; CORT: corticosterone; LNFPIII: lacto-N-fucopentaose III.
Table 4.
Gene expression in the ventral hippocampus of male C57BL6/J mice 6 h post exposure termination in two models of Gulf War Illness.
| Gene | Model 1 Treatment | Model 2 Treatment | ||||
|---|---|---|---|---|---|---|
| Vehicle – LNFPIII | PB/PM – Vehicle | PB/PM – LNFPIII | Vehicle – LNFPIII | PB/DEET/CORT/DFP – Vehicle | PB/DEET/CORT/DFP – LNFPIII | |
| Fold ± SEM | Fold ± SEM | Fold ± SEM | Fold ± SEM | Fold ± SEM | Fold ± SEM | |
| CD206 | 0.70 ± 0.39 | 0.91 ± 1.24 | 1.32 ± 0.56 | 1.28 ± 0.61 | 0.97 ± 0.37 | 0.75 ± 0.15 |
| COX2 | 1.94 ± 0.58 | 1.60 ± 0.72 | 1.38 ± 0.40 | 1.44 ± 0.37 | 1.18 ± 0.49 | 2.13 ± 0.42 |
| F4/80 | 1.20 ± 0.16 | 1.42 ± 0.16* | 1.70 ± 0.35* | 1.00 ± 0.19 | 1.47 ± 0.22 | 0.98 ± 0.16 |
| FIZZ1 | 1.44 ± 1.05 | 2.10 ± 0.53 | 1.01 ± 0.14 | 1.27 ± 0.40 | 1.64 ± 0.49 | 0.85 ± 0.44 |
| GFAP | 0.60 ± 0.23 | 1.53 ± 0.85 | 0.79 ± 0.18 | 0.91 ± 0.28 | 1.01 ± 0.37 | 1.24 ± 0.40 |
| HMGB1 | 1.15 ± 0.19 | 1.26 ± 0.15 | 0.99 ± 0.10 | 1.53 ± 0.25 | 1.44 ± 0.25 | 1.16 ± 0.18 |
| LIF | 1.11 ± 0.37 | 1.14 ± 0.37 | 0.98 ± 0.25 | 1.36 ± 0.12 | 0.81 ± 0.20 | 1.66 ± 1.56 |
| NLRP3 | 1.04 ± 0.21 | 1.13 ± 0.23 | 0.90 ± 0.96 | 1.43 ± 0.31 | 1.37 ± 0.49 | 1.23 ± 0.31 |
| TNF-α | 1.25 ± 0.47 | 1.87 ± 0.38 | 1.78 ± 0.40 | 1.56 ± 0.43 | 2.30 ± 0.67 | 1.72 ± 0.54 |
| Arg1 | 0.70 ± 0.14 | 1.96 ± 2.32 | 2.36 ± 2.42 | 1.77 ± 0.63 | 1.34 ± 2.16 | 1.28 ± .0.80 |
| NOS2 | 1.02 ± 0.33 | 1.07 ± 0.20 | 1.20 ± 0.12 | 1.46 ± 0.23 | 1.04 ± 0.28 | 0.81 ± 0.20 |
| Arg1:NOS2 | 1.05 ± 0.02 | 0.98 ± 0.03 | 0.98 ± 0.03 | 1.00 ± 0.02 | 1.00 ± 0.03 | 0.99 ± 0.03 |
| BDNF | 1.07 ± 0.31 | 0.94 ± 0.28 | 0.84 ± 0.32 | 1.31 ± 0.36 | 0.87 ± 0.59 | 1.14 ± 0.08 |
| NGF | 0.87 ± 0.11 | 0.81 ± 0.30 | 0.76 ± 0.14 | 1.42 ± 0.22 | 1.20 ± 0.28 | 1.44 ± 0.23 |
Data were analyzed by the ΔΔCt method with 18S as the HKG and are presented as fold change from vehicle-vehicle control. The Arg1:NOS2 ratio was calculated based on the ΔCt values. n = 6/group for Model 1 and n = 5-7/group for Model 2.
indicates significant difference from control and are bolded (p ≤ 0.05).
Italicized values indicate trend (p ≤ 0.10) for treatment compared to control within both models. 18S was used as the housekeeping gene. Abbreviations: PB: pyridostigmine bromide; PM: Permethrin; DEET: N,N-Diethyl-methylbenzamide; DFP: diisopropylfluorophosphate; CORT: corticosterone; LNFPIII: lacto-N-fucopentaose III.
In Model 2, PB/DEET/CORT/DFP caused significant and robust upregulation of IL-1β (Fig. 4g), CCL-2 (Fig. 4i), YM-1 (Fig. 4l), and IL-6 (Fig. 4h) (p’s ≤ 0.001). Trending increases by PB/DEET/CORT/DFP were apparent for TNFα (p = 0.07, Table 4) and HMGB1 (p = 0.09, Table 4). LNFPIII did not prevent these effects. However, in the presence of LNFPIII, the increases of F4/80 (p= 0.09, Table 4) and YM-1 (p ≤ 0.05, Fig. 4l) by PB/DEET/CORT/DFP were lessened.
Here, we also examined the ratio between anti-inflammatory Arg1 and inflammatory NOS2 to determine the nitric oxide status (Rath et al., 2014). While there were no significant changes in either model for Argl or NOS2 hippocampal mRNAs (Table 4), PB/PM treatment caused a trending reduction in the Argl/NOS2 ratio (p = 0.06, Table 4) that was unaffected by LNFPIII.
Finally, to determine if neuroinflammation and neurochemical disbalance is associated with effects on key neurotrophic factors, BDNF and NGF mRNA in the vHIP was measured; at this time point, neither BDNF, nor NGF were affected by GWI chemicals or LNFPIII in either model (Table 4).
4. Discussion
It has been well documented that veterans with GWI experienced a myriad of chemical exposures during the 1990-1991 Gulf War. Thus, it is imperative to take advantage of models with similar chemical conditions to understand the pathogenesis of GWI and identify possible therapeutic targets (White et al., 2016). Here, we utilized two chemically different GWI models (O’Callaghan et al., 2015; Zakirova et al., 2015) to further characterize the models’ immediate monoamine profiles after GWI chemicals exposure, discern the neuroinflammatory environments, and examine how a novel immunomodulatory treatment, LNFPIII, can modulate the aforementioned parameters. To characterize the neurochemical profiles of the two models utilized in this study, monoamines were examined from various brain regions and the spleen with the use of HPLC-ECD. Neuroinflammation was also evaluated in the ventral hippocampus (vHip) with qPCR. Perturbations were evident in both models for monoamines, both centrally and peripherally, and for brain inflammatory markers. LNFPIII treatment modulated many of these effects.
A number of behavioral symptoms experienced by GWI veterans could be explained, at least in part, by monoamine dyshomeostasis. Several reports have implicated alterations in cognition and memory, motor function, and mood in the pathology of GWI (Carreras et al., 2018; Parihar et al., 2013). Here, evidence for alterations in central levels of 5-HT, DA, as well as changes in inflammatory markers, could explain the some of the symptoms experienced by GWI veterans, especially if these perturbations are lasting and/or have lasting consequences.
Increased serotonin utilization was evident in multiple structures including the NAc, STR, AMY, dHip, and vHip. In both models, GWI chemicals led to increased metabolism of 5-HT in the NAc and vHip; these areas are highly innervated by serotoninergic projections that regulate affect, as well as memory (Hensler, 2006). LNFPIII modulated these serotoninergic effects in both models. For example, while in the NAc of both models increases in 5-HIAA were not modulated by LNFPIII, it prevented increases in 5-HT turnover by causing a compensatory increase in 5-HT, albeit to a lesser extent in Model 2. Similar effects on 5-HT were observed in the vHip. The less pronounced and subtle effects of LNFPIII in Model 2 could be attributed to the more impactful nature of this model. Thus, a larger dose of the LNFPIII, as in previous studies using LNFPIII treatment (50 μg), could be entertained in future studies using Model 2 (Tundup et al., 2015; Zhu et al., 2012). Persistence of these acute effects by GWI chemicals exposure may lead to mood disturbances such as anxiety and depression, as well as influence fatigue, which all play part in GWI symptomology (DDGWIRP, 2018; Korte-Bouws et al., 2018; Pavese et al., 2010). Further, it has been shown that persistent alterations in 5-HT affect hippocampal neurogenesis; thus, the increases in hippocampal metabolism of 5-HT in both models observed here may impact learning, memory, and mood regulation in the long term (Alenina and Klempin, 2015; Sahay et al., 2011). By compensating for GWI chemicals effects on 5-HT homeostasis, LNFPIII might aid in mitigating some of these neurological deficits.
Dopamine dyshomeostasis was evident in both models within multiple brains regions (STR, AMY, vHip, and dHip), albeit to a different extent. These areas, all innervated by DA projections, regulate cognition, learning and memory, emotional processing, and motor function (Belujon and Grace, 2017; Kempadoo et al., 2016; Willard et al., 2015). Here, GWI chemicals caused similar increases in DA utilization (turnover) in the dHip in both models. Model specific alterations of DA were also apparent in the STR, AMY, and vHip in Model 1 and NAc and vHip in Model 2. LNFPIII modulations of DA were apparent, but distinct, among the models. For example, in Model 1, LNFPIII prevented elevations in DA in the vHip. However, in Model 2, LNFPIII further increased DA. This suggests that the overlap of DA effects between the two models is brain region specific and that LNFPIII effects are both model- and brain region-specific. Persistent utilization of DA, especially in regions such as the hippocampus or striatum, may lead to behavioral dysfunction. GWI veterans have shown deficits in tasks of memory and cognitive function compared to healthy controls, and many of these results have been recapitulated in animal studies (Abdullah et al., 2011; Anger et al., 1999; Hattiangady et al., 2014; Hubbard et al., 2014; Macht et al., 2019; Parihar et al., 2013; White et al., 2016; Zakirova et al., 2015). Dopamine release promotes learning and memory in the dorsal hippocampus; increased acute utilization of DA in the present study suggests decreased tissue reserve that could impact learning and memory where DA would be needed in the process (Kempadoo et al., 2016). Further, GWI veterans show deficits in psychomotor tasks (Anger et al., 1999; Proctor et al., 2006) that could be linked to increased utilization of DA by GWI chemicals in areas, such as the PFC and STR, in both models. The STR is a crucial area for regulation of movement and interestingly, stress (a major component of Model 2) has been implicated in disrupting striatal DA metabolism, resulting in motor deficits (Haber, 2016; Sudha and Pradhan, 1995).
In the periphery, increases in splenic 5-HT by GWI chemicals in both models were apparent with the effects in Model 1 prevented by LNFPIII. These serotonergic effects suggest GWI chemicals caused acute peripheral serotonin imbalance. Increased utilization of 5-HT plays a role in activating T-cells and modulates immune responses, i.e. cytokine release (Wu et al. 2018). To this end, peripheral increases in IL-6 and TNFα can mediate disturbances in mood, i.e., anxiety and depression (Wohleb et al., 2014; You et al., 2011). In addition, 5-HT alterations in the periphery might be reflective of the homeostasis of this neurotransmitter in the CNS (Herr et al., 2017). Perturbations in splenic norepinephrine were not apparent in Model 1. However, GWI chemicals in Model 2 elevated the NE metabolite MHPG and this effect was not prevented by LNFPIII resulting in an elevation in NE turnover. This is most likely explained by this model’s exposure to corticosterone, as stress has been shown to deplete NE in the spleen, in part by increasing its metabolism (Blandino et al., 2006; Kennedy et al., 2005). Further, this increased metabolism of NE in the spleen could lead to elevations in inflammatory mediators peripherally (Blandino et al., 2006) and could explain some of the central inflammation in this model.
Multiple GWI studies, including with one of the models utilized in the present study, have determined that systemic inflammation and wide spread neuroinflammation, particularly within the hippocampus, is apparent post exposure to GWI chemicals (Broderick et al., 2013; Broderick et al., 2018; O’Callaghan et al., 2015; Parkitny et al., 2015). Here, we evaluated expression of various inflammatory genes in the vHip to compare to the previous research conducted (i.e. O’Callaghan et al. 2015), characterize neuroinflammation in Model 1 de novo, and determine if this coincides with the altered CNS monoamine neurochemistry.
In both models, increased levels of CCL-2, IL-1β, TNFα and YM-1 expression was apparent with the magnitude of the increase being greater in Model 2. Of note, IL-6 was not affected in Model 1, but was significantly upregulated in Model 2. The early effects on CCL-2, IL-1β and IL-6 seen in Model 2 mirror the inflammatory effects observed in a previous study with this model (O’Callaghan et al., 2015). Concomitant with the less robust acute neuroinflammation in Model 1, LNFPIII was an effective anti-inflammatory treatment in this model, but less so in Model 2. Of note, Casp1 was upregulated by GWI chemicals in Model 1, but not when LNFPIII was also present. The upregulation of Caspl, at least in Model 1, is suggestive of inflammasome activation, which could lead to increases in active IL-1β, as the mature form of IL-1β is dependent on Caspl cleavage (Deng et al., 2019; Dinarello et al., 2013). The fact that LNFPIII prevented this effect on Caspl indicates that part of its protective effects is by preventing inflammasome activation. Interestingly, expression of IL-18 was increased by LNFPIII in the presence of GWI chemicals in Model 1. A recent study highlighted the synergistic role that IL-18 and IL-10 play in M2 macrophage polarization (Kobori et al., 2018), suggesting that the increases in brain IL-18 by LNFPIII might work in concert with Casp-1 inhibition to curb inflammation. F4/80, a marker of both M1 and M2 microglia (Greter et al., 2015), was upregulated by GWI chemicals in both models, but LNFPIII only dampened this increase in Model 2. Further, YM-1, a microglia M2 marker, was increased in both models. These increases in M2 microglia markers suggest an immune system attempt to rebalance the responses to GWI chemicals at this early time point (Ansari, 2015). Of note, the Arg1:NOS2 ratio was only marginally affected (decreased) by GWI treatment (Model 1) and LNFPIII did not modulate this ratio. This indicates that nitric oxide likely plays a secondary or minor role in the GWI chemicals induced neuroinflammation and its consequences. Overall, brain qPCR data suggest that (1) both models skew the innate immune system towards inflammation by targeting mostly inflammatory cytokines and less so nitric oxide balancing molecules and (2) the anti-inflammatory effects of LNFPIII in this context are cytokine-centered.
LNFPIII produces its anti-inflammatory effects by targeting the CD14/TLR-4 signaling to activate ERK dependent production of several anti-inflammatory mediators (Atochina et al., 2008; Srivastava et al., 2014; Tundup et al., 2015). The fact that LNFPIII modulated inflammatory effects primarily in Model 1, but not as well in the Model 2 could be attributed to the potent nature of this model (Locker et al., 2017; O’Callaghan et al., 2015). The acute increases in inflammatory cytokines might be too strong to allow for LNFPIII to be potent anti-inflammatory in the short-term in Model 2 and a higher LNFPIII dose might be needed to combat the effects of DFP treatment (O’Callaghan et al., 2015). However, the level of inflammation/neuroinflammation by GWI is more subtle in Model 1 and data from this model suggests that LNFPIII, at the treatment level used here, might be effective if given in an acute GWI-exposure related scenario.
5. Summary/Conclusion
Overall, centrally, in both models, serotonergic and dopaminergic dyshomeostasis was present in multiple brain regions. However, the effects observed varied between models and were more pronounced in Model 1. Peripherally, splenic 5-HT and NE were altered by GWI chemicals, primarily in Model 2. Further, GWI chemicals in both models produced some shared (IL-1β, CCL-2, TNFα and YM-1) and model specific elevations in hippocampal inflammatory markers. LNFPIII treatment modulated many of these acute effects, especially on brain neurochemistry and neuroinflammation (Summary Fig. 5). Many of these acute effects post GWI chemicals treatment, if persistent in the long term, could mediate the symptoms GWI veterans currently experience. LNFPIII shows protective effects at this early time point in restoring neurochemical disbalance and neuroinflammation, highlighting its value as a promising therapeutic that should be further investigated in long-term GWI studies after initial GWI chemicals exposure.
Supplementary Material
Highlights:
Gulf War Illness (GWI) chemicals effects in two different rodent models were tested
GWI chemicals caused broad and model/brain region-specific monoamine disruption
Neuroinflammation was present in the ventral hippocampus in both GWI models
Splenic serotonin was disrupted in both GWI models, but norepinephrine only in one
LNFPIII, a novel immunotherapeutic, reduced or prevented effects of GWI treatments
6. Acknowledgements/Conflicts of interest
The authors declare no conflicts of interest. This research was supported by Department of Defense grant number W81XWH-16-1-0586 to NMF. HEG’s participation in this research was made possible by NIH grant T35OD010433.
Abbreviations
- AMY
amygdala
- CORT
corticosterone
- DEET
N,N-Diethyl-methylbenzamide
- DFP
diisopropylfluorophosphate
- dHip
dorsal hippocampus
- GW
Gulf War
- GWI
Gulf War Illness
- HPLC-ECD
high performance liquid chromatography with electrochemical detection
- IL
interleukin
- LNFPIII
Lacto-N-fucopentaose III
- NAc
nucleus accumbens
- PB
pyridostigmine bromide
- PFC
prefrontal cortex
- PM
permethrin
- qPCR
quantitative polymerase chain reaction
- STR
striatum
- vHip
ventral hippocampus
- DA
dopamine
- HVA
homovanillic acid
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindoleacetic acid
- MHPG
3-methoxy-4-hydroxyphenylglycol
- NE
norepinephrine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, Mouzon B, Mullan M, Mathura V, Mullan M, Ait-Ghezala G, Crawford F, 2011. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Med 13(4), 275–288. [DOI] [PubMed] [Google Scholar]
- Alenina N, Klempin F, 2015. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 277, 49–57. [DOI] [PubMed] [Google Scholar]
- Anger W, Storzbach D, Binder L, Campbell K, Rohlman D, McCauley L, 1999. Evidence of cognitive deficits in Persian Gulf War veterans: interim report from a population-based study. J Int Neuropsychol Soc 5, 203–212. [DOI] [PubMed] [Google Scholar]
- Ansari MA, 2015. Temporal profile of M1 and M2 responses in the hippocampus following early 24h of neurotrauma. J Neurol Sci 357(1–2), 41–49. [DOI] [PubMed] [Google Scholar]
- Atochina O, Da’dara AA, Walker M, Ham DA, 2008. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology 125(1), 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk JN, El-Amin S, Corey R, Rayhan R, Timbol C, 2013. Carnosine treatment for gulf war illness: a randomized controlled trial. Glob J Health Sci 5(3), 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA, 2017. Dopamine System Dysregulation in Major Depressive Disorders. Int J Neuropsychopharmacol 20(12), 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P, Li C, Stanya KJ, Jacobi D, Dai L, Liu S, Gangl MR, Harn DA, Lee CH, 2012. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat Med 18(11), 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P Jr., Barnum CJ, Deak T, 2006. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173(1–2), 87–95. [DOI] [PubMed] [Google Scholar]
- Boyd KC, Hallman WK, Wartenberg D, Fiedler N, Brewer NT, Kipen HM, 2003. Reported Exposures, Stressors, and Life Events Among Gulf War Registry Veterans. Journal of Occupational and Environmental Medicine 45(12), 1247–1256. [DOI] [PubMed] [Google Scholar]
- Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, Fletcher MA, Klimas N, 2013. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain Behav Immun 28, 159–169. [DOI] [PubMed] [Google Scholar]
- Broderick G, Fletcher MA, Gallagher M, Barnes Z, Vernon SD, Klimas NG, 2018. Exploring the Diagnostic Potential of Immune Biomarker Co-expression in Gulf War Illness. Methods Mol Biol 1781, 101–120. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2011. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130(2), 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras I, Aytan N, Mellott T, Choi JK, Lehar M, Crabtree L, Leite-Morris K, Jenkins BG, Blusztajn JK, Dedeoglu A, 2018. Anxiety, neuroinflammation, cholinergic and GABAergic abnormalities are early markers of Gulf War illness in a mouse model of the disease. Brain Res 1681, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW, 2010. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology 31(5), 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry N, Creed F, Silman A, Dunn G, Baxter D, Smedley J, Taylor S, Macfarlane GJ, 2001. Health and exposures of United Kingdom Gulf war veterans. Part II: The relation of health to exposure. Occup Environ Med 58(5), 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A, Filipov NM, 2007. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem 100(5), 1177–1187. [DOI] [PubMed] [Google Scholar]
- Czirr E, Wyss-Coray T, 2012. The immunology of neurodegeneration. J Clin Invest 122(4), 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DDGWIRP, 2018. The Gulf War Illness Landscape in: Programs, D.o.D.C.D.M.R. (Ed.). [Google Scholar]
- Deng J, Yu XQ, Wang PH, 2019. Inflammasome activation and Th17 responses. Mol Immunol 107, 142–164. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G, 2013. Interleukin-18 and IL-18 binding protein. Front Immunol 4, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta ST, Engel CC Jr., Collins JF, Baseman JB, Dever LL, Taylor T, Boardman KD, Kazis LE, Martin SE, Horney RA, Wiseman AL, Kernodle DS, Smith RP, Baltch AL, Handanos C, Catto B, Montalvo L, Everson M, Blackburn W, Thakore M, Brown ST, Lutwick L, Norwood D, Bernstein J, Bacheller C, Ribner B, Church LW, Wilson KH, Guduru P, Cooper R, Lentino J, Hamill RJ, Gorin AB, Gordan V, Wagner D, Robinson C, DeJace P, Greenfield R, Beck L, Bittner M, Schumacher HR, Silverblatt F, Schmitt J, Wong E, Ryan MA, Figueroa J, Nice C, Feussner JR, Group VAC, 2004. Benefits and harms of doxycycline treatment for Gulf War veterans’ illnesses: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 141(2), 85–94. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, 2006. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res 6(1-2), 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH, 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 33(3), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CC, Tarelli R, 2011. Parkinson’s disease and systemic inflammation. Parkinsons Dis 2011, 436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA, Allison M, Koperski S, Koslik HJ, Devaraj S, Ritchie JB, 2014. Coenzyme Q10 benefits symptoms in Gulf War veterans: results of a randomized double-blind study. Neural Comput 26(11), 2594–2651. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Croxford AL, 2015. Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation. Front Immunol 6, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, 2016. Corticostriatal circuitry. Dialogues Clin Neurosci 18(1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK, 2014. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, 2006. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 30(2), 203–214. [DOI] [PubMed] [Google Scholar]
- Herr N, Bode C, Duerschmied D, 2017. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med 4, 48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, Haley RW, Rypma B, 2014. Central Executive Dysfunction and Deferred Prefrontal Processing in Veterans with Gulf War Illness. Clin Psychol Sci 2(3), 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V, 2006. Innate Immunity Gone Awry: Linking Microbial Infections to Chronic Inflammation and Cancer. Cell 124(4), 823–835. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER, 2016. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113(51), 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SL, Nickerson M, Campisi J, Johnson JD, Smith TP, Sharkey C, Fleshner M, 2005. Splenic norepinephrine depletion following acute stress suppresses in vivo antibody response. J Neuroimmunol 165(1–2), 150–160. [DOI] [PubMed] [Google Scholar]
- Kobori T, Hamasaki S, Kitaura A, Yamazaki Y, Nishinaka T, Niwa A, Nakao S, Wake EL, Mori S, Yoshino T, Nishibori M, Takahashi EL, 2018. Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis. Front Immunol 9(334), 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte-Bouws GAH, van Heesch F, Westphal KGC, Ankersmit LMJ, van Oosten EM, Gunturkun O, Korte SM, 2018. Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats. Pharmaceuticals (Basel) 11(3), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Lin Z, de La Serre CB, Wagner JJ, Ham DH, Pepples LM, Djani DM, Weber MT, Srivastava L, Filipov NM, 2016. Time-dependent behavioral, neurochemical, and metabolic dysregulation in female C57BL/6 mice caused by chronic high-fat diet intake. Physiol Behav 157, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Dodd CA, Filipov NM, 2013. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol Teratol 39, 26–35. [DOI] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP, 2017. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. Journal of neurochemistry 142(3), 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht VA, Woodruff JL, Maissy ES, Grillo CA, Wilson MA, Fadel JR, Reagan LP, 2019. Pyridostigmine bromide and stress interact to impact immune function, cholinergic neurochemistry and behavior in a rat model of Gulf War Illness. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed T, Hattiangady B, Shuai B, Shetty AK, 2014. Parvalbumin and neuropeptide Y expressing hippocampal GABA-ergic inhibitory interneuron numbers decline in a model of Gulf War illness. Front Cell Neurosci 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JV, LeBouf RF, Kelly KA, Michalovicz LT, Ranpara A, Locker AR, Miller DB, O’Callaghan JP, 2018. The Neuroinflammatory Phenotype in a Mouse Model of Gulf War Illness is Unrelated to Brain Regional Levels of Acetylcholine as Measured by Quantitative HLLIC-UPLC-MS/MS. Toxicological Sciences 165(2), 302–313. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM, 2015. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J Neurochem 133(5), 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Abdullah L, Evans J, Reed JM, Montague H, Mullan MJ, Crawford FC, 2014. Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34(2), 109–127. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 38(12), 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L, Middleton S, Baker K, Younger J, 2015. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunol 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ, 2010. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133(11), 3434–3443. [DOI] [PubMed] [Google Scholar]
- Pott Godoy MC, Ferrari CC, Pitossi FJ, 2010. Nigral neurodegeneration triggered by striatal AdIL-1 administration can be exacerbated by systemic IL-1 expression. J Neuroimmunol 222(1–2), 29–39. [DOI] [PubMed] [Google Scholar]
- Proctor SP, Heaton KJ, Heeren T, White RF, 2006. Effects of sarin and cyclosarin exposure during the 1991 Gulf War on neurobehavioral functioning in US army veterans. Neurotoxicology 27(6), 931–939. [DOI] [PubMed] [Google Scholar]
- Rath M, Muller I, Kropf P, Closs EI, Munder M, 2014. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol 5, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Timbol CR, Adewuyi O, Walitt B, VanMeter JW, Baraniuk JN, 2013. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War illness. PLoS One 8(3), e58493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R, 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472(7344), 466–U539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava L, Tundup S, Choi BS, Norberg T, Ham D, 2014. Immunomodulatory glycan lacto-N-fucopentaose III requires clathrin-mediated endocytosis to induce alternative activation of antigen-presenting cells. Infect Immun 82(5), 1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR, 2012. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environ Health Perspect 120(1), 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha S, Pradhan N, 1995. Stress-induced changes in regional monoamine metabolism and behavior in rats. Physiol Behav 57(6), 1061–1066. [DOI] [PubMed] [Google Scholar]
- Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, Henderson WG, Kang HK, Eisen SA, Murphy FM, 2009. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc 15(5), 717–729. [DOI] [PubMed] [Google Scholar]
- Tundup S, Srivastava L, Norberg T, Watford W, Harn D, 2015. A Neoglycoconjugate Containing the Human Milk Sugar LNFPIII Drives Anti-Inflammatory Activation of Antigen Presenting Cells in a CD14 Dependent Pathway. PLoS One 10(9), e0137495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard AM, Bouchard RS, Gittis AH, 2015. Differential degradation of motor deficits during gradual dopamine depletion with 6-hydroxydopamine in mice. Neuroscience 301, 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goni F, 2015. Immunotherapeutic approaches for Alzheimer’s disease. Neuron 85(6), 1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP, 2014. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry 75(12), 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Denna TH, Storkersen JN, Gerriets VA, 2019. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res 140, 100–114. [DOI] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y, 2011. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res 225(1), 135–141. [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, Ait-Ghezala G, 2015. Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness. PLoS One 10(3), e0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zella SMA, Metzdorf J, Ciftci E, Ostendorf F, Muhlack S, Gold R, Tonges L, 2019. Emerging Immunotherapies for Parkinson Disease. Neurol Ther 8(1), 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Trikudanathan S, Zozulya AL, Sandoval-Garcia C, Kennedy JK, Atochina O, Norberg T, Castagner B, Seeberger P, Fabry Z, Harn D, Khoury SJ, Guleria I, 2012. Immune modulation by Lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin Immunol 142(3), 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T, Derfuss T, de Stefano N, Giovannoni G, Palavra F, Tomic D, Vollmer T, Schippling S, 2016. Optimizing treatment success in multiple sclerosis. J Neurol 263(6), 1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.