Abstract
Refractory hypertension is a severe phenotype of antihypertension treatment failure. Treatment resistant hypertension, a less severe form of difficult-to-treat hypertension, has been associated with significantly worse health outcomes. However, no studies currently show how health outcomes may worsen upon progression to refractory hypertension. Refractory hypertension and treatment resistant hypertension were studied in 3147 hypertensive participants in the Chronic Renal Insufficiency Cohort (CRIC). The hypertensive phenotype (i.e., no treatment resistant or refractory, treatment resistant, or refractory hypertension) was identified at the baseline visit and health outcomes were monitored at subsequent visits. Outcome risk was compared using Cox proportional hazards models with time-varying covariates. A total of 136 (4.3%) individuals were identified with refractory hypertension at baseline. After adjusting for participant characteristics, individuals with refractory hypertension had increased risk for the composite renal outcome across all study years (50% decline in estimated glomerular filtration rate or end stage renal disease; hazard ratio for study years 0–10 = 1.73 [95% confidence interval; 1.42–2.11]) and the composite cardiovascular disease outcome during later study years (stroke, myocardial infarction, or congestive heart failure; hazard ratio for study years 0–3 = 1.25 [0.91–1.73], for study years 3–6 = 1.50 [0.97–2.32]), and for study years 6–10 = 2.72 [1.47–5.01]) when compared to individuals with treatment resistant hypertension. There was no significant difference in all-cause mortality between those with refractory vs. treatment resistant hypertension. We provide the first evidence that refractory hypertension is associated with worse long-term health outcomes compared to treatment resistant hypertension.
Keywords: refractory hypertension, treatment resistant hypertension, antihypertension treatment failure, chronic kidney disease, prognosis
Graphical Abstract
Introduction
Treatment resistant hypertension (TRH) affects nearly 20% of U.S. adults currently being treated for hypertension1. However, the current definition of TRH (i.e., controlled blood pressure [BP; <140/90 mm Hg] when prescribed ≥ 4 antihypertensive medication classes, inclusive of a diuretic or uncontrolled BP [≥140 and/or ≥90 mm Hg] when prescribed ≥ 3 antihypertensive medication classes, inclusive of a diuretic)2 includes refractory hypertension, a novel phenotype of antihypertensive treatment failure3, 4. Refractory hypertension (RfH; defined in population studies as uncontrolled BP when taking ≥5 antihypertensive medication classes, inclusive of a diuretic) represents a notable 3–31% of those originally diagnosed with TRH3–8. However, the importance of differentiating individuals with RfH from individuals with TRH is speculative given that little is currently known about the prognosis of those with RfH separate from those with TRH.
TRH is associated with higher risks of all-cause mortality and cardiovascular disease (CVD; e.g., stroke, congestive heart failure [CHF], myocardial infarction) compared to non-resistant hypertension 9, 10. However, these estimates of outcome risk include RfH within the definition of TRH10. Although health outcomes in RfH have not been studied, in population studies, individuals with RfH have been shown to have higher body mass index than their treatment resistant counterparts6 as well as higher prevalence of comorbidities such as diabetes and albuminuria3, 6, 8. RfH is also associated with decreased kidney function (estimated glomerular filtration rate [eGFR] < 60 ml/min/1.73m2) and black race 3, 6, 8. As such, we would expect the prognosis to be worse for those with RfH compared to TRH, although no current evidence supports this hypothesis11.
To provide the first evaluation of the relationship between RfH and renal, cardiovascular, and mortality outcomes, we used data from the Chronic Renal Insufficiency Cohort (CRIC) Study. Individuals with decreased kidney function have a higher prevalence of RfH compared to the general population3, 6, 8. Due to the low prevalence of RfH in the general population8, the CRIC Study’s focus on participants with impaired kidney function provides an ideal population to study RfH. The aims of this study were two-fold. First, we aimed to evaluate if prognosis for adults with RfH differs from those with treatment resistant and those with non-treatment resistant or RfH. Second, because previous estimates of outcome risk in patients with TRH included those with RfH, we also aimed to provide corrected estimates of outcome risk for those with treatment resistant, but not refractory, hypertension. Overall, through this analysis, we aimed to assess if refractory hypertensives require consideration as a separate phenotype from TRH due to markedly different health outcomes.
Methods
The data for this study can be obtained by request from the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases’ Central Repository (https://repository.niddk.nih.gov/home/). Code for all statistical analyses is available upon request from the corresponding author.
Study Population
Study design and rationale of the CRIC Study have been described previously12, 13. Briefly, the CRIC Study is an ongoing, multicenter, prospective cohort study designed to evaluate risk factors and for the progression of chronic kidney disease (CKD) and CVD in adults with mild to moderate CKD. The study recruited 3939 adults between 2003 and 2008 from seven clinical centers in the U.S. (Baltimore, MD; Philadelphia, PA; Cleveland, OH; Detroit, MI; Chicago, IL; New Orleans, LA; and Oakland, CA). Inclusion criteria included age 21 to 74 years and estimated glomerular filtration rate (eGFR) range of 20 to 70 ml/min/1.73 m2. Exclusion criteria included diagnosis of polycystic kidney disease and use of immunosuppression for glomerulonephritis. The CRIC study was approved by all participating centers’ institutional review boards, and all patients provided informed consent. De-identified data was obtained for this study, and as such, these analyses were deemed non-human subjects research by the local Institutional Review Board (Springfield Committee for Research Involving Human Subjects).
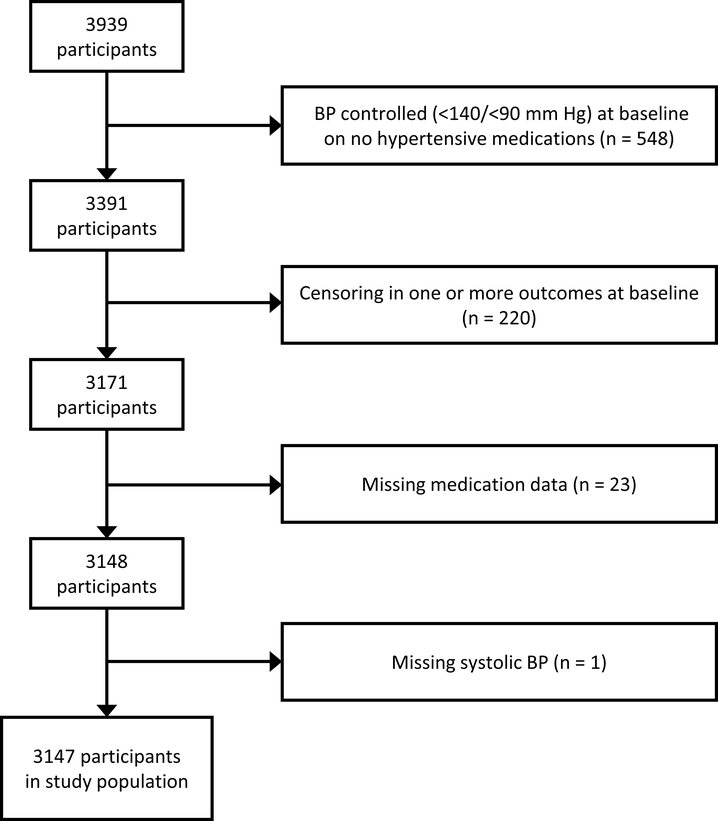
Of the 3939 participants enrolled in CRIC, 548 patients with controlled BP (<140/90 mm Hg) on zero hypertensive medications at baseline (representing both hypertensive and normotensive individuals) were excluded from the analysis. An additional 220 participants were excluded due to censoring in any outcomes at the baseline visit. One participant was excluded for missing systolic blood pressure, and 23 were excluded for missing medication data, leaving 3147 participants in the final analysis data set at the baseline visit (Figure 1). Participants were subsequently lost to follow-up over the course of yearly study visits (see Table S1 for full follow-up participation).
Figure 1:
Flow chart showing patient inclusion/exclusion criteria. Arrows and boxes to the right show the number of patients excluded for various reasons. Boxes on the left show the number of patients in the study after each exclusion criteria is applied.
Blood pressure measurement
Participant’s BP was measured with a Tycos Classic Aneroid sphygmomanometer using a standard protocol (i.e., refrain from caffeine, smoking, and exercise at least 30 minutes before, five-minute rest immediately before, quiet room, appropriate size cuff, seated with back supported, legs uncrossed, feet flat on floor and taken using the right arm whenever possible with arm supported on a table). The average of 3 BP measurements was used.
Refractory/Treatment resistant hypertension definition
TRH was defined as BP ≥140 and/or ≥90 mm Hg despite being prescribed 3 or 4 antihypertensive drug classes, including a diuretic (uncontrolled TRH; Table 1) or BP <140/90 mm Hg while prescribed 4 or more antihypertensive drug classes, including a diuretic (controlled TRH; Table 1)2.
Table 1:
Hypertension phenotypes considered.
| Hypertensive phenotype | Abbreviation | Definition |
|---|---|---|
| Refractory hypertension | RfH | BP ≥ 140/90 while prescribed ≥ 5 antihypertension medication classes, including a diuretic |
| Treatment resistant hypertension | TRH | BP ≥ 140/90 while prescribed 3 or 4 antihypertension medication classes, including a diuretic |
| OR | ||
| BP <140/90 while prescribed ≥ 4 antihypertension medication classes, including a diuretic | ||
| Responsive hypertension | Non-TRH/RfH | BP ≥ 140/90 while prescribed 1 or 2 antihypertension medication classes, including a diuretic |
| OR | ||
| BP ≥ 140/90 while prescribed any antihypertension medication, not including a diuretic | ||
| OR | ||
| BP ≥ 140/90 while not prescribed antihypertension medication | ||
| OR | ||
| BP <140/90 while prescribed 1, 2, or 3 antihypertension medication classes, including a diuretic | ||
| OR | ||
| BP <140/90 while prescribed any antihypertension medication, not including a diuretic | ||
Definitions of RfH differ between clinic and population studies due to the relative rarity of mineralocorticoid receptor antagonist (i.e., spironolactone and eplerenone, which are required under the most evolved clinic definition4) use outside of hypertension specialty clinics8. Thus, RfH was defined as BP ≥140/90 despite being prescribed ≥5 antihypertensive drug classes, including a diuretic, as in other population studies (Table 1)3, 6, 8.
Because medication adherence and white coat hypertension were not routinely assessed during all visits, apparent TRH (aTRH) and apparent RfH (aRfH) are more appropriate labels for these definitions. However, for the remainder of this paper, we will use TRH and RfH for simplicity. Individuals without TRH or RfH will be referred to as non-TRH/RfH (Table 1)
We note that a threshold of 140/90 mm Hg was used in the definitions of hypertension, TRH, and RfH. This threshold is consistent with the definition used in the CRIC study13, in previous outcomes studies in CRIC10, and in the previous Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) guidelines14. However, the previous JNC 715 and current American College of Cardiology/American Heart Association16 guidelines advocate for lower treatment targets (130/80 mm Hg) for individuals with CKD or all hypertensives, respectively. To explore the impact of newer blood pressure targets on our results, we performed a sensitivity analysis using 130/80 mm Hg as the threshold for hypertension in all definitions.
Outcomes
Three outcomes were chosen for this analysis: 1) a composite of cardiovascular outcomes, including stroke, myocardial infarction (MI), and congestive heart failure (CHF); 2) a composite of renal outcomes, including 50% decline in eGFR from baseline or end-stage renal disease (ESRD); and 3) all-cause mortality. In all analyses, participants were censored at their last follow up visit or at the time of death. Deaths in CRIC are ascertained by reports from next of kin, retrieval of death certificates or obituaries, review of hospital or outpatient records, and searching Social Security Death vital status and state death files, if available. Heart failure and major cardiovascular events in CRIC are identified by asking study participants biannually if they were hospitalized and by reviewing electronic health records. At least two study physicians review all events and deaths using medical records and determine the likelihood of events based on modified clinical Framingham criteria. ESRD is identified by self-report and corroborated using data from the United States Renal Data System. Becase eGFR was only measured at fixed intervals, ascertainment of time to eGFR halving was imputed assuming a linear decline in kidney function between annual visits17.
Covariate Definitions
In the CRIC study, individuals self-identified race. Diabetes was defined as self-reported use of anti-diabetes medication, fasting plasma glucose ≥ 126 mg/dl, or non-fasting plasma glucose ≥ 200 mg/dl. eGFR was estimated by an internally derived equation utilizing serum creatinine, and cystatin C18. Current smoking was defined as both currently smoking cigarettes and having smoked 100 cigarettes in one’s lifetime. A history of cardiovascular disease was defined as previously having coronary artery disease, prior revascularization, heart failure, stroke, and/or peripheral vascular disease. Antihypertensive medications were grouped according to class (i.e., nitrates, vasodilators, aldosterone antagonists, epithelial sodium channel [ENaC] inhibitors, renin-angiotensin system [RAS] blockers, sympatholytics, diuretics, beta blockers, and calcium-channel antagonists). We note that because aldosterone antagonists are considered as their own class for this study, we consider RAS blockers instead of renin-angiotensin-aldosterone system (RAAS) blockers combined. In addition, diuretics include loop and thiazide diuretics. K-sparing diuretics were included as either aldosterone antagonists (i.e., spironolactone, eplerenone) or ENaC inhibitors (i.e., amiloride, triamterene) and did not satisfy the diuretic prescription requirement for RfH and TRH in this analysis.
Statistical Analysis
For the cross-sectional analysis of baseline measurements, two comparisons were made: non-TRH/RfH vs TRH, and TRH vs RfH. Continuous baseline characteristics were compared with t-tests and categorical characteristics with Chi-square tests. Median follow-up durations for each outcome were estimated using the reverse Kaplan-Meier method.
Relative risks for each outcome between those with non-TRH/RfH, TRH, and RfH were estimated with Cox proportional hazard models. Hypertensive classification (i.e., non-TRH/RfH vs. TRH vs. RfH) was included as a time-varying predictor, allowing individuals to change their hypertensive phenotype through time in accordance with their BP control and the intensity of their prescribed antihypertensive treatment. Additional baseline covariates included in the Cox models included: age, sex, race/ethnicity, college education, diabetes, body mass index (BMI), eGFR (log-transformed), current smoking, 24-hour urine protein (log-transformed), low-density lipoprotein (LDL), history of cardiovascular disease, and study site. The proportional hazard assumption was tested for each individual covariate in the model and globally by methods outlined by Grambsch and Therneau19. In the event of a violation of the proportional hazard assumption for any individual covariate, a step function was introduced to stratify time into intervals demarcated by the following year ranges: years 0 to 3, years 3+ to 6, and years 6+ to 10, where the intervals are non-overlapping (i.e., left-open and right-closed intervals). Age, BMI, and the log of eGFR were all included as time-varying coefficients in this way to satisfy the proportional hazard assumption. In addition, the coefficients for hypertension classification were estimated using these intervals when analyzing the CVD composite outcome due to a failure of the proportional hazard assumption related to RfH in this outcome only. All additional analyses use this interval estimation of the hypertension classification coefficients for consistency.
Missing data was present in observations of BMI (0.3%, 8/3147), 24-hour urine protein (5.1%, 160/3147), and LDL (0.4%, 12/3147). Missing values were imputed prior to estimation of the Cox models. Imputation was done by multiple imputation using chained equations as described in Van Burren & Groothuis-Oudshoorn20. Imputation models included the Nelson-Aalen estimator of cumulative hazard as a predictor variable as recommend by White & Ryston21. Coefficients for log hazard were pooled before exponentiation to determine hazard ratios as recommended by Marshall et al.22.
Because individuals with uncontrolled TRH (i.e., uncontrolled BP when prescribed 3 or 4 antihypertensive drug classes, including a diuretic) may progress to a refractory diagnosis upon treatment intensification, have higher BP levels, and have higher health risks than those with controlled TRH23, these individuals may be more similar to refractory hypertensives than their controlled TRH counterparts. To assess the relationship between uncontrolled TRH and RfH, we also repeated all analyses using only uncontrolled TRH, while excluding those with controlled TRH at any point in time.
Results
Prevalence
Of the 3147 hypertensive participants in CRIC considered in this study, 2006 (63.7%) had baseline BP control consistent with non-TRH/RfH hypertension, 1005 (31.9%) had TRH, and 136 (4.3%) had RfH. Among participants with RfH at baseline, 32 (23.5%) still had RfH at their final follow-up visit (Table S2). In those with TRH, 490 (48.8%) exited the study with TRH, while 71 (7.1%) progressed to RfH upon exit (Table S2). Participants with non-TRH/RfH at baseline largely maintained this phenotype at their final visit (n = 1592; 79.4%) with only 371 (18.5%) and 43 (2.1%) progressing to TRH and RfH, respectively (Table S2). Over the course of the study, 2109 participants (67.0%) transitioned between phenotypes 1 or fewer times (Table S3).
Baseline participant characteristics
Baseline characteristics are provided in Table 2. In general, participants in CRIC were over 50, majority male, predominately Caucasian or African American, and obese. Compared to those with TRH, those with RfH at baseline were more likely to be black, have a history of cardiovascular disease, congestive heart failure, and stroke, and have lower serum potassium. Compared to those with non-TRH/RfH hypertension, individuals with TRH at baseline were more likely to be older, black, a non-smoker, and have lower educational attainment. In addition, compared to those with non-TRH/RfH, those with TRH at baseline were more likely to have lower eGFR, higher BMI, higher HbA1c, higher proteinuria, higher urine albumin-to-creatinine ratio, and higher HDL, LDL, and total cholesterol. They are also more likely to report a history diabetes, cardiovascular disease, congestive heart failure, stroke, atrial fibrillation, and chronic obstructive pulmonary disease. We also note that there was a trend towards higher levels of serum aldosterone when comparing RfH to TRH and TRH to non-TRH/RfH at baseline, although the former trend was of borderline statistical significance (p = 0.07 and 0.02, respectively).
Table 2:
Baseline characteristics of CRIC participants according to hypertensive phenotype.
| Hypertensive Phenotype | p-value | ||||
|---|---|---|---|---|---|
| Characteristic | non-TRH/RfH (n = 2006) |
TRH (n = 1005) |
RfH (n = 136) |
non-TRH/RfH. vs. TRH | TRH vs. RfH |
| Age, years, mean (SD) | 57.9 (11.0) | 61.0 (9.2) | 60.9 (8.9) | <0.01 | 0.90 |
| Women, n (%) | 891 (44) | 438 (44) | 56 (41) | 0.69 | 0.66 |
| Race, n (%) | <0.01 | 0.04 | |||
| Non-Hispanic white | 898 (45) | 296 (29) | 28 (21) | ||
| Non-Hispanic black | 788 (39) | 531 (53) | 89 (65) | ||
| Hispanic | 251 (13) | 136 (14) | 16 (12) | ||
| Other | 69 (3) | 42 (4) | 3 (2) | ||
| Education, n (%) | <0.01 | 0.67 | |||
| Less than high school | 392 (20) | 264 (26) | 38 (28) | ||
| High school graduate | 374 (19) | 212 (21) | 31 (23) | ||
| Some college | 585 (29) | 313 (31) | 44 (32) | ||
| College graduate and Above | 655 (33) | 216 (21) | 23 (17) | ||
| Diastolic BP, mm Hg, mean (SD) | 72.4 (12.4) | 70.6 (13.6) | 78.8 (16.3) | <0.01 | <0.01 |
| Systolic BP, mm Hg, mean (SD) | 126.6 (19.7) | 135.4 (23.3) | 158.7 (14.8) | <0.01 | <0.01 |
| eGFR, mL/min per 1.73 m2, median (IQR) | 44.7 (34.0–55.4) | 36.9 (28.4–47.4) | 34.9 (26.8–49.2) | <0.01 | 0.27 |
| Serum creatinine, mg/dL, mean (SD) | 1.8 (0.6) | 2.0 (0.7) | 2.1 (0.7) | <0.01 | 0.12 |
| HbA1c, %, mean (SD) | 6.6 (1.5) | 7.0 (1.6) | 7.1 (1.7) | <0.01 | 0.39 |
| Serum potassium, mmol/L, mean (SD) | 4.4 (0.5) | 4.3 (0.6) | 4.2 (0.6) | 0.11 | 0.01 |
| 24-hour urine protein, g, median (IQR) | 0.2 (0.1–0.8) | 0.3 (0.1–1.3) | 0.9 (0.2–2.6) | <0.01 | 0.07 |
| HDL, mg/dL, mean (SD) | 48.2 (15.7) | 45.3 (13.9) | 46.3 (14.6) | <0.01 | 0.41 |
| LDL, mg/dL, mean (SD) | 103.9 (35.4) | 97.3 (34.2) | 102.9 (41.3) | <0.01 | 0.08 |
| Total cholesterol, mean (SD) | 185.5 (46.5) | 177.7 (44.2) | 183.0 (50.0) | <0.01 | 0.19 |
| BMI, kg/m2, mean (SD) | 31.6 (7.4) | 34.1 (8.0) | 34.0 (8.0) | <0.01 | 0.87 |
| Urine albuminin/creatinine ratio, ug/mg, mean (SD) | 597.2 (1605.9) | 836.9 (1785.1) | 1090.5 (1461.9) | <0.01 | 0.12 |
| Serum aldosterone, ng/dL, mean (SD) | 13.8 (17.6) | 15.6 (24.3) | 23.9 (126.1) | 0.02 | 0.07 |
| Diabetes, n (%) | 889 (44) | 651 (65) | 94 (69) | <0.01 | 0.37 |
| History of cardiovascular disease, n (%) | 526 (26) | 490 (49) | 88 (65) | <0.01 | <0.01 |
| Congestive heart failure, n (%) | 102 (5) | 170 (17) | 34 (25) | <0.01 | 0.03 |
| Current smoker, n (%) | 284 (14) | 115 (11) | 13 (10) | 0.04 | 0.61 |
| Stroke, n (%) | 163 (8) | 145 (14) | 29 (21) | <0.01 | 0.05 |
| Atrial fibrillation, n (%) | 282 (14) | 223 (22) | 38 (28) | <0.01 | 0.16 |
| Asthma, n (%) | 246 (12) | 135 (14) | 20 (15) | 0.3 | 0.93 |
| Chronic obstructive pulmonary disease, n (%) | 54 (3) | 39 (4) | 7 (5) | 0.05 | 0.59 |
| General medication use, n (%) | |||||
| Aspirin | 812 (40) | 550 (55) | 72 (53) | <0.01 | 0.76 |
| NSAIDs | 999 (50) | 600 (60) | 81 (60) | <0.01 | 1.00 |
| SSRIs | 196 (10) | 98 (10) | 11 (8) | 1.00 | 0.64 |
| Statins | 1089 (54) | 688 (68) | 93 (68) | <0.01 | 1.00 |
| Number of antihypertensive classes prescribed, median (range) | 2 (0, 6) | 4 (3, 8) | 5 (5, 7) | <0.01 | <0.01 |
| Antihypertensive medication use, n (%) | |||||
| Nitrates | 36 (2) | 113 (11) | 29 (21) | <0.01 | <0.01 |
| Vasodilators | 73 (4) | 202 (20) | 69 (51) | <0.01 | <0.01 |
| Aldosterone antagonists | 44 (2) | 78 (8) | 12 (9) | <0.01 | 0.79 |
| ENaC inhibitors | 82 (4) | 81 (8) | 10 (7) | <0.01 | 0.91 |
| RAS blockers | 1417 (71) | 823 (82) | 120 (88) | <0.01 | 0.09 |
| Sympatholytics | 231 (12) | 392 (39) | 105 (77) | <0.01 | <0.01 |
| Diuretics | 861 (43) | 1005 (100) | 136 (100) | -* | -* |
| Beta blockers | 782 (39) | 796 (79) | 129 (95) | <0.01 | <0.01 |
| Calcium channel antagonists | 685 (34) | 652 (65) | 115 (85) | <0.01 | <0.01 |
Diuretics are required in the definition for both TRH and RfH
Baseline medication use
Compared to those with TRH, those with RfH at baseline were more likely to be prescribed second-line antihypertensive medications, including nitrates, vasodilators, sympatholytics, beta blockers and calcium channel antagonists. There was no apparent difference amongst difficult-to-treat phenotypes in non-antihypertensive medication use at baseline (i.e., aspirin, nonsteroidal anti-inflammatory drugs [NSAIDs], selective serotonin reuptake inhibitors [SSRIs], and statins). When compared to those with non-TRH/RfH hypertension, those with TRH at baseline were more likely to be prescribed all antihypertensive medication classes as well as aspirin, NSAIDs, and statins.
Outcomes
The median follow-up time for the renal composite (50% eGFR decline/ESRD) outcome was 7.02 years (95% CI: 6.95–7.09) with 1121 reported composite renal events. The median follow-up time for the CVD composite (stroke/MI/CHF) outcome was 7.87 years (7.81–7.93), with CHF alone contributing to over half (466) of the 799 reported composite CVD events (Table S4). The median follow-up time for all-cause mortality was 7.99 years (7.93–8.06) with 678 reported deaths.
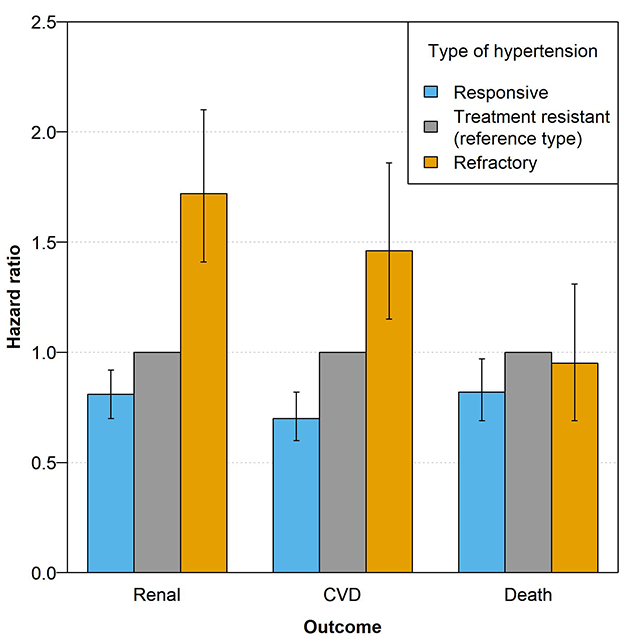
In unadjusted analyses, the risk of renal and CVD outcomes was higher in individuals with RfH compared to those with TRH and in individuals with TRH compared to those with non-TRH/RfH, but all-cause mortality was only higher in those with TRH compared to those with non-TRH/RfH (Table S5). When adjusting for patient characteristics, hazard ratios were attenuated, but there remained a significantly higher risk of renal outcomes in RfH vs. TRH and in TRH vs. non-TRH/RfH (Table 3). Similarly, the adjusted risk for cardiovascular outcomes was significantly higher in RfH vs. TRH in years 6–10 (although there was a non-significant trend towards higher risk in RfH in years 3–6) and in TRH vs. non-TRH/RfH in years 0–6 (Table 3). Also, the hazard ratio for RfH vs. TRH in years 0–3 for the CVD composite outcome was shown to be potentially higher, although not statistically significant, in the RfH phenotype (Table 3). After adjustment, there was no difference in the risk of all-cause mortality in RfH vs. TRH, despite there being a significantly higher risk of mortality in TRH vs. non-TRH/RfH (Table 3).
Table 3:
Adjusted hazard ratios* (HR) for outcomes in patients with no treatment resistant or refractory, treatment resistant, and refractory hypertension.
| Outcome | non-TRH/RfH | TRH | RfH |
|---|---|---|---|
| CVD Composite (stroke/MI/CHF) | |||
| Events per 1000 person years | 28.8 | 65.1 | 122.1 |
| HR (95% CI) – Years 0 – 3 | 0.69 (0.55–0.85) | Ref. † | 1.25 (0.91–1.73) |
| HR (95% CI) – Years 3 – 6 | 0.59 (0.46–0.77) | Ref. | 1.50 (0.97–2.32) |
| HR (95% CI) – Years 6 – 10 | 1.06 (0.73–1.56) | Ref. | 2.72 (1.47–5.01) |
| Renal Composite (50% eGFR decline/ESRD) | |||
| Events per 1000 person years | 52.8 | 95.8 | 190.6 |
| HR (95% CI) – Years 0 –10 | 0.81 (0.71–0.93) | Ref. | 1.73 (1.42–2.11) |
| All-Cause Mortality | |||
| Events per 1000 person years | 21.9 | 44.2 | 61.5 |
| HR (95% CI) – Years 0 – 10 | 0.82 (0.70–0.97) | Ref. | 0.95 (0.69–1.31) |
Hazard ratio adjusted for sex, race, education, diabetes, current smoking, log(24-hour urine protein), LDL, history of CVD, clinic site, age (time stratified), BMI (time stratified), and log(eGFR) (time stratified).
Reference category for hazard ratios.
Of the 1005 individuals with TRH at baseline, 408 (40.6%) had uncontrolled TRH. When the analyses were restricted to individuals with uncontrolled TRH, we found similar adjusted hazard ratios for all outcomes when comparing RfH to uncontrolled TRH and uncontrolled TRH to non-TRH/RfH (Table 4).
Table 4:
Adjusted* hazard ratios (95% CI) for outcomes in patients with no treatment resistant or refractory, uncontrolled treatment resistant, and refractory hypertension.
| Outcome | non-TRH/RfH | Uncontrolled TRH | RfH |
|---|---|---|---|
| CVD Composite (stroke/MI/CHF) | |||
| Years 0 – 3 | 0.72 (0.55–0.94) | Ref. † | 1.32 (0.90–1.92) |
| Years 3 – 6 | 0.63 (0.46–0.86) | Ref. | 1.27 (0.70–2.32) |
| Years 6 – 10 | 1.02 (0.64–1.63) | Ref. | 3.51 (1.71–7.19) |
| Renal Composite (50% eGFR decline/ESRD) | |||
| Years 0 –10 | 0.80 (0.68–0.93) | Ref. | 1.66 (1.31–2.10) |
| All-Cause Mortality | |||
| Years 0 – 10 | 0.89 (0.73–1.09) | Ref. | 1.15 (0.80–1.67) |
Hazard ratio adjusted for sex, race, education, diabetes, current smoking, log(24-hour urine protein), LDL, history of CVD, clinic site, age (time stratified), BMI (time stratified), and log(eGFR) (time stratified).
Reference category for hazard ratios.
When we used a threshold of 130/80 mm Hg to define hypertension, TRH, and RfH instead of 140/90 mm Hg, we found similar results (Tables S6 and S7). Under this threshold, risk for the cardiovascular and renal composite outcomes were higher in more severe hypertensive phenotypes (i.e., RfH vs. TRH vs. non-TRH/RfH; Table S5). Although all-cause mortality risk was greater in individuals with TRH vs. non-TRH/RfH, there was no difference between individuals with RfH and TRH (Table S5).
Discussion
This study is highly novel as it is the first quantify the long-term health impacts of RfH and provide context for this diagnosis relative to TRH. We have shown that RfH is associated with markedly greater risk of CKD progression and cardiovascular events, especially in later study years, when compared to those with TRH, while no increase in mortality was observed. Despite separating out the relatively high risk patients with RfH, we still observed significantly higher risks for all health outcomes in participants with TRH when compared to no TRH or RfH. The negative health impacts observed here have been hypothesized previously but not confirmed11.
Although there is a paucity of studies evaluating health outcomes in RfH, and our study is limited to individuals with CKD, other longitudinal studies of TRH have found similar associations between TRH and increased cardiovascular10, 23–26, renal10, 25, and all-cause mortality10, 23–26 risk. Uncontrolled TRH, by definition, is more similar to RfH than controlled TRH. Previous studies found that cardiovascular risk was greater in uncontrolled TRH compared to controlled TRH with no difference in mortality23. This finding mirrors our own that cardiovascular and renal events are increased in RfH compared to uncontrolled TRH with no corresponding increase in all-cause mortality (Table 4). Thus, our results point to RfH as a high risk hypertension phenotype despite the similarity in definition with uncontrolled TRH, with health risks increasing from non-resistant/refractory hypertension to TRH to RfH.
We also found similar risk profiles under thresholds of 140/90 mm Hg (Tables 3 and 4) and 130/80 mm Hg (Table S6 and S7). The lack of differentiation in outcome risk between thresholds may indicate that hypertension severity may not lie on a strict continuum, a conjecture supported by the observation that individuals with RfH have significantly higher BP levels than even their TRH counterparts (Table 2). In this regard, the choice of BP threshold may have little to do with identifying individuals with TRH and RfH. Rather, the choice of BP target may only impact individual outcome risks within hypertension phenotypes and not the relative risks between phenotypes. However, future studies are needed to further elucidate how changes in treatment targets impact outcomes in severe hypertensive classes like TRH and RfH.
When compared to the prevalence of RfH observed in CRIC, population studies have generally found prevalence to be lower (i.e., 0.5–1.4%)3, 6, 8. Given that patients with CKD are known to be at greater risk for RfH3, 6, 8, the relatively high prevalence of RfH reflects the patient population considered in CRIC. In addition, this relatively high prevalence is in line with estimates obtained from hypertension specialty clinics (i.e., 2.7–9.5%)4, 5, where CKD is a common comorbidity.
In this study, baseline patient characteristics were similar between those with treatment resistant and RfH. Only race, serum potassium, and a history of cardiovascular disease and congestive heart failure were significantly different when comparing these two phenotypes at baseline. This result is in contrast to population-based studies where age, sex, BMI, albuminuria, and diabetes were also found to be significantly different between RfH and TRH3, 6, 8. In comparison with population studies, CRIC’s patient population was restricted to those with CKD. Restriction of the analyses to individuals with CKD may have introduced selection bias, eliminating some of the demographic and clinical characteristics that were previously observed to be associated with RfH. In addition, we found a nonsignificant trend towards higher serum aldosterone in patients with RfH at baseline that could indicate the presence of undiagnosed primary aldosteronism. Because primary aldosteronism is associated with increased CVD27–29 and renal health30 risks, relatively high rates of undiagnosed primary aldosteronism could, at least in part, explain some of our results regarding negative health outcomes in individuals with RfH. RfH may also reflect poor adherence to medications, which could not be measured in this study, and may also partly explain some of the elevated risk observed.
This study benefits from several strengths. There was a relatively large sample size of individuals with hypertension in the CRIC Study, particularly those with RfH. In the general population, this is a relatively rare phenotype, and a much larger sample size would be required to identify a sufficient number of patients with RfH. Also, the duration of follow-up within CRIC is long, providing robust longitudinal data on long-term health outcomes.
However, this study is also limited in several ways. First, the focus is specifically on individuals with CKD, and despite the large overall sample size, refractory hypertension is relatively rare. Future studies are needed to confirm outcomes of RfH in other patient populations. We also did not rigorously explore social determinants of health. We included education level, which was not different between those with TRH and those with RfH. However, African-American participants were more likely to have RfH, which could reveal potential latent confounding in the role of RfH in shaping health outcomes due to unaccounted for social determinants of health. Self-identification of race by participants may limit these racial conclusions though. In addition, as noted above, white-coat hypertension, masked hypertension, and medication nonadherence cannot be excluded in this analysis leading to potential misclassification of a participant’s hypertension phenotype, although the prevalence of white-coat hypertension in CRIC has previously been found to be low31. Similarly, therapeutic inertia could lead to further misclassification between hypertension phenotypes if individuals with uncontrolled BP were not prescribed additional medications as needed. Future cohort studies should attempt to disentangle the impact of therapeutic nonadherence, therapeutic inertia, and white-coat hypertension on risk assessments for refractory hypertension. Finally, the definition of RfH has changed over time3–6, 8, 9, 11. However, using the most evolved definition (i.e., uncontrolled BP when taking 5 or more antihypertensive drug classes, including a long-acting thiazide or thiazide-like diuretic and a mineralocorticoid receptor antagonist [MRA])4 is often prohibitive given the low utilization of mineralocorticoid receptor antagonists in the general population3, 8. This definition is also limited in populations with CKD, like in CRIC, because MRAs may be discontinued due to hyperkalemia risk in those patients with eGFR <45 mL/min per 1.73 m2. As a result, we chose to use a definition for refractory hypertension that is used in population-based studies6, 8. More guidance is needed about how to reconcile definitions of RfH across patient populations and whether the specific definition of RfH impacts our understanding of patient outcomes.
Perspectives
This study provides the first evidence that, in cohort of individuals with CKD, the prognosis of those with RfH is worse than in those with TRH. As such, RfH should be considered as a quantitatively worse phenotype from TRH. Specifically, the cardiovascular and renal health risks associated with RfH underscore the need for early identification of these individuals, despite their relative rarity, in order to better monitor and mitigate long-term risk through alternate treatment strategies. Future studies should attempt to confirm these findings in non-CKD populations.
Supplementary Material
Novelty and Significance.
What is New?
This is the first study to separately consider refractory hypertension and treatment resistant hypertension to make clear the health risks associated with each.
What is Relevant?
This study showed that health outcomes worsened when considering refractory hypertension vs. treatment resistant hypertension vs. non- treatment resistant or refractory hypertension.
Summary?
Refractory and treatment resistant hypertension were evaluated in a 3147 individuals with mild-to-moderate chronic kidney disease who were at higher risk for these severe forms of hypertension. Renal and cardiovascular outcomes, but not mortality, were worse for those with refractory compared to treatment resistant hypertension. Those with treatment resistant hypertension experienced higher risks for all outcomes (renal, cardiovascular, and mortality) when compared to those without refractory or treatment resistant hypertension.
Acknowledgments
Sources of Funding
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 412 RR-02413.
Disclosure
J.M.F. has received grant support from Vascular Dynamics, Bayer, Quantam Genomics, ReCor Medical, Indorsia and GlaxoSmithKline and has served as a consultant for NuSirt, Allergan, and BackBeat Hypertension. He also serves on the data safety and monitoring board for Rox Medical. Dr. Rahman has received grant support from Bayer and Duke Clinical Trials Center, and honoraria from Relypsa and Reata (not relevant to current work). The other authors declare no conflict of interest.
References
- 1.Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment-resistant hypertension in the united states. Hypertension. 2019;73:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public E, Publications Committee of the Council on H, Council on C, Stroke N, Council on Clinical C, Council on G, Precision M, Council on Peripheral Vascular D, Council on Quality of C, Outcomes R, Stroke C. Resistant hypertension: Detection, evaluation, and management: A scientific statement from the american heart association. Hypertension. 2018;72:e53–e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun DA, Booth JN 3rd, Oparil S, Irvin MR, Shimbo D, Lackland DT, Howard G, Safford MM, Muntner P. Refractory hypertension: Determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014;63:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory hypertension: Evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension. 2015;66:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S, Calhoun DA. Refractory hypertension: Definition, prevalence, and patient characteristics. J Clin Hypertens (Greenwich). 2012;14:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armario P, Calhoun DA, Oliveras A, Blanch P, Vinyoles E, Banegas JR, Gorostidi M, Segura J, Ruilope LM, Dudenbostel T, de la Sierra A. Prevalence and clinical characteristics of refractory hypertension. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modolo R, de Faria AP, Sabbatini AR, Barbaro NR, Ritter AM, Moreno H. Refractory and resistant hypertension: Characteristics and differences observed in a specialized clinic. J Am Soc Hypertens. 2015;9:397–402 [DOI] [PubMed] [Google Scholar]
- 8.Buhnerkempe MG, Botchway A, Prakash V, Al-Akchar M, Nolasco Morales CE, Calhoun DA, Flack JM. Prevalence of refractory hypertension in the united states from 1999 to 2014. J Hypertens. 2019;37:1797–1804 [DOI] [PubMed] [Google Scholar]
- 9.Dudenbostel T, Siddiqui M, Gharpure N, Calhoun DA. Refractory versus resistant hypertension: Novel distinctive phenotypes. J Nat Sci. 2017;3. [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER 3rd, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr., Rahman M, Investigators CS. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: Report from the chronic renal insufficiency cohort study. Hypertension. 2016;67:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudenbostel T, Siddiqui M, Oparil S, Calhoun DA. Refractory hypertension: A novel phenotype of antihypertensive treatment failure. Hypertension. 2016;67:1085–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort Study I. The chronic renal insufficiency cohort (cric) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–153 [DOI] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort Study G. Chronic renal insufficiency cohort (cric) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA. 2014;311:507–520 [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ, National Heart L, Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 16.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI, Investigators CS. Association of kidney disease outcomes with risk factors for ckd: Findings from the chronic renal insufficiency cohort (cric) study. Am J Kidney Dis. 2014;63:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI, Investigators CS. Estimating gfr among participants in the chronic renal insufficiency cohort (cric) study. Am J Kidney Dis. 2012;60:250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526 [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in r. Journal of Statistical Software. 2011;45:1–67 [Google Scholar]
- 21.White IR, Royston P. Imputing missing covariate values for the cox model. Stat Med. 2009;28:1982–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: Current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvin MR, Booth JN 3rd, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P, Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr., Crowley K, Goto S, Ohman EM, Bakris GL, Perlstein TS, Kinlay S, Bhatt DL, Investigators RR. Resistant hypertension: A frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204–1214 [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M, Group ACR. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: Results from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat). Hypertension. 2014;64:1012–1021 [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Huo T, Delia Johnson B, Bittner V, Kelsey SF, Vido Thompson D, Noel Bairey Merz C, Pepine CJ, Cooper-Dehoff RM. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: A report from the nhlbi-sponsored wise study. J Am Heart Assoc. 2014;3:e000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50 [DOI] [PubMed] [Google Scholar]
- 28.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248 [DOI] [PubMed] [Google Scholar]
- 29.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85 [DOI] [PubMed] [Google Scholar]
- 30.Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645 [DOI] [PubMed] [Google Scholar]
- 31.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan YH, Keane MG, Kusek JW, Makos GK, Miller ER, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR, Wright JT, Xie DW, Rahman M, Cohort CRI. Masked hypertension and elevated nighttime blood pressure in ckd: Prevalence and association with target organ damage. Clin J Am Soc Nephro. 2016;11:642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.