Abstract
Coronavirus disease 2019 (COVID-19) has been recognized as a worldwide pandemic. However, the clinical course of COVID-19 remains poorly characterized. Although some cases of pneumothorax have been reported, they all had pulmonary complications or were managed with mechanical ventilation. We herein report a case of pneumothorax that developed even though the patient had no pulmonary underlying diseases and had never been managed with mechanical ventilation. In the present case, a lung bulla was found on chest computed tomography during treatment for COVID-19. We concluded that COVID-19 affected the formation of the lung bulla and induced the complication of pneumothorax.
Keywords: bulla, corticosteroids, COVID-19 pneumonia, organizing pneumonia, pneumothorax, severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) has been identified as a worldwide pandemic. Severe acute respiratory dysfunction often occurs in patients with COVID-19. However, the clinical course in COVID-19 patients has not been fully documented, most notably in the later stages of the disease.
Several complications have been reported among COVID-19 patients. For example, one report showed that pneumothorax developed in 1% of COVID-19 patients (1). Another report suggested that two cases of pneumothorax may have developed due to mechanical ventilation with high positive end-expiratory pressure (PEEP) causing the rupture of pre-existing blebs (2). Another report described a case of pneumothorax in a 62-year-old patient diagnosed with COVID-19 who had no smoking history or any associated underlying medical conditions, including chronic obstructive pulmonary disease (3). However, whether or not pneumothorax can occur in COVID-19 patients who have no underlying risk factors, such as the presence of pulmonary diseases or treatment using mechanical ventilation, has been unclear.
We herein report a case of pneumothorax that developed in association with COVID-19 pneumonia. The patient had no smoking history and no underlying pulmonary disease before the development of COVID-19 pneumonia and had not received mechanical ventilation during the treatment of the pneumonia.
Case Report
A 77-year-old man presented with a chief complaint of a fever lasting 4 days and was admitted to our hospital for the evaluation and treatment. He was confirmed positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcription-polymerase chain reaction prior to admission and was diagnosed with COVID-19 pneumonia. The patient had no smoking history and no pulmonary comorbidities, including chronic obstructive pulmonary disease or interstitial pneumonia.
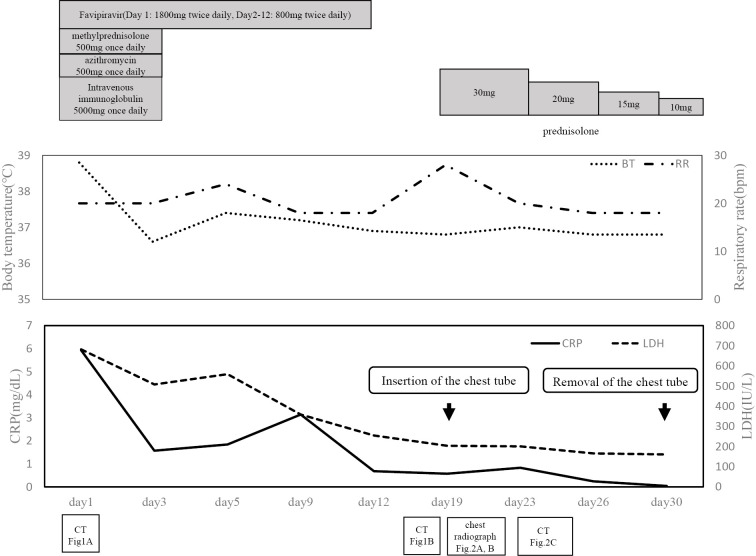
His height was 171.4 cm, and his body weight was 60.3 kg. At admission, his vital signs included a body temperature of 38.8 °C, blood pressure of 93/61 mmHg, heart rate of 88 beats per minute, respiratory rate of 22 breaths per minute (bpm), and percutaneous oxygen saturation (SpO2) of 97% while receiving supplemental oxygen (3 L/min). Peripheral blood and serum tests revealed elevated levels of C-reactive protein and lactate dehydrogenase (Table). Overall, his condition included mild to severe inflammation and respiratory failure. Chest computed tomography (CT) was notable for diffuse bilateral ground-glass opacity shadows (Fig. 1A). He was treated with favipiravir (1,800 mg twice daily on day 1, followed by 800 mg twice daily on days 2-12), azithromycin (500 mg once daily), and intravenous immunoglobulin (5,000 mg once daily). Treatment with immunoglobulin and azithromycin was continued for 3 days after admission. Furthermore, methylprednisolone (500 mg once daily, for 3 days from admission) was also intravenously administered. After treatment using these drugs and oxygen inhalation, the patient's body temperature and hypoxia improved. In addition, his inflammation improved, and reverse transcription-polymerase chain reaction tests for SARS-CoV-2 were negative by day 16.
Table.
Laboratory Examination Findings on Admission.
| Hematology | Blood chemistry | Serological test | |||
| WBC | 8,470 (/μL) | TP | 6.7 (g/dL) | CRP | 5.93 (mg/dL) |
| Seg | 91.7 (%) | T-bil | 1.4 (mg/dL) | KL-6 | 380 (U/mL) |
| Eos | 0 (%) | AST | 73 (IU/L) | Hb-A1c (NGSP) | 7.6 (%) |
| Baso | 0.2 (%) | ALT | 35 (IU/L) | GLU | 208 (mg/dL) |
| Mono | 2.8 (%) | LDH | 682 (IU/L) | BNP | 29.3 (pg/mL) |
| Lymph | 5.3 (%) | CK | 638 (IU/L) | Arterial blood gas analysis | |
| RBC | 543×105(/μL) | BUN | 46.1 (mg/dL) | pH | 7.485 (%) |
| Hb | 16.0 (g/dL) | Cre | 1.05 (mg/dL) | pCO2 | 30.5 (mmHg) |
| Hct | 46.7 (%) | Na | 138 (mEq/L) | pO2 | 70.6 (mmHg) |
| Plt | 14.3×105(/μL) | K | 4.1 (mEq/L) | Lac | 2.0 (mmol/L) |
| Cl | 101(mEq/L) | HCO3- | 22.7 (mmol/L) | ||
WBC: white blood cell, Seg: segmented leukocyte, Eos: eosinophil, Baso: basophil, Mono: monocyte, Lymph: lymphocyte, RBC: red blood cell, Hb: hemoglobin, Hct: hematocrit, Plt: platelet, TP: total protein, T-bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, CK: creatine kinase, BUN: blood urea nitrogen, Cre: creatinin, CRP: C-reactive protein, KL-6: Krebs von den Lungen-6, Hb-A1c: hemoglobin A1c, NGSP: Glycohemoglobin Standardization Program, GLU: glucose, BNP: brain natriuretic peptide, pH: potential of hydrogen, Lac: lactate
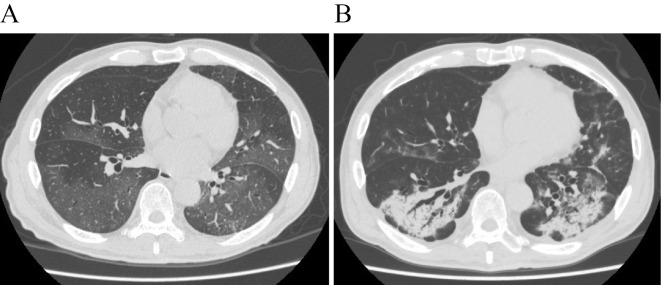
Figure 1.
Chest computed tomography (CT) performed at the time of admission revealing diffuse, bilateral ground-glass opacities (A). Chest CT performed on day 19 was notable for the expansion of the consolidation and bilateral bronchiectasis in the lower lobes (B).
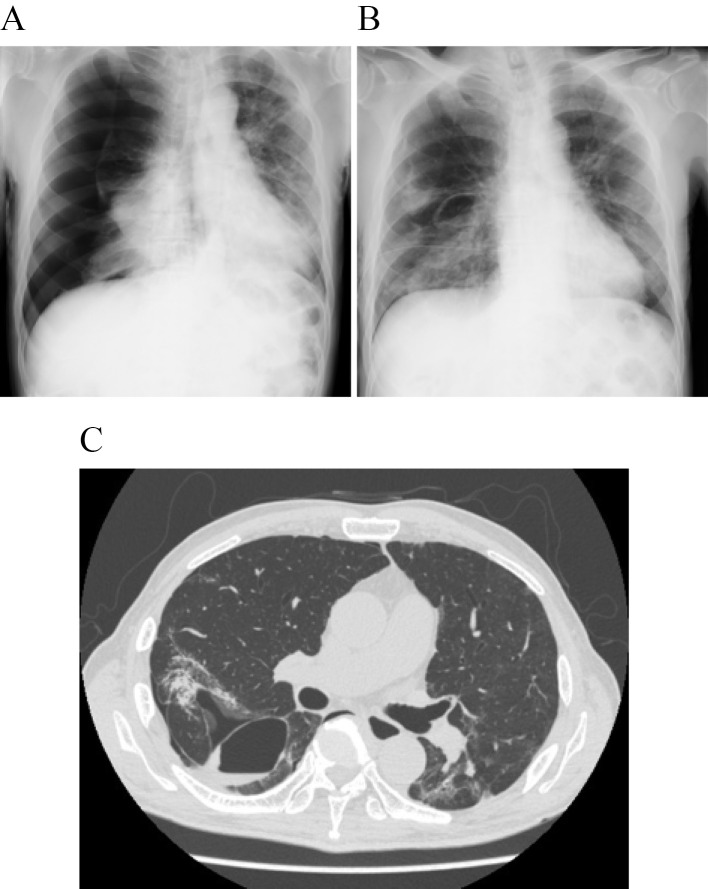
On day 16, bilateral lung consolidation appeared on chest X-ray. Chest CT performed on day 19 was notable for the expansion of the consolidation and bilateral bronchiectasis in the lower lobes (Fig. 1B). We recognized these features as being similar to those in cases of organizing pneumonia (OP), and corticosteroid treatment was reinitiated. One hour after the first dose of prednisolone (30 mg), the patient reported new-onset chest pain, and his respiratory rate increased to 28 bpm. Chest X-ray revealed right-sided pneumothorax (Fig. 2A). This finding was associated with decreased SpO2 levels (84%), and a chest tube was inserted immediately. On day 21, chest X-ray clearly showed a bulla in the right lung (Fig. 2B). Continuous air-leak through a chest tube was observed during the following 12 days, and treatment with prednisolone continued. Chest CT performed at this time revealed improvement in the pneumothorax and bulla formation in the superior basal segment (segment 6; S6) of the right lung (Fig. 2C). Tests for SARS-CoV-2 remained negative. The respiratory rate improved to 20 bpm, and the patient was discharged from the hospital after removal of the chest tube (Fig. 3).
Figure 2.
A and B: Chest X-ray at the onset of pneumothorax (A) and after the insertion of a chest tube that revealed re-expansion of the lung and a right-sided small bulla (B). C: Repeat chest CT performed after 12 days of air drainage showing notable improvement of the pneumothorax and bulla formation in right S6.
Figure 3.
Clinical course of the patient described in the present case report.
Discussion
This case is remarkable for several reasons. First, pneumothorax developed in this patient who did not have any risk factors for pneumothorax, including mechanical ventilation, a smoking history, and pulmonary comorbidities. Second, a lung bulla was detected on chest X-ray and CT after the development of pneumothorax despite not being observed on either at the start of COVID-19 treatment.
There have been several reports of pneumothorax developing in patients undergoing treatment for COVID-19. Among them, one report described two cases of pneumothorax during treatment for COVID-19 (2). Both patients in this report had been intubated and received mechanical ventilation. In addition, a previous study reported that pneumothorax developed in 8% of patients with COVID-19 after treatment with mechanical ventilation (4). Another report showed the development of pneumothorax in a patient in whom a giant bulla had been observed on chest CT after receiving mechanical ventilation using PEEP (5). Therefore, these findings suggest that mechanical ventilation using PEEP may cause the development of pneumothorax, partly through the development and rupture of existing bullae or cysts.
In contrast, the present case had no smoking history and no pulmonary comorbidities. The patient did not receive mechanical ventilation, and no lung bullae or cysts had been observed on chest CT at admission. However, a lung bulla was detected after the development of pneumothorax. These findings suggest that, in this case, a lung bulla might have developed via lung injury caused by COVID-19 pneumonia and might be associated with the development of pneumothorax.
Pathological characteristics of postmortem biopsies of patients who died from SARS-CoV-2 have been reported (6, 7), including pronounced desquamation of pneumocytes and hyaline membrane formation, which are consistent with a diagnosis of acute respiratory distress syndrome, acute fibrinous pneumonia, and OP. In the present case, chest CT performed on day 19 was notable for the expansion of the consolidation and bilateral bronchiectasis in the lower lobes, and we recognized these features as being similar to those in a case of OP (8). However, we did not perform bronchoscopy due to concerns over the medical staff's safety and welfare. Future evaluations of this nature may reveal pathological changes associated with COVID-19 pneumonia.
There is currently no proven effective therapy for COVID-19. Clinical trials of several antiviral and antiretroviral drugs are currently ongoing. Several studies have reported that treatment with remdesivir might be effective in the early stages of COVID-19 pneumonia (9, 10). However, the impact of corticosteroid therapy in this setting has been controversial. In patients who were diagnosed with SARS-CoV or Middle East Respiratory Syndrome, corticosteroids were found to delay the clearance of viral RNA (11, 12). Furthermore, corticosteroid treatment was associated with increased mortality among patients diagnosed with pneumonia secondary to influenza infection (13). However, in our case, we treated a patient with methylprednisolone and prednisolone in addition to favipiravir, azithromycin, and immunoglobulin. This is because treatment with systemic steroids from the beginning of treatment has been suggested to improve SpO2 in COVID-19 patients (14, 15). A recent study also reported that the use of dexamethasone in patients with COVID-19 resulted in reduced rates of 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone (16).
Although the mechanism underlying the development of a bulla and pneumothorax in the present case was unclear, the check-valve mechanism-associated formation of bullae and the subsequent development of pneumothorax has been suggested in a previous patient with OP treated with corticosteroids (8). Corticosteroid therapy may also cause a delay in the wound-healing process of fragile lung tissue, and these histological changes may induce the formation of bullae through the check-valve mechanism. A previous report described a COVID-19 patient with pneumothorax who was also treated with corticosteroid for six days from the beginning of hospitalization (3). In the present case, corticosteroid therapy started at the beginning of hospitalization might have been associated with the development of the bulla and thus caused pneumothorax. More attention needs to be focused on the clinical efficacy and adverse effects of corticosteroids in the treatment of COVID-19.
Conclusion
This is the first case of COVID-19 pneumonia complicated with acute pneumothorax in a patient who did not have any risk factors for pneumothorax, including mechanical ventilation, a smoking history, and the presence of lung bullae and other pulmonary comorbidities.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the patient who consented to participate in our study.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507-513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aiolfi A, Biraghi T, Montisci A, et al. Management of persistent pneumothorax with thoracoscopy and blebs resection in COVID-19 patients. Ann Thorac Surg. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med 27: taaa062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anesth 125: e28-e37, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 21: 541-544, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420-422, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copin MC, Parmentie E, Duburcq T, et al. ; Lille COVID-19 ICU and Anatomopathology Group. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med 23: 1-3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadota T, Shimizu K, Tsurushige C, et al. Organizing pneumonia complicated by cyst and pneumothorax formation. Intern Med 51: 3155-3158, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382: 2327-2336, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. Lancet 395: 1569-1578, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 31: 304-309, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 197: 757-767, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Ni YN, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 23: 99, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther 5: 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther 5: 57, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report. N Engl J Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]