Abstract
Treatment with tocilizumab (TCZ) to block interleukin-6 (IL-6) signalling is predicted to mitigate cytokine release syndrome (CRS) caused by coronavirus disease 2019 (COVID-19). However, the adverse effects of TCZ on patients with COVID-19 remain unclear. We herein report a patient with COVID-19 treated with TCZ who developed acute hypertriglyceridaemia. Despite favipiravir treatment, acute respiratory distress syndrome developed in a 45-year-old patient with COVID-19; thus, TCZ was initiated. The triglyceride levels greatly increased after TCZ administration. Physicians should consider the negative impact of TCZ on the lipid profile in patients with COVID-19, although COVID-19-induced CRS itself may be an aggravating factor.
Keywords: COVID-19, tocilizumab, acute hypertriglyceridaemia
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has rapidly spread worldwide, becoming a global-scale public health concern. Approximately 25% of patients with COVID-19 develop critical conditions, such as acute respiratory distress syndrome, which contributes to multiple organ failure (1). Interleukin-6 (IL-6)-driven cytokine release syndrome (CRS) plays a major role in the pathological progression of COVID-19 (2). Because COVID-19 with CRS can be ameliorated by the inhibition of the IL-6 signalling pathway, treatments that block IL-6 might be effective in these patients.
A preliminary open-label study of 21 patients with COVID-19 treated with tocilizumab (TCZ), an IL-6 receptor antibody, showed promising results. In addition, no adverse effects were observed during the study (3). However, careful observation is needed when administering TCZ because limited data are available on its short-term adverse effects.
We herein report a COVID-19 patient treated with TCZ exhibiting a sharp increase in serum triglyceride (TG) levels.
Case Report
A 45-year-old Japanese man with no pre-existing medical conditions visited a general hospital because of a fever and general fatigue that lasted for 2 days in early April 2020. As a polymerase chain reaction (PCR) test for SARS-CoV-2 was negative, he received a diagnosis of a common cold. However, he visited the hospital after three days because of persistent symptoms. Chest computed tomography (CT) revealed bilateral peripheral infiltration in the lungs; therefore, COVID-19 was highly suspected at this stage, despite the negative PCR result. He was thus admitted to the hospital, and ciclesonide along with supportive care was administered.
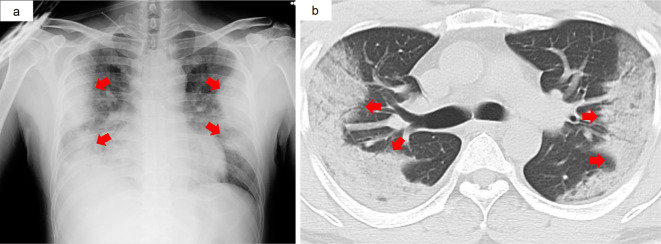
Based on a positive PCR result for SARS-COV-2 after two consecutive false negative results, favipiravir and nafamostat treatments were initiated (eight days had passed since the onset of disease). Despite antiviral treatment and supportive care, his condition exacerbated, and he was referred to the University Hospital of the Ryukyus on day 10 after the onset of symptoms. Fig. 1 shows the CT scan images, and Table presents the laboratory findings on admission.
Figure 1.
Chest images of a 45-year-old man with COVID-19 presenting with a fever and dyspnoea for several days. a: Bilateral peripheral air space opacities were observed by chest X-ray. b: Computed tomography showed a consolidation pattern in the subpleural areas of the bilateral upper lobe. These images were taken upon admission at the University Hospital of the Ryukyus (i.e. 10 days after the onset of symptoms).
Table.
Laboratory Findings on Admission.
| Haematology | Biochemistry | |||
| White blood cell (/μL) | 6,300 | Aspartate aminotransferase (U/L) | 38 | |
| Neutrophil (%) | 92 | Alanine aminotransferase (U/L) | 48 | |
| Lymphocyte (%) | 6.4 | Lactate dehydrogenase (U/L) | 293 | |
| Monocyte (%) | 1.6 | Alkaline phosphatase (U/L) | 339 | |
| Haemoglobin (g/dL) | 10.4 | γ-glutamyl transpeptidase (U/L) | 191 | |
| Haematocrit (%) | 29 | Creatine kinase (U/L) | 58 | |
| MCV (fl) | 83.6 | Total protein (g/dL) | 4.5 | |
| Platelets (/μL) | 162,000 | Albumin (g/dL) | 2.2 | |
| Total bilirubin (mg/dL) | 1.4 | |||
| Serology | Blood urea nitrogen (mg/dL) | 5 | ||
| C-reactive protein (mg/dL) | 15.26 | Creatinine (mg/dL) | 0.55 | |
| Procalcitonin (ng/mL) | 0.258 | Triglyceride (mg/dL) | 95 | |
| Interleukin-6 (pg/mL) | 160 | HDL-C (mg/dL) | 19 | |
| LDL-C (mg/dL) | 50 | |||
| Coagulation | Amylase (U/L) | 61 | ||
| PT-INR | 1.17 | |||
| APTT (sec) | 44.6 | Arterial blood gas analysis* | ||
| D-dimer (μg/mL) | 0.3 | pH | 7.506 | |
| PaCO2 (mmHg) | 31.5 | |||
| PaO2 (mmHg) | 68.7 | |||
| HCO3- (mEq/L) | 24.3 |
* On 4 litres of oxygen via mask, respiration rate 30/minute
APTT: activated partial clotting time, HCO3-: serum bicarbonate concentration, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, MCV: mean corpuscular volume, PaCO2: partial pressure of carbon dioxide in arterial blood, PaO2: partial pressure of oxygen in arterial blood, PT-INR: prothrombin time-international normalized ratio
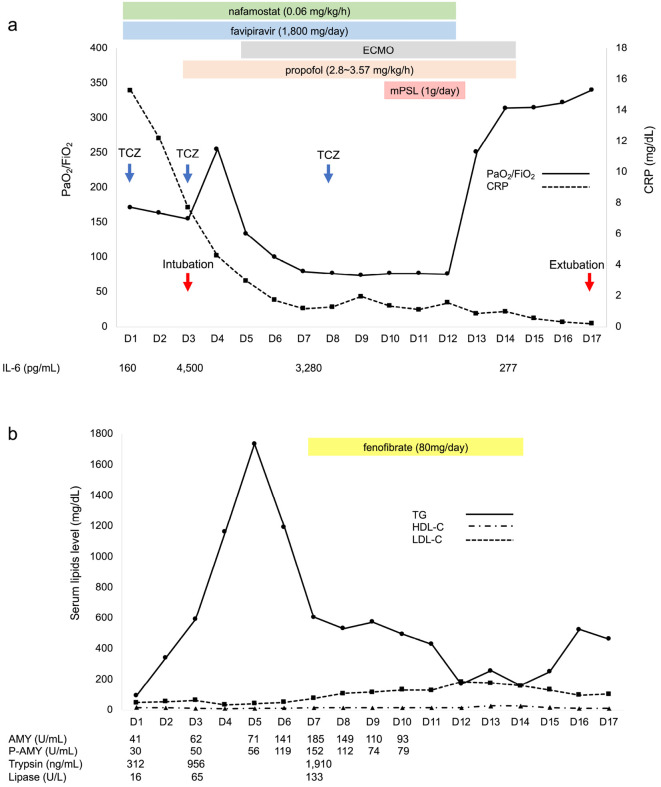
As shown in Fig. 2a, intravenous TCZ (8 mg/kg) was administered as part of compassionate therapy after obtaining informed consent from the patient on the first day of admission at our hospital. Nonetheless, hypoxia persisted, and the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) continued to deteriorate on day 3 (from 185 to 155); he was thus intubated and transferred to the intensive-care unit. Propofol was administered for sedation. His serum TG levels showed a sharp increase from 95 to 300 mg/dL in the 2 days following the administration of TCZ. Because the patient had severe acute respiratory distress syndrome, a second dose of TCZ was administered on day 3 in the intensive-care unit. A transient increase in PaO2/FiO2 was observed on day 4. However, it quickly dropped the following day. Therefore, extracorporeal membrane oxygenation (ECMO) was initiated on day 5. However, the serum TG levels greatly increased to 1,763 mg/dL on the same day. The serum pancreatic amylase level gradually increased in accordance with the TG elevation and peaked on day 7. In addition, the serum trypsin and lipase levels significantly increased during the same period. Fenofibrate was commenced on the same day despite the gradual decrease in serum TG levels starting on day 6, as hypertriglyceridaemia would have caused acute pancreatitis (AP). As severe respiratory failure persisted, TCZ was administered for a third time on day 8. In addition, a 3-day course of methylprednisolone (1 g/day) was administered between days 10 and 12. A small increase in serum TG levels was observed despite treatment with fenofibrate on day 9. On day 13, there was a substantial increase in the PaO2/FiO2 ratio, and this upward trend continued. ECMO was safely discontinued on day 14. The patient was extubated and discharged from the ICU. The propofol dose was 2.8-3.57 mg/kg/h during sedation, and enteral feeding was not initiated. As his TG levels were stable, fenofibrate was discontinued on day 15; however, his TG levels increased again the following day. Throughout the clinical course, the serum low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels remained low (median: 93 mg/dL, range: 35-185 mg/dL; median: 15.5 mg/dL, range 10-29 mg/dL, respectively), except for the LDL-C levels from days 12 to 14, which increased following fenofibrate administration.
Figure 2.
Clinical course of the patient after admission. a: PaO2/FiO2 plummeted after a transient improvement with two doses of TCZ; thus, ECMO was initiated on day 5. There was a significant increase in PaO2/FiO2 after mPSL administration. The serum CRP levels showed a steady decrease in response to TCZ treatment; The IL-6 levels increased and then showed a downward trend. b: The serum TG levels showed a sharp increase after two consecutive administrations of TCZ over a few days after admission. The serum P-AMY, trypsin, and lipase levels also increased. Prior to fenofibrate initiation, the TG levels decreased sharply despite continuous propofol infusion. On day 9, the TG levels showed a mild increase after the third TCZ administration. AMY: amylase, CRP: C-reactive protein, ECMO: extracorporeal membrane oxygenation, IL-6: interleukin-6, mPSL: methylprednisolone, P-AMY: pancreatic amylase, PaO2/FiO2: the ratio of arterial oxygen partial pressure to fractional inspired oxygen, TCZ: tocilizumab, TG: triglyceride
The patient was discharged from the hospital without oxygen support on day 38.
Discussion
The present case shows that the administration of TCZ for CRS with COVID-19 can affect the lipid profile. Despite the lack of robust data, a wide range of compassionate therapies, including TCZ, have been initiated for patients with severe COVID-19, as no established medication is available. Therefore, it is important to raise awareness of the parameters that should be monitored by clinicians.
Treatment-related increases in circulating lipid levels have been observed during the long-term administration of TCZ in patients with rheumatoid arthritis (RA) (4-6). Cacciapaglia et al. observed a significant increase in TG levels in 40 patients with RA treated with TCZ (TG, 139±13 mg/dL at baseline vs. 157±15 mg/dL at 24 weeks) compared with those in 20 RA patients who received methotrexate (TG, 126±26 mg/dL at baseline vs. 121±24 mg/dL at 24 weeks) during the first 24 weeks (4). According to Giles et al., in a randomised controlled trial comparing treatment with TCZ and etanercept for RA, patients administered TCZ showed a median increase of 13.6% and 11.1% in TG and LDL-C levels, respectively, compared to those in the etanercept arm at 4 weeks, demonstrating a significant increase (6).
In contrast, a rapid and substantial increase in TG levels was observed for a short period in our patient. Systemic inflammatory response syndrome (SIRS) induced by COVID-19 was likely involved in the drastic change in TG levels. During SIRS, proinflammatory cytokines, including IL-6, induce fatty tissue lipolysis and hepatic fatty acid synthesis, resulting in increased TG levels (7). The increase in TGs might be more dramatic in SIRS than in chronic inflammatory conditions, such as RA. Furthermore, the inhibition of the IL-6 receptor-mediated clearance of IL-6 by TCZ leads to increased serum IL-6 levels (Fig. 2a), which in turn can increase TG production. Theoretically, the use of TCZ in the treatment of chimeric antigen receptor T cell-induced CRS can induce similar phenomena. However, there have been no reports of hypertriglyceridaemia (8), although its occurrence might simply have been overlooked.
Hypocholesterolaemia frequently occurs in critically ill patients with SIRS and sepsis (7). Besides IL-6, other inflammatory cytokines, such as IL-1β and tumour necrosis factor-α (TNF-α), also inhibit the production of apolipoproteins, leading to a decrease in LDL-C. This may explain why low levels of LDL-C were observed in our patient despite IL-6 signalling inhibition by TCZ. The pathogenic mechanism underlying the HDL decrease during infection and inflammation needs to be elucidated in future studies (7).
The efficacy and safety of TCZ administration to treat COVID-19 have not been widely examined. However, small and single-arm studies have suggested that IL-6 receptor blockers can ameliorate CRS due to COVID-19 without causing adverse effects (3, 9, 10). Although the optimal number of doses remains unknown, Luo et al. suggested that up to three repeated doses of TCZ could benefit critically ill patients with approximately 10-fold elevated IL-6 levels (9). Therefore, repeated administration of TCZ was performed in our patient owing to severe respiratory failure.
The intravenous administration of propofol can cause acute hypertriglyceridaemia, which can contribute to AP (11). Propofol may have been one factor aggravating hypertriglyceridaemia in this case. However, the TG levels in this patient had already increased by 12 hours after the administration of TCZ. Furthermore, the concentration of TGs decreased despite the continuation of propofol and prior to the administration of fenofibrate. Based on these results, the hyperglyceridaemia observed in our patient was likely due to TCZ. However, several unpublished studies on the safety profile of favipiravir seemed to describe an increase in TG levels, although this increase was not significant compared with that in uric acid levels (12).
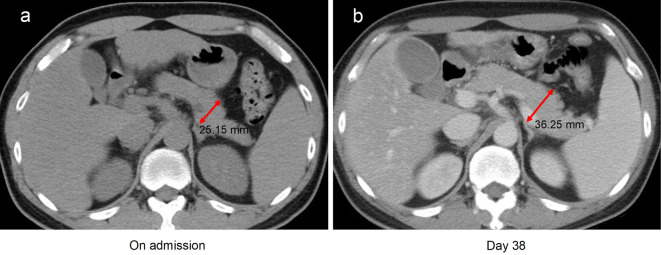
The risk of hypertriglyceridaemia-induced AP should be considered in patients with COVID-19 treated with TCZ. A causal relationship between long-term TCZ therapy and AP among patients with RA has been reported (13). Recently, Morrison et al. reported two cases of acute hypertriglyceridaemia in patients with COVID-19 treated with TCZ (14). They also observed an increase in AP biomarkers, namely amylase and lipase. In our patient, the serum lipase, trypsin, and amylase levels were increased. In addition, abdominal CT on day 25 revealed enlargement of the pancreas, which suggested that AP had developed during the clinical course (Fig. 3).
Figure 3.
Abdominal computed tomography findings in our patient with COVID-19 who developed hypertriglyceridaemia following TCZ administration. Compared with the findings on the day of admission (a), the pancreas was enlarged on day 38 (b). No spread of inflammation to the surrounding tissues and organs was observed. TCZ: tocilizumab
In conclusion, the findings of this case report suggest that physicians should be aware of hypertriglyceridaemia and the associated potential risk of AP during TCZ treatment for CRS associated with severe COVID-19, particularly when repeated doses of TCZ are combined with propofol administration. Further studies evaluating the safety and efficacy of TCZ are needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 232: 1061-1069, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science 368: 473-474, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Xu XL, Han MF, Li TT, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 117: 10970-10975, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacciapaglia F, Anelli MG, Rinaldi A, et al. Lipids and atherogenic indices fluctuation in rheumatoid arthritis patients on long-term tocilizumab treatment. Mediators Inflamm. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman E, Rahat MA, Feld J, et al. Effects of tocilizumab, an anti-interleukin-6 receptor antibody, on serum lipid and adipokine levels in patients with rheumatoid arthritis. Int J Mol Sci 20: 4633, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 72: 31-40, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Brigatto AP, Golucci S, Marson FAL, et al. Lipid profile associated with the systemic inflammatory respose syndrome and sepsis in critically ill patients. Nutrition 55-56: 7-14, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Le RQ, Yuan W, Shord SS, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23: 943-947, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 92: 814-818, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Giambenedetto S, Ciccullo A, Borghetti A, et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaud JC, Berger MM, Pannatier A, et al. Hyperglyceridemia: a potential side effect of propofol sedation in critical illness. Intensive Care Med 38: 1990-1998, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic? J Virus Erad 2: 45-51, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaig T, Douros A, Bronder E, Klimpel A, Kreutz R, Garbe E. Tocilizumab-induced pancreatitis: case report and review of data from FDA adverse event reporting system. J Clin Pharm Ther 41: 718-721, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]