Abstract
Effective use of genetic and genomic data in cancer prevention and treatment depends on adequate communication with patients and the public. Although relevant empirical work has emerged, the scope and outcomes of this communication research have not been characterized. We conducted a comprehensive scoping review of recent published research (2010–2017) on communication of cancer-related genetic and genomic testing (CGT) information. Searches in six databases revealed 9,243 unique records; 513 papers were included. Most papers utilized an observational quantitative design; fewer utilized an experimental design. More attention has been paid to outcomes of CGT results disclosure than to decision making regarding CGT uptake or the process of results disclosure. Psychosocial outcomes were most common across studies. This literature has a strong focus on BRCA1/2, with few papers focused on Lynch syndrome or next-generation technologies. Women, Caucasians, older adults, and those of higher socioeconomic status were overrepresented. Research gaps identified include the need for studies on the process of CGT communication; examining behavioral, decision-making, and communication outcomes; and inclusion of diverse populations. Addressing these gaps can help improve the use of genomics in cancer control and reduce disparities in access to and use of CGT.
Keywords: communication, cancer, genetic testing, return of results, decision-making
INTRODUCTION
Advances in genetic and genomic technologies are transforming cancer prevention and care.1 Genetic-based risk assessment can help define cancer risks for individuals and families and facilitate decision making about risk management options.2–6 Genomic tumor data may also inform choice of therapy for cancer patients.7 Effective use of genetic and genomic data in cancer care depends strongly upon communication with patients and the general public. Given that genetic and genomic information generated for individuals is becoming increasingly complex, understanding how this information is communicated and understood is a key priority for research and clinical practice.
A growing body of empirical literature has emerged to address these questions. Yet the scope and nature of this work are diffuse. A comprehensive examination of the state of the science on communication of cancer-related genetic and genomic testing (hereafter CGT) will add to our understanding of which communication processes and outcomes have been the focus of previous studies and identify research gaps. Prior reviews have examined some aspects of the literature on communication about CGT and have mostly focused on the psychosocial and behavioral outcomes of returning genetic test results in the context of inherited cancer syndromes.8–10 Other reviews have focused specifically on lower risk contexts. For example, Hollands et al. (2016) examined the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and behavioral motivation,11 and McBride et al. (2010) reviewed consumers’ views on direct-to-consumer genetic information.12 Our recent landscape analysis of published reviews and meta-analyses found that the most frequent area of focus among these reviews was the psychological and behavioral impact of testing.13 However, the landscape analysis did not examine or synthesize the characteristics of the individual papers that comprise this literature.
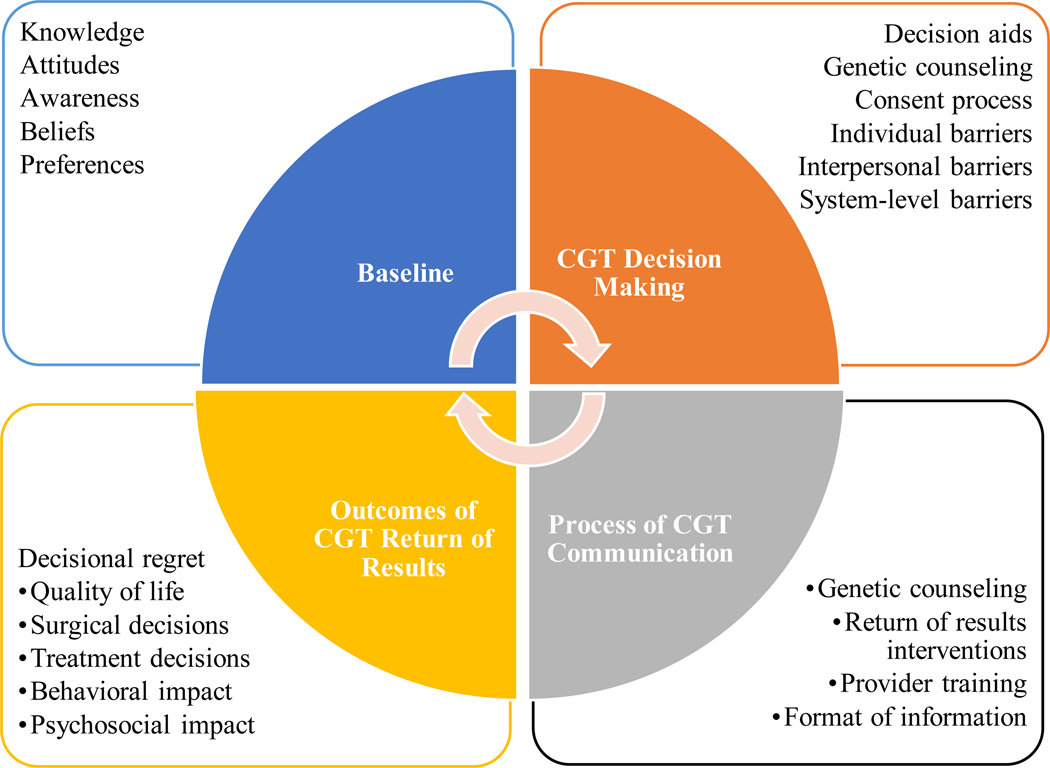
In our landscape analysis, we proposed a model delineating phases of CGT-related communication with patients, consumers, and the general public, which is based on models of patient-provider communication.14 As shown in Figure 1, the phases of this continuum are: 1) baseline knowledge and awareness of CGT, which forms the context for communication; 2) decision making (i.e., any communication about whether or not to have CGT); 3) process of communication about CGT (e.g., return of results); and 4) intermediate and distal outcomes of communication of CGT results (e.g., anxiety, health behavior change).13 As discussed above, prior reviews have mostly focused on the last phase. However, developing a complete understanding of communication outcomes requires a thorough analysis of all phases of the continuum.
Figure 1.
Phases of cancer-related genetic and genomic testing (CGT) communication.
The purpose of this review was to comprehensively examine published empirical research on communication about CGT between providers and patients or with consumers across the four phases of our continuum. Because our purpose was to describe the state of this research, we conducted a scoping review, a type of review that maps the relevant literature,15 rather than a systematic review focused on a more targeted question. We investigated the characteristics of studies examining the communication of CGT information, as well as the populations included in these studies, to identify both areas of strength and research gaps within the literature.
METHODS
Literature Search
We utilized Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.16 We conducted a comprehensive electronic literature search of articles published between January 2010 and January 2017 in the following databases: Medline, Embase, CINAHL, PsycINFO, Cochrane Library, and ERIC. This timeframe was selected both to allow a focus on recent research and to capture the period when next-generation sequencing technologies became available. We used controlled vocabulary (MeSH, EMTREE, and PsycInfo Subject Headings) where possible, combined with key words due to the lack of standard search terms in this literature. The broad categories for search terms included cancer, genetic/genomic communication, provider/direct-to-consumer, and patient/public. The full Medline search strategy is presented in Supplemental Appendix A.
Inclusion and Exclusion Criteria
Detailed inclusion and exclusion criteria were developed through an iterative process of review and testing. Inclusion criteria were: English-language, included cancer-related genetic and/or genomic information, and relevant to at least one phase of the CGT communication. Articles were excluded if they focused only on providers or family communication; presented normative analyses; or did not present any empirical data. Articles were not restricted based on study design or outcome.
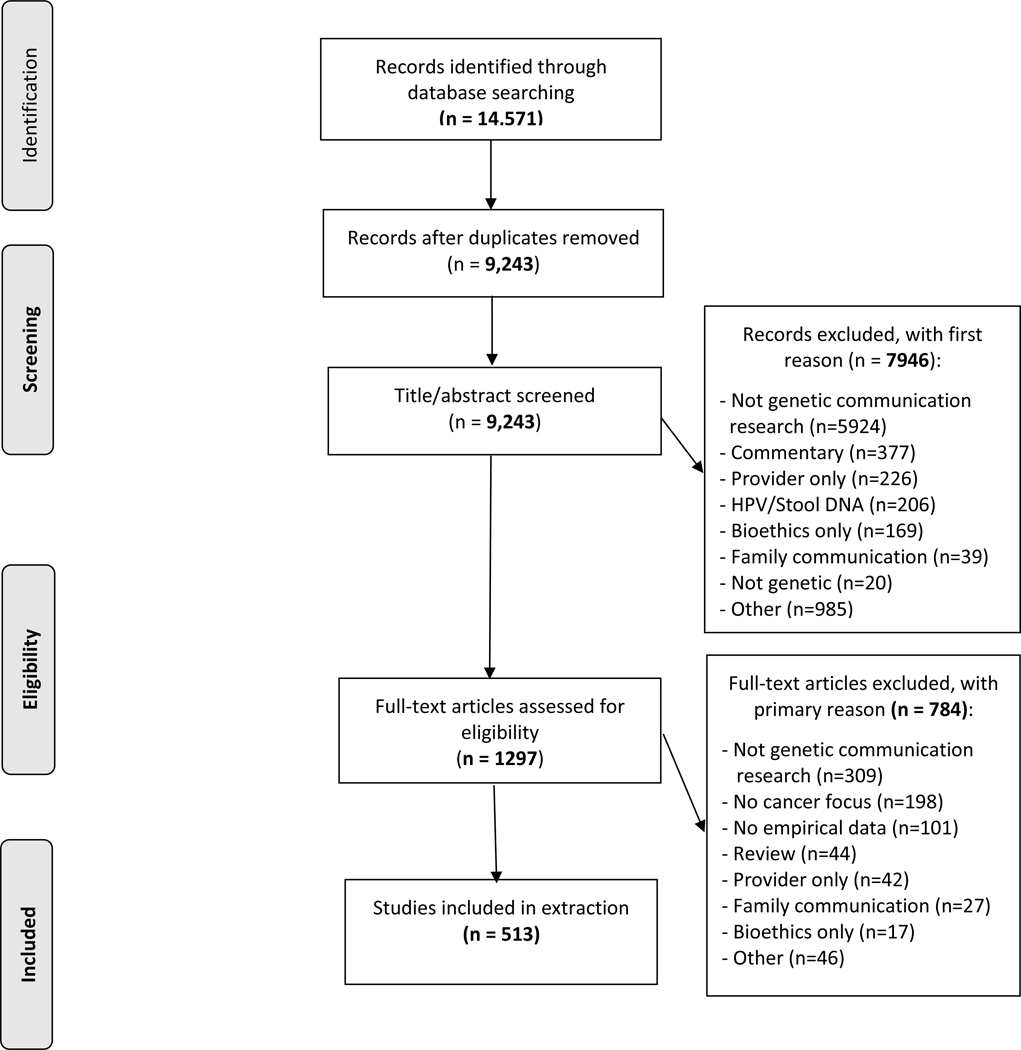
A total of 9,243 unique papers were initially identified (Figure 2). All titles and abstracts were reviewed by a trained coder, with 20% reviewed by a second coder. The level of agreement between coders for the decision to advance the paper to full-text review was adequate17 (Cohen’s kappa = 0.70; inter-coder agreement = 92%). For dual-coded articles, if at least one coder indicated “yes,” the article progressed to the next stage of review. All full-text articles (n=1,297) were then evaluated by two independent coders; disagreements were resolved by a third coder. 513 articles were included in the final sample (Supplemental Table).
Figure 2.
PRISMA flow diagram.
Data Extraction
We developed a detailed data extraction protocol through several iterative rounds of review and testing (Supplemental Appendix B). The protocol included five major sections: (1) general study information; (2) quantitative outcomes and/or qualitative themes; (3) cancer and genetic area of focus; (4) participant characteristics; and (5) return of results processes. A single coder extracted information from each article, with a second coder extracting information for 10% of the articles. A third coder reconciled all discrepancies and determined a final code. Across 84 codes, only six had inter-coder reliability that fell beneath pre-determined thresholds (i.e., Cohen’s kappa of 0.60 and 80% inter-coder agreement). The consensus coder also reviewed the use of these six codes.
RESULTS
Study Characteristics
Over half (57%) of included studies were conducted in the United States (Table 1). A range of study designs was used; the majority of papers (54%) utilized a quantitative, observational design (e.g., longitudinal, cross-sectional survey). The next most common study design was qualitative (e.g., interviews, focus groups; 23%), whereas about 14% described randomized intervention trials or quasi-experiments (i.e., had a comparison group). The median sample size was 214 for quantitative, observational studies; 27 for qualitative studies; and 200 for intervention studies.
Table 1.
Characteristics of included studies (n=513).
| Characteristic | n | % |
|---|---|---|
| Countriesa | ||
| United States | 292 | 57.0 |
| The Netherlands | 51 | 10.0 |
| United Kingdom | 31 | 6.0 |
| Canada | 30 | 6.0 |
| Other(s) | 121 | 23.3 |
| Study Designs | ||
| Quantitative, observational | 279 | 54.4 |
| Qualitative | 120 | 23.4 |
| Randomized intervention or quasi-experiment | 70 | 13.6 |
| Mixed-methods | 32 | 6.2 |
| Case studies | 12 | 2.3 |
| Cancer genetic or genomic (CGT) continuum phasea | ||
| Baseline knowledge and awareness | 160 | 31.1 |
| Decision making about uptake of CGT | 112 | 21.8 |
| Process of CGT communication | 106 | 20.7 |
| Outcomes of CGT results disclosure | 243 | 47.3 |
| Cancer only condition studied | 421 | 82.1 |
| Cancer typea | ||
| Breast and/or ovarian cancer | 276 | 53.8 |
| Cancer – general | 73 | 14.2 |
| Multiple cancers or cancer syndromes | 55 | 10.7 |
| Colorectal cancer | 51 | 10.0 |
| Type of CGT informationa | ||
| BRCA1/BRCA2 | 261 | 50.9 |
| Lynch syndrome/HNPCC | 40 | 7.8 |
| Exome or genome sequencing | 30 | 5.8 |
| SNP/polygenic risk score | 19 | 3.7 |
| Gene panel/multi-gene panel | 9 | 1.7 |
| p53/Li-Fraumeni | 7 | 1.4 |
| p16 | 7 | 1.4 |
| Pharmacogenetic/pharmacogenomic | 3 | 0.6 |
| Hypothetical information used | 115 | 22.4 |
| Direct-to-consumer information used | 34 | 6.6 |
Categories not mutually exclusive
CGT Continuum Phases
With regard to phases of the CGT continuum, which were not mutually exclusive, the most common focus was on outcomes of CGT results disclosure (47%).e.g.,18 About 31% of studies focused on examining baseline CGT knowledge and awareness. e.g.,19 Approximately equal proportions of studies focused on decision making about CGT uptake (22%) e.g.,20 and the process of CGT communication (e.g., return of results; 21%).e.g.,21. Illustrative examples of studies in each CGT continuum phase, organized by type of study design, are provided in Supplemental Box 1.
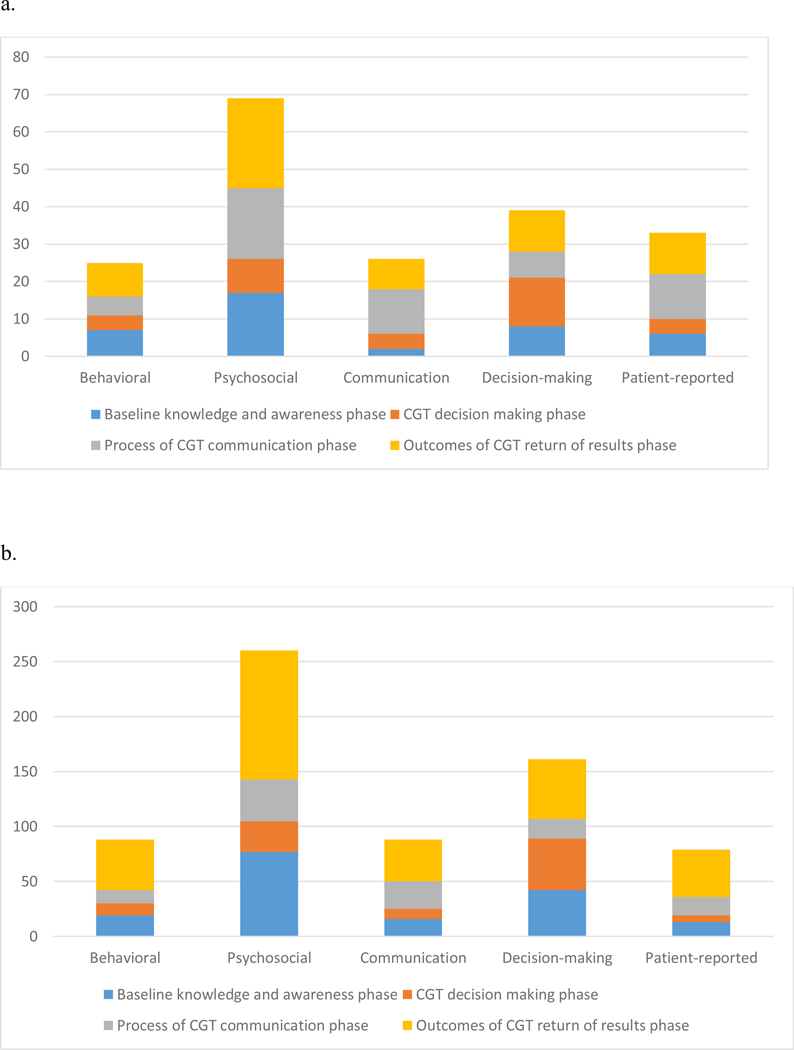
Outcomes Assessed
Figure 3 shows study quantitative outcomes/qualitative themes by CGT continuum phase, organized by study design. Psychosocial outcomes/themes (e.g., anxiety, distress) were most common. In randomized trials and quasi-experiments (Figure 3a) and quantitative, observational studies (Figure 3b), decision making outcomes/themes were more commonly examined than communication outcomes/themes. Behavioral outcomes/themes (e.g., health behaviors, behavioral intentions) were the least common category for all study designs except quantitative observational designs, for which patient-reported outcomes/themes were less common (Figure 3b).
Figure 3.
Outcome and theme categories by study design and phase of CGT continuum.
a. Outcome categories for randomized trials and quasi-experiments (n=70).
b. Outcome categories for quantitative observational studies (n=279).
c. Theme categories for qualitative studies (n=120).
d. Outcome and theme categories for mixed methods studies (n=32).
Cancer and CGT Focus
The vast majority of included papers focused on genetic and genomic information related to cancer only (82%; Table 1). About one quarter (22%) analyzed responses to hypothetical CGT information. Of the studies presenting hypothetical information, the majority (53%) examined baseline CGT knowledge and awareness. The most common types of cancer studied were breast and/or ovarian (54%), followed by colorectal cancer (10%). Consistent with this, the most common type of genetic information discussed was the BRCA1/BRCA2 genes (51%), which affect risk of breast and ovarian cancer. The proportion of papers in each phase discussing BRCA1/2 testing was 39% for baseline knowledge and awareness of CGT, 51% for decision making about uptake of CGT, 53% for process of communication about CGT, and 60% for outcomes of communication of CGT results. Only 8% of papers focused on Lynch syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC). Few papers examined information generated through direct-to-consumer genetic testing (7%).
Next Generation Technologies
Relatively few papers focused on genomic information generated by exome or genome sequencing (5%) or multi-gene panels (2%). Of these papers, 17% related to baseline knowledge and awareness of these technologies, 33% to decision making about uptake of sequencing or multi-gene panels, 27% to the process and 23% to the outcomes of communication of test results. Similar to the overall sample, the most common type of outcome in these papers was psychosocial (74% of papers), followed by decision making (49%). Few papers assessed communication (18%) or behavioral (10%) outcomes.
Characteristics of Study Participants
The most commonly reported characteristics of study participants were sex (reported in 93% of studies) and age (reported in 88%; Table 2). In almost half of studies (45%), all participants were women. In contrast, only 1% of studies had all male participants. Few studies focused on younger adults; 7% of studies included participants with a mean age of 35 years or younger. In addition, few studies included pediatric populations; only 5% of studies included minors under 18 years of age. Only 7% of studies recruited participants based on their parental status. Few studies (3%) reported whether or not participants were from rural/frontier areas. Reporting of socioeconomic status of participants was variable. Although 70% of studies reported on participants’ educational attainment, only 28% reported on income. Among the U.S. studies that reported educational data, only 2% met our a priori threshold of having a sample with 70% of participants with a high school education or less.
Table 2.
Characteristics of participants in included studies (n=513).
| Characteristic | n | % |
|---|---|---|
| Age (n=478)a | ||
| Mean age 35 years or younger | 34 | 7.1b |
| Minors less than 18 included | 24 | 5.0b |
| Parents (n=513) | 35 | 6.8 |
| Sex (n=479)a | ||
| 100% women | 227 | 47.3c |
| 100% men | 7 | 1.4c |
| ≥25% men | 179 | 37.3c |
| Race/ethnicity (n=279)a | ||
| ≥70% White | 177 | 63.4d |
| 0% White | 47 | 16.8d |
| ≥70% Black/African American | 13 | 4.6d |
| 0% Black/African American | 144 | 51.6d |
| ≥70% Hispanic/Latino | 10 | 3.6d |
| 0% Hispanic/Latino | 181 | 64.9d |
| ≥70% Asian/Pacific Islander | 3 | 1.1d |
| 0% Asian/Pacific Islander | 202 | 72.4d |
| ≥70% Native American/Alaska Native | 1 | 0.4d |
| 0% Native American/Alaska Native | 244 | 87.4d |
| Ashkenazi Jewish (n=49) | ||
| 100% Ashkenazi Jewish | 10 | 20.4e |
| Income reported | 145 | 28.3 |
| Education reported | 360 | 70.2 |
| Education reported for U.S. studies | 208 | |
| ≥70% had high school education or less | 4 | 1.9f |
| ≥70% had some college or greater | 140 | 67.3f |
| Rurality reported | 14 | 2.7 |
Not mutually exclusive
Percentage of 478 studies reporting age data
Percentage of 479 studies reporting sex of participants
Percentage of 279 studies reporting race/ethnicity of participants
Percentage of 49 studies reporting whether or not participants were Ashkenazi Jewish
Percentage of U.S. based studies
The race and/or ethnicity of study participants was reported in about half of the studies (54%); most U.S. studies (86%) reported these data. Among studies that reported race/ethnicity, we examined what proportion had 70% or more of participants from a specific racial or ethnic group. In total, 63% of studies met this threshold for White/Caucasian, 5% for Black/African American, 4% for Hispanic/Latino, 1% for Asian/Pacific Islander, and 0.4% for Native American/Alaska Native. We also found that 17% of included studies had no White/Caucasian participants, 52% had no Black/African American participants, 65% had no Hispanic/Latino participants, 72% had no Asian/Pacific Islander participants, and 87% had no Native American/Alaska Native participants.
DISCUSSION
This scoping review examined the characteristics of more than 500 recently published papers focused on the communication of CGT information. Although a range of study designs have been employed in this area of research, the majority of papers utilized an observational quantitative design. This finding suggests a need for intervention trials to examine the effects of different communication formats, channels, and strategies in presenting genetic and genomic information to the public, informing shared decision making about testing, and enabling accessible and patient-centered approaches to returning genetic test results to patients and their families. With respect to the CGT continuum phases, the review also showed that there has been more attention to the outcomes of CGT results disclosure than to decision making regarding testing or the process of results disclosure. At present, studies that examine the outcomes of results disclosure often do not examine the communication process (e.g., what, how, when, and by whom results are delivered) in detail. The field needs a more fine-grained understanding of the effects of the communication processes and contexts on the intermediate and distal outcomes of CGT results disclosure.
Our findings also show a continuing emphasis on psychosocial outcomes in this literature. This finding is consistent with older reviews showing a focus on anxiety and distress outcomes following return of genetic test results.8–10 Although emotional and cognitive psychosocial outcomes are certainly important, the literature needs to expand. Models of patient-provider communication14 and health behavior change suggest that behavioral, decision-making, clinical communication, patient-reported, and psychosocial outcomes are all critical to investigate in order to improve patient health outcomes. Additional research is needed to understand under what circumstances, in which contexts, and in what populations, disclosure of genetic and genomic information affects health behavior change. A greater focus on outcomes such as health behaviors and medical decisions, in concert with more in-depth examination of the processes of return of results communication, can shed further light on the contexts in which CGT information may affect behavior change.
The review also showed a strong focus on breast and ovarian cancer and BRCA1/2 in this literature. In contrast, relatively few papers focused on Lynch syndrome, despite the longstanding availability of this testing, strength of evidence for the clinical importance of testing for genes associated with Lynch syndrome and the growth of universal screening programs for patients diagnosed with colorectal and endometrial cancer.22–24 This is a clear gap for which additional research is needed.25 In addition, despite the rapidly growing importance of next-generation technologies such as multi-gene panel tests and genome sequencing,3,26–29 relatively few studies to date have focused on communication of results generated by these technologies. Although tests for hereditary breast and ovarian cancer and Lynch syndrome have been available longer and currently have the strongest evidence for return of results in clinical settings,32 it is important for researchers investigating CGT communication processes to conduct studies as soon as possible in order to inform how to effectively return results from next generation technologies that are being introduced into clinics so that the results of communication research are clinically relevant in the era of precision medicine. Particularly given the greater complexity, larger amount of information provided, and greater possibility of uncertainty and ambiguity inherent in such results,26,27,29–31 future research is needed that investigates the process and outcomes of communication of these results and, when examining outcomes, moves beyond psychosocial outcomes to examine how such information may affect behavior and how patients communicate about the results with others. Furthermore, although our prior landscape analysis identified a substantial number of prior reviews investigating direct-to-consumer genetic testing,13 relatively few empirical studies included in this scoping review did so. Studies investigating this increasingly important phenomenon are needed to understand CGT communication with patients and the public that occurs without a provider as a learned intermediary.33
Finally, the findings from this scoping review highlight the strong need to diversify the population subgroups included in studies of CGT communication. Reporting of demographic factors should be improved in order to better characterize study samples. However, the participant data that have been reported showed important gaps in inclusion. Age and sex were most commonly reported, and these data showed the need for increasing research with pediatric and young adult populations and with men. Prior reviews have documented persistent barriers to access and use of genetic and genomic information by minority racial and ethnic groups.34 Only about half of the included studies reported on the race/ethnicity of participants, making it more difficult to examine racial and ethnic disparities. The data reported revealed a clear lack of inclusion of participants from racial and ethnic groups other than Caucasians. Aspects of socioeconomic status such as income were rarely reported, making it difficult to examine disparities. Reported educational data showed that very few studies focused on samples with lower levels of education. Although the limited inclusion of individuals from minority racial and ethnic groups has been noted previously, our scoping review also highlights lack of inclusion for those with limited educational attainment and for pediatric and young adult populations. The findings also identify lack of inclusion of participants from minority racial and ethnic groups as a consistent and pervasive problem throughout the literature on CGT communication. As the cost of genomic technologies decreases and these technologies become more accessible, guidance is needed regarding effective and tailored communication approaches across population subgroups.34,35 The issue of inclusion in CGT communication research is particularly pressing given the goals of precision medicine initiatives such as the All of Us Research Program to reach diverse populations.
The results of this review should be considered in light of its limitations. The lack of standard MeSH headings and key words in this literature made it difficult to locate relevant studies. Establishing consistent key terms for CGT communication processes (e.g., genetic communication, cancer) would facilitate future reviews. Because of our interest in describing the characteristics of the peer-reviewed literature, unpublished studies were not included. Studies from different countries described race/ethnicity and socioeconomic status differently; we focused on describing results from the U.S. which were more likely to report such characteristics. Because this is a scoping review, we were not able to synthesize the findings to generate overall effect sizes or summarize the effect of a specific communication process, as is done with a systematic review or meta-analysis. Our aims were to present a map of the literature on CGT communication and identify important research gaps; future reviews can hone in on a subset of studies to investigate a particular research question in detail.
In summary, this scoping review highlights areas of strength in recent literature on communication of CGT information. In particular, much is known regarding communication about the genes BRCA1/2, and psychosocial outcomes of testing have been a strong area of emphasis. Research gaps were also identified, including the need for additional studies on communication processes; expanding the types of outcomes studied; increased research related to Lynch syndrome and next-generation technologies; and inclusion of diverse samples with complete descriptions of participant characteristics. Addressing these research gaps can help improve the health of cancer patients and those at-risk of cancer, and reduce disparities in access to and use of CGT across population subgroups.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support was provided by the National Cancer Institute’s Behavioral Research Program through HHSN261201700078P. We also acknowledge the direct financial support for the research reported in this publication provided by the Huntsman Cancer Foundation. We would like to acknowledge assistance from the CTSA Systematic Review Core at the University of Utah. We also thank Courtney Tern and Angela Falisi for their work as coders, and Bradford Hesse and Charlisse Caga-Anan for their invaluable contributions to the study design.
REFERENCES
- 1.Kensler TW, Spira A, Garber JE, Szabo E, Lee JJ, Dong Z. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev Res 2016;9(1):2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judkins T, Leclair B, Bowles K, et al. Development and analytical validation of a 25-gene next generation sequencing panel that includes the BRCA1 and BRCA2 genes to assess hereditary cancer risk. BMC Cancer 2015;15:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. J Natl Comp Cancer Network 2014;12(9):1339–1346. [DOI] [PubMed] [Google Scholar]
- 4.Hiraki S, Rinella ES, Schnabel F, Oratz R, Ostrer H. Cancer risk assessment using genetic panel testing: considerations for clinical application. J Genet Couns 2014;23:604–617. [DOI] [PubMed] [Google Scholar]
- 5.Beery TA, Williams JK. Risk reduction and health promotion behaviors following genetic testing for adult-onset disorders. Genet Test 2007;11(2):111–123. [DOI] [PubMed] [Google Scholar]
- 6.Burke W, Psaty BM. Personalized medicine in the era of genomics. J Am Med Assoc 2007;298(14):1682–1684. [DOI] [PubMed] [Google Scholar]
- 7.Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol 2013;31(15):1803–1805 [DOI] [PubMed] [Google Scholar]
- 8.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med 2008;10(1):19–32. [DOI] [PubMed] [Google Scholar]
- 9.Butow PN, Lobb EA, Meiser B, Barratt A, Tucker KM. Psychological outcomes and risk perception after genetic testing and counselling in breast cancer: A systematic review. Med J Aust 2003;178:77–81. [DOI] [PubMed] [Google Scholar]
- 10.Kaphingst KA, McBride CM. Patient responses to genetic information: studies of patients with hereditary cancer syndromes identify issues for use of genetic testing in nephrology practice. Semin Nephrol 2010;30(2):203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollands GJ, French DP, Griffin SJ. The impact of communicating genetic risks of disease on risk-reducing health behaviour. BMJ 352(8049):1/2p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride CM, Wade CH, Kaphingst KA. Consumers’ views of direct-to-consumer genetic information. Ann Rev Genom Human Genet 2010;11:427–446. [DOI] [PubMed] [Google Scholar]
- 13.Peterson E, Chou W-YS, Gaysynsky A, et al. Communication of cancer-related genetic and genomic information: a landscape analysis of reviews. TBM 2018;8:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein RM, Street RL. Patient-centered communication in cancer care: promoting healing and reducing suffering. Bethesda, MD:National Cancer Institute, 2007. [Google Scholar]
- 15.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Method 2005;8(1):19–32 [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine. 2009;6(7). [PMC free article] [PubMed] [Google Scholar]
- 17.Fleiss J, Levin B, Paik M. Statistical methods for rates and proportions. 3rd ed New York: John Wiley & Sons, 2003. [Google Scholar]
- 18.Aktan-Collan K, Kaariainen H, Jarvinen H, et al. Psychosocial consequences of predictive genetic testing for Lynch syndrome and associations to surveillance behaviour in a 7-year follow-up study. Fam Cancer 2013;12(4):639–646. [DOI] [PubMed] [Google Scholar]
- 19.Agurs-Collins T, Ferrer R, Ottenbacher A, Waters EA, O’Connell ME, Hamilton JG. Public Awareness of Direct-to-Consumer Genetic Tests: Findings from the 2013 U.S. Health Information National Trends Survey. J Cancer Educ 2015;30(4):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderfer MA, Zelley K, Lindell RB, et al. Parent decision-making around the genetic testing of children for germline TP53 mutations. Cancer 2015;121(2):286–293. [DOI] [PubMed] [Google Scholar]
- 21.Thomassen G, Sarangi S. Evidence-based familial risk explanations in cancer genetic counselling. Health Risk Soc 2012;14(7–8):607–626. [Google Scholar]
- 22.Vindigni S, Kaz A. Universal screening of colorectal cancers for Lynch syndrome: challenges and opportunities. Dig Dis Sci 2016;61(4):969–976. [DOI] [PubMed] [Google Scholar]
- 23.Hampel H Point: justification for Lynch syndrome screening among all patients with newly diagnosed colorectal cancer. J Natl Comp Cancer Network 2010;8:597–601. [DOI] [PubMed] [Google Scholar]
- 24.Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/familial high-risk assessment: Colorectal version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Comp Cancer Network 2016;14:1010–1030. [DOI] [PubMed] [Google Scholar]
- 25.Slater MD, Long M, Bettinghaus EP, Reineke JB. News coverage of cancer in the United States: a national sample of newspapers, television, and magazines. J Health Commun 2008;13(6):523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol 2013;31(10):1267–1270. [DOI] [PubMed] [Google Scholar]
- 27.Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. 2014; https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Accessed 05/03/18.
- 28.Easton DF, Pharoah PDP, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372(23):2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe Schneider K, Anguiano A, Axell L, et al. Collaboration of Colorado cancer genetic counselors to integrate next generation sequencing panels into clinical practice. J Genet Couns 2014;23:640–646. [DOI] [PubMed] [Google Scholar]
- 30.Slavin TP, Niell-Swiller M, Solomon I, et al. Clinical application of multigene panels: challenges of next-generation counseling and cancer risk management. Front Oncol 2015;5:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills R, Haga SB. Genomic counseling: next generation counseling. J.Genet Couns 2014;23:689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury MJ, Bowen MS, Clyne M, et al. From public health genomics to precision public health: a 20-year journey. Genet Med 2017;ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes. 2018; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm599560.htm. Accessed 5/7/18
- 34.Kaphingst KA, Goodman MS. Importance of race and ethnicity in indivdiuals’ use of and responses to genomic information. Pers Med 2016;13(1):1–4. [DOI] [PubMed] [Google Scholar]
- 35.McBride CM, Bowen D, Brody LC, et al. Future health applications of genomics: Priorities for communication, behavioral, and social sciences research. Am J Prev Med 2010;38(5):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray S, O’Grady C, Karp L, et al. Risk information exposure and direct-to-consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer. Cancer Epi Biomarkers Prev 2012;18(4):1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez CV, Bouffet E, Malkin D, et al. Attitudes of parents toward the return of targeted and incidental genomic research findings in children. Genet Med 2014;16(8):633–640. [DOI] [PubMed] [Google Scholar]
- 38.Chalela P, Pagan JA, Su D, Munoz E, Ramirez AG. Breast cancer genetic testing awareness, attitudes and intentions of Latinas living along the US-Mexico border: a qualitative study. J Commun Medicine Health Educ 2012;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genetic Couns 2010;19(6):618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuppermann M, Wang G, Wong S, et al. Preferences for outcomes associated with decisions to undergo or forgo genetic testing for Lynch syndrome. Cancer 2013;119(1):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaphingst KA, McBride CM, Wade C, Alford SH, Brody LC, Baxevanis AD. Consumers’ use of web-based information and their decisions about multiplex genetic susceptibility testing. J Med Internet Res 2010;12(3):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meiser B, Gleeson M, Kasparian N, et al. There is no decision to make: experiences and attitudes toward treatment-focused genetic testing among women diagnosed with ovarian cancer. Gynecol Oncol 2012;124(1):153–157. [DOI] [PubMed] [Google Scholar]
- 43.Leventhal KG, Tuong W, Peshkin BN, et al. “Is it really worth it to get tested?”: primary care patients’ impressions of predictive SNP testing for colon cancer. J Genetic Couns 2013;22(1):138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albada A, van Dulmen S, Ausems MG, Bensing JM. A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial. Genet Med 14(5):535–542. [DOI] [PubMed] [Google Scholar]
- 45.Salemink S, Dekker N, Kets CM, van der Looij E, van Zelst-Stams WA, Hoogerbrugge N. Focusing on patient needs and preferences may improve genetic counseling for colorectal cancer. J Genet Couns 2013;22(1):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aasen T, Skolbekken JA, Author A, et al. Preparing for and communicating uncertainty in cancer genetic counselling sessions in Norway: an interpretative phenomenological analysis. Health Risk Soc 2014;16(4):370–389. [Google Scholar]
- 47.Hitch K, Joseph G, Guiltinan J, Kianmahd J, Youngblom J, Blanco A. Lynch syndrome patients’ views of and preferences for return of results following whole exome sequencing. J Genet Couns 2014;23(4):539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smit AK, Espinoza D, Newson AJ, et al. A pilot randomized controlled trial of the feasibility, acceptability, and impact of giving information on personalized genomic risk of melanoma to the public. Cancer Epi Biomarker Prev 2017;26(2):212–221. [DOI] [PubMed] [Google Scholar]
- 49.Pieterse AH, Ausems MG, Spreeuwenberg P, van Dulmen S. Longer-term influence of breast cancer genetic counseling on cognitions and distress: smaller benefits for affected versus unaffected women. Patient Educ Couns 2011;85(3):425–431. [DOI] [PubMed] [Google Scholar]
- 50.Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. “It’s not like judgment day”: public understanding of and reactions to personalized genomic risk information. J Genet Couns 2012;21(3):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanderson SC, Linderman MD, Suckiel SA, et al. Psychological and behavioural impact of returning personal results from whole-genome sequencing: the HealthSeq project. Eur J Hum Genet 2017;25(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.