Abstract
A 47-year-old man presented with dysgeusia, anorexia, and diarrhea. An endoscopic evaluation showed widespread gastrointestinal nodular inflammation and polyps. The pathological findings were consistent with Cronkhite-Canada Syndrome (CCS). Prednisolone therapy resulted in clinical improvement. However, CCS relapse complicated with gastric obstruction was observed during drug tapering. Although his symptoms disappeared after the reintroduction of steroids, he developed membranous nephritis. Additional cyclosporine A (CyA) treatment dramatically improved his proteinuria and residual gastrointestinal polyposis. The clinical symptoms resolved with steroid treatment, while CyA was effective for both CCS lesions and membranous nephropathy. CyA might therefore be a potential treatment option for CCS associated with membranous nephropathy.
Keywords: gastric obstruction, membranous glomerulonephritis, prednisolone, cyclosporine
Introduction
Cronkhite-Canada Syndrome (CCS) is a rare, non-hereditary disorder described for the first time in 1955 (1). CCS is characterized by widespread hamartomatous gastrointestinal (GI) polyposis and variable abnormalities of ectodermal tissue, including hyperpigmentation, alopecia, and onychodystrophy (2). Prominent symptoms include weight loss, protein-losing enteropathy, diarrhea, abdominal pain, nausea, vomiting, dysgeusia, and atrophic tongue. The etiology of CCS remains unknown; however, the pathogenesis may be rooted in autoimmune or infectious disease responses (3). The diagnosis of CCS is based on the clinical history, physical examination, endoscopy-based observation of GI polyposis, and histopathology. Common complications include GI hemorrhaging with anemia, malnutrition, intussusception, and rectal prolapse, although several unusual complications or concomitant diseases have also been reported, including recurrent severe acute pancreatitis, myelodysplastic syndrome, giant-cell bone tumor, multiple rib fractures, schizophrenia, portal thrombosis, and membranous nephropathy (MN) (3). Although there is no consensus concerning the treatment of CCS, corticosteroid therapy with adjunctive nutritional support has been recommended (4). However, steroid-resistant CCS has been reported (5, 6).
We herein report a case of CCS associated with gastric outlet obstruction and MN development. This is the first report on CCS causing gastric obstruction and the sixth report on CCS with MN. In the present case, steroid therapy led to partial remission, whereas additional treatment with cyclosporine A (CyA) after the development of MN resulted in remission of both the residual polypoid lesions and MN.
Case Report
A 47-year-old man presented with a 3-month history of dysgeusia, anorexia, and diarrhea. The patient had no relevant family or remarkable medical history except for nephrotic syndrome treated with prednisolone (PSL) until he was 18 years old.
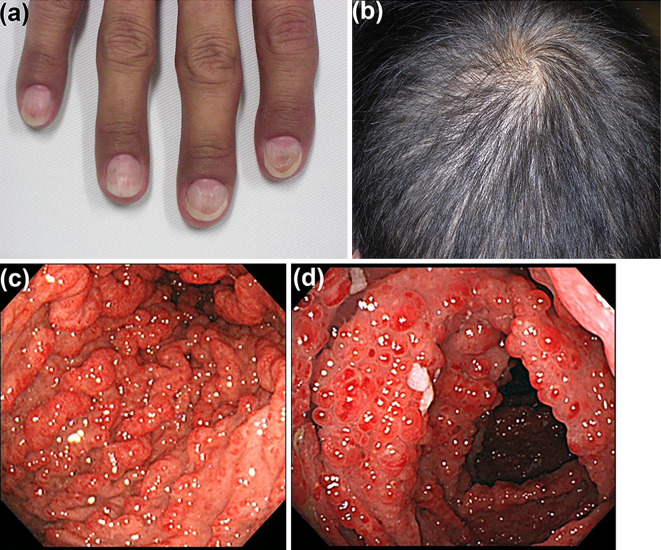
A physical examination revealed onychodystrophy (Fig. 1a), alopecia (Fig. 1b), and skin hyperpigmentation on his hands and feet. Laboratory tests showed hypoalbuminemia (33 g/L). Esophago-gastroduodenoscopy (EGD) and total colonoscopy (TCS) revealed multiple edematous reddish polypoid lesions in the stomach, duodenum, and the entire colon and rectum (Fig. 1c, d). Biopsies from the gastric and colonic polyps and the non-polypoid mucosa produced pathological evidence of cystically dilated glands associated with inflammation and edema of the lamina propria. The patient was diagnosed with CCS given his typical clinical history and examination results and consistent pathological findings.
Figure 1.
A physical examination of the CCS patient when presenting to the previous clinic included onychodystrophy (a), diffuse alopecia (b), and multiple edematous reddish polypoid lesions in the stomach (c), and colon (d). CCS: Cronkhite-Canada Syndrome
He was initially treated with oral PSL 40 mg daily, and his symptoms improved. However, after steroid tapering to 5 mg daily over 6 months, he was referred to our hospital because of symptom relapse, including anorexia, nausea, diarrhea with abdominal distension, body weight loss, onychodystrophy, alopecia, and skin hyperpigmentation. The laboratory results were as follows: hemoglobin, 107 g/L; serum total protein, 46 g/L; albumin, 25 g/L; total bilirubin, 6.8 μmol/L; aspartate aminotransferase, 16 IU/L; alanine aminotransferase, 11 IU/L; alkaline phosphatase, 145 IU/L; γ-glutamyl transpeptidase, 7 U/L; lactate dehydrogenase, 193 IU/L; blood urea nitrogen, 2.4 mmol/L; serum creatinine (Cr), 43.3 μmol/L; total cholesterol, 4.31 mmol/L; amylase, 80 IU/L; and C-reactive protein, <0.001 g/L. A urinalysis was negative for protein, occult blood, and glucose.
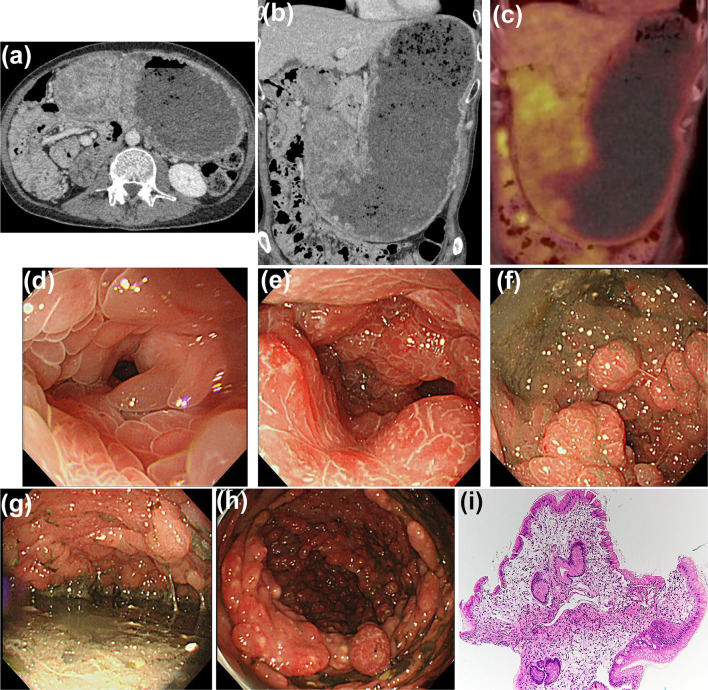
Computed tomography showed marked wall thickening of the gastric antrum accompanied by dilatation (Fig. 2a, b), evidenced by the absence of an intense fluorodeoxyglucose uptake (Fig. 2c). EGD revealed that the obstruction had been caused by marked gastric wall thickening due to severe confluent edematous sessile gastric polyps (Fig. 2d–g). TCS revealed multiple reddish polyps (Fig. 2h) and an edematous adjacent mucosa along the entire colon. A histopathological examination of the biopsied polyp specimens revealed a severely edematous stroma and mononuclear or eosinophilic cell infiltration without malignancy (Fig. 2i). These findings were consistent with CCS relapse.
Figure 2.
Findings at CCS relapse. CT revealed massive gastric dilatation and marked wall thickening (a, b). No uptake of intense FDG was observed in the gastric wall thickening (c). EGD revealed gastric outlet obstruction due to the numerous edematous and reddish polyps (d-g). Furthermore, TCS revealed multiple reddish polyps along the entire colon (h). Histopathology of biopsied gastric polyp specimens demonstrated a substantially edematous stroma and inflammatory cell infiltration without malignancy (i, H&E, ×40). CCS: Cronkhite-Canada Syndrome, CT: computed tomography, FDG: fluorodeoxyglucose, EGD: esophago-gastroduodenoscopy, TCS: total colonoscopy, H&E: Hematoxylin and Eosin staining
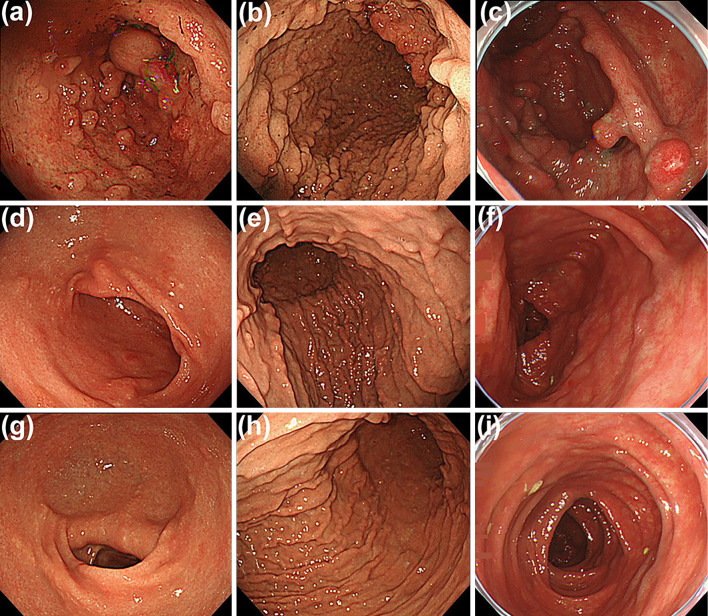
The patient was administered intravenous PSL at a daily dose of 40 mg with intravenous nutritional therapy. PSL was tapered to 10 mg for 4 weeks, the gastric sessile polyps gradually shrank, and the obstruction, clinical symptoms, and nutritional condition improved. However, since the gastric and colonic polyps did not revert completely, the patient was required to maintain oral PSL (10 mg/day) after the disappearance of his symptoms (Fig. 3a-c).
Figure 3.
Endoscopic findings during treatment. Multiple polypoid lesions were still seen in the gastric antrum (a) and body (b) as well as the colon (c) at the diagnosis of MN. Five months after the additional treatment of CyA, these polypoid lesions had almost disappeared from the stomach (d, e) and colon (f). One and a half years later, endoscopic images revealed no evidence of recurrence (g and h, stomach; i, colon). MN: membranous glomerulonephritis, CyA: cyclosporine A
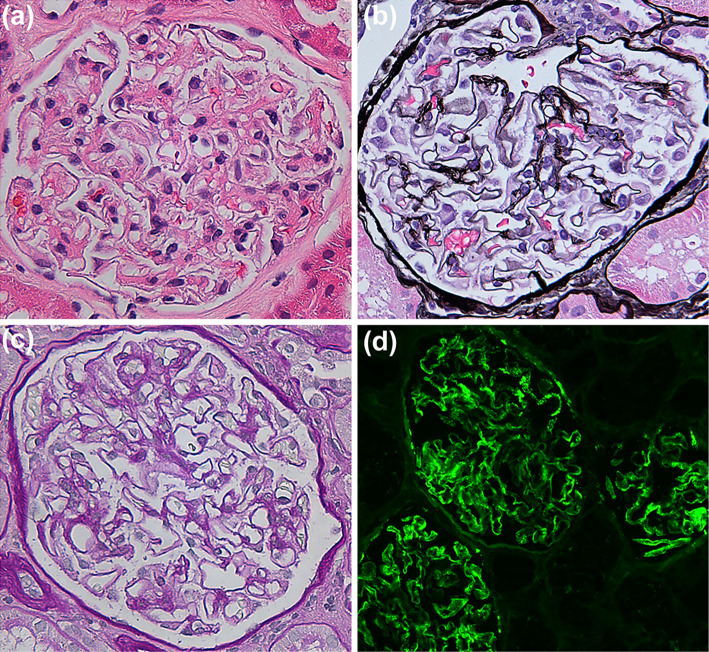
Twenty months after his relapse, the patient remained symptom-free; however, his urine test for protein was positive at his annual general checkups. A thorough medical examination found a high urinary protein-to-creatinine ratio (UPCR; 4.14 g/gCr) with hypoalbuminemia (30 g/L), no serum anti-nuclear antibodies, and normal levels of serum complements C3 and C4. His medications at that time included PSL 10 mg daily. Renal biopsy specimens showed thickening of the glomerular basement membranes with subepithelial spikes by periodic acid-Schiff methenamine silver staining (Fig. 4a-c). An immunofluorescence microscopic evaluation revealed staining along with glomerular capillary walls for immunoglobulin IgG, IgA, and κ chain (Fig. 4d). Based on these findings, he was diagnosed with MN during PSL treatment with CCS. After treatment with intravenous methylprednisolone (0.5 g/day) for 3 days followed by both oral PSL (35 mg/day) and CyA (100 mg/day), his proteinuria improved (UPCR, 0.29 g/gCr).
Figure 4.
Pathological findings of renal biopsy specimens. Glomerular basement membrane thickening with subepithelial spikes was observed (a, H&E, ×400; b, PAM, ×400; c, PAS, ×400). An immunofluorescence microscopic evaluation revealed strong granular peripheral capillary loop reactivity for IgG (d, ×200). H&E: Hematoxylin and Eosin staining, PAM: periodic acid-Schiff methenamine silver stain, PAS: periodic acid-Schiff stain
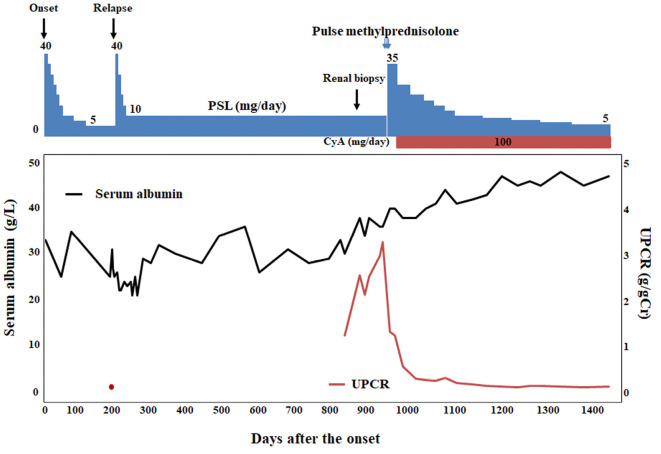
Five months after treatment with both PSL and CyA, endoscopic abnormalities dramatically improved and the polypoid lesions eventually almost disappeared (Fig. 3d-f). After an additional 1.5 years of follow-up, the patient remained asymptomatic, with no proteinuria and no evidence of relapse of the polypoid lesions (Fig. 3g-i) under maintenance treatment with CyA and PSL 5 mg daily. The clinical course of the present case is summarized in Fig. 5.
Figure 5.
Clinical course of the present patient with CCS associated with MN. PSL: prednisolone, CyA: cyclosporine A, UPCR: urinary protein-to-creatinine ratio
Discussion
CCS is a rare, non-hereditary syndrome of unknown etiology with diffuse GI polyposis associated with ectodermal changes. It may develop in all ethnic groups, and more than 500 cases have been described in the literature with an estimated incidence of 1 per 1 million persons per year and 75% of cases detected in Japan (7, 8). According to a Japanese nationwide survey including 210 patients with CCS (4), the average age at the diagnosis is 63.5 years with an approximately 1.8:1 male-to-female distribution, and both upper and lower GI polyposis accompanied by ectodermal abnormalities have been confirmed in all cases. Polyposis is observed in the stomach, small intestine, and colon but generally not in the esophagus. Esophageal polyps were reported to be involved in some patients with CCS, although their biopsies showed nonspecific inflammation or squamous papilloma (4). The pathological features of the polyp are a benign, juvenile-like or hamartomatous appearance with cystic dilated and distorted glands, massive submucosal edema, especially in the lamina propria, and mild infiltration of inflammatory cells, including eosinophils (3, 9). The GI non-polypoid mucosa is also significantly altered and comprises impaired crypt architecture, including dilation and branching, edema, and the presence of mixed inflammatory infiltrates.
Gastric sessile polyps reportedly appear smaller and more confluent than their colonic counterparts (4); however, in the present case, massive gastric polyps had caused the gastric obstruction. This is the first case showing CCS associated with gastric outlet obstruction, although colonic intussusception is one of the major complications. In the present case, there was no evidence of GI cancer involvement, which is reportedly a significant complication of CCS. Mechanical obstruction and reduced motility, which were associated with the massive gastric CCS polyposis and wall thickening, appeared to have caused the gastric outlet obstruction in the present case. Given the MN development, the present patient may have had altered immunocompetence, which might have promoted the development of massive progression of the gastric lesions. However, gastric obstruction has never been reported, even in the previously reported cases of CCS associated with MN (10-14). Further studies are therefore required to elucidate its pathogenesis.
MN is a very rare complication of CCS, and only five cases (10-14) have been reported previously (Table). All previous patients were male, and the mean age was 50.4 (range, 17-71) years old. In two cases, MN was observed before the diagnosis of CCS. Another three patients developed MN within one year after the diagnosis of CCS. In the present case, MN was diagnosed 29 months after the onset of CCS. This may suggest that monitoring proteinuria in patients with CCS should be considered, even a few years after disease onset. During adolescence, the present patient suffered from nephrotic syndrome, the cause of which was unclear to us because of the lack of medical record data. MN is one of the most common causes of nephrotic syndrome in adults as well as the most common cause of paraneoplastic glomerulonephritis. In fact, three cases with CCS complicated by MN had malignant tumors (two colorectal cancers and one gastric cancer), for which surgery was required. However, no malignancies were found in the other three cases, including the present case. The development of MN in patients with CCS is not always linked to malignancy. MN is now thought to be an autoimmune disease mainly caused by autoantibodies recognizing glomerular podocyte target antigens, such as the M-type phospholipase A2 receptor (PLA2R) (15). Serum autoantibodies to PLA2R in patients with MN are mainly of the IgG4 subclass (15). In addition, several studies have demonstrated the infiltration of IgG4-positive plasma cells in CCS polypoid lesions (16, 17). Although we did not examine the IgG4 levels in the present case, a common autoantibody may play a role in the development of both MN and CCS.
Table.
Characteristics of the Present and Previously Reported Patients with CCS Associated with MN.
| References | Age/Sex | Onset of MN at the diagnosis of CCS | Treatments for CCS | Additional treatments for MN | Patient outcome† |
| 10 | 41/M | 5 years before | Low anterior resection due to rectal adenocarcinoma | None | Remission‡ |
| 11 | 64/M | 10 months after | Right hemicolectomy due to ascending colon mucinous adenocarcinoma and oral PSL (40 mg/day, tapered later) | Reintroduction of PSL | Remission‡ |
| 12 | 17/M | A few months after | CyA | CyA | Remission‡ |
| 13 | 59/M | 30 years before | Total gastrectomy due to gastric tubular adenocarcinoma | None | ND |
| 14 | 71/M | 12 months after | PSL and azathioprine (75 mg/day) | CyA and rituximab in addition to azathioprine | Remission§ |
| The present case | 47/M | 29 months after | PSL | Pulse methylprednisolone (tapered later) and CyA | Remission |
†Remission was defined as both regression of clinical symptoms of CCS and proteinuria. ‡No endoscopic findings after treatment were described. §In this case, symptoms of CCS improved after additional CyA treatment, whereas his proteinuria did not resolve. Rituximab resulted in a regression of his MN and endoscopic findings. CCS: Cronkhite-Canada Syndrome, MN: membranous nephropathy, CyA: cyclosporine A, PSL: prednisolone, M: male, ND: not described
Although steroid therapy is thought to be the ideal medical treatment for CCS, rapid steroid tapering has been reported to be associated with early relapse (4). In the present case, a slow reduction in the dose appeared to avoid a relapse, although the GI polypoid lesions did not completely resolve, and the patient eventually developed MN. According to the previous studies reporting on treatment of CCS associated with MN (Table), reinduction of PSL (11), CyA (12, 14), and rituximab (14), which is a chimeric monoclonal antibody targeting the pan B-cell marker CD20, have been considered as therapeutic options. In addition, CyA treatment may be effective for patients with steroid-resistant CCS (5). In the present case, intravenous pulse therapy with methylprednisolone followed by CyA maintenance therapy successfully led to the improvement of his proteinuria and noticeable regression of the residual polypoid lesions. Why CyA treatment was effective in the present case is unclear. CyA is known to inhibit the activity of Th2 cells, which contribute to IgG4 production (18, 19). Given the potential mechanistic role of IgG4 in both MN and CCS, CyA appears to be a promising treatment option in patients with CCS associated with MN; however, further studies are required to validate its efficacy.
In conclusion, we described for the first time a patient with CCS associated with gastric outlet obstruction and MN. CyA treatment was effective for both CCS and MN in the present case. The present findings provide valuable insight into the clinical course and management of CCS. Calcineurin inhibitors such as CyA might have potential therapeutic efficacy for CCS associated with MN.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Cronkhite LW, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med 252: 1011-1015, 1955. [DOI] [PubMed] [Google Scholar]
- 2.Zhao R, Huang M, Banafea O, et al. Cronkhite-Canada syndrome: A rare case report and literature review. BMC Gastroenterol 16: 1-5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopáčová M, Urban O, Cyrany J, et al. Cronkhite-Canada syndrome: review of the literature. Gastroenterol Res Pract 2013: 1-9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe C, Komoto S, Tomita K, et al. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol 51: 327-336, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamakawa K, Yoshino T, Watanabe K, et al. Effectiveness of cyclosporine as a treatment for steroid-resistant Cronkhite-Canada syndrome; two case reports. BMC Gastroenterol 16: 1-7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweetser S, Ahlquist DA, Osborn NK, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci 57: 496-502, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Schulte S, Kütting F, Mertens J, et al. Case report of patient with a Cronkhite-Canada syndrome: Sustained remission after treatment with corticosteroids and mesalazine. BMC Gastroenterol 19: 1-5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao EJ, Hyder SM, DeNucci TD, Fine S. A Successful steroid-sparing approach in Cronkhite-Canada syndrome. ACG Case Reports J 6: e00055, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calva D, Howe JR. Hamartomatous polyposis syndromes. Surg Clin North Am 88: 779-817, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Ogata Y, Akagi Y, et al. Cronkhite-Canada syndrome associated with advanced rectal cancer treated by a subtotal colectomy: report of a case. Surg Today 31: 521-526, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi Y, Yoshikawa M, Tsukamoto N, et al. Cronkhite-Canada syndrome with colon cancer, portal thrombosis, high titer of antinuclear antibodies, and membranous glomerulonephritis. J Gastroenterol 38: 791-795, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Vernia P, Marcheggiano A, Marinaro V, Morabito S, Guzzo I, Pierucci A. Is Cronkhite-Canada syndrome necessarily a late-onset disease? Eur J Gastroenterol Hepatol 17: 1139-1141, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Karasawa H, Miura K, Ishida K, et al. Cronkhite-Canada syndrome complicated with huge intramucosal gastric cancer. Gastric Cancer 12: 113-117, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Firth C, Harris LA, Smith ML, Thomas LF. A case report of Cronkhite-Canada syndrome complicated by membranous nephropathy. Case Reports Nephrol Dial 8: 261-267, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck LH, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11-21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegert-Johnson DL, Osborn N, Smyrk T, Boardman LA. Cronkhite-Canada syndrome hamartomatous polyps are infiltrated with IgG4 plasma cells. Digestion 75: 96-97, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Sweetser S, Ahlquist DA, Osborn NK, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci 57: 496-502, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol 28: 203-213, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Lee JU, Kim LK, Choi JM. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front Immunol 9: 2747, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]