Abstract
No licensed vaccine exists against Visceral Leishmaniasis (VL), a disease caused by Leishmania donovani parasite. We have previously reported both macrophages and dendritic cells play important role in the protection induced by a live attenuated centrin gene-deleted L. donovani (LdCen−/−) parasite vaccine. The role of neutrophils in orchestrating the initial innate response to pathogens is widely recognized. To investigate the early interaction of LdCen−/− with neutrophils, we immunized mice intradermally in the ear pinna with LdCen−/−. Compared to LdWT infection, LdCen−/− parasites induced higher recruitment of neutrophils to the ear dermis and ear draining lymph nodes (dLN) as early as 6 to 18h after immunization which were predominantly proinflammatory in nature. Neutrophils from ear dLN of LdCen−/− immunized mice exhibited heightened expression of costimulatory molecules and attenuated expression of co-inhibitory molecules necessary for higher T cell activation. Further phenotypic characterization revealed heterogeneous neutrophil populations containing Nα, Nβ subtypes in the ear dLN. Of the two, the parasitized Nα subset from LdCen−/− immunized mice exhibited much stronger antigen specific CD4+T cell proliferation ex-vivo. Adoptive transfer of neutrophils bearing LdCen−/− parasites induced an increased Th1 response in naïve mice. Importantly, neutrophil depletion significantly abrogated antigen specific CD4+T cell proliferation in LdCen−/− immunized mice and impaired protection against virulent challenge. Conversely, replenishing of neutrophils significantly restored the LdCen−/− induced host protective response. These results suggest that neutrophils are indispensable for protective immunity induced by LdCen−/− parasite vaccine.
Introduction
Visceral Leishmaniasis (VL) is one of the most lethal neglected tropical diseases with approximately 20,000 to 40,000 deaths worldwide each year (1–3).
The causative Leishmania spp. of VL, Leishmania donovani (L. donovani) is a blood-borne obligate intracellular protozoan parasite. Infection is initiated when metacyclic promastigotes are deposited into the host skin through the bite of a female sand fly which is followed by the rapid recruitment of neutrophils to the inoculation site where they internalize the parasites (4, 5). Neutrophils play a major role in immunity as a first line of defense against several pathogens (6). Neutrophils can kill the pathogen through a combination of phagocytosis, release of cytotoxic granules, and neutrophil extracellular traps (7). There is increasing evidence that neutrophils may also modulate the adaptive immune response indirectly through the release of chemokines and cytokines that recruit dendritic cells (DCs) to sites of inflammation or by direct engagement with T cells and their activation (8, 9). Paradoxically, neutrophils can also secrete cytokines such as IL-10 and TGF-β with known suppressive activity on T cells (10–12).
A neutrophil-induced pathogen-specific T cell response was observed in several vaccines such as modified vaccinia Ankara virus, poxvirus, and a live attenuated tuberculosis vaccine (13–15). These studies showed that neutrophils play an important role in the generation of a CD8+T cell response to a modified vaccinia Ankara virus, or a CD4+T cell response to live attenuated tuberculosis vaccine. Neutrophil depletion during tuberculosis vaccination abrogated the induction of Th1-specific responses and impaired bacterial load reduction in vaccinated animals, highlighting the protective role of neutrophil in vaccine immunity (14). In contrast, vaccination with killed L. major+CpG followed by sand fly mediated challenge infection showed an extended presence of otherwise short-lived neutrophils up to 8 days post-infection that impaired the vaccine efficacy (16).
Of the several vaccination approaches against Leishmania, live attenuated vaccines showed great potential (17). Our laboratory has developed a live attenuated centrin gene-deleted L. donovani (LdCen−/−) strain which induced protection against virulent L. donovani infection in mice, hamsters, and dogs through induction of a host protective T cell response (18–22). Recently, we reported that LdCen−/− parasites promote classical activation (M1 dominant) in macrophages that leads to the generation of protective Th1 responses in BALB/c mice (23). Although, macrophages have long been considered to be the major innate effector cells responsible for killing Leishmania parasites, recent studies have reported that following Leishmania infection, neutrophils are the predominant infiltrating phagocytic cells to the inoculation site and play prominent role during the initial phase of infection (5, 24–27). Nevertheless, little is known about the role of neutrophils in live attenuated Leishmania parasite vaccine induced immunity.
Hence, in the present study we investigated the role of neutrophils in the development of protective immunity by live attenuated LdCen−/− vaccines. We demonstrated that following intradermal immunization with LdCen−/−, large number of neutrophils were recruited very early in the infection to the inoculation site and corresponding dLN and initiated an effector CD4+Th1 response. Importantly, depletion of neutrophils significantly abrogated LdCen−/− induced antigen specific CD4+T cell activation in vivo which further resulted in reduced protection against virulent L. donovani challenge. Our studies for the first time show neutrophils play a major role in protective immune response induced by a live attenuated Leishmania vaccine.
Material and Methods
Animals and parasites:
5- to 6-week-old female C57BL/6 mice obtained from the National Cancer Institute, NIH, Bethesda, MD were used in the experiments. We have used 6–8-week-old female mice for all our experiments. All mice were maintained in the FDA/CBER AAALAC-accredited facility under standard environmental conditions for this species. Among parasites, the LdWT (MHOM/SD/62/1S), Centrin gene-deleted (LdCen−/−) parasites were used (18, 19). The parasites were cultured according to the previously described procedure (19, 28). Red fluorescent protein (RFP)-expressing LdWT parasites were developed using the pA2RFPhyg plasmid for the integration of an RFP/hygromycin B resistance gene expression cassette into the parasite’s 18S rRNA gene locus, as described previously (29). LdCen−/− parasites expressing mCherry were generated using the pLEXSY-mCherry-sat2 plasmid, following the manufacturer’s protocol (Jena Bioscience). The parasites were cultured according to a procedure previously described (30).
Ethics statement:
The animal protocol for this study has been approved by the Institutional Animal Care and Use Committee at the Center for Biologics Evaluation and Research, US FDA (ASP 1995#26). Further, the animal protocol is in full accordance with the “Guide for the Care and Use of Laboratory Animals” as described in the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals 2015.
(http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf).
Infection of mice and isolation of tissue resident macrophages
The mice were injected intradermally in the ear pinna either with PBS (uninfected naïve mice) or 106 stationary phase LdWT/ LdCen−/− promastigotes. In each study, a minimum of six mice were used per group. Age-matched naive mice were used as a control. At 6h post infection, mice were sacrificed and tissue resident macrophages (Cd11b+F4/80+ Ly6G− Ly6C− MHCII−) from different groups of mice were sort selected using the BD FACS Aria-IITM. Single-cell suspensions were prepared from ear dermis, and then labeled for fluorochrome tagged anti–TCR-β, anti-NK1.1, anti-Cd19 Abs using fluorochrome tagged magnetic beads, and passed through LS columns to select out these cell types. Flow through enriched population was collected and stained with Cd11b-BV510, F4/80-PAC, Ly6G-APC, Ly6C- FITC, MHCII- APC-CY7 Ab and further sort selected.
Infection of mice and isolation of neutrophils, macrophages and dendritic cells
The mice were injected intradermally in the ear pinna either with PBS (uninfected naïve mice) or 106 stationary phase LdWT/ LdCen−/− promastigotes. In some experiment’s mice were infected with LdWTRFP/ LdCen−/−mCherry parasites. In each study, a minimum of six mice were used per group. At different time post infection mice were sacrificed and total neutrophils (Cd11b+ Ly6G+ Ly6Cint) or parasitized neutrophils (Cd11b+ Ly6G+ Ly6CintRFP+/mCherry+), macrophages (Cd11b+Ly6C−Ly6G−CD11c−MHCII+) and DCs (Cd11b+Ly6C−Ly6G−CD11c+MHCII+) from different groups of mice were sort selected using the BD FACS Aria-IITM. Single-cell suspensions were prepared from ear dermis, ear dLN and spleen then labeled for fluorochrome tagged anti–TCR-β, anti-NK1.1, anti-Cd19 Abs using fluorochrome tagged magnetic beads, and passed through LS columns to select out these cell types. Flow through enriched population was collected and stained with neutrophil, macrophages and dendritic cell specific markers and further sort selected.
RT-PCR for cytokines:
Total RNA was extracted from 1. thioglycolate elicited peritoneal neutrophils 2. tissue resident macrophages recruited in the ear dermis of either uninfected (PBS injected) or LdWT or LdCen−/− infected mice 3. neutrophils recruited in ear dermis and ear dLN following ID injection of either PBS/ LdWT or LdCen−/− parasites 4. Nα neutrophils recruited in ear dLN following ID injection of either PBS/ LdWT or LdCen−/− parasites 5. macrophages and DCs recruited in ear dLN following ID injection of either PBS/ LdWT or LdCen−/− parasites by using an RNAqueous-Micro kit (AM1931; Ambion) which also eliminates any contaminating DNA by using on-column PureLink DNase treatment during RNA purification. Four hundred nanograms of total RNA was reverse transcribed into cDNA by using random hexamers with a high-capacity cDNA reverse transcription kit (Applied Biosytems). Cytokine-Chemokine gene expression levels were determined using the TaqMan gene expression master mix and premade TaqMan gene expression assays (Applied Biosystems) using a CFX96 Touch Real-Time System (Bio-Rad, Hercules, CA). The data were analyzed with CFX Manager Software. Expression of the following genes was determined using TaqMan gene expression assays (Applied Biosystems) in the CFX96 Touch Real-Time System: CXCL-1 (Mm04207460_m1); CXCL2 (Mm00436450_m1); CCL2 (Mm00441242_m1); CCL3 (Mm99999057_m1) IL-12 (Mm00434174_m1); TNF-α (Mm00443258_m1); IL-10 (Mm00439614_m1); GAPDH (Mm99999915_g1); Arginase-1 (Mm00475988_m1); PDL-1 (Mm03048248_m1). Expression values were determined by the 2−ΔΔCt method. Samples were normalized to GAPDH expression and determined relative to expression values from untreated samples or PBS injected mice as appropriate.
Neutrophil collection:
Peritoneal exudate cells from mice were obtained 5 h after injection with 3% thioglycolate (Sigma-Aldrich, St. Louis, MO). The isolation of peritoneal neutrophils was performed with the MiniMACS system (Miltenyi Biotech). Neutrophil purity (>92%) was validated by fluorescence-activated cell sorting (FACS) and prior to treatment or coculture with parasites. Neutrophils were plated in poly-L-Lysine coated culture plate.
Neutrophil Extracellular Trap (NET) quantitation and visualization:
Neutrophils that release DNA and elastase to form filamentous structures were considered as producing NETs.
a. Quantification of DNA released from neutrophils:
DNA released from neutrophils was quantified as described previously (31). Briefly, thioglycolate elicited neutrophils (2 × 106) were incubated with either Leishmania promastigotes (LdWT/LdCen−/−) or zymosan at a multiplicity of infection (MOI) of 5:1 in RPMI 1640 supplemented with 10 mM HEPES, penicillin/streptomycin, and 2% heat-inactivated mouse serum at 37°C in a humidified incubator with 5% CO2 for 6h. After incubation, 1 U/ml micrococcal nuclease (Worthington Biochemical, Lakewood, NJ) in the presence of 1 mM Ca2+ was added to the culture which were then maintained for 2h at 37 °C. The nuclease activity was stopped with 5 mM EDTA, and samples were collected. Released DNA was quantified in the culture supernatant using the QUBIT assay (Invitrogen/Molecular Probes).
b. Neutrophil elastase activity assay:
Thioglycolate elicited purified peritoneal neutrophils (2 × 106) were adhered on polylysine (0.01%) treated coverslips in serum free medium and were infected with promastigotes at a 1:5 cell/parasite ratio at 37°C for 6h. The activities of neutrophil elastase (NE) were measured by kits (Molecular Probes, Eugene, OR) as per manufacture’s protocol.
c. Scanning Electron Microscopy:
Neutrophils (2 × 106) were adhered on polylysine (0.01%) treated coverslips. LdWT or LdCen−/− parasites were added at a 1:5 cell/parasite ratio and was incubated for 6h at 37°C, 5% CO2. Cultures were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, post fixed with 1% osmium tetroxide and 0.8% potassium ferricyanide, and dehydrated with an ascending ethanol series. After dehydration and critical-point drying, the specimens were coated with carbon and analyzed in a scanning electron microscope.
In Vitro Migration Assay:
6.5-mm diameter trans well dishes (Corning Costar) with 3-μm pore filters were used for in vitro migration assay. Briefly, thioglycolate elicited peritoneal macrophages (1.0 × 106) were infected with either LdWT or LdCen−/− parasites for 6h. Murine neutrophils (isolated and purified from peritoneal cavity after thioglycolate stimulation) using MiniMACS system (Miltenyi Biotech as described above) were then added to the upper chamber, and supernatants of parasite infected peritoneal macrophages were added to the bottom chamber. Migration in medium alone acts as the negative control. After 3h of incubation, 7 mM EDTA was added to the bottom wells for 10 minutes to release any adhered cells from the well and filter. Cells from the lower chambers were then stained with Trypan Blue and counted on a hemocytometer.
Intradermal inoculation with LdWT or LdCen−/− parasites:
C57BL/6 mice were infected intradermally in the ear with 106 LdWT or LdCen−/− parasites or fluorescent LdWTRFP/LdCen−/−mCherry using a 29-gauge needle (BD Ultra-Fine) in a volume of 10μl. The uninfected naïve/control mice received PBS. To obtain chronically infected mice, C57BL/6 mice were infected 24 weeks previously with 105 L. donovani metacyclic promastigotes via tail vein. The CD4+T cells isolated from these mice were used for coculture experiment either with parasitized total neutrophils or with parasitized Nα neutrophils at a 1:20 neutrophils/CD4+T cell ratio.
Processing of ear tissue and ear dLN and spleen:
Ear tissue was prepared as previously described (32). Briefly, the two sheets of ear dermis were separated from the control/infected mice, deposited in DMEM containing 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.2 mg/ml Liberase CI purified enzyme blend (Roche Diagnostics Corp.), and incubated for 1h and 30 min at 37°C. Digested tissue was placed in a grinder and processed in a tissue homogenizer (Medimachine; Becton Dickenson). Retromaxillary (ear) lymph nodes were removed, and mechanically dissociated using tweezers and a syringe plunger. Tissue homogenates were filtered through a 70 µm cell strainer (Falcon Products). The single cell suspension of splenocytes were prepared after mechanical grinding a followed by ACK lysis.
Neutrophil depletion (for Supplementary Fig 7)
Depletion of neutrophils was carried out by employing i.p. injection of, 1 mg of 1A8 (In Vivo monoclonal antibody anti-mouse Ly6G/Ly6C, BioXCell), or GL113 (In Vivo monoclonal antibody IgG2b isotype control, BioXCell), 1 d prior to parasite injection and every subsequent 48h after i.v injection with the parasites till day 5. At day 5, the efficiency and specificity of the depletions were evaluated on lymph node and splenic cell preparations as previously described (14, 32).
Adoptive Transfer:
Neutrophils were purified from the BM of donor mice by purification with neutrophil isolation kit (Miltyeni) and either not infected or infected with fluorescent LdWT or LdCen−/− parasites (1:15 cell/parasite ratio) for 5h. The higher number of parasites were used for increased rate of infection. The neutrophils were extensively washed to remove extracellular parasites followed by 2h rest. The percent infection of the neutrophils was determined by flow cytometry. Subsequently, 2X106 neutrophils from different groups were injected i.v. into the recipient mouse. The recipient mouse previously received 100 μl BrdU-PBS 1 day prior to the adoptive transfer.
BrdU Labeling and Detection:
T cell proliferation in vivo was measured with BrdU following a protocol previously described (15). Briefly mice were injected twice intraperitoneally 12h apart with 100 μl BrdU-PBS (0.8 mg/ml) and infected with either LdWT or LdCen−/− parasite bearing neutrophils via tail vein. 5d post infection, LNs and spleens were removed and stained for BrdU (BrdU flow kit BD) and for CD3, CD8, CD4, CD44 markers.
In some experiments T cell proliferation with BrdU was measured in neutrophil depleted/ GL113 treated infected mice. 1mg 1A8/GL113 were injected in mice along with BrdU-PBS intraperitoneally. The mice were infected intravenously next day either with LdWT/LdCen−/− parasites. The neutrophil depletion was maintained for 5 days with administration (i. p.) of antibody on alternate days. 5d post infection, LNs and spleens were removed and stained for BrdU (BrdU flow kit BD) and for CD3, CD4, CD44 markers. The kit contains 7AAD which was added in this experiment to denote the nucleated cells.
Intracellular staining and flow cytometry
The ear dermis/ ear dLN/ lymph nodes/ spleen was removed from animals and single cell suspension was prepared. For surface staining, cells were blocked at 4°C with rat anti-mouse CD16/32 (5 μg/ml) from BD Pharmingen for 20 min. Cells were then stained with anti-mouse Ly6G, anti-mouse CD11b, anti-mouse CD3, anti-mouse CD4, anti-mouse CD44, anti-mouse CCR7, anti-mouse PD-1 (eBioscience) for 30 min (each with 1:200 dilution; at 4°C). The cells were then stained with Live/Dead fixable aqua (Invitrogen/Molecular Probes) to stain dead cells. Cells were washed twice with wash buffer and fixed with a Cytofix/Cytoperm kit (BD Bioscience) for 20 min at room temperature. Intracellular staining was performed with anti-mouse IFN-γ, anti-mouse IL-2, anti-mouse IL-10 (eBioscience) (each with 1:300 dilution; at 4°C). Cells were washed twice with permeabilization buffer and acquired on an LSR II (BD Biosciences) equipped with 407-, 488-, 532-, and 633-nm laser lines using FACS Diva 6.1.2 software. Data were analyzed with FlowJo software version 9.7.5 (Tree Star). For analysis, first doublets were removed using width parameter; dead cells were excluded based on staining with the Live/Dead aqua dye. Lymphocytes were identified according to their light-scattering properties. CD4 and CD8 T cells were identified as CD3+ lymphocytes uniquely expressing either CD4 or CD8.
Ex vivo Neutrophil and T cell co-culture studies:
Parasitized total neutrophils or parasitized Nα neutrophil was flow sorted from ear dLN 48h post infection, followed by an in vitro co-culture experiment with CD4+T cells (at a 1:20 neutrophils/CD4+T cell ratio) isolated and purified from 24 weeks L. donovani exposed mice. After 5d of incubation at 37°C in a 5% CO2 humidified chamber, T cell proliferation was measured by studying the dilution of CFSE in CD4 stained CD44 hi T cells via Flow cytometry.
Immunization and challenge studies
In 1st set of experiment, C57BL/6 (n=19) mice were injected intraperitoneally (i.p) with 200 μg of anti-mouse Ly6G antibodies (BioXcell; clone 1A8) or GL113 (control IgG, BioXCell) according to the protocol described before (14). The depletion started 1 day before the intradermal immunization with 3X106 stationary phase LdCen−/− promastigotes and was maintained for 21 days with administration of antibodies on alternate days. At day 22, 3 animals from each experimental group were euthanized, and the lymph nodes and spleens were collected and evaluated by flow cytometry. At day 22, 6 immunized animals from each group were sacrificed and splenocytes were isolated and Leishmania Ag-specific cytokines (IFN-γ and IL-10) were measured by sandwich ELISA kit (ebioscience) as described above.
In 2nd set of experiment (n=16) the depletion started 1 day before the intradermal immunization and was maintained for 10 days with administration of antibodies on alternate days. At day 10 and day 22 three animals from each experimental group were euthanized and the neutrophils levels were checked in the lymph nodes and spleen of each group of mice.
The remaining animals from 1st and 2nd set of experiments (n= 10) were then challenged via tail vein with 105 virulent L. donovani (LdWT) metacyclic parasites at day 22. Infective-stage metacyclic promastigotes of L. donovani were isolated from stationary cultures by density gradient centrifugation as described previously (33). Age-matched naive mice used as controls were also similarly challenged with 105 virulent L. donovani metacyclic parasites. At 8 week of post challenge period, parasite load was measured from spleens of challenged mice by culturing the separated host cell preparations by limiting dilutions as previously described (19). In case of 1st set of experiment IFN-γ and IL-10 were measured from the Leishmania Ag–stimulated splenocyte culture supernatants 3-week postimmunization+ 8-week post challenge by sandwich ELISA (eBioscience).
Statistical Analysis
Statistical analysis of differences between means of groups was determined by unpaired two-tailed Student t test, using GraphPad Prism 5.0 software. A p value < 0.05 was considered significant, and a p value < 0.005 was considered highly significant.
Results
1. LdCen−/− parasites induce a strong effector function in neutrophils in vitro
We assessed the neutrophil recruitment in vitro in response to infection with either LdWT/LdCen−/− parasites using a trans-well migration assay. Specifically, chemotaxis of neutrophils towards cell-free supernatants from LdWT or LdCen−/− infected (6h post infection) peritoneal macrophages was examined. Media alone served as a negative control. Neutrophils exposed to supernatants from macrophages infected with LdWT or LdCen−/− showed significantly more migration compared to media alone (Fig 1A). Notably, significantly higher number of neutrophils migrated when exposed to supernatants derived from LdCen−/− than LdWT infected macrophage (Fig 1A). Next, we investigated the phenotype of neutrophils following infection with LdWT and LdCen−/− parasites in vitro. First, we compared phagocytosis of fluorescent LdWTRFP and LdCen−/−mCherry parasites by neutrophils at 37°C. Control phagocytic reaction with each parasite was performed at 4℃. After 4h of incubation at 37°C there were no significant differences in the percentage of parasitized neutrophils between LdWTRFP and LdCen−/−mCherry infections, and both were significantly higher than their respective controls (Fig 1B).
Figure 1: LdCen−/− infection induced strong effector function in neutrophils compared to LdWT infection in vitro.
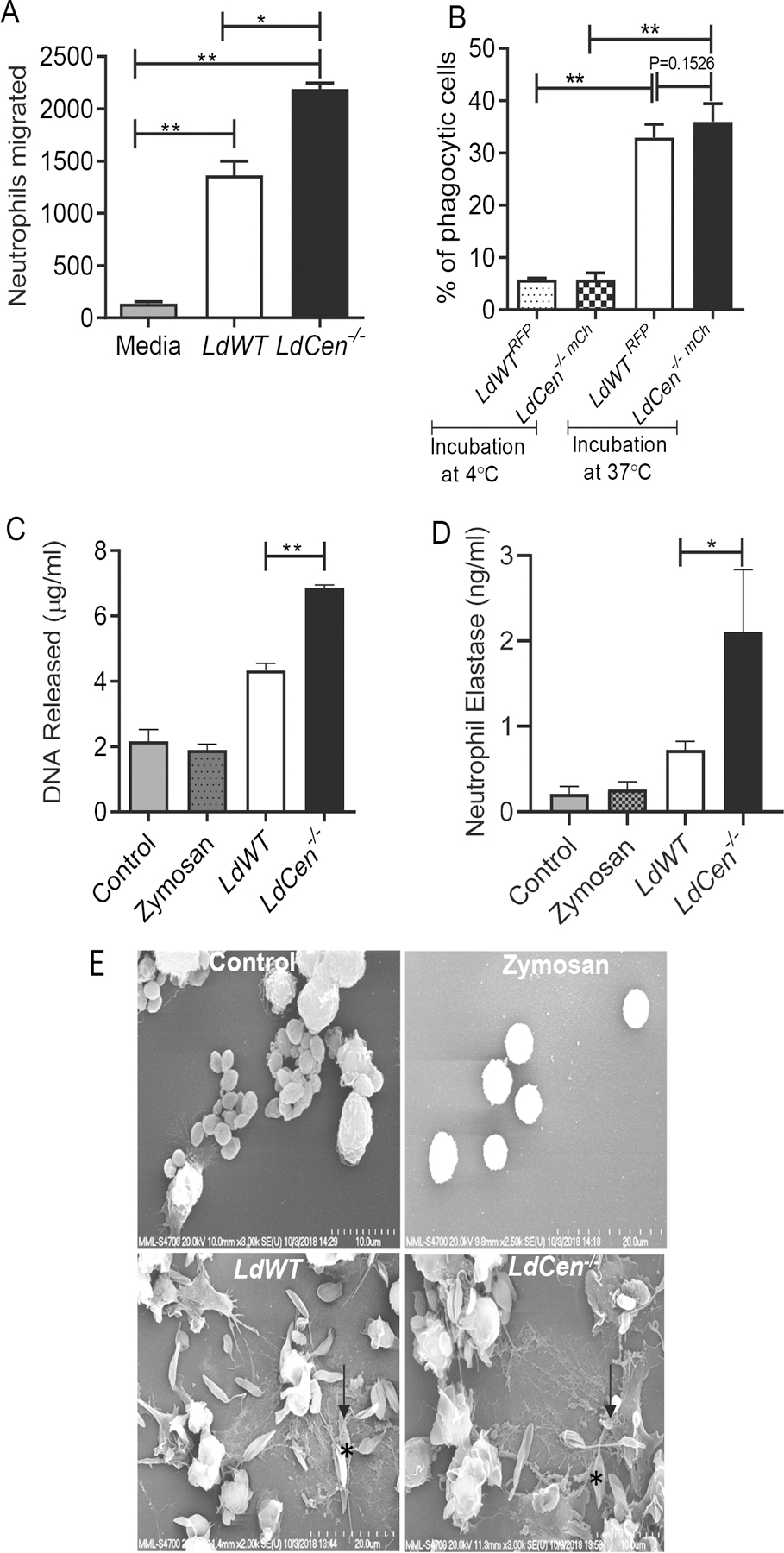
(A) Absolute number of neutrophils that migrated after stimulation with supernatants of LdWT or LdCen−/− infection (6h) of macrophages was measured by chemotaxis assay. The data represent the mean values ± standard deviations (SD) of results from 3 independent experiments that all yielded similar results. * P < 0.05; ** P < 0.005. (B) Peritoneal neutrophils were cocultured with LdWTRFP or LdCen−/−mcherry for 4h (1:5 cell: parasite ratio) either at 4℃ or at 37℃. Bar diagrams represent percentages of RFP+/mCherry+ neutrophils as determined by flow cytometry. Data are pooled from 3 independent repeats and are shown as means ± standard deviations (** P < 0.005) between the groups. (C) Neutrophils were incubated either with LdWT or LdCen−/− promastigotes or with zymosan. DNA released was quantified after 6h of incubation. The data presented are means ± SD of 3 independent experiments. ** P < 0.005 between the groups. (D) Peritoneal neutrophils were lysed and assayed for NE activity as described in Material Methods. The NE activity was expressed as the absorbance observed at 450 nm. Data are pooled from 3 independent repeats and are shown as means ± standard deviations (*P < 0.05) between the groups. (E) Scanning electron microscopy for visualization of NET. Naïve neutrophils were either remain uninfected or incubated with zymosan or promastigotes (as indicated by*) for 6h. NET fibers (as indicated by black arrow) were found in infected (LdWT or LdCen−/− ) neutrophils. The micrographs are representative of 3 independent experiments in which at least 100 cells per sample were analyzed.
Neutrophils that release DNA and elastase to form filamentous structures are considered as producing neutrophil extracellular traps, that mediate microbicidal activity (NETs) (24). We therefore, assessed the potential of LdCen−/− parasite infection to induce the formation of NETs. Neutrophils were incubated for 6h with either LdWT or with LdCen−/− parasites and the formation of NETs was assessed by quantification of DNA release. We found that the release of DNA by parasite infected neutrophils was 2–3-fold higher than the uninfected control and zymosan (phagocytic control) treated neutrophils (Fig 1C). However, the DNA release was significantly higher in LdCen−/− compared to LdWT infected neutrophils (Fig 1C). Neutrophil elastase (NE) enzymatic activity in uninfected, zymosan treated, and LdWT or LdCen−/− infected culture supernatants 6h post infection showed that NE activity was 2-fold higher in LdCen−/− compared to LdWT infection (Fig 1D). Finally, analysis of Leishmania promastigote–NET interaction by scanning electron microscopy further showed distinct networks of NETs (arrowhead) with promastigotes (*) (Fig 1E).
2. Kinetics of neutrophil recruitment to ear dermis, ear dLN and spleen following LdCen−/− intradermal infection
To investigate the neutrophil recruitment kinetics in vivo we examined the ear dermis, ear dLN and spleen of C57BL/6 mice following intradermal injection with PBS or LdWT or LdCen−/− parasites from 6h and up to 14 days post infection. The gating strategy for selecting neutrophils from ear dermis, ear dLN and spleen is shown in Supplementary Fig 1A, C, D respectively. Analysis of cells isolated from the ear dermis revealed a significant and rapid increase in CD11b+ Ly-6G+Ly-6Cint neutrophils beginning at 6h and peaking at 18h in LdCen−/− and LdWT infected mice compared to PBS treated control mice, followed by a gradual drop up to 14 days post-infection (Fig 2A). We observed a significantly higher number of neutrophils in LdCen−/− infection compared to LdWT infection at 6h and 18h time points (Fig 2A). Comparison of LdWTRFP and LdCen−/−mCherry infected mice ear dermis showed similar percentages of RFP+/m-Cherry+ neutrophils (Supplementary Fig 1B, Fig 2B) indicating that both parasites have similar in vivo infective capacities. Kinetics of neutrophil influx in the ear dLN in response to LdWT and LdCen−/− infection was also determined between 6h and 14d after infection. Significantly higher neutrophil influx was noted in the ear dLN of LdCen−/− infected mice compared to LdWT infected mice starting at 18h and peaked at 72h post infection (Fig 2C) followed by a drop in the number of neutrophils up to 14 days post-infection (Fig 2C). Quantification of parasite-infected neutrophils in the ear dLN at 48h after infection revealed similar percentages of RFP+/m-Cherry+ neutrophils (Fig 2D). Analysis of spleen revealed a significant increase in neutrophil numbers in LdWT/LdCen−/− infected mice starting at 72h and continued till 7d post infection followed by a decline (Fig 2E). LdCen−/− infected mice showed significantly higher splenic neutrophil influx compared to LdWT infected mice both at 72h and 7d post infection (Fig 2E).
Figure 2: Kinetics of ear dermis, ear draining lymph nodes and splenic neutrophil recruitment following LdCen−/− intradermal infection.
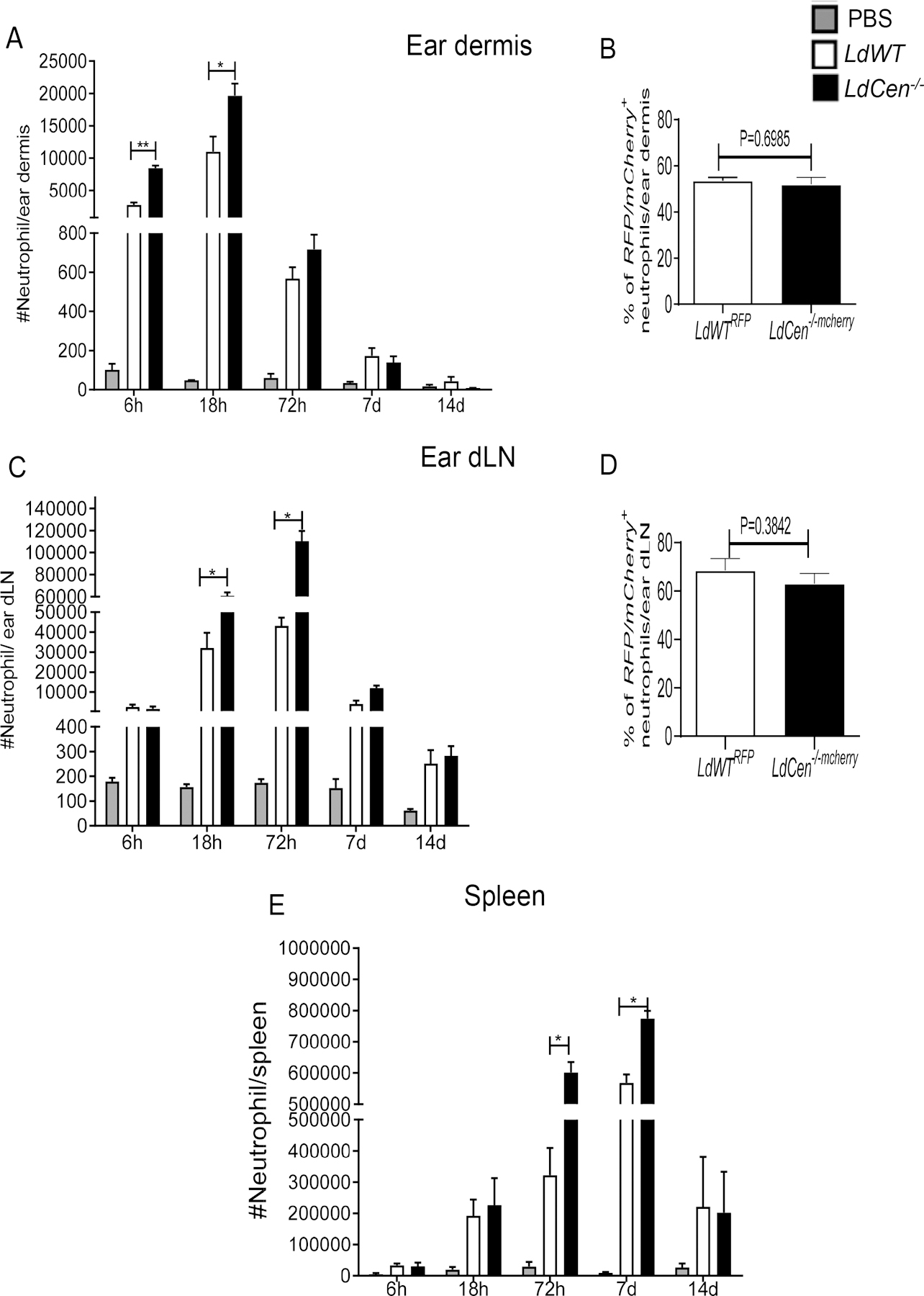
Mice were injected intradermally either with PBS or LdWT or LdCen−/− parasites for different time periods as indicated. Kinetics of neutrophil recruitment in ear dermis, ear draining lymph node (dLN) and spleen was analyzed by flow cytometry. Neutrophils were defined as a subpopulation of CD11b+myeloid cells having the following markers: Ly6CintLy6G+. Changes in the total number of neutrophils per (A) ear dermis (C) ear dLN and (E) spleen. Values shown are the mean numbers of cells per ear dermis/ear dLN / spleen ± standard deviations of results. 6 ears, ear dLN and spleen at each time point, pooled data from 3 independent experiments (n=6). (*P < 0.05, ** P < 0.005) (B) Changes in the subsets of parasitized (RFP+/ mCherry+) neutrophils in ear dermis at 6h post infection and (D) ear dLN at 48h post infection expressed as a percentage of the total RFP+/ mCherry+ neutrophils. The data presented are means ± SD of 3 independent experiments (n=6).
We also investigated the sequence of recruitment of other myeloid cells such as macrophages and dendritic cells (DCs) to the ear dermis, ear dLN and spleen of C57BL/6 mice following intradermal injection with LdWT or LdCen−/− parasites from 6h and up to 14 days (Supplementary Fig 2). The numbers of macrophages (Cd11b+Ly6G−Ly6C−CD11c−MHCII+) and DCs (Cd11b+Ly6G−Ly6C−CD11c+MHCII+) were lower compared to neutrophils at early time points (between 6–72h) in ear dermis (Supplementary Fig 2A–2C); ear dLN (Supplementary Fig 2D–2F) and spleen (Supplementary Fig 2G–2I) and remained relatively unchanged compared to PBS treated control mice until 7 days post-infection, marking the onset of their massive accumulation in these organs. Interestingly, a significant increase in macrophages and DCs in all the organs tested was observed in LdCen−/− infected mice compared to LdWT at 7d and 14d post infection (Supplementary Fig 2A–2I).
3. Increased CXCL1/CXCL2 production by ear tissue resident macrophages following LdCen−/− infection recruits a significantly higher number of neutrophils to the ear dermis
To investigate the mechanism of differential recruitment of neutrophils at the injection site and subsequently to distal organs following LdCen−/− infection compared to LdWT infection, we studied CXCL1 and CXCL2 expression in the tissue resident Ly6C− macrophages from ear dermis that has been implicated in neutrophil recruitment (34, 35). We sort selected tissue resident macrophages from the ear dermis of PBS or parasite injected mice 6h post infection, by gating on Cd11b+F4/80+ Ly6G− Ly6C−MHCII− live single cells (Fig 3A) and assessed the expression levels of CXCL1, CXCL2. There were no significant differences in the number of ear dermal tissue resident macrophages between LdWT and LdCen−/− infected mice (Fig 3B). However, ear tissue resident macrophages from LdCen−/− infected mice produced 2.1-fold and 3.3-fold higher mRNA levels of CXCL1 (Fig 3C) and CXCL2 (Fig 3D), respectively compared to LdWT infection suggesting that the elevated chemokines may result in enhanced recruitment of neutrophils in LdCen−/− infected mice as observed in Fig 2.
Figure 3. Increased CXCL1/CXCL2 production by ear tissue resident macrophages following LdCen−/−intradermal infection.
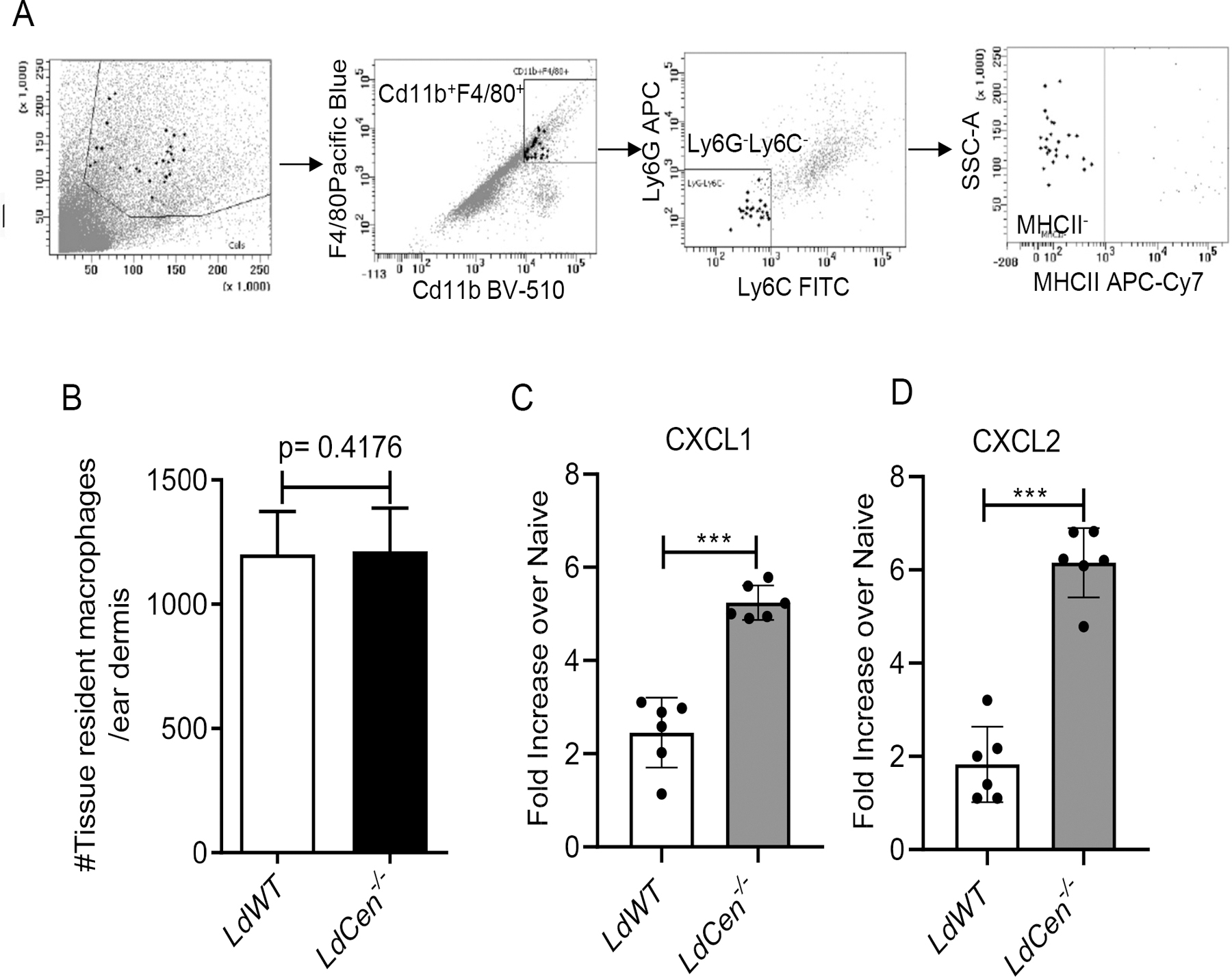
(A) Mice were either not infected (PBS injected) or infected intradermally in the ear pinna with LdWT or LdCen−/− parasites for 6h. Ear tissue resident macrophages were sort selected from different groups of mice (n=6) by gating live single cells for Cd11b+ F4/80+ Ly6G− Ly6C−MHCII−. The sorting strategy is displayed. (B) The absolute number of tissue resident macrophages obtained per ear dermis of the infected mice were calculated. The experiment was repeated 3 times with pooled digests from 6 ear dermis per experiment. Means and standard errors of the means for 6 mice in each group are shown. Data are representative of 3 independent experiments. (C, D) Changes in the expression of CXCL1 and CXCL2 mRNA in sort selected ear tissue resident macrophages were determined by qPCR as described in Material and Methods. Data are presented as fold change from uninfected naive mice. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=6). *** P < 0.0005.
4. LdCen−/− intradermal infection induced significantly higher pro-inflammatory neutrophils and decreased anti-inflammatory neutrophils in ear dermis and ear dLN compared to LdWT
We next characterized the neutrophil phenotype recruited to the ear dermis and ear dLN in LdCen−/− infected mice and compared to LdWT infected mice. At 18h and 72h post infection we sort selected total neutrophils from ear dermis (Supplementary Fig 3A) and ear dLN (Supplementary Fig 3B) of either PBS or LdWT parasite or LdCen−/− parasite injected mice by gating on live single Cd11b+Ly6G+Ly6Cint cells and assessed the cytokine and chemokine gene expression profiles. We observed in ear dermis both at 18h and 72h post infection, neutrophils from LdCen−/− infected mice showed a significant increase in the expression of proinflammatory cytokine and chemokine genes such as, IL-12, TNF-α, CCL2 and CCL3 respectively (Fig 4A–4D), with a concomitant down-modulation of an anti-inflammatory cytokine, IL-10 (Fig 4E) compared to neutrophils from LdWT infected mice. Expression of these genes was substantially lower at 72h post infection compared to 18h post infection (Fig 4A–4E). Likewise, in ear dLN at 18h and 72h post infection neutrophils showed a significant increase in the expression of the proinflammatory cytokine and chemokine genes (IL-12, TNF-α, CCL2 and CCL3) (Fig 4F–4I) along with a substantial decrease of IL-10 expression (Fig 4J) compared to LdWT infected mice. In contrast to the ear dermis, the expression of all the proinflammatory and anti-inflammatory genes significantly increased 72h post infection compared to 18h post infection in the ear dLN (Fig 4F–4J).
Figure 4: LdCen−/− intradermal infection induced significantly higher pro-inflammatory neutrophils and decreased anti-inflammatory neutrophils in ear dermis and ear dLN compared to LdWT.
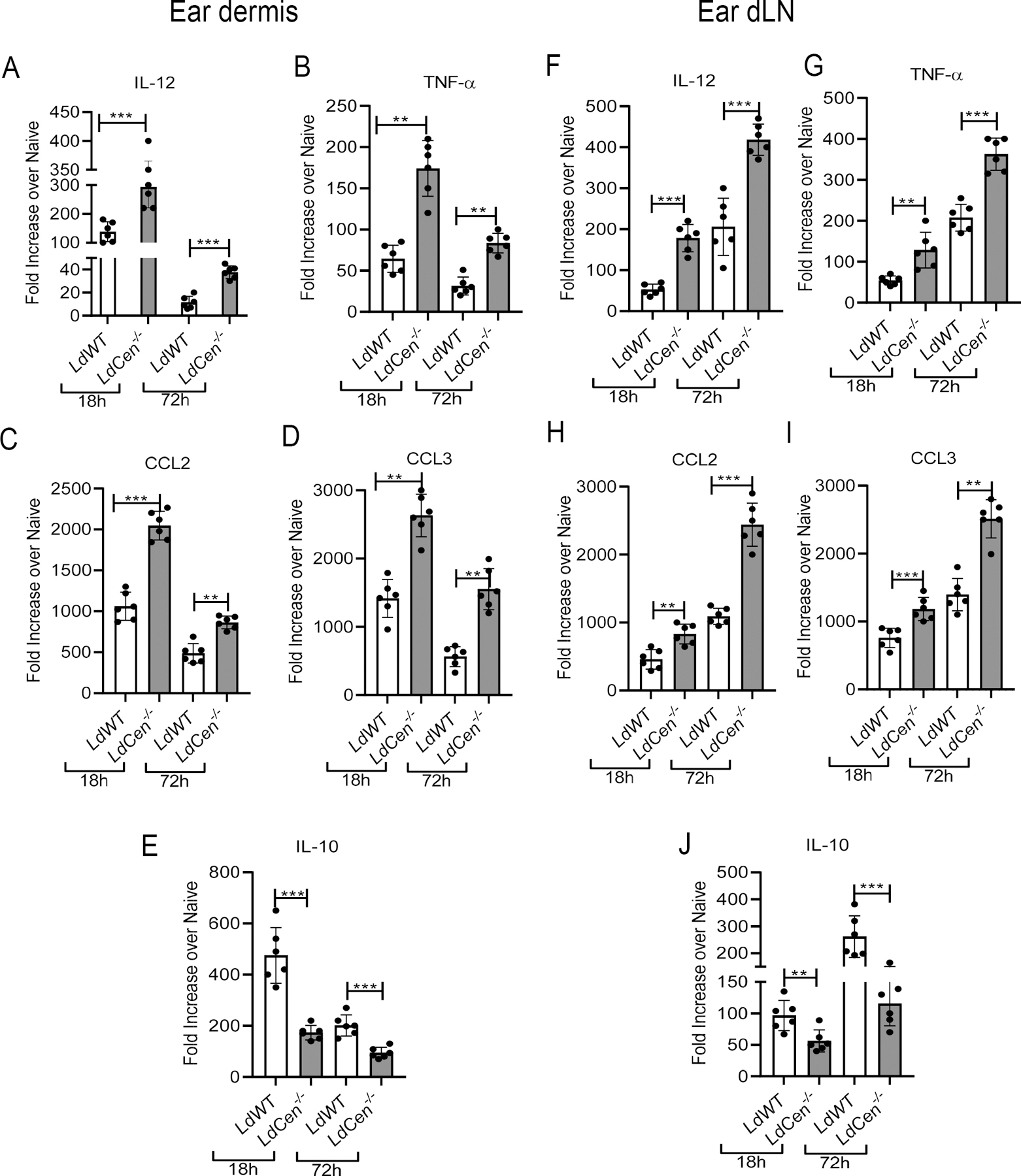
(A-E) Neutrophils (Cd11b+Ly6G+Ly6Cint) were flow sorted from the ear dermis or (F-J) ear dLN 18h and 72h post infection respectively. Normalized expression levels of (A, F) IL-12p70, (B, G) TNF-a, (C, H) CCL2, (D, I) CCL3 and (E, J) IL-10, in ear dermis and ear dLN were estimated at indicated time points by qPCR. Data are presented as fold change from uninfected naive mice. The experiment was repeated 3 times with pooled digests from 6 −8 ear dermis and ear dLN per experiment. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=6). * P < 0.05; ** P < 0.005; ***P< 0.0005
We also performed a comparative analysis to check the cytokine-chemokine expression pattern between neutrophil, macrophages and DCs recruited in the ear dLN 48h post infection with either LdWT/LdCen−/− parasites. The macrophages and DCs were sort selected from different groups of mice as shown in Supplementary Fig 3C. We found neutrophils from ear dLN of LdCen−/− infected mice produced significantly higher cytokine such as IL-12 (Supplementary Fig 3D) and chemokine viz., CCL3 (Supplementary Fig 3E) compared to macrophages or DCs.
5. Increased expression of costimulatory molecules and attenuated expression of co-inhibitory molecules in the neutrophils recruited to ear dLN following LdCen−/− intradermal infection
To test the neutrophil mediated T cell activation, we first assessed the expression of costimulatory molecule in the neutrophils recruited in the ear dLN of C57BL/6 mice following intradermal injection with PBS (uninfected naïve mice) / LdWT parasites/ LdCen−/− parasites 48h post infection. The gating strategy and individual flow plots is shown in Fig 5A. Neutrophils from LdCen−/− infected mice ear dLN showed a significant increase in MHCII (Fig 5B) and CD80 (Fig 5D) compared to LdWT infected mice. We did not find differences in the level of MHCI expression between LdWT and LdCen−/− infected mice (Fig 5C). Additionally, we measured the expression of co-inhibitory/exhaustion markers such as PDL-1 and Arginase-1 in sort selected neutrophil population from ear dLN by RT-PCR. Ear dLN neutrophils from LdCen−/− infected mice exhibited significantly reduced expression of PDL-1 and Arginase-1 compared to LdWT (Fig 5E). Independently, we also measured PD-1 expression, the receptor for PDL1 on T cells, from ear dLN of LdWT and LdCen−/− infected mice 48h post-infection. Antigen specific CD4+T cells from ear dLNs of LdCen−/− infected mice showed significant attenuation of PD1 compared to LdWT (Fig 5F, Supplementary Fig 4A), further indicative of a higher CD4+T cell activation in LdCen−/− infected mice.
Figure 5: Increased expression of costimulatory molecules and attenuated expression of co-inhibitory molecules in the neutrophils recruited to ear dLN following LdCen−/− intradermal infection:
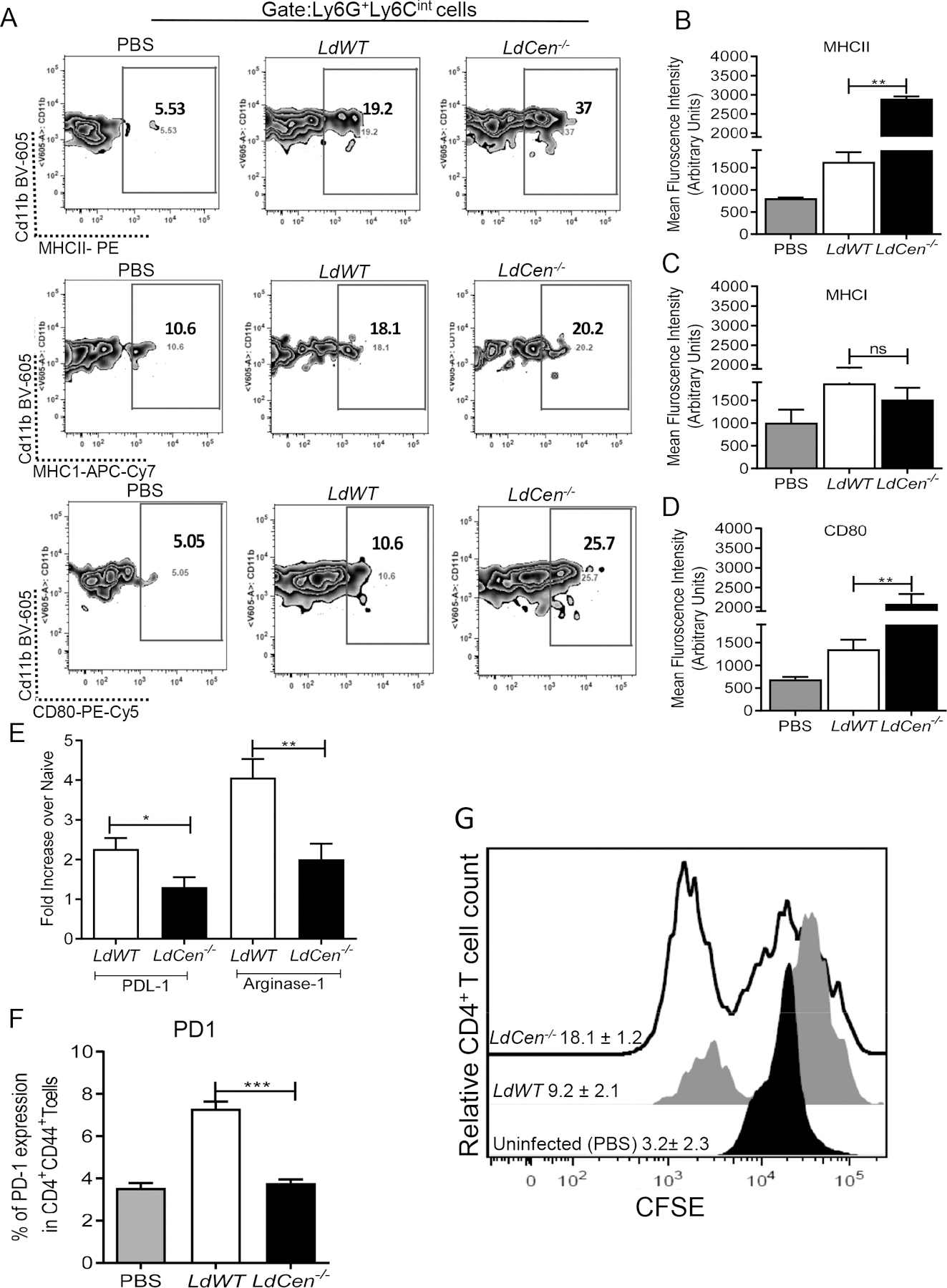
Mice were injected intradermally in the ear pinna either with PBS /LdWT parasites/ LdCen−/− parasites and the expression of MHCII, MHC1, CD80 in the neutrophils recruited in ear dLN were analyzed by flow cytometry. (A) The gating strategy and the individual flow plots have been shown for ear dLN (B, C, D) Mean fluorescence intensity of the costimulatory molecules (MHCII, MHC1, CD80) expression in ear dLN has been represented by the bar diagram. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=6). (E) Changes in the expression of co-inhibitory molecules PDL-1 and Arginase-1 mRNA in sort selected neutrophils from ear dLN 48h post infection were estimated by qPCR. Data are presented as fold change from uninfected naive mice. The experiment was repeated 3 times with pooled digests from 6 −8 ear dLN per experiment. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=4). * P < 0.05; ** P < 0.005. (F) Percentage of antigen specific CD4 T cells (CD4+CD44+) in ear dLN expressing PD-1. Columns show mean ± SEM of triplicates (n=4). *** P<0.0005. (G) Antigen specific CD4 T cell proliferation was estimated from parasitized neutrophil-CD4 T cell coculture assay by studying CFSE dilution of gated CD4+CD44+T cells and is represented by the staggered offset histogram overlay. Cell proliferation was analyzed in triplicate experiments (n=6), and histograms representative of mean values were overlaid for the figure.
To test whether neutrophils showing higher MHCII and lower PDL-1 expression in LdCen−/− infected mice ear dLN, are competent in priming of CD4+T cells, we sort selected uninfected neutrophils from PBS-injected mice or parasitized neutrophils from LdWT RFP/LdCen−/−mCherry infected mice ear dLN 48h post infection as shown in the representative sorting strategy (Supplementary Fig 4B). The sort selected neutrophils were cultured with CFSE-labelled CD4+T cells isolated from the spleen of mice previously infected with L. donovani and recovered from infection. Following 5d of co-culture, neutrophils from LdCen−/− infected mice induced significant increase in antigen specific CD4+T cell proliferation compared to those cocultured with neutrophils from LdWT infected mice as illustrated by the offset histogram depicting the individual percentage of proliferating CD4+T cells from different groups (Fig 5G).
6. Identification of distinct subpopulations of neutrophils in Leishmania infection
Heterogeneous neutrophil populations, based on their size and complexity, have been reported to perform distinct functions during viral infection (13). That prompted us to explore and characterize the heterogeneity of neutrophils recruited to the ear dLN following intradermal infection with LdCen−/− or LdWT 48h post infection. Flow cytometric analysis revealed two clear subsets of murine neutrophils (Fig. 6A) according to their forward and side scatter properties, as indicators of size and granularity, and the expression of the neutrophil marker Ly6G and named as Nα and Nβ, as described in a recent study demonstrating neutrophil heterogeneity during viral infection (13). We observed that number of Nα is higher compared to Nβ in the ear dLN and that the absolute number of Nα is significantly higher in LdCen−/− compared to LdWT infected mice (Fig 6B). To compare the phagocytic efficiency of Nα and Nβ population, we infected mice with LdWTRFP or LdCen−/− mCherry parasites. Almost all the parasitized neutrophils were of Nα type and there was no difference in the parasitized Nα type neutrophils between LdWT and LdCen−/− infections (Fig 6C) indicating that Nα neutrophils have the most phagocytic potential.
Figure 6: Identification and functional characterization of subpopulations of neutrophils during Leishmania infection.
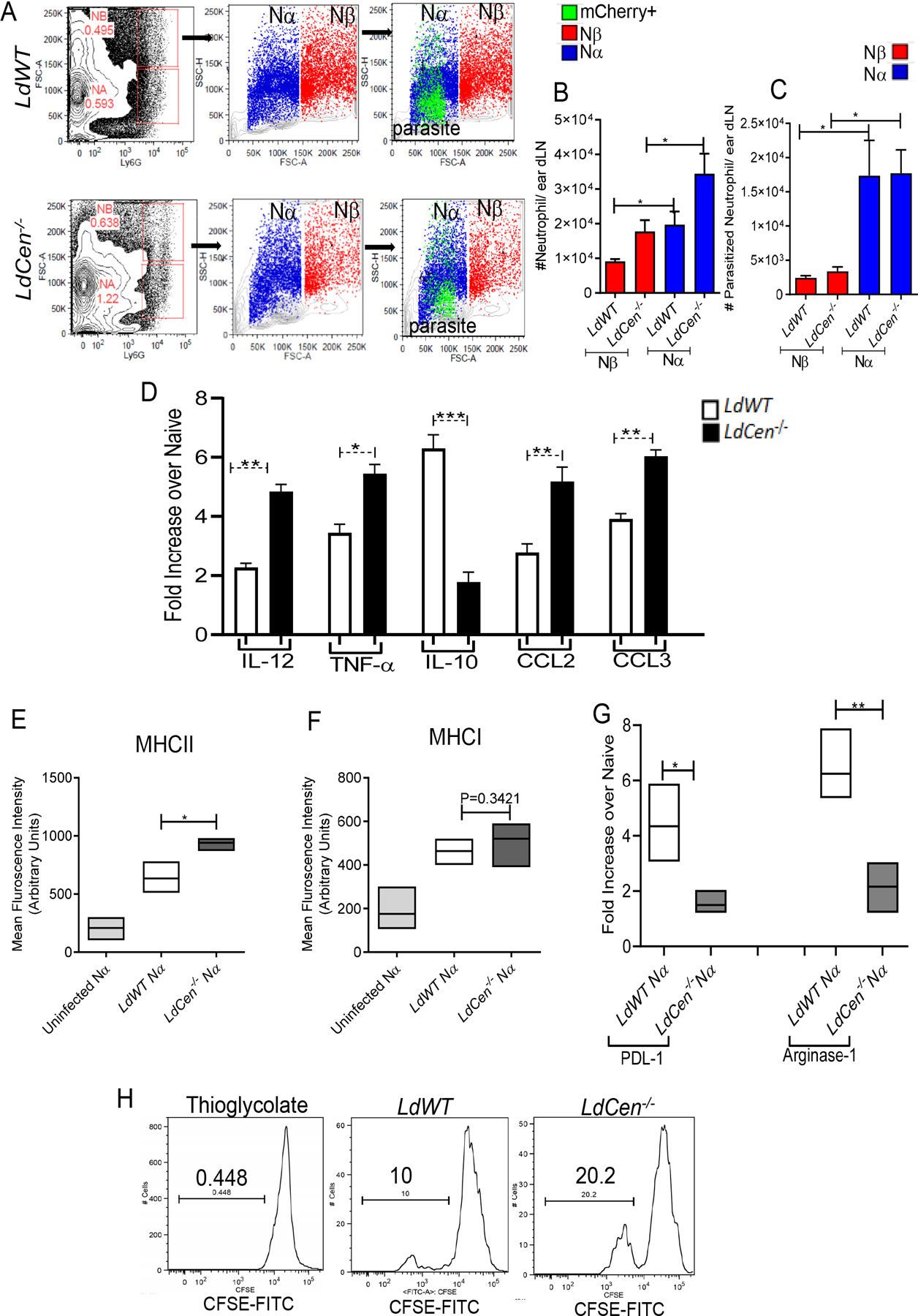
(A) FACS plots determined by forward scatter (FSC) and Ly6G and side scatter (SSC) and FSC of ear dLN homogenates of LdWT and LdCen−/− infected mice (n=6). Nα (blue) and Nβ (red) neutrophil subsets are shown as dot plots. Infected neutrophils (Nα mCherry+; light green) from ear dLN homogenates of LdWTRFP and LdCen−/−mCherry infected mice. (B) Absolute number of total Nα and Nβ neutrophils or (C) parasitized (RFP/mCherry+) Nα and Nβ neutrophils in the ear dLN at 48h post infection in LdWT and LdCen−/− infected mice. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=6). * P < 0.05. (D) Changes in the expression of mRNA encoding cytokines-chemokines in sort selected Nα population from uninfected, LdWT and LdCen−/− infected mice ear dLN were estimated by qPCR. Data are presented as fold change from uninfected naïve mice. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=6). * P < 0.05; ** P < 0.005; *** P < 0.005. (E, F) Mean Fluorescence Intensity of MHC1I and MHC1 expression in Nα neutrophils of uninfected and LdWT or LdCen−/− infected mice at 48h post infection in ear dLN. Columns show mean ± SEM of 5 mice. * P < 0.05 (G) The expression of mRNA encoding co-inhibitory molecules PDL1 and Arginase-1 in Na neutrophils sorted from ear dLN 48h post infection by qPCR. Data are presented as fold change from naive mice. The data represent the mean values ± standard deviations of results from 3 independent experiments that all yielded similar results (n=4). * P < 0.05; ** P < 0.005. (H) Nα can directly activate CD4+T cell proliferation ex-vivo. Antigen specific CD4 T cell proliferation was estimated from parasitized Nα neutrophil-CD4 T cell coculture assay by studying CFSE dilution of gated CD4+CD44+T cells and is represented by the staggered offset histogram overlay. Cell proliferation was analyzed in triplicate experiments (n=6).
7. Only Nα neutrophils induced robust antigen specific CD4 T cell proliferation ex-vivo
To demonstrate that Nα neutrophils possess functional characteristics, consistent with the results observed with total neutrophils, we first analyzed the expression of cytokines and chemokines in sort selected Nα neutrophils from LdWT and LdCen−/− infected mice ear dLN. The sorting strategy is shown in Supplementary Fig 5A. Nα neutrophils from LdCen−/− infected mice showed a significantly higher level of proinflammatory cytokines-chemokines and attenuated IL-10 expression compared to LdWT infection (Fig 6D). Similarly, flowcytometric analysis of Nα neutrophils from LdCen−/− infected mice showed a significantly higher level of MHCII expression compared to LdWT (Fig 6E). consistent with a higher antigen presenting potential observed in LdCen−/− infected mice. However, there was no significant difference in MHCI expressing Nα neutrophils between LdWT and LdCen−/− infected mice (Fig 6F) suggesting a predominantly MHCII restricted CD4+T cell priming by Nα neutrophils during LdCen−/− infection as was also observed in total neutrophils in Fig 5A, 5B, 5C.
We next analyzed the expression of co-inhibitory molecules such as PDL-1 and Arginase-1 in sort selected Nα population from LdWT and LdCen−/− infected mice ear dLN at 48h post infection (Fig 6G). Notably, Nα neutrophils from LdCen−/− infected mice ear dLN exhibited significantly attenuated expression of PDL-1 and Arginase-1 compared to LdWT (Fig 6G) which may lead to a heightened activation of T cells.
Since Nα neutrophils showed the expression of markers consistent with antigen presenting activity, we analyzed the ability of Nα neutrophils to activate CD4+T cells. To this end, we sort selected uninfected Nα neutrophils from thioglycolate-injected mice or parasitized Nα from LdWTRFP or LdCen−/−mCherry infected mice ear dLN 48h post infection as shown by the representative sorting strategy in Supplementary Fig 5B. Nα neutrophils from different groups of mice were incubated with CFSE-labelled antigen experienced CD4+T cells for 5 days. The Nα enriched fraction from LdCen−/− infected mice showed significant increase in antigen specific CD4+T cell proliferation compared to corresponding population from LdWT as shown by the representative histograms (Fig 6H) and quantitative bar diagram indicating % cells showing CFSE dilution on gated CD44+CD4+ cells (Supplementary Fig 5C). These results suggest that Nα neutrophil subset of neutrophils from LdCen−/− infected mice significantly induced MHCII restricted CD4+Th1 cell activation and proliferation unlike LdWT infection and is similar to the data with total neutrophils shown in Fig 5.
Comparative phenotypic characterization of Nα and Nβ population showed that all the key cytokines-chemokines costimulatory and co-inhibitory molecules were predominantly expressed in Nα neutrophils compared to Nβ cells in both LdWT and LdCen−/− infected mice (data not shown).
8. Adoptive transfer of LdCen−/− infected neutrophils induced significantly higher antigen specific Th1 cell proliferation compared to LdWT and neutrophil depletion abrogates the same in mice
To test, whether adoptive transfer of LdCen−/− infected neutrophils can similarly induced antigen specific T cell proliferation in vivo, compared with neutrophils bearing LdWT infected parasites, we purified neutrophils from bone marrow of naïve mice that were positive (95%) for neutrophil associated marker (Cd11b and Ly6G) with negligible contamination with monocytes/DCs. The purified neutrophils were either not infected or infected with fluorescent LdWT /LdCen−/− parasites for 5h in vitro, followed by extensive washing and allowed to rest for 2h to ensure internalization of the parasites prior to intra venous (i.v.) injection
Before adoptive transfer, we checked the percent infection in these neutrophils and found the presence of >75% infected neutrophils following LdWT/LdCen−/− infection (Supplementary Fig 6A). Additionally, we checked CCR7 expression, in the uninfected/ LdWT infected / LdCen−/− infected neutrophils. CCR7 is a crucial homing receptor used by neutrophils to migrate to lymph nodes (36). Accordingly, we found significantly higher CCR7 expression on LdWT infected or LdCen−/− infected neutrophils (Fig 7A, 7B) compared to uninfected neutrophils. However, CCR7 expression was significantly higher in neutrophils infected with LdCen−/− compared to LdWT parasites (Fig 7A, 7B).
Figure 7: Adoptive transfer of LdCen−/− infected neutrophils, induced significantly higher antigen specific CD4+T cell proliferation compared to LdWT.
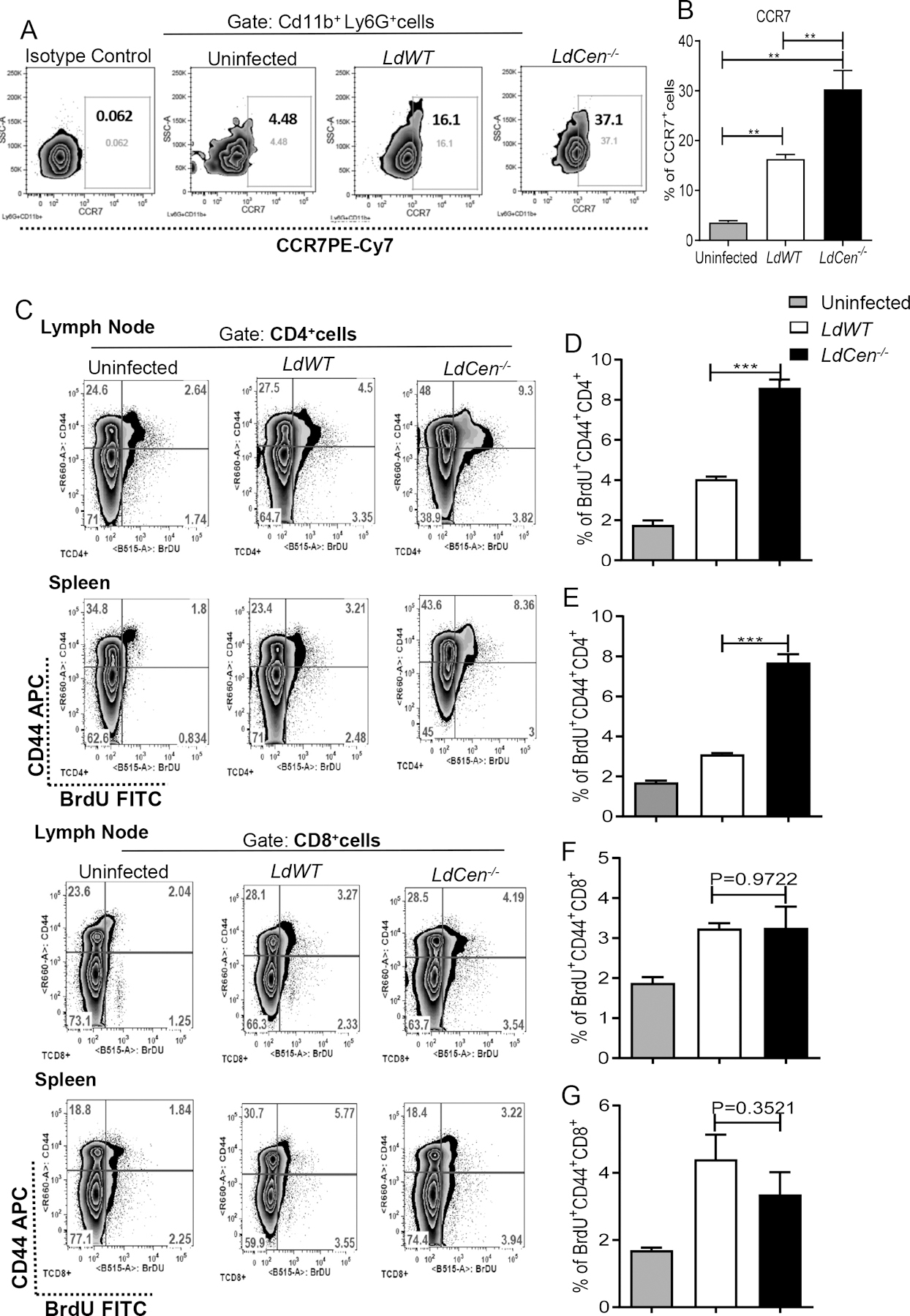
(A, B) CCR7 expression levels in uninfected/ LdWT infected / LdCen−/− infected neutrophils before adoptive transfer as measured by flow cytometry and shown by the (A) representative flow plots and the (B) bar diagram. Graphs show mean ± standard deviations of results from 3 independent experiments that all yielded similar results, ** P < 0.005. (C, D, E, F, G) Adoptive transfer of LdCen−/− parasite bearing neutrophil induced heightened antigen specific CD4+T cell proliferation in vivo compared to LdWT. Antigen specific T cell proliferation in vivo was measured with BrdU as described in Material and Methods. (C) The representative flow plots for CD4+CD44+ and CD8+CD44+T cells from lymph nodes and spleen (D, E, F, G) Bar diagrams represent the percentage of BrdU+CD44+CD4+T cells/ BrdU+CD44+CD8+T cells in the lymph nodes and spleen on day 5 after i.d. injection of parasitized neutrophils. The data represent the mean values ± standard deviations of results from 3 independent experiments (n=6). *** P < 0.0005.
Uninfected/ LdWT infected / LdCen−/− infected neutrophils were injected into mice that were previously injected with BrdU. Lymph nodes and spleen were removed, and proliferative antigen-specific T cell responses were measured via BrdU staining on day 5. The gating strategy and individual flow plots have been shown in Fig 7C. We observed that adoptive transfer of LdCen−/− infected neutrophils resulted in a significantly higher proliferative CD4+T cell response in the lymph nodes (Fig 7D) and spleen (Fig 7E) as compared to LdWT infected neutrophils. In contrast, adoptive transfer of neutrophils containing LdWT infected or LdCen−/− infected parasites exhibited identical proliferation of CD8+ T cells in the lymph nodes (Fig 7F) and spleen (Fig 7G).
Further, antigen experienced BrdU+CD44+CD4+ T cells from lymph nodes of the mice that received LdCen−/− infected neutrophils produced significantly higher levels of proinflammatory cytokines IL-2 and IFN-γ along with an overall higher IFN-γ/IL-10 ratio compared to mice which received LdWT infected neutrophils (Supplementary Fig 6B, 6C, 6D). The frequency of all the cytokine secreting BrdU+CD44+CD8+T cells was comparatively lower than CD4+T cells in all the groups observed and not statistically different between the LdWT and LdCen−/− groups (Supplementary Fig 6E, 6F).
We next confirmed the specificity of CD4 T cell activation by neutrophils via depleting them using an anti-neutrophil monoclonal antibody (1A8) as depicted in supplementary Fig 7A. Administration of 1A8, depleted ~90% of Cd11b+Ly6G+ neutrophils in lymph nodes (Supplementary Fig 7B, 7C) and spleen (Supplementary Fig 7D, 7E) compared to isotype control (GL113) treated groups. Antigen specific CD4+T cell proliferation was significantly attenuated in the lymph nodes and spleen after neutrophil depletion in LdCen−/− infected mice whereas no significant reduction was observed in LdWT infected mice after neutrophil depletion (Supplementary Fig 7F–7I).
9. Neutrophil depletion abrogates the LdCen−/− induced host protective immunity
To ascertain the critical role of neutrophils in LdCen−/− mediated protection against virulent Leishmania challenge in vivo, mice were depleted of neutrophils using anti-neutrophil antibody (1A8). GL113 treated mice served as a control for the experiment (Schematic in Fig 8A). Neutrophil depletion was initiated 1 day prior to the intradermal immunization with LdCen−/− promastigotes and was continued for 21 days with administration (i. p.) of antibodies on alternate days. Significant depletion of neutrophils in 1A8 treated mice lymph nodes and spleen was observed on day 22 (Supplementary Fig 8A–8D). The remaining immunized mice were challenged on day 22 with virulent L. donovani parasites (i. v.) and were monitored for 8-weeks. Age matched naïve mice were challenged with virulent L. donovani parasites (i. v.). Neutrophil depletion with 1A8 significantly reduced the IFN-γ/ΙL−10 ratio in splenocytes from LdCen−/− immunized mice compared to GL113 treated immunized mice at 3-week post immunization and after 8-week post-challenge time points (Fig 8B) indicating a poor Th1 response. Parasite burden in the spleen after 8 weeks of challenge with LdWT parasites in the neutrophil depleted and non-depleted mice showed that neutrophil depletion reversed LdCen−/− mediated parasite control as indicated by a significantly increased spleen parasite burden at 8-week post challenge period compared to GL113 treated immunized challenged mice (Fig 8C). These results suggest that absence of neutrophils leads to the generation of a defective Th1 response which renders the immunized mice susceptible towards virulent Leishmania challenge.
Figure 8. Neutrophil depletion abrogates the LdCen−/− induced host protective immunity; albeit neutrophil repletion before challenge partially restore protection.
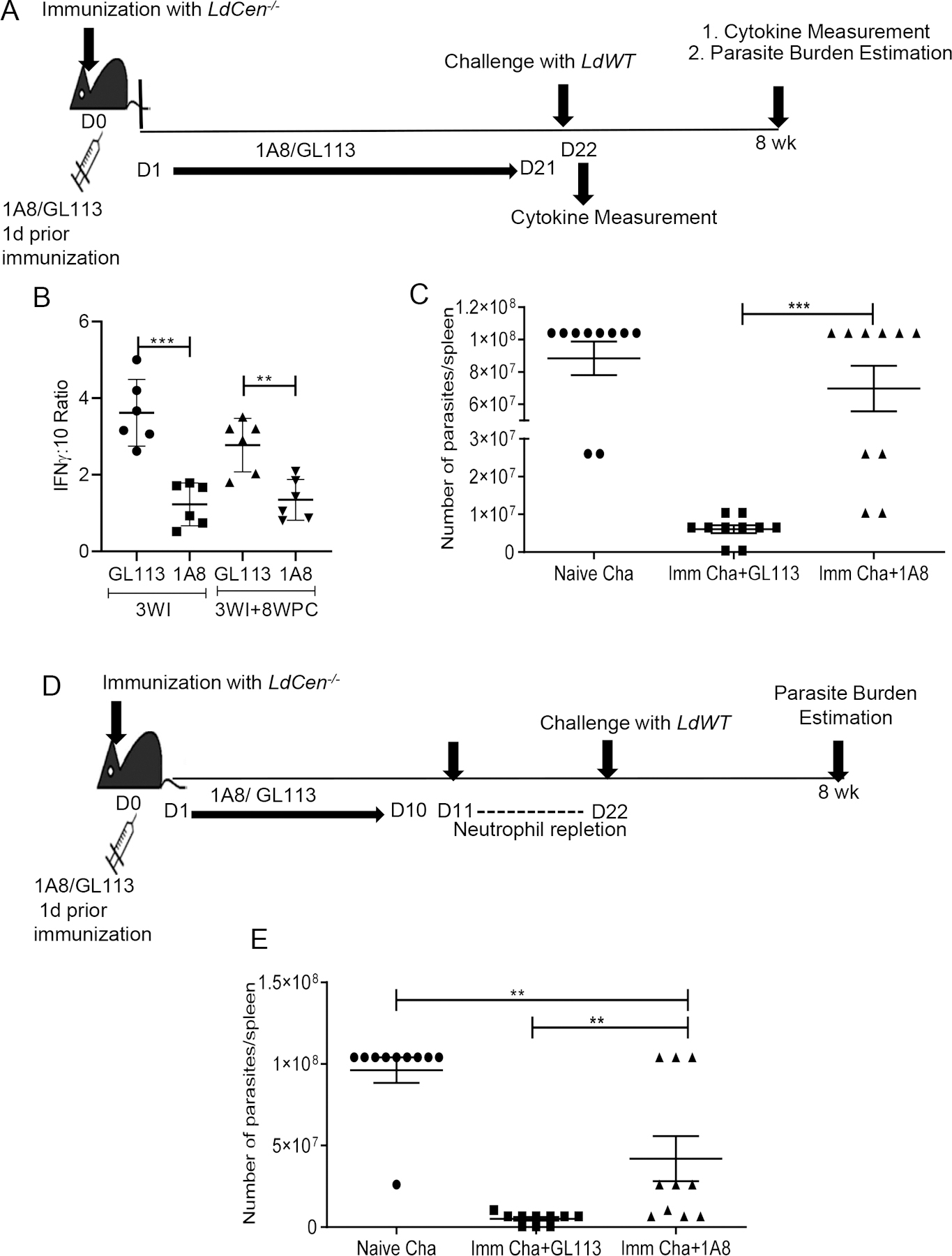
Mice were either treated with neutrophil depleting 1A8 antibody or GL113, 1 day prior to immunization with LdCen−/− as described in Materials and Methods. (A) Schematic diagram showing the treatment regimen. (B) Leishmania Ag-specific cytokines were measured from splenocytes of GL113 or 1A8 treated LdCen−/− immunized mice at the time of challenge (3WI, 3 wk post immunization) and after challenge (3WI + 8WPC: 8 wk post challenge) by sandwich ELISA. The ratio of IFNγ :IL-10 is shown. The data represent the mean values ± SEM of results from 2 independent experiments. Mean and SEM of 6 mice in each group are shown. ** P < 0.005; *** P <0. 0005. (C) Splenic parasite burden was measured at 8 wk post challenge in different groups of immunized challenged and naïve challenged mice. The data represent the mean values ± SEM of results from 2 independent experiments. Mean and SEM of 10 mice in each group are shown *** P < 0.0005.
In a separate experiment mouse were either treated with 1A8 antibody or GL113, 1 day prior to immunization with LdCen−/− but the neutrophil level was allowed to return to normal as described in Materials and Methods. (D) Schematic diagram showing the treatment regimen. (E) Splenic parasite burden was measured at 8 wk post challenge in different groups of immunized challenged and naïve challenged mice. The data represent the mean values ± SEM of results from 2 independent experiments. Mean and SEM of 10 mice in each group are shown ** P < 0.005.
10. Neutrophil repletion restores the LdCen−/− induced host protective immunity
To further establish the role of neutrophils in vaccine induced protection we depleted the neutrophils at early time point followed by neutrophil repletion before challenge and determined whether it could restore the protection (Schematic in Fig 8D). Specifically, we depleted the neutrophils from mice one day before the immunization and continued the depletion till day 10 and found there was significant depletion of neutrophils in 1A8 treated mice (Supplementary Fig 8E, 8F). After Day 10, IA8 antibody treatment was stopped and neutrophil levels were allowed to reach homeostatic levels for 12 days. At day 22, before challenge, we checked the level of neutrophils in the spleen of GL113 treated and 1A8 treated immunized mice and found neutrophil levels were restored in the later and were similar in both groups of mice (Supplementary Fig 8G, 8H). At Day 22, the animals were challenged with wild type parasites and parasite burden was measured at 8-weeks post challenge. The neutrophil repletion in the 1A8 treated immunized mice reverted the control of parasitemia in majority of the LdCen−/− immunized mice after 8 weeks post challenge and was significantly reduced compared to naïve challenged mice (Fig 8E).
Discussion:
Neutrophils may play either deleterious or protective roles at the onset of Leishmania infection depending on the Leishmania species (7). For example, following L. major infection, an early wave of neutrophils contributes to the development of a Th2 response in a susceptible BALB/c strain of mice while the absence of neutrophils during the first week of infection significantly reduced the parasite number (37). Likewise, persistent recruitment of neutrophils to the inflammatory site accompanying with Nlrp3 inflammasome dependent IL-1β production has been identified as essential component in the development of a non-healing form of cutaneous leishmaniasis in conventionally resistant mice (38). In sharp contrast, following infection with L. donovani, neutrophils play a crucial role in resistance to infection via generation of an IFN-γ dominant Th1 response (39). Following neutrophil depletion, a shift from an IFN-γ dominant Th1 response to a CD4+ Th2 response was observed which significantly enhanced parasite growth in visceral organs of BALB/c mice following L. donovani challenge (39). Interestingly, neutrophil-induced pathogen-specific T cell response was also observed in several vaccine studies (13–15). In the context of paradoxical roles attributed to neutrophils in Leishmania infection, we under took studies to define the role of neutrophils in live attenuated Leishmania (LdCen−/−) vaccine induced immunity.
Neutrophil activation for enhanced microbial activity is a critical requirement, as it leads to the successful elimination of Leishmania parasites. However, it is well documented that Leishmania parasites are highly resistant to neutrophil mediated microbicidal activity. For example, L. donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps (24, 40–42). However, compared to LdWT parasites, LdCen−/− infection induced higher NET activity in neutrophils indicating the generation of robust microbicidal response in LdCen−/− infected neutrophil.
We performed spacio-temporal characterization of neutrophil population and analyzed their functions induced locally in the ear dermis, in the lymph nodes draining the LdCen−/− injection site as well as in the spleen using the mouse ear as a surrogate model of intradermal vaccination and compared the responses to LdWT infection. We observed during early period of infection by either LdWT or LdCen−/− parasites, neutrophils accounted for the dominant influx of myeloid cells in the dermal site, ear draining lymph nodes and spleen whereas other myeloid cell populations peaked later. Importantly, during early time points neutrophil influx was significantly higher with the LdCen−/− parasites compared to LdWT in ear, ear dLN and spleen. Thus, neutrophils seem to play an important role in LdCen−/− mediated immune response and that neutrophil mediated response was evident before macrophage/DC’s arrival to the infection site. Indeed, the presence of parasitized neutrophils in the draining lymph node, further indicates that neutrophils carry the LdWT/LdCen−/− parasites to the lymph node at early point following infection where they may activate naïve T cells (43).
Recruitment of neutrophils to the site of infection is orchestrated by the type of cells present at the site and the chemotactic stimuli they generate (34). In that regard, the key role of tissue resident macrophages in neutrophil recruitment through their synthesis of chemokines such as CXCL1 and CXCL2 has been documented in several studies (34, 44, 45). In our study we found that as early as 6h post infection, the Ly6C− resident macrophage population from the ear dermis of LdCen−/− infected mice produced a significantly higher CXCL1, CXCL2 levels that facilitate the recruitment of a large number of neutrophils compared to LdWT infection.
Neutrophil derived cytokines play an important role in dictating the fate of Leishmania parasites. Following infection with L. major, neutrophils induced host protecting proinflammatory cytokines in resistant C57BL/6 mice (26). In contrast, neutrophils have been shown to induce a disease promoting Th2 response in susceptible BALB/c mice which provide the required microenvironment for parasite survival (37). In our study, neutrophils responding to LdCen−/− parasites in the ear and ear dLN augmented pro-inflammatory cytokines and diminished the secretion of anti-inflammatory cytokines which might play a critical role in shaping the protective response to the LdCen−/− infection. In addition to cytokines, neutrophil-derived chemokines play an important role in recruiting inflammatory and immune cells (8) (46) (11). We observed that LdCen−/− infection significantly induced chemokines including CCL2 and CCL3, which may play a role in coordinating downstream immune responses. Overall, increased expression of cytokines and chemokines in neutrophils from ear dermis, and ear dLN at different time points and earlier than other myeloid cells are consistent with the increased neutrophil influx into these organs.
By their ability to produce immunomodulatory cytokines, neutrophils, have been shown to exert a major impact in driving MHCII dependent proliferation of CD4+T cells in vitro (47). Therefore, the strong type 1 cytokine response and NETosis in neutrophils induced by LdCen−/− infection along with the attenuation of immunosuppressive cytokines (IL-10, TGFβ) can predict the activation of T cells. Importantly, it has been reported recently that MHC-II+ neutrophils from subjects with VL failed to stimulate T-cell proliferation and cause T cell exhaustion due to higher PDL1 and subsequent high PD-1expression by the lymphocytes of the same subject (25). Hence, the heightened expression of costimulatory molecules (MHC-II, CD80) and attenuated expression of co inhibitory molecules in neutrophils from ear dLN and corresponding lower expression of PDL-1 receptor, PD-1 in T cells from LdCen−/− infected mice may enable heightened CD4+T cell activation. Indeed, our study indicates that parasitized neutrophils from LdCen−/− infected mice can efficiently prime the antigen experienced CD4+T cells in ex-vivo cultures.
In our study we also discovered that neutrophil induced CD4+T cell activation is being mediated by a specific subset of such cells during Leishmania infection. Based on the size and complexity neutrophils have recently been characterized as two different subtypes, viz: Nα and Nβ (13). We identified Nα as a predominant population in LdCen−/− immunization that expresses a higher level of costimulatory molecules compared to Nβ and thus functionally pertinent to LdCen−/− induced immunity. The enhanced MHCII expression accompanied by significant attenuation of co-inhibitory molecules in Nα from LdCen−/− infected mice resulted in significantly higher CD4+T cell activation compared to Nα population from LdWT infected mice. Taken together these results strongly suggest a role for Nα subset of neutrophils in direct activation of CD4+T cells by LdCen−/− immunization. It is important to note that there was no difference in the MHCI expression in Nα neutrophils between LdWT and LdCen−/− infected mice. Thus, it appears that LdCen−/− infected Nα neutrophils utilize a MHCII restricted pathway for antigen presentation consistent with other studies (47–50). Notably, presence of Nα and Nβ subtypes was also observed during attenuated New York vaccinia virus (NYVAC-C3) infection in mice (13). However, in contrast to our study, in the context of viral infection, Nβ neutrophils have higher levels of APC markers and a greater capacity to induce virus antigen-specific CD8+T-cell activation than Nα cells. These differences could be due to the differences in the pathogens.
Studies from both mice and humans indicate that neutrophils can promote T cell activation (48–50). For example, neutrophils have been shown to contribute to the initial activation of Ag-specific CD4+T cells in the lungs during Mycobacterium tuberculosis infection (51). In this study, we demonstrated that adoptive transfer of LdCen−/− parasite bearing neutrophils in mice resulted in significantly higher antigen specific CD4+Th1 cell proliferation in the lymph nodes and spleen. Of note, heightened CCR7 expression in the LdCen−/− parasite bearing neutrophils compared to LdWT suggests that these cells have a higher homing potential to the lymph nodes and can efficiently interact with the T cells as was shown in studies with Ccr7−/− mice (36). Neutrophils being short lived cells, their role in regulating T cell activation beyond the early anti-microbial activity is often questioned (52). Recent demonstration of the role of neutrophils in shaping adaptive immunity in bacterial (53) and parasitic (54) infections has rekindled an interest in neutrophils as potent mediators of adaptive immunity (55). Even though it could be argued that prolonged culture of neutrophils in our ex-vivo CD4+ T cell experiment could have resulted in apoptotic cells causing the activation of T cells, data from our in vivo experiments negate such possibility and clearly substantiate the role of neutrophils in the activation of adaptive CD4+T cell response following LdCen−/− infection as reported earlier (48–50).
Finally, the importance of neutrophils in LdCen−/− parasite mediated immunity was further substantiated through the observation that neutrophil depleted LdCen−/− immunized mice failed to induce complete protection against virulent L. donovani challenge presumably due to a defect in the generation of an antigen specific Th1 response. On the contrary, neutrophil repletion before virulent challenge partially restored the vaccine induced protection further indicating that neutrophils play an essential role in generating live attenuated Leishmania vaccine induced immunity.
In summary, our findings demonstrate that development of protective immunity following immunization with LdCen−/− parasite vaccines critically depends on the innate immune responses orchestrated by the neutrophils that are recruited to the inoculation site before other myeloid cells. Our data points to the hierarchical response that exists among various myeloid cells both in the magnitude of influx and the attendant immune signals. Neutrophils provide initial stimulatory cues to effector CD4+Th1 cells, involved in protection against virulent Leishmania challenge. In addition, we also observed that a subset set of neutrophils may be playing an important role in developing the vaccine induced immunity. Collectively, our results highlight the novel role of neutrophils and advance our understanding of vaccine induced protective immunity as previously demonstrated in studies with macrophages (23) and dendritic cells (56). This finding may have implications regarding future vaccine designs against other forms of leishmaniasis and other parasitic vaccines.
Supplementary Material
Key Points:
Neutrophils play critical roles in Leishmania vaccine induced protective immunity.
Depletion of neutrophils impairs Leishmania vaccine efficacy.
Acknowledgments
We thank Drs. John Hobson, Shaden Kamhawi, Fabiano Oliviera, Thiago Soares desouza vieira for critical review of the manuscript. We thank Drs. Adovi Akue and Mark K Kukuruga of CBER flow core for sorting the immune cells.
Footnotes
Funding for these studies was provided by intramural funds of the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration.
The findings of this study are an informal communication and represent the authors’ own best judgments. These comments do not bind or obligate the FDA.
References
- 1.Spear RC 2017. Review of “Mathematical Models for Neglected Tropical Diseases: Essential Tools for Control and Elimination, Part B” Edited by Basanez Maria-Gloria and Anderson Roy M. Parasit Vectors 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leta S, Dao TH, Mesele F, and Alemayehu G 2014. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis 8: e3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebremichael Tedla D, Bariagabr FH, and Abreha HH 2018. Incidence and Trends of Leishmaniasis and Its Risk Factors in Humera, Western Tigray. J Parasitol Res 2018: 8463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsen ED, Hay C, Henard CA, Popov V, Garg NJ, and Soong L 2013. Leishmania amazonensis amastigotes trigger neutrophil activation but resist neutrophil microbicidal mechanisms. Infect Immun 81: 3966–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, and Sacks D 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321: 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolaczkowska E, and Kubes P 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 7.Hurrell BP, Regli IB, and Tacchini-Cottier F 2016. Different Leishmania Species Drive Distinct Neutrophil Functions. Trends Parasitol 32: 392–401. [DOI] [PubMed] [Google Scholar]
- 8.Tecchio C, Micheletti A, and Cassatella MA 2014. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller I, Munder M, Kropf P, and Hansch GM 2009. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 30: 522–530. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Cassatella MA, Costantini C, and Jaillon S 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11: 519–531. [DOI] [PubMed] [Google Scholar]
- 11.Kalyan S, and Kabelitz D 2014. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol 44: 627–633. [DOI] [PubMed] [Google Scholar]
- 12.Borregaard N, Sorensen OE, and Theilgaard-Monch K 2007. Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28: 340–345. [DOI] [PubMed] [Google Scholar]
- 13.Di Pilato M, Mejias-Perez E, Zonca M, Perdiguero B, Gomez CE, Trakala M, Nieto J, Najera JL, Sorzano CO, Combadiere C, Pantaleo G, Planelles L, and Esteban M 2015. NFkappaB activation by modified vaccinia virus as a novel strategy to enhance neutrophil migration and HIV-specific T-cell responses. Proc Natl Acad Sci U S A 112: E1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trentini MM, de Oliveira FM, Kipnis A, and Junqueira-Kipnis AP 2016. The Role of Neutrophils in the Induction of Specific Th1 and Th17 during Vaccination against Tuberculosis. Front Microbiol 7: 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, Descours B, Reboulleau D, Bonduelle O, Verrier B, Van Rooijen N, Combadiere C, and Combadiere B 2012. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity 37: 917–929. [DOI] [PubMed] [Google Scholar]
- 16.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, and Sacks DL 2009. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathog 5: e1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestre R, Cordeiro-da-Silva A, and Ouaissi A 2008. Live attenuated Leishmania vaccines: a potential strategic alternative. Arch Immunol Ther Exp (Warsz) 56: 123–126. [DOI] [PubMed] [Google Scholar]
- 18.Selvapandiyan A, Debrabant A, Duncan R, Muller J, Salotra P, Sreenivas G, Salisbury JL, and Nakhasi HL 2004. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J Biol Chem 279: 25703–25710. [DOI] [PubMed] [Google Scholar]
- 19.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, and Nakhasi HL 2009. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol 183: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 20.Fiuza JA, Santiago Hda C, Selvapandiyan A, Gannavaram S, Ricci ND, Bueno LL, Bartholomeu DC, Correa-Oliveira R, Nakhasi HL, and Fujiwara RT 2013. Induction of immunogenicity by live attenuated Leishmania donovani centrin deleted parasites in dogs. Vaccine 31: 1785–1792. [DOI] [PubMed] [Google Scholar]
- 21.Fiuza JA, Gannavaram S, Santiago Hda C, Selvapandiyan A, Souza DM, Passos LS, de Mendonca LZ, Lemos-Giunchetti Dda S, Ricci ND, Bartholomeu DC, Giunchetti RC, Bueno LL, Correa-Oliveira R, Nakhasi HL, and Fujiwara RT 2015. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 33: 280–288. [DOI] [PubMed] [Google Scholar]
- 22.Fiuza JA, Dey R, Davenport D, Abdeladhim M, Meneses C, Oliveira F, Kamhawi S, Valenzuela JG, Gannavaram S, and Nakhasi HL 2016. Intradermal Immunization of Leishmania donovani Centrin Knock-Out Parasites in Combination with Salivary Protein LJM19 from Sand Fly Vector Induces a Durable Protective Immune Response in Hamsters. PLoS Negl Trop Dis 10: e0004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya P, Dey R, Dagur PK, Kruhlak M, Ismail N, Debrabant A, Joshi AB, Akue A, Kukuruga M, Takeda K, Selvapandiyan A, McCoy JP Jr., and Nakhasi HL 2015. Genetically Modified Live Attenuated Leishmania donovani Parasites Induce Innate Immunity through Classical Activation of Macrophages That Direct the Th1 Response in Mice. Infect Immun 83: 3800–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabriel C, McMaster WR, Girard D, and Descoteaux A 2010. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol 185: 4319–4327. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Davis RE, Srivastva S, Nylen S, Sundar S, and Wilson ME 2016. A Subset of Neutrophils Expressing Markers of Antigen-Presenting Cells in Human Visceral Leishmaniasis. J Infect Dis 214: 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, and Tacchini-Cottier F 2007. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol 82: 288–299. [DOI] [PubMed] [Google Scholar]
- 27.Charmoy M, Auderset F, Allenbach C, and Tacchini-Cottier F 2010. The prominent role of neutrophils during the initial phase of infection by Leishmania parasites. J Biomed Biotechnol 2010: 719361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, and Duncan R 2010. Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol Microbiol 77: 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chagas AC, Oliveira F, Debrabant A, Valenzuela JG, Ribeiro JM, and Calvo E 2014. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog 10: e1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvapandiyan A, Duncan R, Debrabant A, Bertholet S, Sreenivas G, Negi NS, Salotra P, and Nakhasi HL 2001. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J Biol Chem 276: 43253–43261. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, and Saraiva EM 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A 106: 6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro-Gomes FL, Peters NC, Debrabant A, and Sacks DL 2012. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog 8: e1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spath GF, and Beverley SM 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 99: 97–103. [DOI] [PubMed] [Google Scholar]
- 34.Kim ND, and Luster AD 2015. The role of tissue resident cells in neutrophil recruitment. Trends Immunol 36: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Grone HJ, Garbi N, Kastenmuller W, Knolle PA, Kurts C, and Engel DR 2014. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, Magistrelli G, Masternak K, Chevailler A, Delneste Y, and Jeannin P 2011. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 117: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 37.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, and Louis JA 2000. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 165: 2628–2636. [DOI] [PubMed] [Google Scholar]
- 38.Charmoy M, Hurrell BP, Romano A, Lee SH, Ribeiro-Gomes F, Riteau N, Mayer-Barber K, Tacchini-Cottier F, and Sacks DL 2016. The Nlrp3 inflammasome, IL-1beta, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol 46: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, and Tacchini-Cottier F 2008. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect Immun 76: 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro-Gomes FL, and Sacks D 2012. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front Cell Infect Microbiol 2: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laufs H, Muller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, Solbach W, and Laskay T 2002. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect Immun 70: 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yorek MS, Poudel B, Mazgaeen L, Pope RM, Wilson ME, and Gurung P 2019. Leishmania major degrades murine CXCL1 - An immune evasion strategy. PLoS Negl Trop Dis 13: e0007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itano AA, and Jenkins MK 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol 4: 733–739. [DOI] [PubMed] [Google Scholar]
- 44.De Filippo K, Henderson RB, Laschinger M, and Hogg N 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180: 4308–4315. [DOI] [PubMed] [Google Scholar]
- 45.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, and Hogg N 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937. [DOI] [PubMed] [Google Scholar]
- 46.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, Proudfoot AE, and Tacchini-Cottier F 2010. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog 6: e1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Culshaw S, Millington OR, Brewer JM, and McInnes IB 2008. Murine neutrophils present Class II restricted antigen. Immunol Lett 118: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, and Lore K 2017. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood 129: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abi Abdallah DS, Egan CE, Butcher BA, and Denkers EY 2011. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol 23: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, and Hansch GM 2000. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology 101: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blomgran R, and Ernst JD 2011. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol 186: 7110–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Wang W, Yang F, Xu Y, Feng C, and Zhao Y 2019. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal 17: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hampton HR, Bailey J, Tomura M, Brink R, and Chtanova T 2015. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun 6: 7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, and Robey EA 2008. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosales C 2020. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol 108: 377–396. [DOI] [PubMed] [Google Scholar]
- 56.Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, Selvapandiyan A, McCoy JP Jr., Duncan R, Satoskar AR, and Nakhasi HL 2014. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J Immunol 193: 3513–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


