Abstract
Objective
We recently reported that routine cardiac computed tomography (CT) scans for radiofrequency catheter ablation (RFCA) of atrial fibrillation (AF) could steadily detect coronary artery lesions (CALs) and could accurately detect myocardial ischemia in 9% of patients with AF who underwent RFCA of AF. The aim of this study was to identify the independent risk factor (s) of myocardial ischemia in those patients.
Methods
Patient characteristics, blood test, CALs, Ordinal coronary calcium scoring (OCCS), and myocardial Ischemia (MI) were evaluated in 757 consecutive patients who underwent RFCA of AF.
Results
There were 685 and 72 patients without and with myocardial ischemia, respectively. A univariate analysis and multivariate statistical analysis revealed that a male gender (Odds ratio 2.11), a high number of co-existing coronary risk factors (NCCRF ≥3) (Odds ratio 2.03), an elevated brain natriuretic peptide level (BNP ≥100 pg/mL) (Odds ratio 3.37), an enlarged left atrial volume (≥90 mL) (Odds ratio 2.91), and a high OCCS (≥4) (Odds ratio 13.0) were independent risk factors of myocardial ischemia in patients undergoing RFCA of AF.
Conclusion
The high OCCS (≥4) by cardiac CT was the strongest independent risk factor of myocardial ischemia in those patients. However, physicians may be able to find the high risk patients of myocardial ischemia by evaluating a male gender, in the presence of a high NCCRF (≥3) and elevated BNP (≥100 pg/mL) without OCCS by cardiac CT in patients undergoing RFCA of AF.
Keywords: atrial fibrillation, brain natriuretic peptide, catheter ablation, computed tomography, myocardial ischemia, ordinal coronary calcium score
Introduction
The number of patients with atrial fibrillation (AF) has been increasing, and radiofrequency catheter ablation (RFCA) of AF has proven to be a useful strategy worldwide (1). A close relationship between AF and myocardial ischemia has been reported (2-4). Almost all institutions routinely perform cardiac computed tomography (CT) before RFCA of AF to evaluate the cardiac anatomy, especially of the left atrium (LA) and pulmonary veins (LA-PVs). We recently reported that, in patients with AF who underwent RFCA of AF, 1) both 64- and 320-line routine cardiac CT scans for RFCA of AF at the timing of the image acquisition of LA-PVs could evaluate coronary artery lesions in 93% of those patients, 2) the prevalence of myocardial ischemia was 9%, 3) significant relationships between the CHADS2 score and prevalence of severe coronary stenosis (>50%) and unevaluable coronary artery lesions, and myocardial ischemia were observed, and 4) the positive predict values of myocardial ischemia in patients with severe coronary stenosis (>50%) and unevaluable coronary artery lesions also significantly increased in accordance with the CHADS2 score (p=0.003) (5). Thus, the aim of this study was to identify the independent risk factor(s) for myocardial ischemia in patients undergoing RFCA of AF.
Materials and Methods
Study population and laboratory analysis
This study was approved by the institutional review committee and ethics review board of our hospitals. From April 2016 to September 2018, 757 consecutive patients with AF and without previous histories of coronary artery disease who were admitted to our hospitals to undergo RFCA of AF were evaluated. The type of AF was determined according to the 2018 JCS/JHRS guidelines on Non-Pharmacotherapy of Cardiac Arrhythmias (6). In brief, the paroxysmal, persistent, and long-lasting AF are defined as AF occurs from a few seconds to days and then stops on its own within 7 days, AF will last for more than seven days and will not correct itself on its own, and AF is a consistently high, erratic heartbeat that cannot be corrected, respectively. Major coronary risk factors were defined as hypertension, diabetes mellitus, dyslipidemia, family history of cardiovascular disease (CVD), ex- or current smoking, and obese [body mass index (BMI) >25 kg/m2]. Patients that could not use contrast because of renal dysfunction (serum creatinine ≥1.5 mg/dL) and those who were receiving hemodialysis were excluded. All patients had their history recorded and underwent a physical examination and laboratory analysis.
Computed tomography
All patients gave their written informed consent before imaging. The imaging technique has been described previously (5). In brief, after the sublingual administration of 0.3 mg of nitroglycerin, following a test bolus, 50-60 mL of nonionic contrast medium (Omnipaque 300, Amersham Health, Oslo, Norway) was injected at 20-22 mgI/kg/second via an antecubital vein. The imaging which was the ideal timing of the image acquisition of the LA-PVs started after confirming the pulmonary arteries had been contrasted by visual observation. Scanning was performed with a single breath hold in the cranio-caudal direction at the level of the aortic arch using a simultaneous acquisition of eight sections (each 0.5 mm), with a prospective electrocardiogram (ECG)-triggering set at 75% of the RR interval, beam collimation of 10 mm, and table speed 16.75 mm/0.5 second or fixed table (0.5 or 0.275 second tube rotation time, 120 kV, 500 mA or 750 mA) by 64-line or 320-line CT scans. Non-contrast cardiac CT was routinely performed before the contrast cardiac CT. Contiguous 0.5 mm axial CT (Aquilion, 64-line, 320-line, TOSHIBA, Tokyo, Japan) slices were reconstructed from the CT data using a soft tissue algorithm and the resulting DICOM data were recorded onto a CD-ROM. When the patients' heart rates were more than 70 bpm, the oral administration of 20 mg of the beta-blocker metoprolol tartrate and/or 0.5 mg of the minor tranquilizer etizolam was used.
Evaluation of coronary artery lesions by cardiac CT and the detection of myocardial ischemia
The coronary lesions were evaluated in all patients who underwent cardiac CT scans during the image acquisition of the LA-PVs for the RFCA of AF. Mild and moderate to severe stenoses were defined as ≤50% and >50% stenosis, respectively. Further, moderate and severe stenoses were defined as 50% to 75% stenosis and ≥75% stenosis, respectively, of that except for the left main trunk. A stenosis ≥50% of the left main trunk was defined as severe stenosis. Unevaluable coronary artery lesions were defined as severe coronary calcifications and/or banding artifacts (7) that made the evaluation of the coronary artery lesions impossible. The patients with moderate to severe coronary stenosis (>50%) and/or unevaluable coronary artery lesions were evaluated for myocardial ischemia before or after the RFCA of AF. To evaluate the myocardial ischemia, they underwent examinations combined with exercise stress testing, 201Tl-schintigraphy, and/or FFR measurements (8), as previously described (5). Myocardial ischemia was defined as positive exercise stress testing, 201Tl-schintigraphy, and/or fractionated flow reserve (FFR) measurements.
Evaluation of the ordinal coronary calcium scoring by non-contrast cardiac CT
Two cardiologist readers independently performed a visual assessment of the coronary artery calcium (CAC), as previously described (9). In brief, the ordinal coronary calcium scoring (OCCS) was scored by the thoracic radiologists as 0, 1, 2, or 3 to correspond to absent, mild, moderate, or severe CAC, respectively, in each coronary artery, including the left main trunk, left anterior descending, left circumflex, and right coronary artery. Mild CAC was defined as the involvement of less than one to two thirds of the vessel length, moderate as involvement of one to two thirds of the vessel length, and severe as involvement of greater than two thirds of the vessel length. The scores were summed to yield an OCCS of 0-12 for each scan (10). The total OCCS was then categorized as absent, mild, moderate, and severe, as 0, 1-3, 4-5, and 6≤, respectively.
Statistical Analysis
The numerical results are expressed in the text as the mean±standard deviation. Paired data were compared by a Fisher's exact test and Student's t-test or the Wilcoxon signed-rank test. The trend in the proportions and correlation between the prevalence of myocardial ischemia and the brain natriuretic peptide (BNP), number of co-existing coronary risk factors (NCCRF), OCCS, or LA volume, was determined by a Cochran-Armitage analysis. A multivariate logistic regression analysis was carried out to evaluate the association between ischemia and the male sex, age, CHADS2 score, diabetes mellitus, history of cerebrovascular apoplexy (CVA)/transient ischemic attacks (TIAs), NCCRF, BNP, diameter of the LA by echocardiography, LA volume by CT, and OCCS by CT. Factors with at least a borderline significance (p<0.15) according to a univariate analysis were included in the multivariate analysis. All analyses were performed with the SAS version 9.2 software program (SAS Institute, Cary, USA). A p of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics and laboratory analysis
Cardiac CT scans for RFCA of AF were performed in 757 patients [515 males and 242 females with a mean age of 68.9±9.2 years, body surface area (BSA) of 1.69±0.19 m2, and BMI of 23.2±3.5 kg/m2] (Table 1) with AF and without a previous history of CVD at our hospitals. No patients suffered from any cardiac CT-related complications except for a contrast-induced eruption. In all patients, the mean CHADS2 score was 2.05±1.31 points. The numbers of patients were 71 (9%), 207 (27%), 242 (32%), 137 (18%), 59 (8%), and 41 (5%) for those with a CHADS2 score of 0, 1, 2, 3, 4, and ≥5 point(s), respectively. The mean NCCRF was 1.94±1.22 factors. The numbers of patients were 86 (11%), 197 (26%), 251 (33%), 137 (18%), 66 (9%), 20 (3%), and 0 (0%) for those with an NCCRF of 0, 1, 2, 3, 4, 5, and 6, respectively. The prevalence of hypertension, diabetes mellitus, dyslipidemia, a family history of CVD, ex- or current smoking, obesity (BMI>25 kg/m2), a history of a CVA/TIA, paroxysmal AF, persistent AF, and long-lasting AF was 519 (69%), 161 (21%), 232 (31%), 137 (18%), 212 (28%), 205 (27%), 124 (16%), 460 (61%), 259 (34%), and 38 (5%), respectively. The values of the BNP, serum creatinine, left ventricular ejection fraction (LVEF), and the diameter of the LA by echocardiography, and LA volume and LA volume index by cardiac CT, were 93.7±109.9 pg/mL, 0.90±0.24 mg/dL, 62.8±9.8%, 39.2±6.7 mm, 89.5±29.3 mL, and 53.3±18.0 mL/m2, respectively. The number of 64- and 320-line CT scans were 465 (61%) and 292 (39%), respectively. The mean OCCS was 1.40±2.05. The numbers of patients were 466 (62%), 169 (22%), 80 (11%), and 42 (6%) for those with OCCS scores of 0 (absent), 1 to 3 (mild), 4 to 5 (moderate), and ≥6 (severe), respectively.
Table 1.
Patient Characteristics.
| All (n = 757) |
N-IS (n = 685) |
IS (n = 72) |
p value (N-IS vs. IS) |
||
|---|---|---|---|---|---|
| Male | 515 (68%) | 455 (66%) | 60 (83%) | 0.003 | |
| Age (years) | 68.9±9.2 | 68.7±9.3 | 70.7±8.2 | 0.071 | |
| Body surface area (m2) | 1.69±0.19 | 1.68±0.18 | 1.72±0.20 | 0.087 | |
| Body mass index (kg/m2) | 23.2±3.5 | 23.2±3.5 | 23.4±3.8 | 0.639 | |
| CHADS2 score (point (s)) | 2.05±1.31 | 1.98±1.27 | 2.72±1.43 | <0.001 | |
| 0 | 71 (9%) | 69 (10%) | 2 (3%) | - | |
| 1 | 207 (27%) | 199 (29%) | 8 (11%) | - | |
| 2 | 242 (32%) | 216 (32%) | 26 (36%) | - | |
| 3 | 137 (18%) | 123 (18%) | 14 (19%) | - | |
| 4 | 59 (8%) | 50 (7%) | 9 (13%) | - | |
| 5 | 26 (3%) | 20 (3%) | 6 (8%) | - | |
| 6 | 15 (2%) | 8 (1%) | 7 (10%) | - | |
| Co-existence | Hypertension | 519 (69%) | 463 (68%) | 56 (78%) | 0.077 |
| Diabetes mellitus | 161 (21%) | 136 (20%) | 25 (35%) | 0.003 | |
| Dyslipidemia | 232 (31%) | 203 (30%) | 29 (40%) | 0.063 | |
| Family history of CVD | 137 (18%) | 120 (18%) | 17 (24%) | 0.202 | |
| Ex- or current smoking | 212 (28%) | 186 (27%) | 26 (36%) | 0.108 | |
| Obese (Body mass index >25 kg/m2) | 205 (27%) | 184 (27%) | 21 (29%) | 0.676 | |
| History of CVA/TIA | 124 (16%) | 102 (15%) | 22 (31%) | 0.001 | |
| Number of co-existing coronary risk factors | 1.94±1.22 | 1.89±1.21 | 2.42±1.25 | <0.001 | |
| 0 | 86 (11%) | 81 (12%) | 5 (7%) | - | |
| 1 | 197 (26%) | 185 (27%) | 12 (17%) | - | |
| 2 | 251 (33%) | 230 (34%) | 21 (29%) | - | |
| 3 | 137 (18%) | 118 (17%) | 19 (26%) | - | |
| 4 | 66 (9%) | 53 (8%) | 13 (18%) | - | |
| 5 | 20 (3%) | 18 (3%) | 2 (3%) | - | |
| 6 | 0 (0%) | 0 (0%) | 0 (0%) | - | |
| Type of atrial fibrillation | Paroxysmal | 460 (61%) | 414 (60%) | 46 (64%) | 0.695 |
| Persistent | 259 (34%) | 235 (34%) | 24 (33%) | - | |
| Long-lasting | 38 (5%) | 36 (5%) | 2 (3%) | - | |
| Laboratory analysis | Brain natriuretic peptide (pg/mL) | 93.7±109.9 | 89.4±112.0 | 134.6±75.6 | <0.001 |
| Serum creatinine (mg/dL) | 0.90±0.24 | 0.90±0.24 | 0.90±0.20 | 0.879 | |
| Left ventricular ejection fraction (%) | 62.8±9.8 | 63.0±9.9 | 60.6±9.3 | 0.042 | |
| Diameter of left atrium (mm) | 39.2±6.7 | 38.9±6.7 | 41.6±5.5 | 0.002 | |
| Computed tomography | 64-line | 465 (61%) | 415 (61%) | 50 (69%) | 0.142 |
| 320-line | 292 (39%) | 270 (39%) | 22 (31%) | 0.142 | |
| Left atrium volume (mL) | 89.5±29.3 | 88.4±29.8 | 99.5±22.3 | 0.002 | |
| Left atrial volume index (mL/m2) | 53.5±18.0 | 53.0±18.2 | 58.4±14.3 | 0.015 | |
| Coronary artery lesions | |||||
| Mild (≤50% stenosis) | 541 (71%) | 541 (79%) | 0 (0%) | <0.001 | |
| Moderate (>50%, <75%) | 72 (10%) | 62 (9%) | 10 (14%) | 0.235 | |
| Severe (≥75%) | 97 (13%) | 42 (6%) | 55 (76%) | <0.001 | |
| Unevaluable coronary artery lesions | 47 (6%) | 40 (6%) | 7 (10%) | 0.136 | |
| Ordinal coronary artery calcium score | 1.40±2.05 | 1.22±1.86 | 3.15±2.78 | <0.001 | |
| Absent (0) | 466 (62%) | 444 (65%) | 22 (31%) | - | |
| Mild (1-3) | 169 (22%) | 156 (23%) | 13 (18%) | - | |
| Moderate (4-5) | 80 (11%) | 57 (8%) | 23 (32%) | - | |
| Severe (≥6) | 42 (6%) | 28 (4%) | 14 (19%) | - | |
N-IS: the non-ischemic patient group, IS: the ischemic patient group, CVD: cardiovascular disease, CVA: cerebrovascular apoplexy, TIA: transient ischemic attack
Comparison between the patients with and without myocardial ischemia
The prevalence of mild, moderate, severe, and unevaluable coronary artery lesions due to severe coronary calcifications and/or banding artifacts, was 541 (71%), 72 (10%), 97 (13%), and 47 (6%), respectively (Table 1). There were 685 and 72 patients without [the non-ischemic patient group (N-IS group)] and with [the ischemic patient group (IS group)] myocardial ischemia, respectively. There were no statistical differences among the age, BSA, BMI, prevalence of the co-existence of hypertension, dyslipidemia, a family history of CVD, ex- or current smoking, obesity (BMI>25 kg/m2), prevalence of paroxysmal AF, persistent AF, and long-lasting AF, and serum creatinine. The prevalence of a male gender, diabetes mellitus, history of a CVA/TIA, and the mean values of the CHADS2 score, and NCCRF was significantly higher in the IS group than N-IS group. Moreover, the values of the BNP level, diameter of the LA by echocardiography, LA volume, LA volume index, and OCCS by cardiac CT were significantly higher and larger in the IS group than N-IS group. The LVEF by echocardiography was significantly lower in the IS group than N-IS group.
Independent risk factors of myocardial ischemia in patients with AF who underwent RFCA of AF
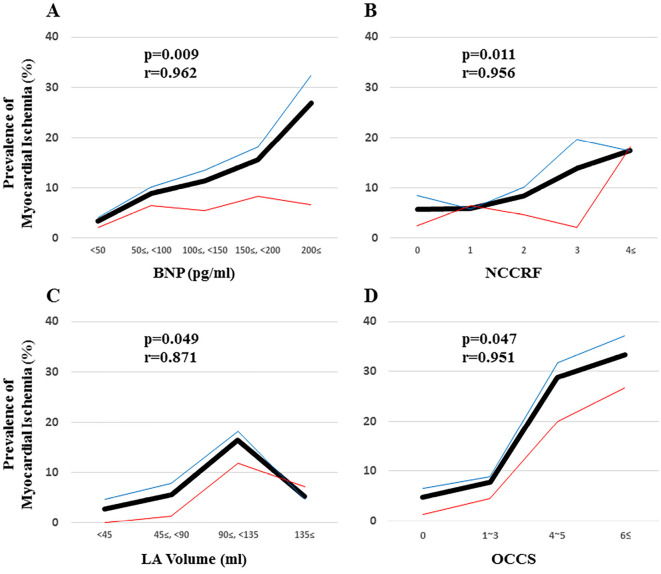
A univariate analysis and multivariate statistical analysis revealed that a male gender (Odds ratio 2.11), NCCRF (≥3) (Odds ratio 2.03), an elevated BNP (≥100 pg/mL) (Odds ratio 3.37), an elevated LA volume (≥90 mL) (Odds ratio 2.91), and the OCCS (≥4) (Odds ratio 13.0) by cardiac CT, were independent risk factors for myocardial ischemia in patients with AF who underwent RFCA of AF (Table 2). Fig. 1 demonstrates a significant correlation between myocardial ischemia and the BNP (A: p=0.009, r=0.962), NCCRF (B: p=0.011, r=0.956), LA volume (C: p=0.049, r=0.871), and OCCS (D: p=0.047, r=0.951) by cardiac CT.
Table 2.
Univariate and Multivariate Analysis.
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Rate of Ischemia | p value | OR (95% CI) | p value | ||
| Male | 11.7% | 0.003 | 2.11 (1.02-4.36) | 0.045 | |
| Age (≥65 years old) | 11.8% | 0.061 | |||
| Co-existence | |||||
| Hypertension | 10.8% | 0.083 | |||
| Diabetes mellitus | 15.5% | 0.006 | |||
| Dyslipidemia | 12.5% | 0.080 | |||
| Family history of CVD | 12.4% | 0.200 | |||
| Ex- or current smoking | 12.3% | 0.128 | |||
| Obese (Body mass index >25 kg/m2) | 10.2% | 0.677 | |||
| History of CVA/TIA | 17.7% | 0.001 | |||
| Number of co-existing coronary risk factors (≥3) | 15.2% | <0.001 | 2.03 (1.10-3.76) | 0.025 | |
| Laboratory analysis | |||||
| Brain natriuretic peptide (≥100 pg/mL) | 18.6% | <0.001 | 3.37 (1.87-6.06) | <0.001 | |
| Left ventricular ejection fraction (<62%) | 8.6% | 0.299 | |||
| Diameter of the left atrium (≥40 mm) | 13.5% | <0.001 | |||
| Computed tomography | |||||
| Left atrium volume (≥90 mL) | 14.6% | <0.001 | 2.91 (1.25-6.75) | 0.013 | |
| Left atrial volume index (≥55 mL/m2) | 12.4% | 0.032 | |||
| Ordinal coronary calcium score (≥4) | 18.0% | <0.001 | 13.0 (3.51-48.4) | <0.001 | |
CVD: cardiovascular disease, CVA: cerebrovascular apoplexy, TIA: transient ischemic attack, OR: Odds ratio, CI: confidence interval
Figure 1.
The association between myocardial ischemia and the brain natriuretic peptide (BNP) level (A), number of co-existing coronary risk factors (NCCRF) (B), left atrial (LA) volume (C), and ordinal coronary calcium scoring (OCCS) (D) by cardiac computed tomography (CT), which were the independent risk factors of myocardial ischemia in patients with atrial fibrillation (AF) who underwent radiofrequency catheter ablation (RFCA) of AF. In accordance with the BNP level (A: p=0.009, r=0.962), NCCRF (B: p=0.011, r=0.956), LA volume (C: p=0.049, r=0.871), and OCCS (D: p=0.047, r=0.951) by cardiac CT, the prevalence of myocardial ischemia significantly increases. The bold black line, narrow blue line, and narrow red line indicate all, male, and female patients, respectively.
Discussion
In this study, there were 72 patients (10%) with myocardial ischemia. A univariate analysis and multivariate statistical analysis revealed that a male gender (Odds ratio 2.11), a high NCCRF (≥3) including hypertension, diabetes mellitus, dyslipidemia, family history of CVD, ex- or current smoking, and obesity (BMI>25 kg/m2) (Odds ratio 2.03), an elevated BNP level (≥100 pg/mL) (Odds ratio 3.37), an enlarged LA volume (≥90 mL) (Odds ratio 2.91), and a high OCCS (≥4) (Odds ratio 13.0) by cardiac CT, were independent risk factors of myocardial ischemia in patients undergoing RFCA of AF. (Table 2 and Fig. 1).
Underlying mechanism of a BNP elevation and LA volume enlargement in the IS group
It is already known that AF (11) and/or myocardial ischemia (12) elevate the BNP. It has also been reported that AF (13) and diabetes mellitus (14) cause left ventricular diastolic dysfunction. Moreover, myocardial ischemia is well-known to cause both left ventricular diastolic and systolic dysfunction (15). In this study, the prevalence of diabetes mellitus (20% vs. 35%; p=0.003), LVEF by echocardiography (63.0±9.9% vs. 60.6±9.3%; p=0.042), and LA volume by cardiac CT (88.4±29.8 vs. 99.5±22.3 mL; p=0.002) were significantly higher, lower, and larger, respectively, in the IS group than the N-IS group. Thus, in the IS group, the myocardial ischemia decreased the LVEF, causing so-called LV systolic dysfunction, and AF, diabetes mellitus, and myocardial ischemia, caused LV diastolic dysfunction. Finally, the increase in the LA loading resulting from both the LV diastolic and systolic dysfunction caused LV volume enlargement and a BNP level elevation in the IS group as compared to the N-IS group. Further, an elevated BNP has been reported to be a predictor of cardiovascular/cerebrovascular events and a decrease in the long-term mortality in patients with myocardial ischemia (16). Thus, physicians should aggressively treat and intervene in those patients.
Evaluation of myocardial ischemia in patients with AF who underwent RFCA of AF
The CAC on cardiac CT is well-known to be one of the important predictors of cardiovascular events associated with myocardial ischemia (9, 17). Actually, in this study, the OCCS (≥4) was the strongest independent risk factor of myocardial ischemia (Odds ratio 13.0). However, its evaluation requires radiation exposure, and it may be comparably complicated and take much time. In this study, we demonstrated that a male gender (Odds ratio 2.11), high NCCRF (≥3) (Odds ratio 2.03), and an elevated BNP level (≥100 pg/mL) (Odds ratio 3.37) were independent risk factors for myocardial ischemia in patients with AF who underwent RFCA of AF. Those 3 factors can be easily evaluated from medical interviews and examinations, and blood tests without radiation exposure from cardiac CT. As demonstrated in Fig. 2, about 40% of the male patients with AF who had a high NCCRF (≥3) and elevated BNP (≥100 pg/mL) may have had myocardial ischemia. Thus, to find myocardial ischemia, physicians should aggressively evaluate those 3 risk factors in patients undergoing RFCA of AF.
Figure 2.
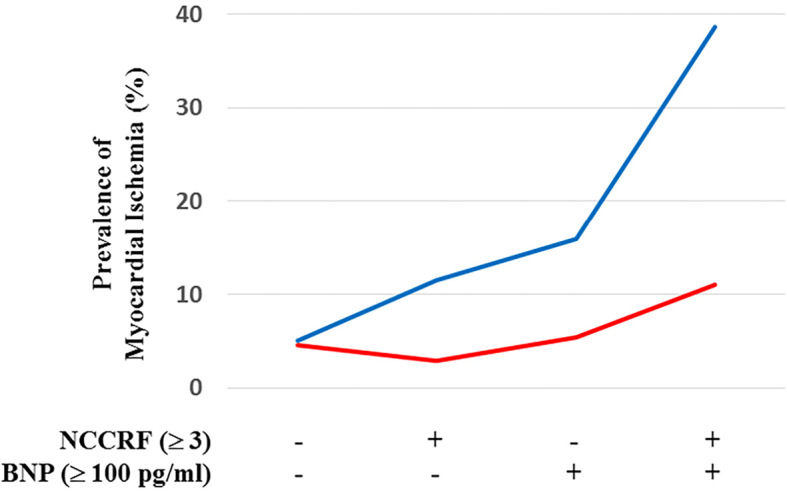
The association between myocardial ischemia and the presence or absence of an elevated brain natriuretic peptide (BNP) level (≥100 pg/mL) and the number of co-existing coronary risk factors (NCCRF) (≥3) in male (blue line) and female (red line) patients who underwent RFCA of AF. In accordance with the presence of an elevated BNP level and NCCRF, the prevalence of myocardial ischemia increases.
Myocardial ischemia and heart failure associated with patients with AF
AF is an important cause of morbidity and mortality worldwide. It has been reported that about 10% and 30% of patients with AF die from myocardial ischemia and heart failure, respectively (18). In our studies (5), about 10% of the patients with AF who underwent RFCA of AF had myocardial ischemia. Moreover, the BNP level in almost all the patients in this study was elevated (Table 1). Thus, preventing death from myocardial ischemia and heart failure should be a major priority in the treatment of the patients with AF. Finally, it may be very important to find and treat myocardial ischemia and heart failure aggressively in patients undergoing RFCA of AF and who have those risk factors.
Limitations of the study
Although our study was a multi-center trial, it was limited by the retrospective design and its relatively small number of patients. Patients with AF who did not undergo RFCA of AF, especially those with long-term AF, were not adequately included in this study. Moreover, we could not reveal any beneficial effects of finding myocardial ischemia in patients undergoing RFCA of AF. Thus, whether our results can safely be extrapolated to a larger number of patients including patients with AF who do not undergo RFCA of AF, especially with long-lasting AF, should be determined in further prospective studies.
Conclusion
In view of these findings, even though without any cardiac CT imaging data including the LA volume and OCCS, physicians should be well aware of this condition when examining patients undergoing RFCA of AF., especially those with a male gender, the presence of a high NCCRF (≥3), and an elevated BNP level (≥100 pg/mL) who may be high risk patients for myocardial ischemia in patients undergoing RFCA of AF. Moreover, those may be one of the most important risk factors for the progression of cardiovascular events including myocardial ischemia and heart failure in patients with AF. Therefore, physicians should aggressively evaluate myocardial ischemia in patients who have those risk factors.
The authors state that they have no Conflict of Interest (COI).
Yoshibumi Antoku, Takahiro Mito, Akihiro Masumoto and Masatsugu Nozoe contributed equally to this work.
Acknowledgement
We thank Mr. John Martin for his linguistic assistance with this paper.
References
- 1.Piccini JP, Fauchier L. Rhythm control in atrial fibrillation. Lancet 388: 829-840, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Kawada S, Watanabe A, Morimoto Y, et al. Radiofrequency catheter ablation prior to percutaneous coronary intervention in patients with atrial fibrillation coexisting with stable coronary artery disease: a single-center pilot study. Heart Vessels 34: 632-640, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Nucifora G, Schuijf JD, Tops LF, et al. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ Cardiovasc Imaging 2: 100-106, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Tomomatsu T, Morishima I, Okumura K, et al. Comparison of frequency and characteristics of patients with atrial fibrillation having ablation with versus without coronary narrowing (>/=50%) by angiography. Am J Cardiol 119: 1770-1775, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Mito T, Takemoto M, Antoku Y, et al. Evaluation of coronary artery disease in patients with atrial fibrillation by cardiac computed tomography for catheter ablation: CADAF-CT trial. Heart Vessels 35: 1037-1043, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurita T, Nogami A; Japanese Circulation Society Foundation/Japanese Heart Rhythm Society Task F. 2018 JCS/JHRS Guideline on Non-Pharmacotherapy of Cardiac Arrhythmias. 2018. https://www.j-circ.or.jp/cms/wp-content/uploads/2018/07/JCS2018_kurita_nogami191120.pdf (in Japanese). [Google Scholar]
- 7.Mori S, Endo M, Asakura H. Improvement in banding artefacts in four-dimensional computed tomography for radiotherapy planning. Phys Med Biol 51: 5231-5244, 2006. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 371: 1208-1217, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr 11: 8-15, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology 257: 541-548, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen CW, Omland T, Clopton P, et al. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol 46: 838-844, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Sabatine MS, Morrow DA, de Lemos JA, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 44: 1988-1995, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Uetake S, Maruyama M, Yamamoto T, et al. Left ventricular stiffness estimated by diastolic wall strain is associated with paroxysmal atrial fibrillation in structurally normal hearts. Clin Cardiol 39: 728-732, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernande L, Bergerot C, Rietzschel ER, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 24: 1268-1275 e1, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Marwick TH, Shah SJ, Thomas JD. Myocardial strain in the assessment of patients with heart failure: a review. JAMA Cardiol 4: 287-294, 2019. [DOI] [PubMed] [Google Scholar]
- 16.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 352: 666-675, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827-832, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Healey JS, Oldgren J, Ezekowitz M, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 388: 1161-1169, 2016. [DOI] [PubMed] [Google Scholar]