Abstract
Severe coronavirus disease (COVID-19) can induce serious complications, including acute respiratory distress syndrome, septic shock, and acute kidney injury. However, few reports have associated COVID-19 with pancreatitis. We herein report the case of a 55-year-old patient who developed acute pancreatitis associated with severe COVID-19 pneumonia and was successfully treated with veno-venous extracorporeal membrane oxygenation (ECMO). Elevated pancreatic enzymes levels and computed tomography findings led to the diagnosis of acute pancreatitis due to COVID-19. Although we found that severe COVID-19 pneumonia can lead to pancreatitis, the underlying pathophysiology remains unknown.
Keywords: coronavirus disease, COVID-19, pancreatitis
Introduction
Typical symptoms of coronavirus disease (COVID-19) include a fever (43.8% of patients at hospital admission), cough (67.8%), and fatigue (38.1%), with more atypical abdominal symptoms including nausea or vomiting (5%) and diarrhea (3.8%) (1). Although severe COVID-19 can lead to serious effects on several organ systems, including acute respiratory distress syndrome (15.6%), septic shock (6.4%), and acute kidney injury (2.9%) (2, 3), there have been few reports of patients with COVID-19 developing pancreatitis (4).
We herein report a patient who developed acute pancreatitis in association with severe COVID-19 pneumonia and was treated successfully with veno-venous extracorporeal membrane oxygenation (ECMO).
Case Report
A 55-year-old patient with no significant medical history had a fever and cough for 1 week prior to seeking care from a local physician. He was diagnosed with COVID-19 pneumonia when the results of a real-time reverse transcriptase-polymerase chain reaction test for COVID-19 nucleic acid were positive; he was subsequently hospitalized (day 8 after the symptom onset). He was administered oral antiviral agents (lopinavir-ritonavir) and intravenous antibiotics (azithromycin and ceftriaxone). On day 13, his respiratory status worsened, with an oxygen saturation of 94% with 8 L of oxygen/min. He was subsequently transferred to our facility for a higher level of care.
The patient was intubated for severe respiratory distress in our emergency room using lung-protective mechanical ventilatory strategies. After noting the ratio of arterial oxygen partial pressure to fractional inspired oxygen as 70, oxygen index of 18, and Murray score of 3.5 for acute lung injury, percutaneous veno-venous ECMO support was initiated using a right femoral 24-Fr drainage cannula and right cervical 16-Fr return cannula. On the same day, we also started intravenous antibiotics (meropenem and vancomycin), an oral antiviral agent (favipiravir), and continuous renal replacement therapy (CRRT) for acute kidney injury.
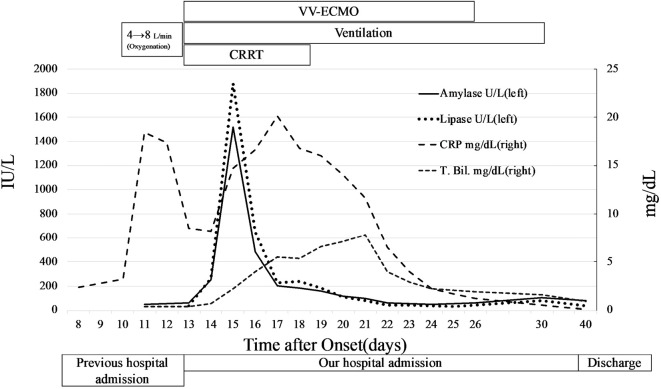
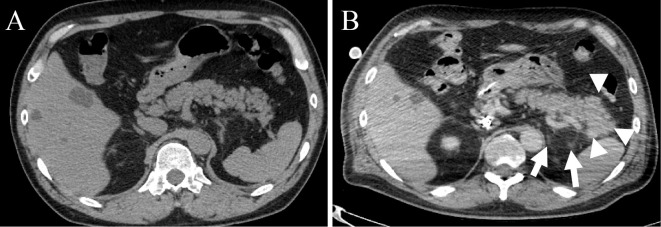
On day 14, the results of routine blood tests showed elevated pancreatic enzymes, including amylase level of 252 U/L (reference range, 44-132 U/L) and lipase level of 263 U/L (reference range, 16-55 U/L) (Fig. 1). Initial investigations revealed a normal corrected serum calcium level and mildly elevated triglycerides (Table). The patient was unable to describe any abdominal pain because of sedation. Acute pancreatitis was suspected, and an ultrasonographic examination was performed. Ultrasonography revealed no pancreatic duct dilation, tumor in the pancreas, or bile duct stones. We did not attempt to acquire an abdominal computed tomography (CT) scan because of the high risk of accidental removal of the ECMO tubes and sudden change in condition when moving the patient to the CT scan room. While the sedative agent was switched from propofol to midazolam due to suspicion of drug-induced pancreatitis, the pancreatic enzyme levels continued to increase. On day 23, contrast-enhanced CT showed diffuse parenchymal enlargement and stranding of the surrounding retroperitoneal fat (Fig. 2). These findings were thought to be changes subsequent to acute pancreatitis. Eventually, he was diagnosed with acute pancreatitis based on elevated pancreatic enzymes and CT findings.
Figure 1.
The patient's clinical course and pancreatic enzyme levels. CRP: C-reactive protein, T. Bil: total bilirubin, VV-ECMO: veno-venous extracorporeal membrane oxygenation, CRRT: continuous renal replacement therapy
Table.
Patient's Initial Blood Test Findings on Admission to Our Hospital.
| WBC | 6,300 | /μL | TP | 5.5 | g/dL |
| RBC | 410 | ×104/μL | Alb | 2.4 | g/dL |
| Hb | 12.5 | g/dL | BUN | 15.6 | mg/dL |
| Ht | 37.9 | % | Cre | 0.85 | mg/dL |
| Plt | 18.5 | ×104/μL | Na | 140 | mEq/L |
| APTT | 34.5 | sec | K | 3.8 | mEq/L |
| PT | 93 | % | Cl | 102 | mEq/L |
| PT-INR | 1.04 | Ca | 8.1 | mg/dL | |
| Fibrinogen | 543 | mg/dL | AST | 78 | U/L |
| FDP | 7.30 | μg/mL | ALT | 70 | U/L |
| Ddimer | 1.72 | μg/dL | LDH | 770 | U/L |
| ALP | 46 | U/L | |||
| T-Bil | 0.4 | mg/dL | |||
| CRP | 8.53 | mg/dL | |||
| Amylase | 63 | U/L | |||
| Lipase | 39 | U/L | |||
| TG | 185 | mg/dL |
Alb: albumin, ALP: alkaline phosphatase, APTT: activated partial thromboplastin time, BUN: blood urea nitrogen, CK: creatine kinase, Cre: creatinine, FDP: fibrin/fibrinogen degradation products, Fib: fibrinogen, Hb: hemoglobin, Ht: hematocrit, Lac: lactate, Plt: platelet, PT: prothrombin time, PT-INR: PT-international normalized ratio, RBC: red blood cell, T-Bil: total bilirubin, TG: triglyceride, TP: total protein, WBC: white blood cell
Figure 2.
Contrast-enhanced computed tomography scan of the patient's pancreas. (A) Pancreas without stones or tumors on the first day in our hospital. (B) Pancreas with diffuse parenchymal enlargement (arrowhead) and stranding of the surrounding retroperitoneal fat (arrow). We believe these findings to be changes subsequent to acute pancreatitis.
As the COVID-19 pneumonia in the patient necessitated conservative fluid management, pneumonia and pancreatitis were treated carefully with arterial pressure-based cardiac output and ultrasonography to avoid hypovolemia. CRRT for acute kidney injury was also initiated, with nafamostat mesylate as the anticoagulant. Amylase and lipase levels slowly decreased and were restored to normal levels on day 22. His respiratory status also improved, and ECMO was withdrawn on day 26, while the respirator was withdrawn on day 30. He eventually required no supplemental oxygenation and was discharged on day 40.
Discussion
The present report describes the case of a previously healthy patient who developed acute pancreatitis in association with severe COVID-19 pneumonia and was treated with veno-venous ECMO. In this case, pancreatitis developed concurrently with worsening COVID-19 pneumonia; blood test results indicated pancreatitis the day after ECMO initiation.
Another recent case report described a patient with COVID-19 pneumonia who experienced abdominal pain and was diagnosed with acute pancreatitis with elevated pancreas-specific plasma amylase (4). In our present case, the patient had severe respiratory distress that compromised his ability to complain of abdominal pain; however, routine blood tests, including pancreatic enzymes, revealed an abnormal pancreatic function. Routine blood tests, including a pancreatic enzyme panel, may be essential for detecting early pancreatitis in patients with severe COVID-19 pneumonia.
Infections with the mumps paramyxovirus, cytomegalovirus, and herpes simplex virus have also been associated with the development of acute pancreatitis, although the underlying pathophysiological mechanisms remain poorly understood (5). At present, pancreatitis induced by viral infection is a diagnosis of exclusion. In this patient, we ruled out other causes of pancreatitis, including alcohol, trauma, biliary obstruction/gall stones, tumor, hypertriglyceridemia, and hypercalcemia. Drug-induced acute pancreatitis is rare but should be excluded in such cases. Of note, we could not rule out drug-induced pancreatitis completely in the present patient. Various drugs have been reported to cause drug-induced pancreatitis, including antiepileptic drugs, antibiotics, antiviral agents, and immunosuppressants (6). Drugs that induce pancreatitis are classified into four types (Class I-IV), and Class I/II drugs have the potential to cause acute pancreatitis (7). Although several medications, including antibiotics (azithromycin, ceftriaxone, meropenem, and vancomycin) and antiviral agents (lopinavir-ritonavir), were administered to this patient, those drugs are not included among these classes. We therefore believe that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may have induced pancreatitis in this patient, although we presently do not fully understand the causal mechanism.
While a conservative fluid administration strategy is recommended during the treatment of COVID-19 pneumonia (8), a more aggressive strategy is advised when treating acute pancreatitis (9). In this case, we detected the pancreatitis early and effectively controlled the intravascular fluid volume through arterial pressure-based cardiac output and ultrasonography, without inducing hypo- or hypervolemia. In addition, we adjusted this patient's pharmacologic regimen to include drugs that were less harmful to the pancreas. Finally, we opted to use nafamostat mesylate as the anticoagulant for CRRT, a medication that is used in Japan to treat pancreatitis and achieve anticoagulation when heparin cannot be used, with evidence suggesting that it also blocks the activation of SARS-CoV-2 (10).
In conclusion, we believe that the early detection of pancreatitis in this patient and the specific treatment strategies described above resulted in the successful outcome. The present report demonstrates that patients with severe COVID-19 pneumonia can develop pancreatitis. Furthermore, similar to the mumps virus, SARS-CoV-2 may induce acute pancreatitis; however, the mechanism underlying this disease process requires further elaboration.
Written informed consent was obtained from the patient for the publication of this case report, and all procedures were performed in accordance with the Declaration of Helsinki.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Guan WJ, Zheng-yi N, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708-1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 41: 1858, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rismanbaf A, Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med 8: e17, 2020. [PMC free article] [PubMed] [Google Scholar]
- 4. Hadi A, Werge M, Kristiansen KT, et al. . Coronavirus disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology 20: 665-667, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parenti DM, Steinberg W, Kang P. Infectious causes of acute pancreatitis. Pancreas 13: 356-371, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Nitsche CJ, Jamieson N, Lerch MM, Mayerle JV. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol 24: 143-155, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 5: 648-661; quiz 644, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal AM, Manrai M, Kochhar R. Fluid resuscitation in acute pancreatitis. World J Gastroenterol 20: 18092-18103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother 64: e00754, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]