Abstract
Background
Despite the recent large number of studies comparing endoscopic and laparoscopic resection for small gastrointestinal stromal tumors (GISTs) (diameter ≤ 5 cm), the results remain conflicting. The objective of this work was to perform a cumulative meta-analysis to assess the advantages and disadvantages of endoscopic resection vs. laparoscopic resection.
Methods
The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched medical databases up to January 2020. Meta-analytical random or fixed effects models were used in pooled analyses. Meta-regression, cumulative meta-analyses, and subgroup analyses were performed to improve the accuracy of the conclusion. Sensitivity analyses were applied to assess the robustness of the results.
Results
A total of 12 cohort studies with 1383 participants comparing endoscopic resection and laparoscopic resection were identified, while three cohort studies with 167 participants comparing endoscopic resection and laparoscopic and endoscopic cooperative surgery were found. We found that endoscopic resection had shorter operation times (weighted mean difference [WMD] = –27.1 min, 95% confidence interval [CI]: –40.8 min to –13.4 min) and lengths of hospital stay (WMD = –1.43 d, 95% CI: –2.31 d to –0.56 d) than did laparoscopic resection. The results were stable and reliable. There were no significant differences in terms of blood loss, hospitalization costs, incidence of complications or recurrence rates. For tumor sizes 2 – 5 cm, endoscopic resection increased the risk of positive margins (relative risk [RR] = 5.78, 95% CI: 1.31 – 25.46). Although operation times for endoscopic resection were shorter than those of laparoscopic and endoscopic cooperative surgery (WMD = –41.03 min, 95% CI: –59.53 min to –22.54 min), there was a higher incidence of complications (RR = 4.03, 95% CI: 1.57 – 10.34).
Conclusions
In general, endoscopic resection is an alternative method for gastric GISTs ≤ 5 cm. Laparoscopic and endoscopic cooperative surgery may work well in combination. Further randomized controlled trials are recommended to validate or update these results.
Keywords: Endoscopic resection, Laparoscopic resection, Gastrointestinal stromal tumors, Meta-analysis
Introduction
Gastrointestinal stromal tumors (GISTs) are common mesenchymal neoplasms of the digestive system.[1] They can occur throughout the digestive tract, with the stomach being the most frequently involved site (60 – 70%).[2,3] Because of their malignant potential, GISTs larger than 2 cm in size are recommended for resection.[4,5] Nevertheless, it has been shown that even GISTs smaller than 2 cm may have a high mitotic index.[6,7] Furthermore, long-term surveillance increases psychological pressures of malignancy and economic costs. For these reasons, early treatment of GISTs is thought to be worthwhile.
Traditionally, surgical resection is the mainstay for treatment of GISTS. With the rapid development of minimally invasive methods, laparoscopic resection (LAP) is believed to have similar long-term effects to those of open surgery for GISTs and to have advantages in terms of blood loss and length of hospital stay.[8,9] Because small GISTs show indolent growth behavior and rarely metastasize to lymph nodes,[10] endoscopic resection (ER), including endoscopic full-thickness resection (EFR), endoscopic submucosal dissection (ESD) and submucosal tunneling endoscopic resection (STER), has also become a viable option for small GISTs.[11,12]
A large number of studies have demonstrated advantages and disadvantages of endoscopic resection over those of laparoscopic resection for small GISTs (≤5 cm).[10,13–22] Nevertheless, the results of head-to-head comparison are conflicting, especially with respect to long-term outcomes. Although laparoscopic and endoscopic cooperative surgery (LECS) that combines laparoscopic and endoscopic methods has become popular in recent years, it remains debatable as to whether it has an advantage over the two original methods.
To resolve these controversies and to improve the generalizability of outcomes, we conducted a meta-analysis to assess the differences between endoscopic resection and laparoscopic resection.
Methods
Data sources and search strategies
This meta-analysis was registered on PROSPERO (No. CRD42020167417).[23] We searched for studies that described the comparison between endoscopic resection and laparoscopic resection for GISTs from medical and biological databases (Medline, EMBASE, and Science Citation Index and Web of Science). A comprehensive search strategy was developed. We used the following keywords
‘endoscopic resection’, ‘endoscopic submucosal dissection’, ‘endoscopic full-thickness resection’, ‘submucosal tunneling endoscopic resection’, ‘laparoscopic resection’, ‘surgery’ or ‘laparoscopic and endoscopic cooperative surgery’ and ‘gastrointestinal stromal tumors’. Duplicate articles were collapsed into a single and unique entry. Additionally, we scanned reference lists of all relevant published studies. Two independent researchers (Xian-Lei Cai and Xue-Ying Li) conducted this work. Discrepancies between the two researchers were resolved by Xiu-Yang Li if required.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) randomized controlled trial (RCT), cohort or case-control study; (2) comparison short-term outcomes or long-term outcomes between ER and LAP / LECS; (3) GISTs (gastric GISTs and intestinal GISTs) smaller than 5 cm; and (4) original studies in English indexed up to January 2020.
Exclusion criteria were as follows: (1) original study did not involve the comparison between ER and LAP; (2) patients undergoing open surgery; (3) other gastrointestinal benign tumors in addition to GISTs; (4) reviews, comments, letters and animal studies; and (5) low-quality studies (we applied the Cochrane collaboration's tool to assess risk of bias for RCTs and used the Newcastle-Ottawa scale to assess the quality of cohort studies with a ‘star system’[24–26]).
Data extraction
All data were extracted by two researchers independently using a standardized form. The characteristics of the included studies were recorded as follows: name of first author, published year, country, study design, gender, age, tumor size, number of ER group, number of LAP group, comparison items (including operation time, estimated blood loss, length of hospital stay, hospitalization costs, complication, microscopic positive margin and recurrence), method of ER, method of LAP, tumor location, tumor mitotic index, types and severity of complication, and time of follow-up.
Statistical analysis
Mean value and standard deviation (SD) were extracted to present the differences in operation time, estimated blood loss, length of hospital stay, and hospitalization costs. This study converted some data from median and interquartile range (IQR) to mean and standard deviation using the method of Hoze et al.[27] Relative risk (RR) and 95% confidence interval (CI) were used to show the differences in incidence of total complication, possibility of positive margin, and recurrence rate.
Meta-regression was applied to assess sources of heterogeneity, considering tumor size. Q-test and I2 statistics were applied to examine the heterogeneity, and the pooled effects were calculated using a random-effects model (P ≤ 0.05 and I2 > 50%)[28] or fixed-effects model (P > 0.05 and I2 ≤ 50%).[29] A cumulative meta-analysis was performed considering year of selected studies. In the order of published year, the trials were added one at a time to compute the pooled estimates sequentially. Because ER is a new technology with a relatively short development time, we assumed that endoscopic techniques would improve over time. The pooled effects of included studies were presented as weighted mean difference (WMD) with 95% CI in common meta-analysis for operation time, estimated blood loss, length of hospital stay and hospitalization costs, and as standardized mean difference (SMD) with 95% CI in cumulative meta-analysis. Forest plots and funnel plots were produced. Publication bias was assessed using the weighted Egger test and Begg's test.[30,31] Sensitivity analysis was used to assess whether the pooled results were undue influenced by an individual study. All analyses were performed using software STATA version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Study characteristics
The selection process was described in a modified PRISMA diagram (Fig. 1). Finally, a total of 12 high-quality cohort studies with 1383 participants comparing ER and LAP were identified, while three cohort studies with 167 participants comparing ER and LECS were found. One was a prospective cohort study;[5] the others were retrospective cohort studies. There were no RCTs or case-control studies. Among these studies, except for the three studies from Korea,[5] Russia,[18] and Japan,[32] all other studies were from China. Tables 1 and 2 display the summaries of each study in this cumulative meta-analysis. Other clinical and pathologic characteristics (method of ER, method of LAP, tumor location, tumor mitotic index, types and severity of complication, and follow-up times) are displayed in Supplementary Table 1.
Figure 1.
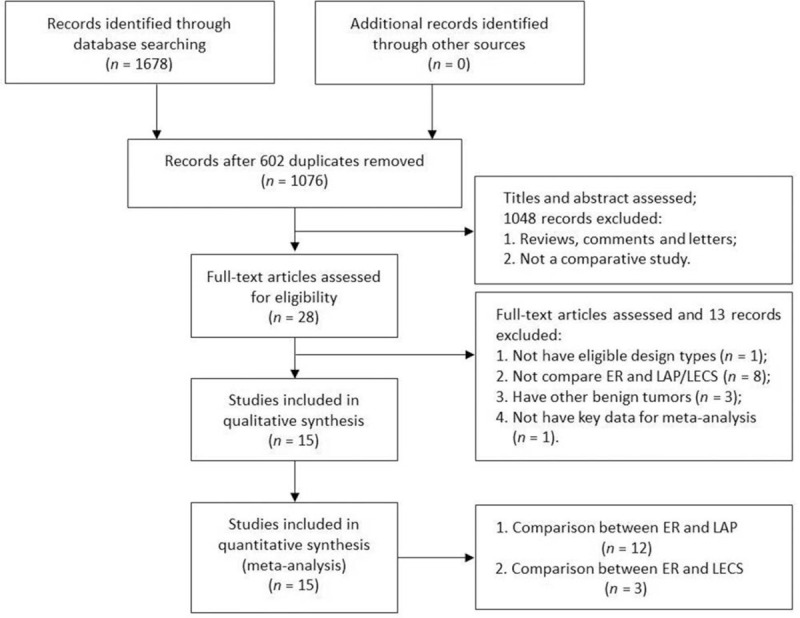
Flow diagram of the studies search process. ER: endoscopic resection; LAP: laparoscopic resection; LECS: laparoscopic and endoscopic cooperative surgery.
Table 1.
Characteristics of cohort studies on comparison between endoscopic resection and laparoscopic resection
| Author | Year | Country | Design | Gender (n) | Age (years) | Tumor diameter (cm) | ER (n) | LAP (n) | Comparison | Quality∗ |
| Wang et al[13] | 2011 | China | Retrospective cohort | M/F: 54/55 | 35–52 | <4.0 | 66 | 53 | Operation timeLOHSHospitalization costComplications | 8 |
| Jeong et al[5] | 2012 | Korea | Prospective cohort | M/F: 36/48 | Mean 55.4 | Mean 3.1 | 27 | 57 | Operation timeLOHSPositive marginsComplicationsRecurrence | 8 |
| Dong et al[14] | 2014 | China | Retrospective cohort | M/F: 7/11 | 32–74 | <5.0 | 10 | 8 | Operation timeLOHSBlood loss | 7 |
| Wu et al[15] | 2015 | China | Retrospective cohort | M/F: 61/31 | Mean 43.5 | 2.5–5.0 | 50 | 42 | Operation timeLOHSComplicationsRecurrence | 8 |
| Meng et al[16] | 2016 | China | Retrospective cohort | M/F: 30/45 | Mean 52.6 | Mean 1.1 | 27 | 48 | Operation timeLOHSHospitalization costBlood lossComplicationsRecurrence | 9 |
| Dai et al[17] | 2017 | China | Retrospective cohort | M/F: 136/199 | Mean 57.0 | <3.5 | 262 | 73 | Operation timeLOHSHospitalization costComplicationsRecurrence | 7 |
| Meng et al[22] | 2017 | China | Retrospective cohort | M/F: 60/66 | Mean 52.2 | <2.0 | 75 | 51 | Operation timeLOHSBlood lossComplicationsRecurrence | 8 |
| Gluzman et al[18] | 2017 | Russia | Retrospective cohort | NA | Mean 61.2 | <5.0 | 22 | 40 | Operation timeLOHSBlood lossComplications | 7 |
| He et al[19] | 2018 | China | Retrospective cohort | M/F: 92/54 | Median 54 | ER: 3.4LAP: 3.7 | 62 | 84 | Operation timeLOHSHospitalization costComplications | 8 |
| Yin et al[20] | 2018 | China | Retrospective cohort | M/F: 42/49 | Mean 57.6 | <5.0 | 46 | 30 | Operation timeLOHSBlood lossPositive marginsComplicationsRecurrence | 7 |
| Chen et al[21] | 2018 | China | Retrospective cohort | M/F: 39/62 | ER: 57LAP:61 | 2.0–5.0 | 35 | 66 | Operation timeLOHSHospitalization costComplicationsRecurrence | 7 |
| Zhao et al[10] | 2019 | China | Retrospective cohort | M/F:60/89 | ER: 57.0LAP:57.7 | <5.0 | 85 | 64 | LOHSComplicationsPositive margins | 7 |
The ‘star system’ of the Newcastle-Ottawa scale. ER: Endoscopic resection; F: Female; LAP: Laparoscopic resection; LOHS: Length of hospital stay; M: Male; NA: Not available.
Table 2.
Characteristics of cohort studies on the comparison between endoscopic resection and laparoscopic and endoscopic cooperative surgery
| Author | Year | Country | Design | Gender (n) | Age (years) | Tumor diameter (cm) | ER (n) | LECS (n) | Comparison | Quality ∗ |
| Balde et al[38] | 2017 | China | Retrospective cohort | M/F: 28/32 | Mean 49.0 | <2.0 | 30 | 30 | Operation timeBlood lossLOHSHospitalization costPositive margins | 9 |
| Yin et al[20] | 2018 | China | Retrospective cohort | M/F: 42/49 | Mean 57.6 | <5.0 | 46 | 15 | Operation timeLOHSBlood lossPositive marginsComplicationsRecurrence | 7 |
| Ojima et al[32] | 2018 | Japan | Retrospective cohort | M/F: 19/27 | ER: 64LECS:73 | ER: 2.3LECS: 2.5 | 25 | 21 | Operation timeBlood lossComplicationsRecurrence | 8 |
‘star system’ of the Newcastle-Ottawa scale. ER: Endoscopic resection; F: Female; LECS: Laparoscopic and endoscopic cooperative surgery; LOHS: Length of hospital stay; M: Male.
Comparison of operation time
A total of 11 trials for operation time were included in the meta-analysis. Meta-regression was performed to assess potential sources of heterogeneity from tumor size of each study. We found that tumor size was not an influencing factor (P = 0.374). Therefore, all eleven estimates were incorporated into the meta-analysis.
When compared with LAP, ER had shorter operation times (WMD = –27.1 min, 95% CI: –40.8 min to –13.4 min, as shown in Fig. 2). There was substantial heterogeneity in the pooled analysis (P < 0.001; I2 = 90.3%). The funnel plot suggested no evidence of publication bias (Begg's test zc = 0.16, P = 0.876; Egger's test t = –0.56, P = 0.592; Supplementary Figure 1A). The sensitivity analysis showed that the meta-analysis results were robust (Supplementary Figure 2A). The result of cumulative meta-analysis is shown in Supplementary Figure 3.
Figure 2.
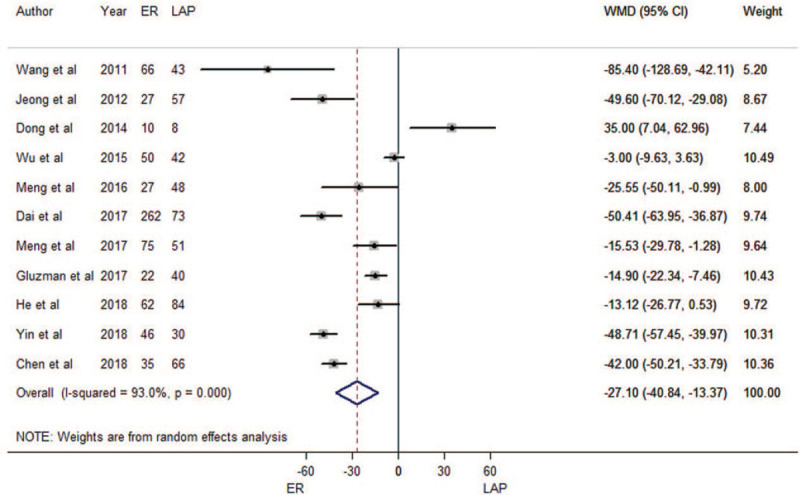
Forest plots of meta-analysis on operation time. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; WMD: weighted mean difference.
Comparison of estimated blood loss
A total of five trials for estimated blood loss during operation were included in the meta-analysis. Meta-regression results indicated that tumor size was not an influencing factor (P = 0.967). Therefore, all five estimates were incorporated into the meta-analysis.
There was no difference between ER and LAP in terms of estimated blood loss (WMD = –9.19 mL, 95% CI: –20.13 mL to 1.74 mL, as shown in Fig. 3). Heterogeneity was detected in the pooled analysis (P < 0.001; I2 = 91.0%). The funnel plot suggested that we could rule out the publication bias (Begg's test zc = 1.22, P = 0.221; Egger's test t = –1.82, P = 0.166, as shown in Supplementary Figure 1B). The sensitivity analysis showed that the meta-analysis results were robust (Supplementary Figure 2B). The result of cumulative meta-analysis is shown in Supplementary Figure 4.
Figure 3.
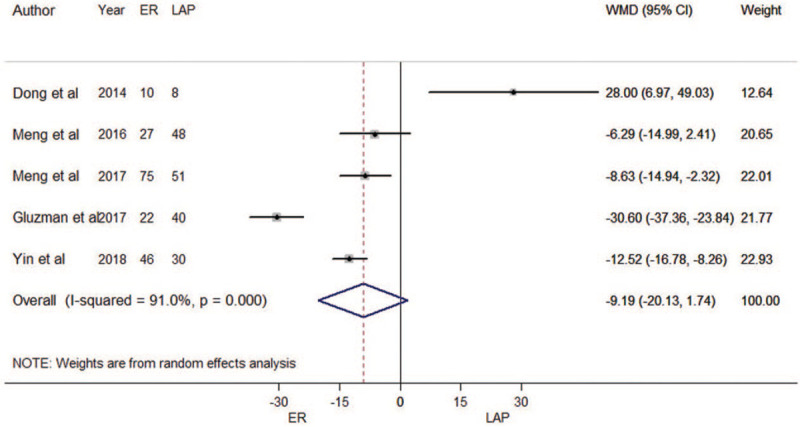
Forest plots of meta-analysis on blood loss. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; WMD: weighted mean difference.
Comparison of length of hospital stay
A total of 12 trials for length of hospital stay were included. The result of meta-regression indicated that tumor size was not an influencing factor (P = 0.059). Therefore, we brought these twelve estimates into the meta-analysis.
When compared with LAP, ER had shorter lengths of hospital stay (WMD = –1.43 d, 95% CI: –2.31 d to –0.56 d; Fig. 4). There was substantial heterogeneity among the included studies (P < 0.001; I2 = 83.1%). The funnel plot suggested no evidence of publication bias (Begg's test zc = 0.21, P = 0.837; Egger's test t = 0.37, P = 0.717; Supplementary Figure 1C). The meta-analysis results were robust (Supplementary Figure 2C). The result of cumulative meta-analysis is shown in Supplementary Figure 5.
Figure 4.
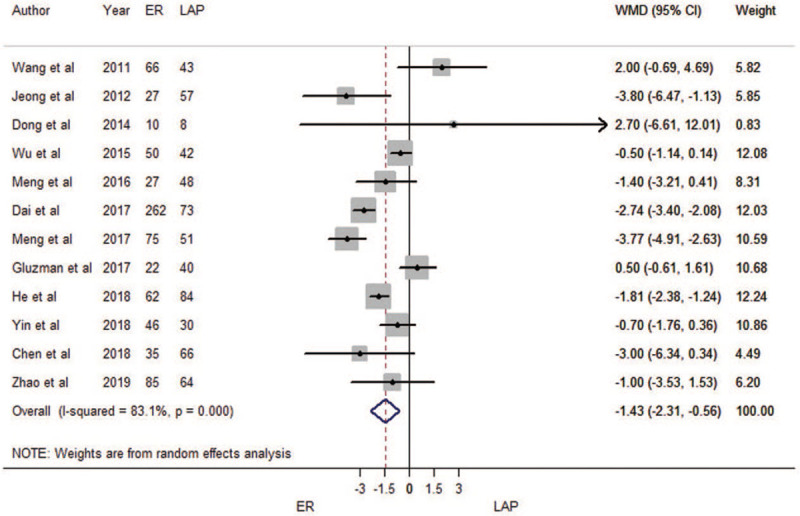
Forest plots of meta-analysis on length of hospital stay. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; WMD: weighted mean difference.
Comparison of hospitalization costs
A total of five trials for hospitalization costs were included in the meta-analysis. Meta-regression showed tumor size was not an influencing factor (P = 0.131). Therefore, all five estimates were incorporated into the meta-analysis.
There was no difference between ER and LAP in terms of hospitalization costs (WMD = –1191.25 RMB yuan, 95% CI: –3109.51 RMB yuan to 727.01 RMB yuan; Fig. 5). Heterogeneity was detected in the pooled analysis (P < 0.001; I2 = 97.6%). The funnel plot suggested that we could rule out the publication bias (Begg's test zc = –0.24, P = 1.000; Egger's test t = –0.08, P = 0.942; Supplementary Figure 1D). The sensitivity analysis showed that results were robust (Supplementary Figure 2D). The result of cumulative meta-analysis is shown in Supplementary Figure 6.
Figure 5.
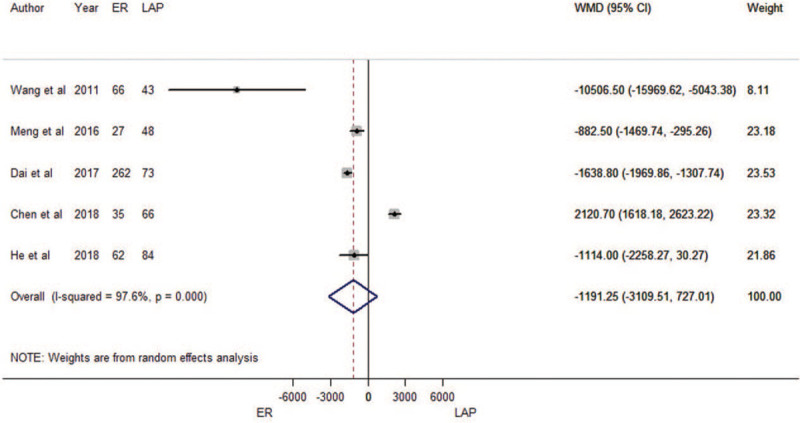
Forest plots of meta-analysis on hospitalization costs. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; WMD: weighted mean difference.
Comparison of total complication rate
Eleven cohort studies were eligible for total complication incidences. The results of meta-regression did not show the source of heterogeneity from tumor size (P = 0.931). Therefore, we brought these 11 estimates into the meta-analysis. There was no difference between ER and LAP in terms of total complication incidence (RR = 1.30, 95% CI: 0.88 – 1.91; Fig. 6). Heterogeneity was not detected in the pooled analysis (P = 0.593; I2 = 0.0%). The funnel plot was symmetrical (Supplementary Figure 1E), and there was no evidence of publication bias (Begg's test zc = 0.31, P = 0.755; Egger's test t = –0.03, P = 0.974). The results were not overly affected by one publication according to sensitivity analyses (Supplementary Figure 2E). The result of cumulative meta-analysis is shown in Supplementary Figure 7.
Figure 6.
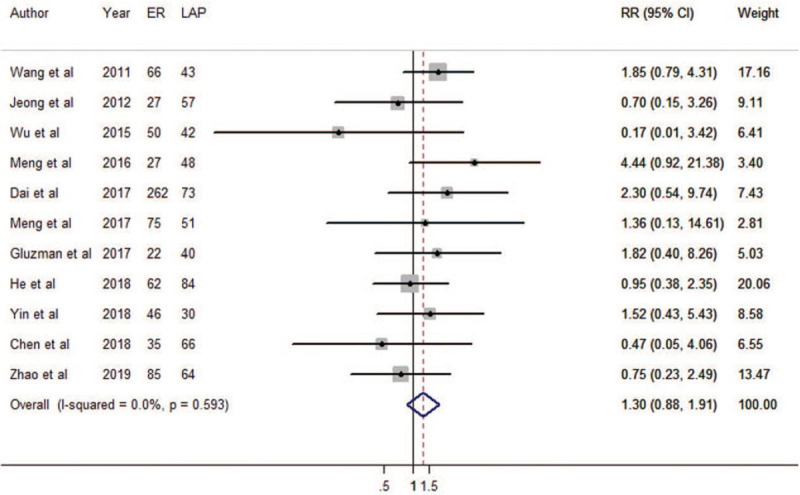
Forest plots of meta-analysis on total complications. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; RR: relative risk.
Comparison of positive margin incidence
Only three trials for positive margin incidence were included. The result of meta-regression indicated that tumor size was not an influencing factor (P = 0.502). Therefore, these three estimates were incorporated into the meta-analysis.
The result of pooled analysis showed that ER gave higher risk of positive margins than did LAP (RR = 5.78, 95% CI: 1.31 – 25.46; Fig. 7). There was no heterogeneity among the included studies (P = 0.751; I2 = 0.0%). Because there were fewer than five studies, we did not draw a funnel plot. The meta-analysis result was not robust because of the small number of included studies (Supplementary Figure 2F). The result of cumulative meta-analysis is shown in Supplementary Figure 8.
Figure 7.
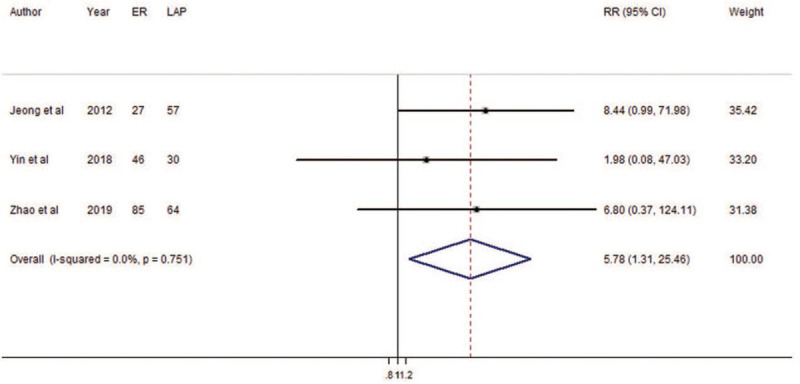
Forest plots of meta-analysis on positive margins. CI: confidence interval; ER: endoscopic resection; LAP: laparoscopic resection; RR: relative risk.
Comparison of recurrence rate
Five cohort studies were eligible for recurrence rate. The results of meta-regression did not show the source of heterogeneity from tumor size (P = 0.547). Therefore, we brought these five estimates into the meta-analysis. There was no difference between ER and LAP in terms of recurrence rate (RR = 0.73, 95% CI: 0.28 – 1.93; Fig. 8). Heterogeneity was not detected in the pooled analysis (P = 0.903; I2 = 0.903%). The funnel plot was symmetrical (Supplementary Figure 1F), and there was no evidence of publication bias (Begg's test zc = –0.24, P = 1.000; Egger's test t = 0.53, P = 0.632). The results were not overly affected by one publication (Supplementary Figure 2G). The result of cumulative meta-analysis is shown in Supplementary Figure 9.
Figure 8.
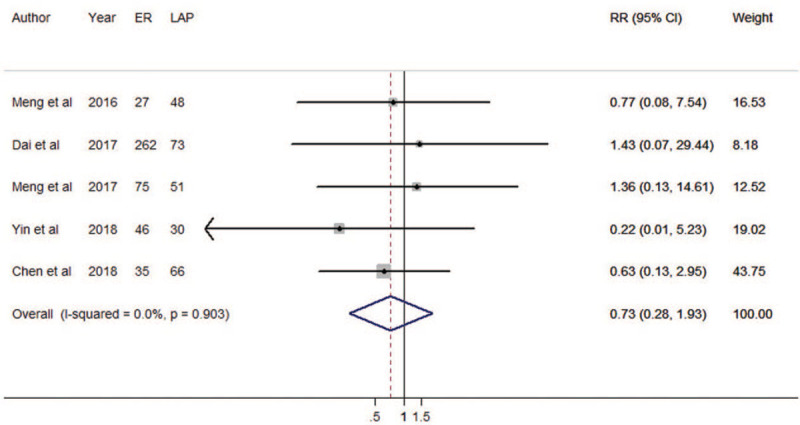
Forest plots of meta-analysis on recurrences. ER: endoscopic resection; LAP: laparoscopic resection; RR: relative risk; CI: confidence interval.
Subgroup analyses stratified by tumor size
Traditionally, 2 cm was regarded as the boundary of treatment options. Hence, we performed subgroup analyses stratified by tumor size of 2 cm (Table 3). We found there were no differences between the two groups in terms of operation times, estimated blood loss, lengths of hospital stay, hospitalization costs, complications or recurrences. However, only the ‘2 – 5 cm’ group presented a comparison on positive margins and returned a statistically significant result.
Table 3.
Subgroup analysis of tumor size for comparison between endoscopic resection and laparoscopic resection
| Items | Tumor size ≤ 2.0 cm | 2.0 cm < Tumor size < 5 cm | ||||
| n | WMD/RR (95% CI) | I2 | n | WMD/RR (95% CI) | I2 | |
| Operation time (min) | 2 | –18.1 (–30.4, –5.7) | 0.0 | 9 | –24.6 (–28.1, –21.2) | 94.3 |
| Blood loss (mL) | 2 | –7.8 (–12.9, –2.7) | 0.0 | 5 | –16.4 (–19.9, –12.8) | 94.6 |
| LOHS (d) | 2 | –3.1 (–4.1, –2.1) | 78.8 | 10 | –1.4 (–1.7, –1.1) | 81.8 |
| Hospitalization cost | 1 | –882.5 (–1469.7, –295.3) | NA | 4 | –1558.8 (–4221.2, 1103.6) | 98.2 |
| Complications | 2 | 3.04 (0.86, 10.77) | 0.0 | 9 | 1.18 (0.78, 1.78) | 0.0 |
| Positive margins | 0 | NA | NA | 3 | 5.78 (1.31, 25.46) | 0.0 |
| Recurrence | 2 | 1.03 (0.20, 5.23) | 0.0 | 3 | 0.61 (0.18, 2.06) | 0.0 |
CI: Confidence interval; LOHS: length of hospital stay; NA: not available; RR: Relative risk; WMD: Weighted mean difference.
Comparison between ER and LECS
Only three trials focused on comparison between ER and LECS. The results of meta-analysis indicated that when compared with LECS, ER had shorter operation time (WMD = – 41.03 min, 95% CI: –59.53 min to –22.54 min, P = 0.001; I2 = 86.3%; Fig. 9A) and higher risk of total complication incidence (RR = 4.03, 95% CI: 1.57 – 10.34, P = 0.146; I2 = 48.0; Fig. 9D). There were no differences between ER and LECS in terms of estimated blood loss (WMD = 4.52 mL, 95% CI: –24.91 mL to 33.96 mL, P = 0.001; I2 = 86.4%; Fig. 9B) and length of hospital stay (WMD = –0.23 d, 95% CI: –1.26 d to 0.79 d, P = 0.892; I2 = 0.0%; Fig. 9C). Because of the small number of included studies, we did not perform analysis of publication bias or sensitivity analysis.
Figure 9.
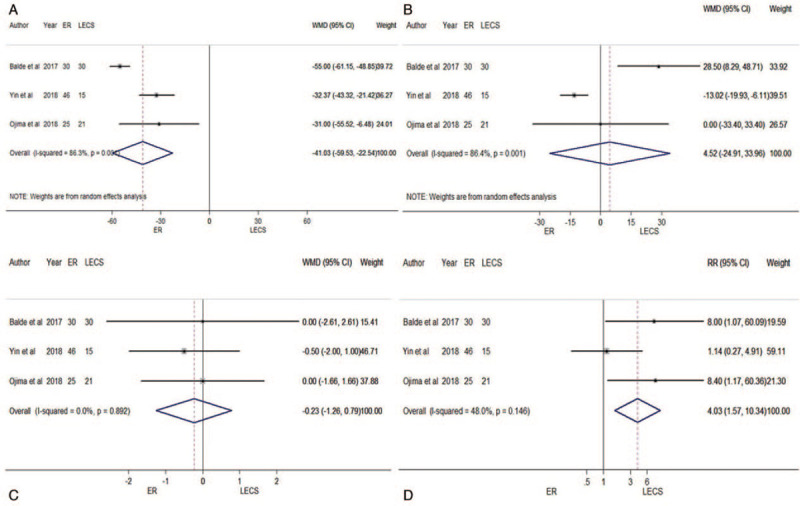
Forest plots of meta-analysis on comparison between ER and LECS: A. operation time; B. blood loss; C. length of hospital stay; D. total complications. CI: confidence interval; ER: endoscopic resection; LECS: laparoscopic and endoscopic cooperative surgery; RR: relative risk; WMD: weighted mean difference.
Discussion
In general, endoscopic resection minimizes the damage to the gastric wall and preserves the integrity of the structure and function of the stomach. It also reduces the anxiety and concern of patients and is more easily accepted by patients.
The results of this meta-analysis suggested that, in terms of short-term effect, endoscopic resection gave shorter operation time and shorter lengths of hospital stay than did laparoscopic resection, with similar outcomes in terms of blood loss, hospitalization costs and total complication with laparoscopic resection. However, endoscopic resection increased the risk of positive margins for the tumor size of 2 to 5 cm. In terms of long-term effects, endoscopic resection and laparoscopic resection had similar recurrence rates. According to the relatively good prognosis of small GISTs, the data of overall survival was insufficient, and it was difficult to carry out relevant meta-analysis. When compared with laparoscopic and endoscopic cooperative surgery, endoscopic resection had shorter operation times but higher risk of total complication incidence. Nevertheless, the paucity of data reduced the accuracy of the results.
The results of cumulative meta-analysis provided abundant and valuable information. For example, in the comparison of operation time, since 2017, all the results suggested that ER reduces the operation time. This finding was stable and reliable, thereby reducing the debate on this issue. Similar results were seen in the comparison of lengths of hospital stay. From 2018, all results suggested that ER reduces the length of hospital stay. For another example, in the comparison of estimated blood loss, at first, ER had more intraoperative blood loss than did LAP; however, with the passage of time, the difference between the two methods narrowed, until there was no significant difference. The forest plots of the cumulative meta-analysis suggested that, with the addition of new research in the future, intraoperative blood loss of ER might be significantly lower than that of LAP, which benefited from the rapid development of endoscopic technology and equipment. The cumulative meta-analysis had its unique advantages; it allowed recognition of trend in the results, and provided more reliable conclusions.
We performed a meta-regression to assess potential sources of heterogeneity from the tumor size of each study. Tumor location was also an important affecting factor of clinical outcome. However, in the original studies, tumors in various locations were combined for statistical purposes, and the clinical data of tumors in each location were not listed separately. It was difficult to perform meta-regression analysis. On the other hand, the proportions of tumors in various locations in the ER and the LAP groups were balanced in the original studies, and there was no statistical difference. In other words, the introduction of bias was relatively small. Therefore, we did not perform meta regression analysis of tumor location.
Our meta-analysis showed that ER gave a higher risk of positive margins than LAP; however, there was no difference between ER and LAP in terms of recurrence rate. We think that the reasons for this are as follows. First, some patients underwent remedial surgery; second, the use of imatinib and radiotherapy[33] could reduce the recurrence rate. Finally, the insufficient number of relevant articles focusing on positive margin and recurrence rate may have affected the accuracy of the results. Moreover, the recurrence of GISTs was more dependent on tumor biology (mitotic index) than on tumor size. Because of the absence of mitotic index data in some original studies, or combing GISTs with different mitotic indexes in the statistical analysis, it was difficult to carry out subgroup analysis based on mitotic index stratification.
In our study, the hospitalization costs and total complication rates were similar between the ER and LAP groups. In general, different complications lead to different costs of treatment, especially serious complications. However, there were no detailed analyses of the cost of various complications in the original studies. It was difficult for us to conduct subgroup meta-analysis of cost based on complication stratification. After systematic review, we found that the safety of ER and LAP were relatively high, and there were no life-threatening complications. Hospitalization costs were also within reasonable ranges, suggesting that both methods were safe and reliable.
Traditionally, for GISTs of more than 2 cm, surgery was recommended. For GISTs less than 5 cm, laparoscopic surgery was considered to be a suitable choice.[10] In recent years, with the further maturity of endoscopic technology, endoscopists have tried to expand the indications of endoscopic resection for GISTs;[34,35] for these reasons, we identified many relevant studies. Our meta-analysis also found that there was no significant difference between ER and LAP in terms of blood loss, hospitalization costs, complications, recurrence rate. ER was more advantageous in terms of operation time and length of hospitalization stay. Nevertheless, ensuring complete resection without tumor rupture and negative margins remains crucial.[1] Positive margins or tumor rupture meant that patients acquired high-risk status directly. The follow-up targeted treatment not only introduced side-effects of drugs, but also increased economic burdens. Therefore, we believe that for 2 – 5 cm GISTs, before choosing endoscopic resection as a treatment, endoscopists should consider the size and location comprehensively and select the appropriate patients carefully.
LECS is a new technology; it combines the advantages of ER and LAP and requires the cooperation of endoscopists and laparoscopic surgeons. It is not only conducive to the guarantee of optimal surgical margins, but also to the retention of residual gastric function.[36,37] In the present study, we found that, although LECS increased operation time over that of ER, it reduced the occurrence of complications. Nevertheless, there were only three relevant articles in this field, insufficient to draw conclusions, and requiring further verification in follow-up studies. ER can be subdivided into many ways, including ESD, EFR, and STER. As for which method is superior, there is no consensus. We believe that doctors should choose the most appropriate method according to the particular situation of the patients. This also means greater requirements for endoscopists, who need to master various kinds of endoscopic resection methods and other modified methods.
This work has several limitations. First, all relevant articles included in the meta-analysis were observational studies. Among them, only one study was a prospective cohort study, and the others were all retrospective reports. No randomized controlled trials in this field had been reported. These conditions reduced the evidence strength of the conclusion. Second, high heterogeneity was detected in the pooled analyses of operation times, estimated blood loss, lengths of hospital stay, and hospitalization costs. We thought that heterogeneity was primarily driven by large amounts of included estimates. Moreover, among all the relevant studies, most were reported by Chinese scholars, and this may introduce further bias. Third, this meta-analysis contained a total of 1550 participants, and overall, the sample size was relatively small. Fourth, though we did not exclude intestinal GISTs in our exclusion criteria, only the study of Gluzman et al.[18] included small intestinal GIST of a small sample (n = 5). Similarly, after excluding Gluzman et al. from the sensitivity analysis, we found that the results did not affect the conclusion. Nevertheless, because the vast majority of patients had gastric GSITs, we believe the results should be interpreted cautiously. In addition, more data are needed to demonstrate the role of ER in other types of GISTs besides gastric GISTs. Fifth, the limited number of studies for comparison between ER and LECS did not allow us to analyze differences such as hospitalization costs, positive margins and recurrence rates or to draw relevant conclusions. The number of relevant studies was insufficient to perform meta-analysis of LAP vs. LECS. If possible, a network meta-analysis among ER, LAP, and LECS could be more meaningful. Taken together, these limitations suggest that the results of our cumulative meta-analysis should be interpreted with caution.
This meta-analysis also had some strengths. First, to the best of our knowledge, this was the first cumulative meta-analysis summarizing the evidence on the comparison between endoscopic resection and laparoscopic resection. Second, the application of cumulative meta-analysis in methodology made the results more intuitive and understandable. Third, meta-regression and publication bias analyses help detect the source of heterogeneity and strengthen the results. Fourth, all the relevant studies were of high quality, and the sensitivity analyses showed that the results were robust and they were not overly affected by one report.
Conclusion
We conclude cautiously that endoscopic resection is an alternative method for properly selected patients with gastric GISTs smaller than 5 cm. ER should be limited before more high-level evidence is obtained from well-designed RCT-clinical trials in the future. For GISTs 2 – 5 cm, endoscopists need to avoid the occurrence of positive margins during endoscopic resection. Laparoscopic and endoscopic cooperative surgery is potentially beneficial for complex GISTs.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Cai XL, Li XY, Liang C, Xu Y, Zhang MZ, Yu WM, Li XY. Endoscopic or laparoscopic resection for small gastrointestinal stromal tumors: a cumulative meta-analysis. Chin Med J 2020;133:2731–2742. doi: 10.1097/CM9.0000000000001069
Xian-Lei Cai and Xue-Ying Li contributed equally to this work.
Funding: This work was supported by grants from the Key Project in Soft Science by the Science and Technology Department of Zhejiang Province (No. 2019C25009), the National Natural Science Funds of Young Scientists of China (No. 81802944), and the Public Welfare Technological Research Program of Zhejiang Province (No. LGF18H160007).
Conflicts of interest: None.
References
- 1.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010; 8 Suppl 2:S1–S41. quiz S42-44. doi:10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iorio N, Sawaya RA, Friedenberg FK. Review article: the biology, diagnosis and management of gastrointestinal stromal tumours. Aliment Pharmacol Ther 2014; 39:1376–1386. doi: 10.1111/apt.12761. [DOI] [PubMed] [Google Scholar]
- 3.Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch 2010; 456:111–127. doi: 10.1007/s00428-010-0891-y. [DOI] [PubMed] [Google Scholar]
- 4.Judson I, Bulusu R, Seddon B, Dangoor A, Wong N, Mudan S. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res 2017; 7:6.doi: 10.1186/s13569-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong IH, Kim JH, Lee SR, Kim JH, Hwang JC, Shin SJ, et al. Minimally invasive treatment of gastric gastrointestinal stromal tumors: laparoscopic and endoscopic approach. Surg Laparosc Endosc Percutan Tech 2012; 22:244–250. doi: 10.1097/SLE.0b013e31825078f2. [DOI] [PubMed] [Google Scholar]
- 6.Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc 2016; 83:318–326. doi: 10.1016/j.gie.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 7.An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc 2017; 31:4522–4531. doi: 10.1007/s00464-017-5511-3. [DOI] [PubMed] [Google Scholar]
- 8.Cui JX, Gao YH, Xi HQ, Cai AZ, Zhang KC, Li JY, et al. Comparison between laparoscopic and open surgery for large gastrointestinal stromal tumors: A meta-analysis. World J Gastrointest Oncol 2018; 10:48–55. doi: 10.4251/wjgo.v10.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z, Li P, Hu Y. Laparoscopic versus open wedge resection for gastrointestinal stromal tumors of the stomach: a meta-analysis. Wideochir Inne Tech Maloinwazyjne 2019; 14:149–159. doi: 10.5114/wiitm.2018.79933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Pang T, Zhang B, Wang L, Lv Y, Ling T, et al. Retrospective Comparison of Endoscopic Full-Thickness Versus Laparoscopic or Surgical Resection of Small (≤ 5 cm) Gastric Gastrointestinal Stromal Tumors. J Gastrointest Surg 2019; doi: 10.1007/s11605-019-04493-6. [DOI] [PubMed] [Google Scholar]
- 11.Tan Y, Tan L, Lu J, Huo J, Liu D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol 2017; 2:115.doi: 10.21037/tgh.2017.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Liao G, Fan C, Yu J, Nie X, Yang S, et al. Long-term outcomes of endoscopic resection of gastric GISTs 2017; 31:4799–4804. doi: 10.1007/s00464-017-5557-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Ren W, Fan CQ, Li YH, Zhang X, Yu J, et al. Full-thickness endoscopic resection of nonintracavitary gastric stromal tumors: a novel approach. Surg Endosc 2011; 25:641–647. doi: 10.1007/s00464-010-1189-5. [DOI] [PubMed] [Google Scholar]
- 14.Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc 2014; 28:1447–1453. doi: 10.1007/s00464-013-3375-8. [DOI] [PubMed] [Google Scholar]
- 15.Wu CR, Huang LY, Guo J, Zhang B, Cui J, Sun CM, et al. Clinical Control Study of Endoscopic Full-thickness Resection and Laparoscopic Surgery in the Treatment of Gastric Tumors Arising from the Muscularis Propria. Chin Med J 2015; 128:1455–1459. doi: 10.4103/0366-6999.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng Y, Cao C, Song S, Li Y, Liu S. Endoscopic band ligation versus endoscopic submucosal dissection and laparoscopic resection for small gastric stromal tumors. Surg Endosc 2016; 30:2873–2878. doi: 10.1007/s00464-015-4571-5. [DOI] [PubMed] [Google Scholar]
- 17.Dai WJ, Liu G, Wang M, Liu WJ, Song W, Yang XZ, et al. Endoscopic versus laparoscopic resection of gastric gastrointestinal stromal tumors: a multicenter study. Oncotarget 2017; 8:11259–11267. doi: 10.18632/oncotarget.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gluzman MI, Kashchenko VA, Karachun AM, Orlova RV, Nakatis IA, Pelipas IV, et al. Technical success and short-term results of surgical treatment of gastrointestinal stromal tumors: an experience of three centers. Transl Gastroenterol Hepatol 2017; 2:56.doi: 10.21037/tgh.2017.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Yan S, Li R, Qiu H, Tu J. A comparative study of treatment of gastrointestinal stromal tumors with laparoscopic surgery: a retrospective study. J buon 2018; 23:820–825. doi:not available. [PubMed] [Google Scholar]
- 20.Yin X, Yin Y, Chen H, Shen C, Tang S, Cai Z, et al. Comparison Analysis of Three Different Types of Minimally Invasive Procedures for Gastrointestinal Stromal Tumors ≤5 cm. J Laparoendosc Adv Surg Tech A 2018; 28:58–64. doi: 10.1089/lap.2017.0305. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Zhang Q, Li FY, Yang L, Zhang DC, Wang LJ, et al. Comparison of treatment outcomes between laparoscopic and endoscopic surgeries for relatively small gastric gastrointestinal stromal tumors. Surg Oncol 2018; 27:737–742. doi: 10.1016/j.suronc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Meng Y, Li W, Han L, Zhang Q, Gong W, Cai J, et al. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J Gastroenterol Hepatol 2017; 32:1693–1697. doi: 10.1111/jgh.13768. [DOI] [PubMed] [Google Scholar]
- 23.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 2012; 1:2.doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet 2015; 386:1845–1852. doi: 10.1016/S0140-6736(15)00045-8. [DOI] [PubMed] [Google Scholar]
- 25.Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, et al. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep 2016; 6:19213.doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10:Ed000142.doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13.doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. doi: not available. [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. doi: not available. [PubMed] [Google Scholar]
- 31.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998; 316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ojima T, Nakamura M, Nakamori M, Takifuji K, Hayata K, Katsuda M, et al. Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery. Surg Endosc 2018; 32:351–357. doi: 10.1007/s00464-017-5683-x. [DOI] [PubMed] [Google Scholar]
- 33.EE O. Radiotherapy for Gastrointestinal Stromal Tumors. Chin Med J 2018; 131:235–240. doi: 10.4103/0366-6999.222344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Gao LQ, Han ZL, Li XF, Wang LH, Liu SD. Effectiveness and safety of endoscopic resection for gastric GISTs: a systematic review. Minim Invasive Ther Allied Technol 2018; 27:127–137. doi: 10.1080/13645706.2017.1347097. [DOI] [PubMed] [Google Scholar]
- 35.Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc 2014; 28:1978–1983. doi: 10.1007/s00464-014-3421-1. [DOI] [PubMed] [Google Scholar]
- 36.Aisu Y, Yasukawa D, Kimura Y, Hori T. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J Gastrointest Oncol 2018; 10:381–397. doi: 10.4251/wjgo.v10.i11.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J Gastroenterol 2015; 21:12482–12497. doi: 10.3748/wjg.v21.i43.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balde AI, Chen T, Hu Y, Redondo NJ, Liu H, Gong W, et al. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc 2017; 31:843–851. doi: 10.1007/s00464-016-5042-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


